Abstract
Background and Aim
Immune checkpoint inhibitors have significantly improved the clinical outcomes of many cancer types, but they induce a range of immune‐related adverse events (irAEs). Although adrenal insufficiency (AI) is a rare irAE, it can lead to serious consequences. This study aimed to determine the clinical features of patients with advanced non‐small‐cell lung cancer (NSCLC) who developed AI following pembrolizumab treatment.
Methods
We retrospectively reviewed and analyzed the clinical data of all patients with NSCLC treated with pembrolizumab at Juntendo University Hospital from February 2017 to December 2020. The diagnosis of AI was established based on the Endocrine Emergency Guidance for the acute management of endocrine complications of checkpoint inhibitor therapy in the UK and the clinical practice guidelines of the Japan Endocrine Society.
Result
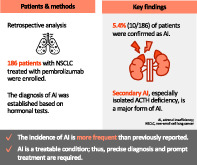
AI was clinically suspected in 59 out of 186 patients treated with pembrolizumab, and 10 (5.4%) cases were confirmed. The symptoms included hyponatremia (n = 9), fatigue (n = 8), and loss of appetite (n = 6). All patients had low adrenocorticotropic hormone (ACTH) levels, and five patients were diagnosed with isolated ACTH deficiency. All patients completely recovered with corticosteroid replacement. The median time to onset of AI was 8.0 (range 3.8–15.2) months. The median progression‐free survival in these patients was 22.4 (95% confidence interval 11.2–not reached) months.
Conclusion
The incidence of AI among patients treated with pembrolizumab is more frequent than previously reported. In addition, secondary AI, especially isolated ACTH deficiency, is a major form of AI induced by pembrolizumab.
Keywords: adrenal insufficiency, immune checkpoint inhibitors, immune‐related adverse events, non‐small‐cell lung cancer
INTRODUCTION
Immune checkpoint inhibitors (ICIs) have substantially improved the prognosis of patients with multiple solid malignancies, including non‐small‐cell lung cancer (NSCLC). ICIs target programmed cell death‐1 (PD‐1)/programmed cell death ligand‐1 (PD‐L1) and cytotoxic T‐lymphocyte‐associated protein 4 (CTLA‐4), which negatively regulate T‐cell activity and aid tumor cells in evading immune surveillance. 1 Despite significant clinical benefits, ICIs have been associated with distinctive side effects called immune‐related adverse events (irAEs). Any organ system could be involved, and the probability of irAE incidence ranges from 54% to 76%. 2 A recent meta‐analysis has indicated that the occurrence of irAEs, particularly endocrine, dermatological, and gastrointestinal irAEs, may be a predictor of ICI efficacy, 1 , 3 and some of the irAEs might be associated with better prognosis. 4 , 5
Adrenal insufficiency (AI) is characterized by deficient production or action of glucocorticoids. 6 This failure can result from loss of function of the adrenal glands or impaired hypothalamic/pituitary regulation of adrenal cortisol synthesis. 7 The clinical presentation of AI varies, depending on the extent of loss of adrenal function and the degree of stress, 7 thus establishing the diagnosis of AI is often difficult. AI can be life‐threatening unless treatment is immediately initiated. 6 In general, the frequency of AI induced by PD‐1 or PD‐L1 inhibitors has been less common than that induced by CTLA‐4 inhibitors or a combination of CTLA‐4 and PD‐1 inhibitors. 8 However, few studies have accurately diagnosed AI, which may lead to underestimation of AI.
In this study, we investigated the frequency, clinical features, and prognosis of patients with NSCLC who developed AI following pembrolizumab treatment in a real‐world setting.
METHODS
Patients
We retrospectively reviewed the clinical data from the medical records of patients with NSCLC treated with pembrolizumab monotherapy or in combination with chemotherapy at Juntendo University Hospital from February 2017 to December 2020. Among them, we selected patients with suspected AI based on symptoms and/or laboratory parameters, such as serum cortisol level. This study was approved by the institutional review board of Juntendo University Hospital (approval number H18‐0083) and was conducted in accordance with the Declaration of Helsinki. The need for consent to participate was waived due to the retrospective nature of the study.
Evaluation of patient characteristics
We reviewed patients' characteristics, including sex, age at the time of pembrolizumab initiation, smoking history, histology, driver mutation status, type and line of treatment, imaging results (computed tomography and pituitary magnetic resonance imaging), and blood test results. Clinical stage was assigned based on the results of chest radiography, chest and abdominal computed tomography, computed tomography or magnetic resonance imaging of the brain, and bone scintigraphy or positron emission tomography. Performance status (PS) was evaluated using the Eastern Cooperative Oncology Group PS scale. Adverse events were evaluated according to the Common Terminology Criteria for Adverse Events version 4.0.
Evaluation of adrenal insufficiency
For AI assessment, we evaluated serum random cortisol levels for screening, and the adrenocorticotropic hormone (ACTH) (cosyntropin) stimulation test was performed to confirm the diagnosis. Serum basal ACTH levels were also measured to distinguish between primary and secondary AIs. Corticotropin‐releasing hormone (CRH), luteinizing hormone‐releasing hormone (LHRH), and thyrotropin‐releasing hormone (TRH) provocation tests and brain‐imaging tests were performed to evaluate the pituitary gland (Figure 1).
FIGURE 1.
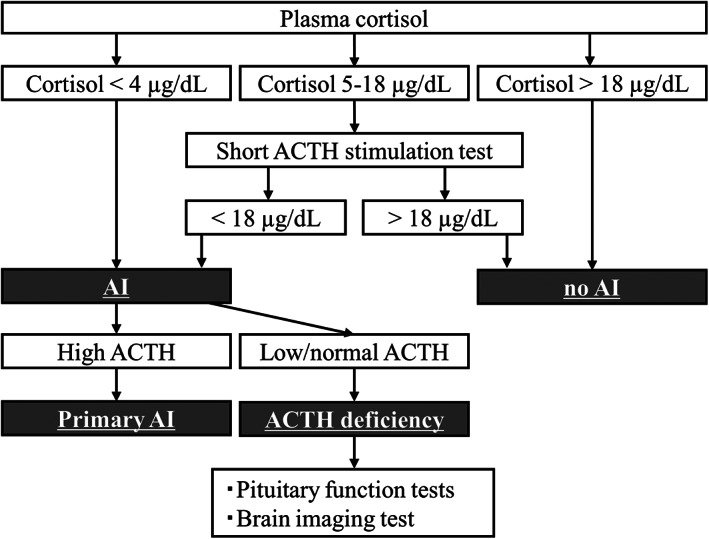
Flow chart of diagnostic criteria of adrenal insufficiency. ACTH, adrenocorticotropic hormone; AI, adrenal insufficiency
Serum basal cortisol and plasma basal ACTH concentrations were measured at 8 AM. The rapid ACTH stimulation test assessed the serum cortisol level at baseline and 30 min (60 min) after intravenous bolus 250 μg injection of cosyntropin. ACTH/cortisol was evaluated at baseline and 30, 60, 90, and 120 min after intravenous bolus 100 μg injection of CRH. LH/follicle stimulating hormone (FSH) was evaluated at baseline and 30, 60, 90, and 120 min after intravenous bolus 100 μg injection of LH/RH. Thyroid‐stimulating hormone (TSH) was evaluated at baseline and 30, 60, 90, and 120 min after intravenous bolus 500 μg injection of TRH.
According to the Endocrine Emergency Guidance of irAE in the UK and Japan Endocrine Society, a serum basal cortisol level at 8 AM above 18 μg/dl excludes AI. We defined AI as a peak serum cortisol level <18 μg/dl in the ACTH stimulation test. Patients with serum cortisol level below 4 μg/dl were also considered to have AI regardless of whether the ACTH stimulation test was performed. If the serum basal ACTH level was below or within the reference range (7.2–63.3 pg/ml), secondary AI was diagnosed. Hyponatremia was defined as a serum sodium concentration lower than 135 mmol/L and eosinophilia count less than 500/μl.
Statistical analysis
Fisher's exact and Mann–Whitney U tests were used to evaluate differences in categorical and continuous variables between the two groups, respectively. Progression‐free survival (PFS) was defined as the period between the start of pembrolizumab and progressive disease or death from any cause. Overall survival (OS) was defined as the period from the time of initial diagnosis to death or the last follow‐up. PFS was evaluated using the Kaplan–Meier method. A p value of less than 0.05 was considered statistically significant. All analyses were performed using the R version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria) and jamovi 2.3.1 (The jamovi project).
RESULTS
Patients characteristics
We selected 186 patients with NSCLC treated with pembrolizumab monotherapy or combination therapy with chemotherapy in our hospital. Among these patients, serum cortisol level was measured in 59 patients as the physician suspected AI. These patients were suspected of having AI owing to their symptoms and/or laboratory findings, such as fever, fatigue, loss of appetite, or hyponatremia. A total of 49 patients with cortisol level ≥18 μg/dl or with causes other than AI were suspected of having AI. Based on the evaluation of cortisol level and rapid ACTH stimulation test, 10 patients were diagnosed with AI (Figure 2).
FIGURE 2.
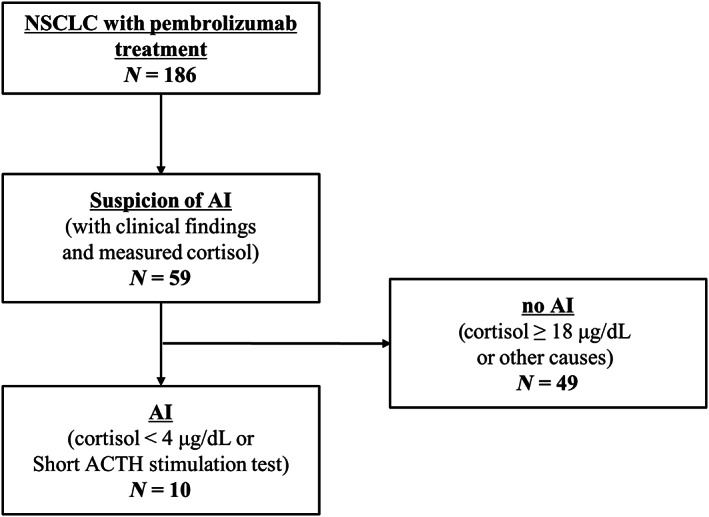
Selection criteria for study participants. ACTH, adrenocorticotropic hormone; AI, adrenal insufficiency; NSCLC, non‐small‐lung cancer
Patient characteristics at the initiation of pembrolizumab are summarized in Table 1. In the group of patients with AI, the mean age was 69 years (range 52–82 years), and six male and four female patients were included. The most common histological type were adenocarcinoma (90%) and three patients showed high PD‐L1 expression (≥50%). Nine patients showed a PS score of 0–1. Pembrolizumab was administered as first‐line treatment in five patients, second‐line treatment in three patients, and third‐line treatment in two patients. Four patients were treated with pembrolizumab monotherapy, whereas six patients were treated with a combination of pembrolizumab and chemotherapy. Most of these characteristics were not statistically different from those of patients without AI. Only the presence of driver mutations differed between the two groups. None of the patients regularly used steroids before AI diagnosis.
TABLE 1.
Baseline characteristics of the study population
| Patients with adrenal insufficiency | Patients without adrenal insufficiency | p value | |
|---|---|---|---|
| n = 10 | n = 176 | ||
| Sex (M/F), n (%) | 6 (60)/4 (40) | 126 (71.6)/50 (28.4) | 0.48 |
| Age (range) | 67.5 (52–80) | 68.0 (40–78) | 0.61 |
| Smoking history (yes/no), n (%) | 9 (90)/1 (10) | 155 (88.1)/21 (11.9) | 1.00 |
| PD‐L1 expression (≥50%/<50% or unknown), n (%) | 3 (30)/7 (70) | 101 (57.4)/75 (42.6) | 0.11 |
| Driver mutation, n (%) | 4 (40) | 16 (9.1) | 0.01 |
| Performance status (0–1/≥2), n (%) | 9 (90)/1 (10) | 154 (87.5)/22 (12.5) | 1.00 |
| Histology (SQ/non‐SQ), n (%) | 1 (10)/9 (90) | 32 (18.2)/144 (81.8) | 0.51 |
| Stage (I or II/III or IV), n (%) | 1 (10)/9 (90) | 26 (14.8)/150 (85.2) | 1.00 |
| Monotherapy/combination therapy, n (%) | 4 (40)/6 (60) | 109 (62.0)/67 (38.0) | 0.19 |
| Treatment of line (first or second/≥third), n (%) | 8 (80)/2 (20) | 158 (89.8)/18 (10.2) | 0.29 |
| Steroid use before incidence of AI, n (%) | 0 (0) | – |
Note: Comparisons performed by Fisher's exact test and the Mann–Whitney U test as appropriate.
Abbreviations: SQ, squamous cell carcinoma; non‐SQ, non‐squamous cell carcinoma; AI, adrenal insufficiency.
Clinical course
In patients with AI (Tables 2 and 3), the most common symptoms were general fatigue and loss of appetite, which more than half of the patients developed. One patient had no symptoms. Laboratory findings revealed hyponatremia in nine patients and eosinophilia in four patients. In the evaluation of hormone levels, all patients showed relatively low plasma ACTH despite cortisol level, leading to the diagnosis of secondary AI. Six patients underwent dynamic tests of pituitary hormones, resulting in isolated ACTH deficiency in five patients and combined ACTH and gonadotropin deficiency in one patient. Brain imaging was performed in eight patients, and no pituitary abnormalities were detected.
TABLE 2.
Clinical characteristics of patients with adrenal insufficiency
| Total | |
|---|---|
| n = 10 | |
| Clinical symptoms | |
| General fatigue, n (%) | 6 (60) |
| Loss of appetite, n (%) | 6 (60) |
| Loss of body weight, n (%) | 2 (20) |
| Central nervous systems, n (%) | 3 (30) |
| Nausea, n (%) | 1 (10) |
| Fever, n (%) | 3 (30) |
| No symptoms, n (%) | 1 (10) |
| Laboratory findings | |
| Hyponatremia, n (%) | 9 (90) |
| Hypoglycemia, n (%) | 2 (20) |
| Eosinophilia, n (%) | 4 (40) |
| Cycles (range) | 7.5 (range 4–14) |
| Onset time (range), month | 8.0 (3.8–15.2) |
| CTCAE grade (2/3/4), n (%) | 1(10)/8(80)/1(10) |
| Serum ACTH | |
| Low ACTH, n (%) | 10 (100) |
| Pituitary deficiencies | |
| Isolated ACTH deficiency, n (%) | 5 (50) |
| ACTH and gonadotropic deficiency, n (%) | 1 (10) |
| No hormonal dynamic test | 4 (40) |
| Pituitary MRI | |
| No abnormality, n (%) | 8 (80) |
| No imaging, n (%) | 2 (20) |
| Treatment | |
| Corticosteroid replacement | 10 (10) |
| Re‐administration of pembrolizumab | |
| Yes | 5 (50) |
| No | 5 (50) |
Note: Definitions: hyponatremia <135 mEq/L; hypoglycemia <65 mg/dl; eosinophilia >500/μL.
Abbreviations: ACTH, adrenocorticotropic hormone; CTCAE, common terminology criteria for adverse event v5.0; MRI, magnetic resonance imaging.
TABLE 3.
Detailed clinical profiles of patients with adrenal insufficiency
| Patient no. | Clinical findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Sex | Treatment | Line | Cycles | Time to event (months) | CTCAE (grade) | Symptoms | Laboratory findings | |
| 1 | 70 | F | Pembrolizumab | 2 | 7 | 4.7 | 3 | Fatigue, nausea, headache | Hyponatremia, hypoglycemia |
| 2 | 70 | M | Pembrolizumab | 1 | 8 | 10.0 | 3 | No symptoms | Hyponatremia |
| 3 | 49 | F | CBDCA + PEM + pembrolizumab | 2 | 14 | 15.4 | 4 | Loss of appetite, fever, arthralgia | Hyponatremia, eosinophilia |
| 4 | 52 | M | CBDCA + PEM + pembrolizumab | 1 | 7 | 11.2 | 3 | Fatigue, loss of appetite, weight loss | Eosinophilia |
| 5 | 80 | F | CBDCA + PEM + pembrolizumab | 1 | 8 | 7.3 | 3 | Fatigue | Hyponatremia |
| 6 | 82 | M | Pembrolizumab | 2 | 14 | 9.8 | 2 | Fatigue, loss of appetite, weight loss | Hyponatremia, eosinophilia |
| 7 | 75 | M | CBDCA + nab‐PTX + pembrolizumab | 1 | 4 | 3.8 | 3 | fatigue, fever, loss of appetite | Hyponatremia, eosinophilia |
| 8 | 60 | M | CBDCA + PEM + pembrolizumab | 1 | 6 | 5.2 | 3 | Loss of appetite, Disturbance of consciousness | Hyponatremia |
| 9 | 56 | M | Pembrolizumab | 5 | 13 | 8.9 | 3 | Fatigue | Hyponatremia |
| 10 | 67 | F | CBDCA + PEM + pembrolizumab | 6 | 7 | 7.0 | 3 | Loss of appetite, Convulsion | Hyponatremia, hypoglycemia |
| Patient no | Hormone level | Pattern of AI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cortisol (μg/dl) | ACTH (pg/ml) | Secondary AI | IAD | Multiple hormone deficiencies | Brain imaging | Other irAE | Hormone replacement therapy (maintenance dose) | Re‐administration of pembrolizumab | |
| 1 | 5 | 12 | ○ | – | ACTH, Gn | No abnormality | Cholangitis, skin toxicity | Dexamethasone 0.5 mg | ○ |
| 2 | 2.4 | 10.1 | ○ | ○ | – | No abnormality | Pneumonitis, thyroid dysfunction, skin toxicity | Prednisolone 20 mg | ○ |
| 3 | 4.8 | ≦3.0 | ○ | ○ | – | No abnormality | Thyroid dysfunction | Hydrocortisone 15 mg | – |
| 4 | 0.6 | <1.5 | ○ | ○ | – | No abnormality | Pneumonitis | Hydrocortisone 15 mg | ○ |
| 5 | 0.6 | 4.2 | ○ | ○ | – | No abnormality | – | hydrocortisone 15 mg | ○ |
| 6 | 3.7 | 29 | ○ | Not tested | Not tested | Not tested | Skin toxicity | Hydrocortisone 5 mg | ○ |
| 7 | 1 | <1.5 | ○ | Not tested | Not tested | No abnormality | Skin toxicity | Hydrocortisone 15 mg | – |
| 8 | 1.5 | <1.5 | ○ | Not tested | Not tested | No abnormality | Thyroid dysfunction, colitis | Hydrocortisone 30 mg | – |
| 9 | 1.9 | 9.7 | ○ | Not tested | Not tested | Not tested | Skin toxicity | Hydrocortisone 20 mg | – |
| 10 | 1.4 | 1.5 | ○ | ○ | – | No abnormality | Skin toxicity | Hydrocortisone 15 mg | – |
Abbreviations: ACTH, adrenocorticotropic hormone; AI, adrenal insufficiency; CBDCA, carboplatin; CDDP, cisplatin; CTCAE, Common Terminology Criteria for Adverse Events version 4.0; F, female; Gn, gonadotropin; IAD, isolated adrenocorticotropic hormone deficiency; M, male; nab‐PTX, nab‐paclitaxel; PEM, pemetrexed.
The median time from the time of initiation of pembrolizumab to diagnosis of AI was 8.0 (range 3.8–15.2) months. The median number of pembrolizumab cycles before AI was 7.5 (range 4–14) cycles. One patient developed AI 4.5 months after pembrolizumab discontinuation. All patients had complicated irAEs, for example skin toxicity and thyroid dysfunction in six and three patients, respectively.
All patients were treated with corticosteroid replacement. Hydrocortisone 15–200 mg/day was initiated in nine patients and prednisolone 20 mg/day in one patient. Subsequently, these medications were tapered and 5–30 mg/day of hydrocortisone was maintained in every patient until the cut‐off date. All patients completely recovered after steroid administration. After the AI was under control, five patients restarted pembrolizumab or combination therapy with corticosteroid replacement therapy without recurrence of AI.
Survival evaluation
The median follow‐up time and PFS in patients with AI were 14.2 (range 6.9–39.1) and 22.4 months (95% confidence interval [CI] 22.4–not reached) months, respectively (Table 4). The median follow‐up time and PFS in patients treated with pembrolizumab monotherapy were 30.6 (range 14.1–39.1) and 16.8 (range 10.1–28.8) months, respectively. With pembrolizumab combination therapy, the median follow‐up time and PFS were 10.2 (range 6.9–20.2) and 9.9 (range 5.5–20.2) months, respectively. On the other hand, the median PFS among patients without AI was 5.8 months (95% CI 4.9–8.8), indicating statistically shorter PFS compared with patients with AI (hazard ratio 0.41, 95% CI 0.17–1.00, p = 0.043). At the cut‐off date, eight patients were alive and two patients had been transferred to another hospital.
TABLE 4.
Progression‐free survivals in patients with or without adrenal insufficiency
| Follow‐up time, months (median) | Progression‐free survival, months (95% CI) | |
|---|---|---|
| All patients (n = 186) | 12.5 (0.1–44.4) | 6.8 (5.1–9.6) |
| Patients with adrenal insufficiency (n = 10) | 14.2 (6.9–39.1) | 22.4 (11.2‐NR) |
| Patients without adrenal insufficiency (n = 176) | 12.5 (0.1–44.4) | 5.8 (4.9–8.8) |
Abbreviations: CI, confidence interval; NR, not reached.
DISCUSSION
In this study, we presented the clinical features of AI induced by PD‐1/PD‐L1 inhibitors and found that AI may be more prevalent than that previously reported.
In our cases, most patients complained of fatigue and loss of appetite, whereas none presented headache or vision disorder. No cases showed brain imaging abnormality. Additionally, all patients presented with secondary AI, with isolated ACTH deficiency being the most common. These features were similar to those previously reported. 9 In contrast, we found that AI developed in 5.3% of patients treated with pembrolizumab, which was more frequent than that in the previously reported studies. Previous phase 3 trials of pembrolizumab monotherapy or combination therapy with chemotherapy in NSCLC demonstrated that the incidence rate of AI was 0.2–0.7%. 10 , 11 , 12 Even if hypophysitis was considered, the incidence of hypophysitis was reported to be significantly less common (0.1–0.7%). 10 , 11 , 12 Conversely, hypophysitis was observed in 8–13% of patients taking CTLA‐4 inhibitors and in 8.5–9.0% of patients taking a combination of CTLA4 and PD‐1 inhibitors. 8 We emphasize that AI induced by pembrolizumab is significantly more frequent than estimated.
In general, AI tends to be underdiagnosed because patients with AI often have nonspecific symptoms, such as fatigue, loss of appetite, weight loss, nausea, and muscle and joint pain. 13 Laboratory findings of patients with AI are also nonspecific, such as hyponatremia, hypoglycemia, or eosinophilia, 13 similar to the result of our study. These nonspecific clinical findings make it difficult to diagnose AI. Furthermore, in the case of irAEs, it took several months for AI to develop after ICI initiation, 8 leading to underdiagnosis. In the present study, the longest period was over a year after pembrolizumab initiation. One patient developed AI 4.5 months after the last pembrolizumab administration, which is similar to the result of a previous study. 14 In our study, the number of patients suspected of AI was also large compared with that in a previous study, 15 which enabled us to identify patients with AI more accurately. Therefore, AI should be suspected at any time during the use of ICIs and even after ICI discontinuation. It is also important to perform hormone tests as needed for precise diagnosis, in consultation with endocrinologists.
Racial differences may also be one of the reasons for the high frequency of AI in this study. Many reports of isolated ACTH deficiency induced by ICIs have been published from Japan. 16 Previous studies have shown that specific human leukocyte antigen alleles are associated with pituitary irAEs. 17 , 18 , 19 Kobayashi et al. reported that the prevalence rates of HLA‐Cw12, HLA‐DR15, HLA‐DQ7, and HLA‐DPw9 were higher in patients with isolated ACTH deficiency induced by ICI than in patients without pituitary irAEs. 17 Differences in HLA may contribute to differences in the frequency of pituitary irAEs.
In patients with cancer, chronic stress activates the hypothalamic–pituitary–adrenal axis, leading to increased levels of blood glucocorticoids continuously. 20 Therefore, we should consider the possibility that it is not strictly accurate for patients with cancer to use the diagnostic criteria of AI same as that for the healthy population. In the Endocrine Emergency Guidance of irAE in the UK, patients with random serum cortisol level <16.3 μg/dl (450 nmol/L) should be treated with corticosteroids for AI. 21 If we had used random serum cortisol level <16.3 μg/dl as the cut‐off, it is possible that 37 more mild cases could have been included. Although further studies are needed to determine the reference value of cortisol in patients with cancer, we should assume that the frequency of adrenal sufficiency will be significantly higher.
There is no consensus on the prognosis of patients with AI, although endocrine irAEs can predict the efficacy of ICIs. 1 Our study shows that the PFS rates in patients with AI treated with pembrolizumab monotherapy and combination therapy were 16.8 (range 10.1–28.8) and 9.9 (range 5.5–20.2) months, respectively. These are favorable PFS rates compared with those of previous phase 3 trials. 11 , 22 In our study, PFS was significantly longer in patients with AI than in those without AI, although this was a small number of AI cases. As OS is an immature data, we need further follow‐up studies. Further accumulation of case numbers is needed to examine the correlation between the incidence of AI and prognosis.
Although the precise mechanisms of pituitary irAE, especially those induced by PD‐1/PD‐L1 inhibitors, have not been fully elucidated, some potential mechanisms have been reported, including the presence of antigens in both tumors and healthy tissue, and increasing levels of preexisting autoantibodies and inflammatory cytokines. 8 Bando et al. showed that ectopic ACTH expression in tumor cells evoked autoimmunity to corticotrope cells, leading to ACTH deficiency. 23 Kobayashi et al. showed that patients with antipituitary antibodies at baseline were prone to develop ICI‐induced isolated ACTH deficiency. 17 In addition, positive conversion of anti‐pituitary antibodies at the onset of pituitary irAEs has also been observed. 17 To determine the pathogenesis, histological evaluations of pituitary glands in patients with PD‐1/PD‐L1 inhibitor‐induced hypophysitis are also required.
Our study has some limitations. Our results are limited by a retrospective approach from a single institution. In addition, the sample size of patients with AI was small. Nevertheless, the assessment of hormone evaluation based on uniform diagnostic criteria is the strength of this study. Further studies are needed to determine the pathological mechanism of AI induced by pembrolizumab.
In conclusion, we strongly suggest that AI is a significantly more frequent irAE than previously reported. AI is a treatable condition, thus precise diagnosis and prompt treatment are required. In addition, secondary AI, especially isolated ACTH deficiency, is a major form of AI induced by pembrolizumab.
AUTHOR CONTRIBUTIONS
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: K.K., M.Y., and N.S. Acquisition of data: K.K., N.S., M.O., K.M, T.A, R.K., and R.S. Analysis and interpretation of the data: K.K., M.O., K.M., T.M., and T.A. Drafting of the manuscript: K.K., Y. M., and N.S. Critical revision of the manuscript for important intellectual content: N.I., T.M., T.A., R.K., T.S., and H.G. Statistical analysis: K.K., M.O., K.M., T.M., T.A., and R.S. Administrative, technical, and material support: N.I., M.O., K.M, T.A., R.K., T.S., and R.S. Study supervision: Y.M., H.G., and T.K.
CONFLICT OF INTEREST
Yoichiro Mitsuishi reports grants from Takeda Pharmaceutical Company Limited, outside the submitted work. Tetsuhiko Asao reports honoraria from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., MSD K.K, Nippon Boehringer Ingelheim Co., Ltd., Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Company Limited outside the submitted work. Ryo Ko reports grants and honoraria from MSD K.K., Nippon Boehringer Ingelheim Co., Ltd., Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Pfizer Inc., and AstraZeneca K.K. outside the submitted work. Takehito Shukuya reports grants and honoraria from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., and MSD K.K., and honoraria from Taiho Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Ono Pharmaceutical Co., Ltd., Bristol‐Myers Squibb Company, Nippon Kayaku Co., Ltd., Takeda Pharmaceutical Company, and Pfizer Inc. outside the submitted work. Kazuhisa Takahashi reports grants and honoraria from Chugai Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Nippon Shinyaku Co., Ltd., Tsumura & Co., Pfizer Inc., Taiho Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Teijin Pharma Limited, Sanofi K.K., Ono Pharmaceutical Co., Ltd., Novartis Pharma K.K., Shionogi & Co., Ltd., Eli Lilly Japan K.K., Bayer Yakuhin, Ltd., Daiichi Sankyo Co., Ltd., Nipro Pharma Corporation, Asahi Kasei Pharma Corporation, Nippon Kayaku Co., Ltd., Takeda Pharmaceutical Company Limited, Kyowa Kirin Co., Ltd., MSD K.K., AstraZeneca K.K., Merck Biopharma Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Bristol‐Myers K.K., Meiji Seika Pharma Co., Ltd., Viatris Inc, Janssen Pharmaceutical K.K., Abbott Japan LLC, and Thermo Fisher Scientific Inc. outside the submitted work. The other authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.com) for English language editing.
Kurokawa K, Mitsuishi Y, Shimada N, Ito N, Ogiwara M, Miura K, et al. Clinical characteristics of adrenal insufficiency induced by pembrolizumab in non‐small‐cell lung cancer. Thorac Cancer. 2023;14(5):442–449. 10.1111/1759-7714.14761
REFERENCES
- 1. Wang D, Chen C, Gu Y, Lu W, Zhan P, Liu H, et al. Immune‐related adverse events predict the efficacy of immune checkpoint inhibitors in lung cancer patients: a meta‐analysis. Front Oncol. 2021;11:631949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu C, Chen YP, Du XJ, et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta‐analysis. BMJ. 2018;363:k4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Das S, Johnson DB. Immune‐related adverse events and anti‐tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grangeon M, Tomasini P, Chaleat S, Jeanson A, Souquet‐Bressand M, Khobta N, et al. Association between immune‐related adverse events and efficacy of immune checkpoint inhibitors in non‐small‐cell lung cancer. Clin Lung Cancer. 2019;20:201–7. [DOI] [PubMed] [Google Scholar]
- 5. Ricciuti B, Genova C, De Giglio A, et al. Impact of immune‐related adverse events on survival in patients with advanced non‐small cell lung cancer treated with nivolumab: long‐term outcomes from a multi‐institutional analysis. J Cancer Res Clin Oncol. 2019;145:479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet. 2014;383:2152–67. [DOI] [PubMed] [Google Scholar]
- 7. Munver R, Volfson IA. Adrenal insufficiency: diagnosis and management. Curr Urol Rep. 2006;7:80–5. [DOI] [PubMed] [Google Scholar]
- 8. Takahashi Y. Mechanisms in endocrinology: autoimmune hypopituitarism: novel mechanistic insights. Eur J Endocrinol. 2020;182:R59–66. [DOI] [PubMed] [Google Scholar]
- 9. Levy M, Abeillon J, Dalle S, Assaad S, Borson‐Chazot F, Disse E, et al. Anti‐PD1 and anti‐PDL1‐induced hypophysitis: a cohort study of 17 patients with longitudinal follow‐up. J Clin Med. 2020;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herbst RS, Baas P, Kim DW, Felip E, Pérez‐Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet. 2016;387:1540–50. [DOI] [PubMed] [Google Scholar]
- 11. Gandhi L, Rodríguez‐Abreu D, Gadgeel S, Esteban E, Felip E, de Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med. 2018;378:2078–92. [DOI] [PubMed] [Google Scholar]
- 12. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD‐L1‐expressing, locally advanced or metastatic non‐small‐cell lung cancer (KEYNOTE‐042): a randomised, open‐label, controlled, phase 3 trial. Lancet. 2019;393:1819–30. [DOI] [PubMed] [Google Scholar]
- 13. Bancos I, Hahner S, Tomlinson J, Arlt W. Diagnosis and management of adrenal insufficiency. Lancet Diabetes Endocrinol. 2015;3:216–26. [DOI] [PubMed] [Google Scholar]
- 14. Boudjemaa A, Rousseau‐Bussac G, Monnet I. Late‐onset adrenal insufficiency more than 1 year after stopping pembrolizumab. J Thorac Oncol. 2018;13:e39–40. [DOI] [PubMed] [Google Scholar]
- 15. Ida H, Goto Y, Sato J, Kanda S, Shinno Y, Morita R, et al. Clinical characteristics of adrenal insufficiency as an immune‐related adverse event in non‐small‐cell lung cancer. Med Oncol. 2020;37:30. [DOI] [PubMed] [Google Scholar]
- 16. Iglesias P, Sánchez JC, Díez JJ. Isolated ACTH deficiency induced by cancer immunotherapy: a systematic review. Pituitary. 2021;24:630–43. [DOI] [PubMed] [Google Scholar]
- 17. Kobayashi T, Iwama S, Sugiyama D, Yasuda Y, Okuji T, Ito M, et al. Anti‐pituitary antibodies and susceptible human leukocyte antigen alleles as predictive biomarkers for pituitary dysfunction induced by immune checkpoint inhibitors. J Immunother Cancer. 2021;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Inaba H, Ariyasu H, Iwakura H, Ueda Y, Kurimoto C, Uraki S, et al. Comparative analysis of human leucocyte antigen between idiopathic and anti‐PD‐1 antibody induced isolated adrenocorticotropic hormone deficiency: a pilot study. Clin Endocrinol (Oxf). 2019;91:786–92. [DOI] [PubMed] [Google Scholar]
- 19. Yano S, Ashida K, Sakamoto R, Sakaguchi C, Ogata M, Maruyama K, et al. Human leucocyte antigen DR15, a possible predictive marker for immune checkpoint inhibitor‐induced secondary adrenal insufficiency. Eur J Cancer. 2020;130:198–203. [DOI] [PubMed] [Google Scholar]
- 20. Cui B, Peng F, Lu J, He B, Su Q, Luo H, et al. Cancer and stress: NextGen strategies. Brain Behav Immun. 2021;93:368–83. [DOI] [PubMed] [Google Scholar]
- 21. Higham CE, Olsson‐Brown A, Carroll P, Cooksley T, Larkin J, Lorigan P, et al. Society for Endocrinology ENDOCRINE EMERGENCY GUIDANCE: acute management of the endocrine complications of checkpoint inhibitor therapy. Endocr Connect. 2018;7:G1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reck M, Rodríguez‐Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med. 2016;375:1823–33. [DOI] [PubMed] [Google Scholar]
- 23. Bando H, Iguchi G, Kanie K, Nishizawa H, Matsumoto R, Fujita Y, et al. Isolated adrenocorticotropic hormone deficiency as a form of paraneoplastic syndrome. Pituitary. 2018;21:480–9. [DOI] [PubMed] [Google Scholar]


