Abstract
Biliary cryptococcosis infection is extremely rare and difficult for preoperative diagnosis. We report a rare case of 61-year-old woman with biliary cryptococcal infection. To explore the general rule and case characteristics of biliary cryptococcal infection and provide a reference for future diagnosis and treatment, we consulted the PubMed database for reported biliary cryptococcal infection from 1985 to 2021. Including the present one, we collected 12 reports, among which half were male and five were younger than 18 years old. Clinical manifestations were mainly jaundice, while in vitro examination revealed bile duct dilatation and bile duct stenosis. In 8 cases (66.67%), symptoms improved or healed after antifungal treatment. Although preoperative misdiagnosis of cryptococcal infection is high, the antifungal treatment is quite effective. Thus, early accurate diagnosis can effectively improve the cure rate of biliary cryptococcosis infected patients.
Keywords: biliary cryptococcosis, obstructive jaundice, Cryptococcus neoformans, literature analysis
Introduction
Cryptococcosis is a global opportunistic fungal disease, that is most common in patients with immunodeficiency or suppressed immune function. It has high morbidity and fatality rates. It can affect any tissue and organ of the human body, but the central nervous system is the most common site, followed by the lungs and skin.1 Cryptococcosis, with cryptococcal infection of the biliary tract as the main clinical manifestation, is extremely rare. Here, we report a case of cryptococcal infection of the biliary tract due to long-term use of glucocorticoids for asthma, in which clinical pharmacist participated the whole treatment process. Also, we present a review the past literature to identify the characteristics of cryptococcal infection.
Clinical Data
A 61-year-old woman complaining of scleral icterus for 2 months was admitted to a local hospital on 3 December, 2019. She was treated with choleretics and hepatoprotective drugs, but the specific drugs were unknown; however, the symptoms recurred easily. She was referred to the outpatient department of our hospital with scleral icterus, yellowing urine, itchy skin, and weakness. Liver function tests showed alanine aminotransferase (ALT) 118U/L, aspartate aminotransferase (AST) 84U/L, alkaline phosphatase (ALP) 272U/L, total serum bilirubin (TBIL) 123.4 μmol/L, direct bilirubin (DBIL) 72.4 μmol/L, indirect bilirubin (IBIL) 51 μmol/L, and γ-glutamyl transferase (γ-GT) of 128U/L. Computed tomography (CT) of the abdomen revealed common bile duct wall thickening and severe biliary obstruction. Magnetic resonance cholangiopancreatography (MRCP) was performed to further characterize the structure of the bile duct in the hilar area is unclear, as well as diffuse irregular mural thickening and lesions of the extrahepatic bile duct. A diagnosis of obstructive jaundice was made, and the patient was hospitalized for future treatment on 19 December, 2019. The patient had been on Salmeterol Xinafoate and Fluticasone Propionate Powder for Inhalation for 30 years; past medical procedures included appendectomy 27 years ago, hysterectomy 20 years ago, and cholecystectomy 10 years ago.
The patient has a history of significant weight loss the months preceding admission to hospital. On admission to our department, few positive findings were noted on physical examination, except that the patient was visibly icteric. No neurological symptoms or evidence of neurological disturbance were detected, and there was no abdominal distension or organomegaly. Vital signs, bowel movement, chest, and cardiovascular observations were normal. Murphy’s sign was negative. Laboratory investigations showed a white blood count of 10.82×109/L with 75% neutrophils. Liver function tests revealed abnormalities consistent with obstructive jaundice. ALT 53U/L, AST 54U/L, ALP 225U/L, TBIL level 412.7mol/L, DBIL 334.7 mol/L, IBIL 78 mol/L, γ-GT 50U/L. Coagulation factors, carcinoembryonic antigen (CEA), and alpha-fetoprotein (AFP) levels were normal. The carbohydrate antigen 19–9 (CA19-9) level was obviously elevated (8358 U/L). Anti-hepatitis viruses, anti-HIV, autoimmune antibodies, and anti-neutrophil plasma antibodies were normal or negative.
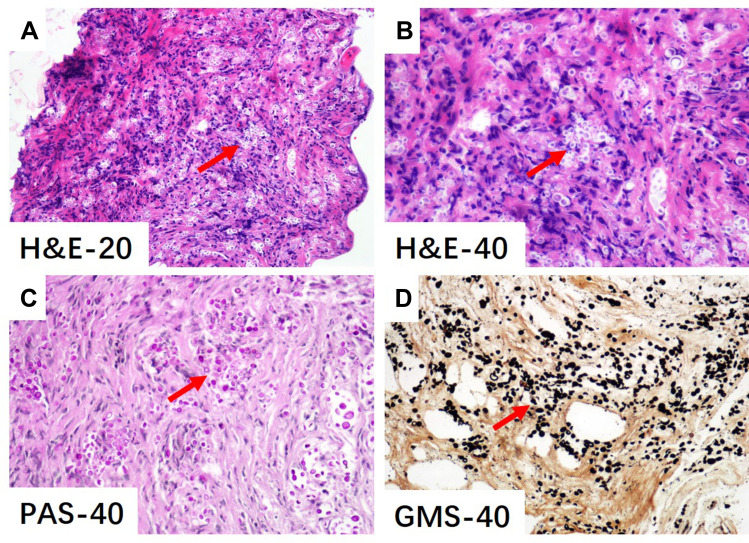
We administered magnesium isoglycyrrhizinate, polyene phosphatidylcholine, amino acids, vitamin C and potassium hydrogen phosphate for symptomatic treatment. For further evaluation Endoscopic Retrograde Cholangiopancreatography (ERCP) was performed at the digestive intervention center, radiography showed a stricture of the bile duct in the hilar area (Bismuth IV) on 20 December, 2019. Two biopsies from the bile duct stricture were collected for pathological examination. Multiple stents were inserted into the bile duct to relieve the obstruction, and nasobiliary duct drainage was performed. There was no evidence of bile duct calculus, cirrhosis, or focal lesions in the liver. Pathological examination after the surgery showed numerous round bodies in the fibrous tissue of the bile duct in the hilar area and suggestive fungal (Cryptococcus) infection by morphology. Hematoxylin and eosin as well as Grocott’s methenamine silver and Periodic acid-Schiff (PAS) staining of the specimen obtained from the stricture place were positive (Figure 1). Immunohistochemistry demonstrated the CD-68, a major antigen expressed on the membrane of mononuclear macrophages and Langerhans cells, was positive. The serum for cryptococcal antigen test was also positive. The diagnosis of biliary cryptococcosis was confirmed.
Figure 1.
Hematoxylin-eosin (H&E) (A, B), Periodic Acid-Schiff (PAS) (C) and Gomori methenamine silver (GMS) (D) staining and the magnification of bile duct tissue for cryptococcus.
Clinicians invited clinical pharmacist consultation the use of drugs for cryptococcosis infection on 29 December, 2019. The patient was treated with oral fluconazole 400 mg/day (800 mg/day on the first day) for 4–6 months. The liver function improved and the temperature normalized, and the patient was discharged with continual oral fluconazole on 4 January, 2020.
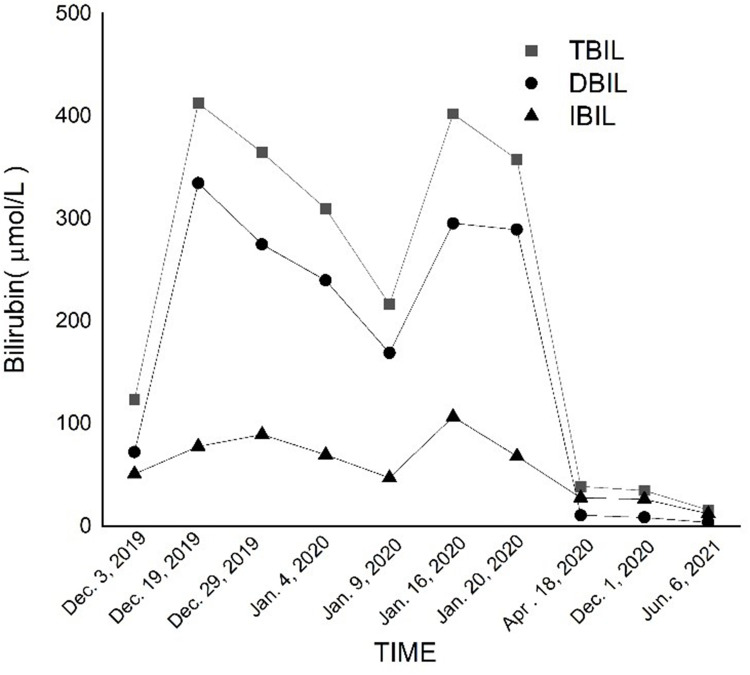
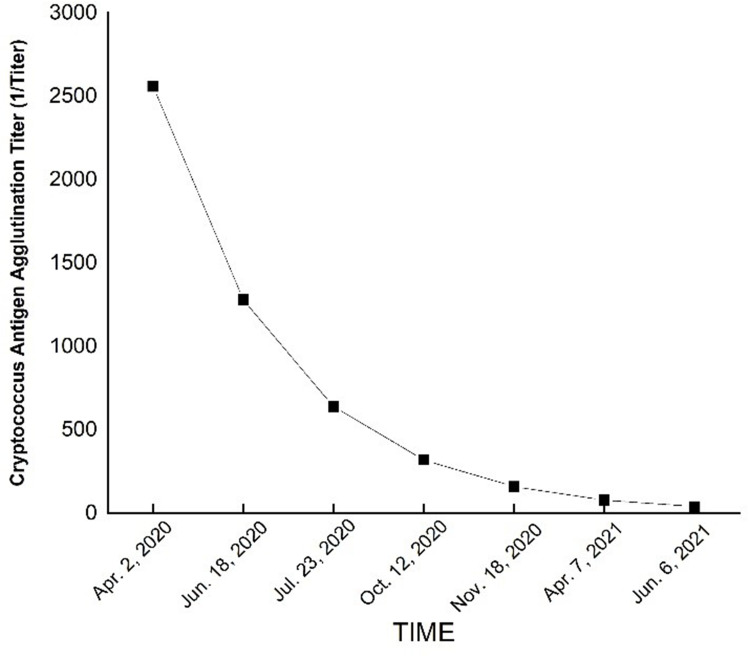
Subsequently, 3 days after discharged, the patient visited the local hospital again for deepened skin and scleral icterus; the patient was experiencing chills and fever, a maximum temperature of 39.2°C, no limb twitching, no unconsciousness, and blood pressure of 80/50 mmHg. She was hospitalized in our department on the basis of biliary infection. Laboratory investigations showed a white blood count of 11.91×109/L with 85.9% neutrophils, C reactive protein (CRP) 32.18 mg/L, Procalcitonin (PCT) 1.32 ng/L, and Interleukin-6 (IL-6) 22.93 pg/mL; liver function showed ALT 59U/L, AST 50U/L, ALP 130U/L, TBIL level 216.5mol/L, DBIL 169.1 mol/L, IBIL 47.4 mol/L, and γ-GT of 45U/L on 9 January, 2020. We administered Biapenem as an anti-infective treatment, and suggested oral fluconazole 400 mg/day because of the patient adjust the fluconazole 200 mg q12h by herself, but the patient did not show any improvement. Liver function indexes were continued to increase, ALT 73U/L, AST 85U/L, ALP 157U/L, TBIL level 402.2mol/L, DBIL 295.4 mol/L, IBIL 106.8mol/L, and γ-GT 56U/L on 16 January, 2020. Again, the patient underwent ERCP at our digestive intervention center, and purulent secretions overflowed from the duodenal papilla. Based on the biopsy results, cholangitis was diagnosed, and biapenem was administered as anti-infective treatment. After 5 days of treatment, the liver function of the patient improved, and the temperature returned to normal. The patient discharged and continue with oral fluconazole 400 mg/day. The biliary stents were replaced on April 2020, December 2020, and June 2021, respectively. Ultimately, the patient achieved complete recovery, and no complications were observed during the 2 years of follow-up. Bilirubin changes showed in Figure 2. Cryptococcus Antigen Agglutination Titer showed in Figure 3. Conditions recommended by clinical pharmacist for treatment showed in Table 1.
Figure 2.
Bilirubin changes of the patient.
Figure 3.
Cryptococcus antigen agglutination titer of the patient.
Table 1.
Conditions Recommended by Clinical Pharmacist for Treatment
| Time | Reason for Consultation | Suggestion of Clinical Pharmacist | After Consultation | Result |
|---|---|---|---|---|
| 30 December 2019 | After the first operation and the Pathological examination suggest Cryptococcus infection | Oral fluconazole 400 mg/day, and 800 mg/day on the first day | Accept the recommended during hospitalization but the patient adjusts oral fluconazole 200 mg q12h by herself after discharge | Improvement |
| 16 January 2020 | Re-admission due to high fever | Oral fluconazole 400 mg qd | Accept the recommended during hospitalization but the patient adjusts oral fluconazole 200 mg q12h by herself after discharge | Improvement |
| 17 April 2020 | Replace the biliary stents | Oral fluconazole 400 mg qd | Accept the recommended during hospitalization but the patient adjusts oral fluconazole 200 mg q12h by herself after discharge | Improvement |
| 02 December 2020 | Replace the biliary stents | Oral fluconazole 400 mg qd | Accept the recommended during hospitalization but the patient adjusts oral fluconazole 200 mg q12h by herself after discharge | Improvement |
| 07 December 2020 | High-fever after replace biliary stents, blood culture showed Enterococcus faecium and Pseudomonas aeruginosa | Oral fluconazole 400 mg qd, Vancomycin 1g IVGTT, Cefoperazone and Sulbactam 3g IVGTT, combine with Amikacin 0.4g IVGTT q12h. | Follow the consultation opinion except for replaced Vancomycin with Linezolid 0.6g IVGTT q12h, but switch back to Vancomycin because of the symptom was not better after 3 days. The patient adjusts oral fluconazole 200 mg q12h by herself after discharge. | Improvement |
| 01 June 2021 | High-fever after replace biliary stents again, but the blood culture was negative. | Oral fluconazole 400 mg qd, Meropenem 1g IVGTT q8h, | Accept the recommended and continue oral fluconazole 400 mg qd. | Improvement |
Literature Review
We searched the PubMed database using the keywords “biliary”, “cryptococcus”, and “biliary cryptococcosis”. After deleting repeat papers and those where cases were unclear, we identified 11 cases from 11 studies2–12 (Table 2). Including the new case described herein, 12 cases include, 6 males and 6 females. In five cases, patients were <18 years old, in three cases they were 18 to 44 years old, and in four cases they were 45 to 65 years old; That is, biliary cryptococci can occur at any age. The main clinical manifestations are jaundice, skin and sclera icterus, and dark urine. General examinations involved ultrasound, CT, MRCP and ERCP, in order to demonstrate biliary dilatation and bile duct obstruction. Bile aspirate culture, biopsy culture, special stains (eg, periodic acid methenamine silver stain, periodic acid Schiff stain and India ink stain), serum cryptococcal antigen titers, molecular sequencing, and PCR were used for diagnosis in laboratory examination. All the cases were immunocompetent except for three cases of HIV infection and one case with long-term use of glucocorticoids (this study). Eight cases cryptococcal infection involved sites other than the bile duct, mainly the central nervous system, lungs, and blood. Four cases of cryptococcal infection involved only the biliary duct. All the cases achieved complete recovery or improvement after antifungal treatment for 2 weeks to 16 months, except for one patient with HIV infection who died of pneumonia.
Table 2.
Clinical Characteristics of Bile Duct Cryptococcosis
| References | Age | Gender | Presenting Symptoms | General Examination | Laboratory Methods | Other Sites Involve | Therapy | Prognosis/Time |
|---|---|---|---|---|---|---|---|---|
| (Kumar et al, 2019)2 | 49 | M | High-colored urine, yellowish discoloration of eyes, pale stools, itchy skin, fever, nausea, vomiting, headache | Scleral icterus, moderate hepatomegaly, ERCP showed CHD. | Bile aspirate smears displayed cryptococcosis, | Blood, CSF | Antifungal, antitubercular, antiretroviral | Died/ 1 month |
| (Burad and Ramakrishna, 2017)3 | 48 | F | Lower back pain, low-grade fever, and an evening rise, yellowing of the eyes and urine | Ultrasound showed hepatomegaly, dilated CBD. CT, EUS showed CBD stricture with a thickened wall. |
The cytology smears suggested cryptococcosis. | Blood, CSF, sputum, vertebra, skin | Amphotericin-B,14days. Fluconazole for 8weeks. | / |
| (Luo et al, 2014)4 | 5 | M | Upper abdominal pain, jaundice, mild hepatosplenomegaly | CT showed CBD with incomplete bile duct obstruction. Ultrasound demonstrated irregular mural thickening of the CBD. | CBD biopsied showed fungal hyphae. Alcian stain for the capsule of C.neoformans was positive. |
/ | Flucomazole,400 mg/d, po | Normal/10 months |
| (Zhang et al, 2014)5 | 5 | M | Low-grade intermittent fever, jaundice, dark urine, clay-colored stool. | Ultrasonography revealed intrahepatic biliary dilatation. CT scan produced a slight irregular peripheral enhancement and a central area of lower attenuation. | Microscopic examination revealed multinucleated giant cells, cuboidal bodies of C.neoformans | NOT | Liposomal amphotericin B, 2 weeks. Fluconazole for 3 months. | Normal/4 months |
| (Cai et al, 2012)6 | 44 | F | Right upper quadrant discomfort and jaundice, icteric eyes and skin with darkened urine, nauseous, colicky pain in the stomach and back | Ultrasonography revealed extra and intrahepatic dilatation. The outer CBD was dilated but the lumen was narrowed. Contrast-enhanced CT scan and an MRCP produced a hilar biliary stricture. |
CBD frozen section samples demonstrated cryptococcosis. Microscopic examination, the periodic acid methenamine silver stain, periodic acid Schiff stain and India ink stain positive. PCR identified pathogen was C. neoformans | NOT | Itraconazole, IVGTT,2 weeks then 10 weeks po | Normal/4 weeks |
| (Pastagia and Caplivski, 2010)7 | 29 | F | Fever, abdominal pain, vaginal bleeding, | Abdominal imaging revealed gallbladder wall thickening with pericholecystic fluid. | Mucicarmine stain revealed Cryptococcus. Biochemical identification, Molecular sequencing revealed C. neoformans. Serum cryptococcal antigen titer 1:512. | Lungs, placenta | Liposomal amphotericin B +flucytosine. Fluconazole | Normal/2 months |
| (Nara et al, 2008)8 | 25 | M | Upper abdominal pain, jaundice, | Enhanced CT scan showed bilateral intrahepatic bile duct dilatation. Cholangiogram showed complete obstruction at the hepatic confluence and irregularity of the left hepatic duct. | Liver mass biopsy showed liver cryptococcosis. Bile and biopsy specimens’ culture, periodic acid Schiff (PAS), Grocott stains showed C. neoformans. Serum Cryptococcus neoformans antigen was positive. | Lungs | Flucomazole,400 mg/d, po,12 months | Normal / 8 months |
| (Singh et al, 2007)9 | 65 | F | Anorexia, nausea, vomiting | Abdominal sonogram demonstrated cholecystitis and cholelithiasis. | Gallbladder biopsy and washings, Blood cultures showed cryptococcus. serum cryptococcal antigen titers were greater than 1:512. | Spinal/cerebrospinal fluid | / | / |
| (Das et al, 2006)10 | 5 | M | High-grade intermittent fever, progressive jaundice, dark urine, clay-colored stool. | Ultrasonography revealed dilatation of intrahepatic biliary. MR and MRCP performed the bile-duct wall thickening and severity of biliary obstruction. | Liver biopsy revealed canalicular cholestasis. Cytological mucicarmine stain of the bile duct wall revealed cryptococcus. | NOT | Amphotericin B, 10 weeks. Fluconazole | Improvement/ 6 weeks |
| (Goenka et al, 1995)11 | 7 | M | High fever, jaundice, pruritus | Ultrasound showed dilatation of intrahepatic biliary. ERCP showed complete block in the CBD. | Liver biopsy showed extrahepatic biliary obstruction. Multiple cryptococci were seen in parenchyma and portal area. | Disseminated | Amphotericin B, with 5-flucytosine | Liver and splenomegaly/ 16 months |
| (Bucuvalas et al, 1985)12 | 15 | F | Right upper quadrant discomfort and jaundice. | Ultrasound, CT showed biliary dilation and a thickened CBD. Intraoperative cholangiogram demonstrated severe stenosis of proximal radicles and obstruction at the cystic duct. | Gram stain of bile, Cultures of the bile and CBD grew C. neoformans. | Urine, blood | Amphotericin B | Improvement/15 days |
Discussion
Cryptococcosis commonly manifests as an opportunistic infection due to a change in the natural climate, the continuous evolution of Cryptococcus itself, and an increase in susceptible individuals.13 Cryptococcal infection is rare in clinical settings, but is mainly observed in patients with HIV infection,14 hematological malignancies, patients receiving immunosuppressive therapy, and patients with T cell-mediated immune function impairment. In the normal population, the infection rate is approximately one in 100,000, but rises to 5–10% in immunosuppressive patients, and 30% in HIV-infected patients. Cryptococcus is mainly spread through pigeon feces and soil, enters the lungs through the respiratory tract, and further spreads through the blood and invades other tissues and organs throughout the body. It usually involves the central nervous system, lungs, skin, prostate, eyes, adrenal glands, and bone marrow;1 biliary infections are very rare.
The rarity of biliary cryptococcal infection makes it difficult to diagnose. The patient described herein was diagnosed with primary sclerosing cholangitis on first visit to a local hospital. After choleretic and hepatoprotective treatments, the symptoms recurred. On admittance to our hospital the patient was diagnosed with cholangiocarcinoma through CT and MRCP examinations, combined with elevated CA199. Previous cases have been diagnosed based on pathology and/or bile culture. In this study, the patient had a rapid onset, was HIV negative, had no history of taking immunosuppressants, and had no special medical history, except for long-term glucocorticoids use, which did not attract the attention of doctors. Auxiliary examination showed that the structure of the bile duct in the hilar area was unclear, the extrahepatic bile duct wall was diffusely diseased, the common bile duct wall was thickened, and that the bile duct was obstructed. The clinical manifestations suggested obstructive jaundice, but considering the age of the patient, bile duct cancer was more likely. Therefore, minimally invasive surgical resection was recommended to remove the obstructive lesion and place a nasobiliary duct tube for drainage, but also to obtain tissue samples to confirm the diagnosis. After the operation, the patient was diagnosed with cryptococcal infection through pathology and special staining. She was administered 400 mg of fluconazole orally once a day (800 mg on the first day) for 6–12 months.14 The liver function and other indicators of the patient improved after taking the drug for 4 days, and she was discharged on the 5th day. The patient reappeared with high fever and shock 2 days later, this may have been related to cholangitis after ERCP. Cholangitis is a common infectious complication of ERCP, with an incidence of approximately 0.5–3%.15,16 Induced cholangitis may be related to inadequate administration of antibacterial drugs before and after surgery, sufficient bile drainage, and the operational skill level of the doctors.17–19 Subsequently, ERCP was performed again to confirm the occurrence of cholangitis. At the same time, the plastic stents were replaced, and biapenem was used for anti-infection treatment. As the possibility of recurrence could not be completely ruled out, follow-up was required. The patient did not show recurrence of symptoms after nearly 2 years, despite continuing to take glucocorticoids.
A review of previously reported biliary cryptococcal infection cases, combined with the diagnosis and treatment of the case described herein suggest that pathological examination and bile culture play a key role in diagnosis. Cryptococcal antigen latex agglutination tests, cryptococcal antigen test, and lateral flow assay (LFA) test is important for the diagnosis of the disease. Here, as the patient had no symptoms of the nervous system, no related examinations were performed. During surgery, stent the placement may cause blockage or displacement, resulting in delayed bile duct infection.20 Therefore, the doctor should choose the stent according to comprehensive factors, such as the cause of the biliary obstruction, the location, and the operating skill level of the doctor.21 Regularly replace the plastic stent, and replace it immediately when clinical manifestations of blockage occur; try to place multiple stents and other measures to reduce the occurrence of cholangitis.22 For patients with cryptococcal infection, early diagnosis should be made to prevent severe liver damage. Early antifungal treatment has an important effect on the prognosis of patients; therefore, such diseases should attract the attention of clinicians.
Acknowledgments
This research was funded by the National Natural Science Foundation of China (grant Nos. 72074218).
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Consent Statement
Written informed consent was provided by the patient for the publication of the case details. Details of the case can be published without institutional approval.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Kronstad JW, Attarian R, Cadieux B, et al. Expanding fungal pathogenesis: cryptococcus breaks out of the opportunistic box. Nat Rev Microbiol. 2011;9(3):193–203. doi: 10.1038/nrmicro2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar N, Gupta R, Sayed S, et al. Cytological diagnosis of cryptococcosis in a biliary specimen: report of a rare case with brief review of literature. Cytopathology. 2019;30(2):249–252. doi: 10.1111/cyt.12626 [DOI] [PubMed] [Google Scholar]
- 3.Burad DK, Ramakrishna B. Cytological diagnosis of biliary cryptococcosis in an immunocompromised patient with mid common bile duct stricture masquerading as cholangiocarcinoma. Cytopathology. 2017;28(2):164–167. [DOI] [PubMed] [Google Scholar]
- 4.Luo Y, Cui MY, Liao B, et al. Diagnostic and post-treatment CT appearance of biopsy proven mixed Cryptococcus and Candida cholangitis. J Xray Sci Technol. 2014;22(6):727–733. [DOI] [PubMed] [Google Scholar]
- 5.Zhang C, Du L, Cai W, Wu Y, Lv F. Isolated hepatobiliary cryptococcosis manifesting as obstructive jaundice in an immunocompetent child: case report and review of the literature. Eur J Pediatr. 2014;173(12):1569–1572. [DOI] [PubMed] [Google Scholar]
- 6.Cai X, Liu K, Liang Y, Yu H, Lv F, Liang X. Isolated biliary cryptococcosis manifesting as obstructive jaundice in an immunocompetent adult. Int J Med Sci. 2012;9(3):200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pastagia M, Caplivski D. Disseminated cryptococcosis resulting in miscarriage in a woman without other immunocompromise: a case report. Int J Infect Dis. 2010;14(5):e441–e443. [DOI] [PubMed] [Google Scholar]
- 8.Nara S, Sano T, Ojima H, et al. Liver cryptococcosis manifesting as obstructive jaundice in a young immunocompetent man: report of a case. Surg Today. 2008;38(3):271–274. [DOI] [PubMed] [Google Scholar]
- 9.Singh CS, Rahman M, Jamil S, Sawhney H, Vernaleo J, Thelmo W. Cholecystitis as the initial manifestation of disseminated cryptococcosis. AIDS. 2007;21(15):2111–2112. [DOI] [PubMed] [Google Scholar]
- 10.Das CJ, Pangtey GS, Hari S, Hari P, Das AK. Biliary cryptococcosis in a child: MR imaging findings. Pediatr Radiol. 2006;36(8):877–880. doi: 10.1007/s00247-006-0197-z [DOI] [PubMed] [Google Scholar]
- 11.Goenka MK, Mehta S, Yachha SK, Nagi B, Chakraborty A, Malik AK. Hepatic involvement culminating in cirrhosis in a child with disseminated cryptococcosis. J Clin Gastroenterol. 1995;20(1):57–60. doi: 10.1097/00004836-199501000-00015 [DOI] [PubMed] [Google Scholar]
- 12.Bucuvalas JC, Bove KE, Kaufman RA, Gilchrist MJ, Oldham KT, Balistreri WF. Cholangitis associated with Cryptococcus neoformans. Gastroenterology. 1985;88(4):1055–1059. doi: 10.1016/S0016-5085(85)80028-7 [DOI] [PubMed] [Google Scholar]
- 13.May RC, Stone NR, Wiesner DL, Bicanic T, Nielsen K. Cryptococcus: from environmental saprophyte to global pathogen. Nat Rev Microbiol. 2016;14(2):106–117. doi: 10.1038/nrmicro.2015.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(3):291–322. doi: 10.1086/649858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan X, Chen S, Zhao Q, et al. The efficacy of temporary placement of nasobiliary drainage following endoscopic metal stenting to prevent post-ERCP cholangitis in patients with cholangiocarcinoma. Saudi J Gastroenterol. 2018;24(6):348–354. doi: 10.4103/sjg.SJG_94_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andriulli A, Loperfido S, Napolitano G, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. 2007;102(8):1781–1788. doi: 10.1111/j.1572-0241.2007.01279.x [DOI] [PubMed] [Google Scholar]
- 17.De Palma GD, Galloro G, Siciliano S, Iovino P, Catanzano C. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: results of a prospective, randomized, and controlled study. Gastrointest Endosc. 2001;53(6):547–553. doi: 10.1067/mge.2001.113381 [DOI] [PubMed] [Google Scholar]
- 18.Kohli DR, Shah TU, BouHaidar DS, Vachhani R, Siddiqui MS. Significant infections in liver transplant recipients undergoing endoscopic retrograde cholangiography are few and unaffected by prophylactic antibiotics. Dig Liver Dis. 2018;50(11):1220–1224. doi: 10.1016/j.dld.2018.05.014 [DOI] [PubMed] [Google Scholar]
- 19.Cotton PB, Connor P, Rawls E, Romagnuolo J. Infection after ERCP, and antibiotic prophylaxis: a sequential quality-improvement approach over 11 years. Gastrointest Endosc. 2008;67(3):471–475. doi: 10.1016/j.gie.2007.06.065 [DOI] [PubMed] [Google Scholar]
- 20.Sawas T, Al Halabi S, Parsi MA, Vargo JJ. Self-expandable metal stents versus plastic stents for malignant biliary obstruction: a meta-analysis. Gastrointest Endosc. 2015;82(2):256–267 e257. doi: 10.1016/j.gie.2015.03.1980 [DOI] [PubMed] [Google Scholar]
- 21.Committee ATA, Pfau PR, Pleskow DK, et al. Pancreatic and biliary stents. Gastrointest Endosc. 2013;77(3):319–327. [DOI] [PubMed] [Google Scholar]
- 22.Costamagna G, Boskoski I. Current treatment of benign biliary strictures. Ann Gastroenterol. 2013;26(1):37–40. [PMC free article] [PubMed] [Google Scholar]