Abstract
Introduction.
Our understanding of the biologic heterogeneity of endometrial cancer has improved, but which patients benefit from single-agent versus combination immune checkpoint blockade remains unclear.
Methods.
We conducted a single-center, randomized, open-label, phase 2 study of durvalumab 1500 mg (Arm 1) versus durvalumab 1500 mg plus tremelimumab 75 mg every 4 weeks (Arm 2) in patients with endometrial carcinoma. The primary endpoints were overall response rate (ORR) and progression-free survival (PFS) at 24 weeks. Patients were stratified by mismatch repair (MMR) status and carcinosarcoma histology. Using a Simon two-stage minimax design, we determined 40 patients per arm would provide 90% power and Type 1 error of 10%.
Results.
Eighty-two patients were enrolled; 77 were evaluable for toxicity (Arm 1: 38, Arm 2: 39) and 75 evaluable for efficacy (Arm 1: 37, Arm 2: 38). Patient were stratified by MMR status (Arm 1: 5, Arm 2: 4 were MMR-deficient). The ORR in Arm 1 was 10.8% (one-sided 90% CI: 4.8–100%); the ORR in Arm 2 was 5.3% (one-sided 90% CI: 1.4–100%). Since the primary endpoint of ORR was not met, 24-week PFS was not compared to historical controls per protocol specification. No new safety signals were identified.
Conclusions.
In these patients with predominantly MMR-proficient endometrial cancer, there was limited response with single-agent and combined immune checkpoint blockade. The pre-specified efficacy thresholds were not met for further evaluation. A deeper understanding of potential mechanisms of resistance to immunotherapy in MMR-proficient endometrial cancer is needed for the development of novel therapeutic approaches.
Keywords: Durvalumab, Tremelimumab, Endometrial cancer, Endometrial carcinosarcoma, Phase 2
1. Introduction
The incidence and disease-related mortality attributed to endometrial cancer are sharply rising. On a global scale, there were 417,000 new cases and 97,000 endometrial cancer–associated deaths in 2020 [1]. In the US, there has been an annual 1.8% increase in endometrial cancer mortality over the past several years, and the mortality rate will soon surpass that of ovarian cancer [2,3]. Growing rates of obesity and sedentary lifestyle have had a much larger effect on the incidence of endometrial cancer (70% of cases) compared with ovarian cancer (4% of cases) [4]. Platinum-taxane combination chemotherapy for advanced/recurrent endometrial cancer is associated with finite efficacy, responses to second-line cytotoxic agents are modest, and tumors often become therapy resistant [5,6]. The recent approval of lenvatinib plus pembrolizumab has given patients further treatment options but at considerable cost and toxicity, making it unsuitable for all patients [7].
The characterization of endometrial cancer into 4 distinct (molecular and prognostic) subtypes has provided insight into how to rationally target this disease. These subtypes include: (1) POLE “ultramutated”; (2) microsatellite instability hypermutated (MSI-H) or mismatch repair deficient (dMMR); (3) copy number low (CNL); and (4) copy number high (CNH) [8]. Since the advent of this classification system, immune checkpoint blockade has proven to be an effective strategy for POLE-“ultramutated” and MSI-H/dMMR (MLH1, MSH2, MSH6, PMS2) endometrial cancers [9,10]. However, the majority of relapsed endometrial cancers are microsatellite stable (MSS) or MMR-proficient (pMMR), CNL, or CNH, with limited responses (<13%) to single-agent programmed cell death protein 1/programmed death-ligand 1 (PD-1/ PD-L1) therapies [11-15]. Combination PD-1/PD-L1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) blockade has shown improved outcomes in various malignancies, including melanoma, renal cell carcinoma, hepatocellular carcinoma, and non-small lung carcinoma [16-19].
Durvalumab is a selective, high-affinity, human IgG1 monoclonal antibody (mAb) that binds to PD-L1 and CD80. Tremelimumab is an IgG2 kappa isotype mAb directed against CTLA-4. Dual inhibition of CTLA-4 plus PD-L1 can enhance T-cell activation and cellular immune responses [20]. Given the non-overlapping mechanisms of action of CTLA-4 and PD-L1 inhibitors and the potential for synergistic activity, we investigated durvalumab with and without tremelimumab in advanced or recurrent endometrial cancer, regardless of MMR status, in this randomized phase 2 study.
2. Methods
2.1. Study design
This was a single-institution, randomized, open-label, phase 2 study of durvalumab monotherapy or durvalumab plus tremelimumab in patients with metastatic, recurrent, or persistent endometrial carcinoma or endometrial carcinosarcoma. Patients were administered either 1500 mg durvalumab via intravenous (IV) infusion every 4 weeks (Arm 1) OR 1500 mg durvalumab and 75 mg tremelimumab via IV infusion every 4 weeks for up to 4 cycles, and then 1500 mg durvalumab every 4 weeks thereafter (Arm 2). The primary endpoints were overall response rate (ORR) and progression-free survival (PFS) at 24 weeks in each treatment arm. Tumor response was evaluated via Response Evaluation Criteria in Solid Tumors (RECIST) 1.1, with response confirmation [21]. Secondary endpoints included clinical benefit rate (CBR), defined as the rate of complete response (CR), partial response (PR), and stable disease (SD) rate in each arm at 24 weeks; duration of response (DOR); ORR evaluated by Immune-related RECIST (irRECIST) criteria in patients treated beyond progression, and safety per the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.03.
Eligible patients had confirmed recurrent or persistent endometrial cancer of the following histologic subtypes: endometrioid, serous, undifferentiated, dedifferentiated, mixed epithelial, mucinous, squamous, transitional cell, adenocarcinoma not otherwise specified, or endometrial carcinosarcoma. All patients were 18 years of age or older; had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; had adequate organ function; and had at least one prior platinum-based chemotherapeutic regimen but no more than three additional cytotoxic regimens. Patients who had received prior anti–PD-1/PD-L1 or anti–CTLA-4 therapy or had inflammatory bowel disease, primary immunodeficiency, allogenic organ transplant, or interstitial lung disease were excluded.
Treatment cycles were 28 days, and treatment was continued until unacceptable toxicity, intolerance, withdrawal, or progression of disease (whichever occurred first). Radiologic assessments occurred every 8 weeks (+/− 7 days) for the first 48 weeks and then every 12 weeks (+/− 7 days) until progression. For patients who remained progression free 2 years after completion of protocol-directed treatment, radiologic assessments occurred every 6 months (+/− 1 month).
MMR status was determined by immunohistochemistry (IHC) staining for MMR proteins (MLH1, MSH2, MSH6, PMS2). M1 (prior to 2019) or ES05 antibodies were used for MLH1, G219–1129 antibody for MSH2, EP49 antibody for MSH6, and A16.4 antibody for PMS2 per our institutional standard guidelines. Microsatellite status was determined by MSI sensor testing of tumor, by known mutations found in MMR genes, or by MSK-IMPACT (Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets)-targeted sequencing [22,23]. Molecular subtype was assigned by our hybrid, hierarchical institutional algorithm, which includes principles from The Cancer Genome Atlas (TCGA) and The Proactive Molecular Risk Classifier of Endometrial Cancer (ProMisE) algorithms [8,24,25]. Briefly, we combined next-generation sequencing parameters, including POLE and TP53 mutation status, MSI score, tumor mutational burden, and fraction of genome altered, with MMR and p53 IHC results. Our institutional database contains molecular subtype for tumors meeting a minimum of 20% tumor purity on MSK-IMPACT for proper classification [26].
The study protocol was approved by the Memorial Sloan Kettering Cancer Center (MSK) institutional review board (IRB) and was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines [27,28]. All patients provided written informed consent prior to study enrollment. The study was registered at ClinicalTrials.gov (NCT03015129). Patients consented to genomic analysis of their tissue samples through a separate MSK IRB-approved protocol (ClinicalTrials.gov, NCT01775072).
2.2. Statistical design and analysis
This single-institution, randomized, phase 2 study was not powered to show superiority of either arm, and the two treatment arms were analyzed separately.
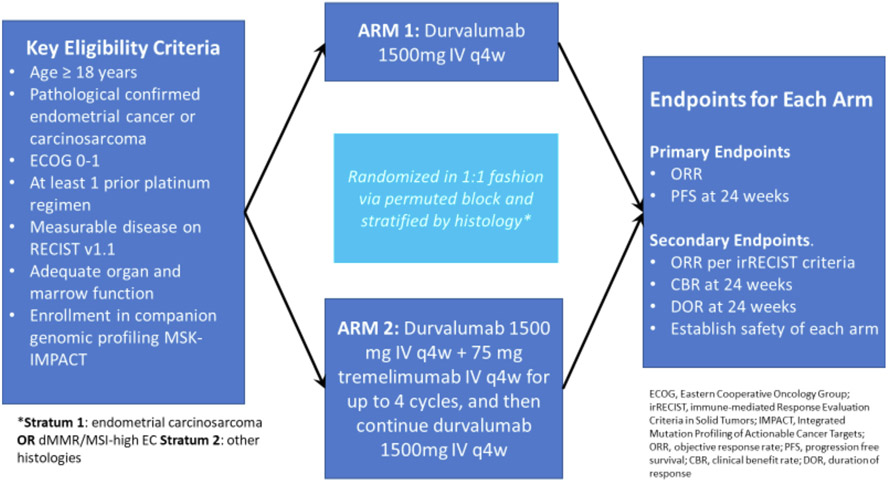
Randomization was completed by random permuted blocks; within each treatment arm, patients were enrolled into two strata, and enrollment of stratum 1 was limited to 10 patients. Stratum 1 included patients with endometrial carcinosarcoma or dMMR/MSI endometrial carcinoma, and stratum 2 included patients with all other pMMR histologies (See Fig. 1).
Fig. 1. Patients and study schema.
ECOG, Eastern Cooperative Oncology Group; RECIST, Response Evaluation Criteria in Solid Tumors; MSK-IMPACT, Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets; ORR, overall response rate; PFS, progression-free survival; irRECIST, immune-related RECIST; CBR, clinical benefit rate; DOR, duration of response.
Based on data from prior immune checkpoint studies in gynecologic malignancies at the time of study inception, we determined an ORR of 10% was not promising and an ORR of ≥25% was considered promising for further study. Using a Simon two-stage minimax design, we determined that 40 patients per treatment arm would provide 90% power and Type 1 error of 10%. Interim analysis was planned after the accrual of 27 patients per arm, whereby 3 or more responders out of 27 would be enough to continue to the second stage. Arm 1 or Arm 2 would be worthy of further investigation if ≥7 patients achieved a CR or PR in each individual arm at final analysis. If the ORR criteria were met, then the PFS rate at 24 weeks would be tested against historical controls from Gynecologic Oncology Group (GOG) studies in similar patient populations [29-31]. The primary and secondary efficacy objectives as CBR were reported with a one-sided 90% CI, which was estimated using exact binomial proportion. The Kaplan-Meier method was used to estimate the median PFS, overall survival (OS), and DOR, as well as their corresponding rates at 24 weeks.
3. Results
3.1. Patient characteristics
Eighty-two patients consented for study enrollment. Three patients failed screening and 2 were randomized but developed rapid progression of disease prior to starting treatment, leaving 77 evaluable patients for toxicity assessment (Arm 1: 38, Arm 2: 39). Two patients received treatment on cycle 1, day 1, but died prior to first radiologic assessment, leaving 75 evaluable patients for efficacy assessment (Arm 1: 37, Arm 2: 38). Demographic and tumor characteristics are outlined in Table 1.
Table 1.
Patient and baseline characteristics.
| Characteristic | Durvalumab (n = 38) |
Durvalumab + Tremelimumab (n = 39) |
|---|---|---|
| Median Age, years (range) | 64 (45-78) | 67 (52-84) |
| ECOG Status | ||
| 0 | 26 (68.4%) | 24 (61.5%) |
| 1 | 12 (31.6%) | 15 (38.5%) |
| Race, n (%) | ||
| White | 27 (71.1%) | 27 (69.2%) |
| African-American | 4 (10.5%) | 5 (12.8%) |
| Asian | 4 (10.5%) | 2 (5.1%) |
| Unknown/Other | 3 (7.9%) | 5 (12.8%) |
| Stage at Diagnosis, n (%) | ||
| Stage I | 9 (23.7%) | 10 (25.6%) |
| Stage II | 4 (10.5%) | 1 (2.6%) |
| Stage III | 11 (28.9%) | 16 (41.0%) |
| Stage IV | 14 (36.8%) | 12 (30.8%) |
| Histology, n (%) | ||
| Endometrioid, Grade 1/2 | 7 (18.4%) | 8 (20.5%) |
| dMMR | 3 (7.9%) | 2 (5.1%) |
| pMMR | 4 (10.5%) | 6 (15.4%) |
| Endometrioid, Grade 3 | 0 | 8 (20.5%) |
| dMMR | 0 | 0 |
| pMMR | 0 | 7 (17.9%) |
| unknown | 0 | 1 (2.6%) |
| Serous | 11 (28.9%) | 6 (15.4%) |
| dMMR | 0 | 1 (2.6%) |
| pMMR | 10 (26.3%) | 5 (12.8%) |
| unknown | 1 (2.6%) * | 0 |
| Clear Cell | 5 (13.2%) | 1 (2.6%) |
| dMMR | 0 | 0 |
| pMMR | 5 (13.2%) | 1 (2.6%) |
| Carcinosarcoma | 6 (15.8%) | 10 (25.6%) |
| dMMR | 0 | 0 |
| pMMR | 5 (13.2%) | 9 (23.0%) |
| unknown | 1 (2.6%) * | 1 (2.6%) |
| Mixed/Dedifferentiated Histology | 9 (23.7%) | 6 (15.4%) |
| dMMR | 2 (5.3%) | 4 (10.3%) |
| pMMR | 7 (18.4) | 1 (2.6%) |
| unknown | 0 | 1 (2.6%) * |
| All Histology Total | ||
| dMMR | 5 (13.2%) | 4 (10.3%) |
| pMMR | 31 (81.6%) | 32 (82.0%) |
| unknown | 2 (5.3%) * | 3 (7.7%) * |
| Prior Cytotoxic Therapy, n (%) | ||
| 1 Line | 13 (34.2%) | 9 (23.1%) |
| 2 Lines | 16 (42.1%) | 16 (41.0%) |
| 3 Lines | 9 (23.7%) | 14 (35.9%) |
| Prior Radiation, n (%) | ||
| Yes | 20 (52.6%) | 26 (66.7%) |
| No | 18 (47.4%) | 13 (33.3%) |
ECOG, Eastern Cooperative Oncology Group; dMMR, mismatch repair deficient; pMMR, mismatch repair proficient.
Microsatellite Stable confirmed with unknown MMR status.
3.2. Efficacy
At the time of data cut-off (December 10, 2021), there were 4 responders among 37 efficacy-evaluable patients in Arm 1 (ORR, 10.8%; one-sided 90% CI: 4.8–100%); 2 patients achieved a CR and 2 achieved a PR as best response (Table 2). Of the 2 patients who achieved a PR, progression of disease was noted at 16 and 24 weeks, respectively, and both had pMMR tumors. The patients who achieved a CR had dMMR tumors, had not progressed at time of data cut-off, and were censored at 73 and 183 weeks. In Arm 2, there were 2 responders among 38 efficacy-evaluable patients (ORR, 5.3%; one-sided 90% CI: 1.4–100%); both patients achieved a CR, had dMMR tumors, and were censored at 60 weeks and 224 weeks. The median DOR was 24 weeks (one-sided 90% CI: 16-Inf) for Arm 1 and had not been reached for Arm 2. Since the primary endpoint of ORR was not met, PFS at 24 weeks was not compared to historical controls per protocol specifications. Additionally, 2 patients had unconfirmed PR (one in each arm) during the first stage of enrollment. In Arm 1, a patient with PR had SD on confirmation. In Arm 2, one patient achieved an initial PR but was removed from the study due to the development of grade 3 immune-mediated adrenal insufficiency and experienced progression at a subsequent time point. The median PFS for Arm 1 was 7.4 weeks (one-sided 90% CI: 7 weeks-Inf), and the median PFS for Arm 2 was 7.9 weeks (one-sided 90% CI: 7 weeks-Inf). The CBR at 24 weeks was 13.5% (one-sided 90% CI: 6.7–100%) in Arm 1 and 10.5% (one-sided 90% CI: 4.7–100%) in Arm 2. ORR for carcinosarcoma patients was 0% in both arms and all patients were MSS or pMMR. Seventeen (43.6%) of 39 patients in Arm 1 completed 4 cycles of durvalumab plus tremelimumab combination therapy.
Table 2.
Efficacy outcomes.
| Objective | Durvalumab | Durvalumab + Tremelimumab |
|---|---|---|
| Primary Objectives (90% CI) | n = 37 | n = 38 |
| ORR | 10.8% (4.8–100%) | 13.5% (6.7–100%) |
| PFS at 24 weeks | 5.3% (1.4–100%) | 13.2% (6.5–100%) |
| Secondary Objectives (90% CI) | n = 37 | n = 38 |
| Median PFS | 7.4 weeks (7–Inf) | 7.9 weeks (7–Inf) |
| CBR | 13.5% (6.7–100%) | 10.5% (4.7–100%) |
| DOR | 24 weeks (16-Inf) | Not Reached |
| irRECIST Objective (90% CI)* | n = 6 | n = 8 |
| ORR | 33.3% (9.3–100%) | 0% |
ORR, overall response rate; PFS, progression-free survival; CBR, clinical benefit rate; DOR, duration of response.
reported for patients who were treated beyond disease progression.
Among 14 patients (Arm 1: 6, Arm 2: 8) treated beyond progression and evaluated by irRECIST criteria, there were no differences in best overall response between irRECIST and RECIST assessments. Of the patients treated beyond progression, one patient (Arm 1) who had achieved SD as best response received clinical benefit and continued treatment approximately 1 year after radiographic progression.
3.3. Safety
The most frequent (≥25%) adverse events (AEs) of any cause are outlined in Table 3. In Arm 1, the most common grade 3 or higher treatment-related AEs (TRAEs) were anemia (n = 3, 8%), hyperglycemia (n = 2, 5%), elevated lipase (n = 2, 5%), diarrhea, elevated alanine transaminase, hyponatremia, and lymphocyte decrease (each n = 1, 3%). In Arm 2, the most common grade 3 or higher TRAEs were colitis (n = 4,10%), hyperglycemia, elevated lipase, and lymphocyte decrease (each n = 2, 5%). There were more immune-mediated TRAEs in the combination arm (Arm 2), including colitis/diarrhea (n = 4), myositis (n = 1), myocarditis (n = 1), pneumonitis (n = 1), and adrenal insufficiency (n = 1).
Table 3.
Adverse events of any cause with an incidence of ≥25%.
| Adverse event | Arm 1 n = 38 |
Arm 2 n = 39 |
||
|---|---|---|---|---|
| Any grade n (%) |
Grade ≥ 3 n (%) |
Any grade n (%) |
Grade ≥ 3 n (%) |
|
| Hyperglycemia | 36 (95) | 4 (11) | 36 (92) | 7 (18) |
| Anemia | 31 (82) | 11 (29) | 34 (87) | 11 (28) |
| Hypoalbuminemia | 26 (68) | 1 (3) | 34 (87) | 1 (3) |
| Hypomagnesemia | 23 (61) | 0 | 30 (77) | 0 |
| Lymphocyte Count Decrease | 20 (53) | 10 (26) | 26 (67) | 15 (38) |
| WBC Decrease | 19 (50) | 0 | 17 (44) | 5 (13) |
| AST Increase | 16 (42) | 1 (3) | 20 (51) | 2 (5) |
| Fatigue | 16 (42) | 0 | 22 (56) | 2 (5) |
| Alkaline Phosphatase Increase | 14 (37) | 1 (3) | 18 (46) | 3 (8) |
| Abdominal Pain | 12 (32) | 0 | 19 (49) | 3 (8) |
| Hyponatremia | 11 (29) | 5 (13) | 15 (38) | 9 (23) |
| Nausea | 11 (29) | 0 | 23 (59) | 2 (5) |
| ALT Increase | 10 (26) | 1 (3) | 14 (36) | 1 (3) |
| INR Increase | 10 (26) | 1 (3) | 12 (31) | 1 (3) |
| Platelet Count Decrease | 10 (26) | 1 (3) | 21 (54) | 1 (3) |
| Arthralgias | 9 (24) | 0 | 4 (10) | 0 |
| Amylase Increase | 8 (21) | 0 | 12 (31) | 0 |
| Hypocalcemia | 8 (21) | 1 (3) | 15 (38) | 3 (8) |
| Pruritis | 8 (21) | 0 | 10 (26) | 0 |
| Constipation | 7 (18) | 0 | 12 (31) | 1 (3) |
| Creatinine Increase | 7 (18) | 0 | 19 (49) | 2 (5) |
| Dyspnea | 7 (18) | 1 (3) | 7 (18) | 0 |
| Pain | 7 (18) | 0 | 11 (28) | 2 (5) |
| Prolonged APTT | 7 (18) | 1 (3) | 14 (36) | 0 |
| Diarrhea | 6 (16) | 1 (3) | 13 (33) | 4 (10) |
| Hyperkalemia | 6 (16) | 1 (3) | 11 (28) | 2 (5) |
| Hypokalemia | 4 (11) | 1 (3) | 13 (33) | 5 (13) |
| Weight loss | 0 | 0 | 10 (26) | 0 |
WBC, white blood count; AST, aspartate aminotransferase; ALT, alanine aminotransferase; INR, international normalized ratio; APTT, activated partial thromboplastin time.
3.4. Molecular analysis
This study included all-comers but stratified patients by MMR status. MMR IHC was not available for 4 patients (Arm 1: 2, Arm 2: 2). Three of these 4 patients had confirmed MSS tumors. Five patients in Arm 1 had dMMR tumors by IHC, and of these, 2 achieved a CR and 1 had SD as best response. Four patients in Arm 2 had dMMR tumors by IHC, and of these, 2 patients achieved a CR and 2 had SD as best response.
4. Discussion
We aimed to explore the efficacy of durvalumab and durvalumab plus tremelimumab in this all-comer, but predominantly pMMR/MSS, endometrial cancer population. Both monotherapy durvalumab (Arm 1) and in combination with tremelimumab (Arm 2) demonstrated modest efficacy of 10.8% and 5.3%, respectively, and neither treatment arm met the pre-specified efficacy endpoints worthy of further evaluation. Although this trial was not designed to compare the two treatment arms, we observed similar low response rates in both arms. Not surprisingly, responses in both Arm 1 and Arm 2 appeared to be driven by the patients with dMMR tumors. There were no new safety signals identified; however, as expected, there were more frequent treatment-related, immune-mediated adverse events in the combination arm.
The continued molecular characterization of endometrial cancer is critical to direct treatment for advanced and recurrent disease. Despite our improved understanding of the biologic heterogeneity of endometrial cancer, the incidence and disease-associated mortality of endometrial cancer is rising, and until very recently, there were limited therapeutic options beyond carboplatin and paclitaxel chemotherapy [3,8,32,33]. Immune checkpoint inhibitor monotherapy in pMMR/MSS endometrial cancer has shown modest activity, with response rates of 3–13% and median PFS of 1.4–1.9 months [12,14,15,34]. Our study, which included a predominately pMMR/MSS population, is consistent with these previously reported trials. With regard to pMMR endometrial cancer, potentially a small subset may benefit from checkpoint inhibitor therapy, and identifying markers for response in this subset is a priority.
In contrast, deep and durable responses to immune checkpoint inhibitors have been demonstrated in patients with dMMR/MSI-H tumors with pembrolizumab, with an ORR of 48%, a median PFS of 13.1 months, and with 88% of patients having a response duration of longer than 1 year [35]. Similarly, findings from the GARNET trial demonstrated an ORR of 45.5%, a median duration of response that had not been reached, and probabilities of response at 12 and 24 months of 93.3% and 83.7%, respectively [9]. Even with dMMR disease, however, there are still critical unanswered questions regarding duration of therapy in complete responders and mechanisms of intrinsic and acquired resistance.
For the majority patients with pMMR endometrial cancer and for those with dMMR/MSI-H disease who do not benefit from immune checkpoint inhibition, combination treatment strategies should be considered. The recent addition of lenvatinib (a multi-kinase inhibitor of VEGFR, FGFR, PGFRα, RET, and KIT) to pembrolizumab has been practice changing for the treatment of pMMR/MSS endometrial cancer, with an improved median PFS (6.6 months vs 3.8 months, HR: 0.6), median OS (17.4 months vs 12 months, HR: 0.68) and ORR (30% vs 15%) compared to standard chemotherapy [7]. This further bolsters the rationale for investigating targeted combination immune checkpoint therapies.
Further characterization of endometrial cancer is essential to identify which patients are most likely to benefit from specific treatments, and a separate molecular and immune analyses with these patients is planned. In conclusion, data from our randomized trial suggest that durvalumab and durvalumab plus tremelimumab have meager efficacy in patients with predominantly pMMR endometrial cancers and that other combination immune checkpoint strategies should be investigated. There are planned molecular and immune analyses to better understand mechanisms of resistance to these available therapies, and investigation of therapeutic approaches to improve checkpoint inhibitor therapy is urgently needed.
HIGHLIGHTS.
Durvalumab has limited efficacy in all-comers but predominately mismatch repair-proficient (pMMR) endometrial cancer.
Combination durvalumab and tremelimumab similarly has limited efficacy in this predominately pMMR population.
Further molecular integration is needed to identify patients who may benefit from combination targeted/immunotherapies.
Funding
This research was funded in part by the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
CRediT authorship contribution statement
Maria M. Rubinstein: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. Eric Rios Doria: Writing – original draft, Writing – review & editing. Jason Konner: Investigation, Writing – review & editing. Stuart Lichtman: Investigation, Writing – review & editing. Qin Zhou: Formal analysis, Writing – review & editing. Alexia Iasonos: Formal analysis, Writing – review & editing. Debra Sarasohn: Writing – review & editing. Tiffany Troso-Sandoval: Investigation, Writing – review & editing. Claire Friedman: Investigation, Writing – review & editing. Roisin O'Cearbhaill: Investigation, Writing – review & editing. Karen Cadoo: Investigation, Writing – review & editing. Chrisann Kyi: Investigation, Writing – review & editing. Seth Cohen: Investigation, Writing – review & editing. Krysten Soldan: Investigation, Writing – review & editing. Eric Billinson: Data curation, Writing – review & editing. Imogen Caird: Data curation, Writing – review & editing. Dasom Jang: Data curation, Writing – review & editing. Khalil Eid: Data curation, Writing – review & editing. Pooja Shah: Data curation, Writing – review & editing. Joyce Guillen: Data curation, Writing – review & editing. Carol Aghajanian: Investigation, Writing – review & editing. Dmitriy Zamarin: Conceptualization, Investigation, Methodology, Writing – review & editing. Vicky Makker: Conceptualization, Investigation, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
Dr. Rubinstein reports grant support paid to the institution from Merck, Zentalis, and AstraZeneca. Dr. Iasonos reports consulting fees from Mylan. Dr. Friedman reports grant support paid to the institution from Genentech/Roche, Bristol Myers Squibb (BMS), Merck, AstraZeneca, and Daiichi; consulting fees from Seagen and BMS; and waived compensation for advisory board participation from Merck and Genentech. Dr. O'Cearbhaill reports meeting/travel support from the Gynecologic Oncology Foundation, Curio, and Hitech Health; participation in the advisory boards of Tesaro/GlaxoSmithKline (GSK), Regeneron, Seattle Genetics, Fresenius Kabi, Bayer, and CarinaBiotech (non-compensated); non-compensated steering committee participation for Tesaro/GSK and AstraZeneca; and grant support paid to the institution from Bayer/Celgene/Juno, Tesaro/GSK, Merck, the Ludwig Cancer Institute, Abbvie/StemCentrx, Regeneron, TCR2 Therapeutics, Marker Therapeutics, Syndax Pharmaceuticals, Genmab/Seagen Therapeutics, Sellas Therapeutics, Genentech, KitePharma, and the Gynecologic Oncology Foundation. Dr. Cadoo reports grant support paid to the institution from The Irish Cancer Society, MSD, and Immunogen; consulting fees from Nextcure, MJH Life Sciences, and GSK; honoraria from SGK, AstraZeneca, and MSD; meeting/travel support from Roche, Pfizer, and MSD; and board/committee participation for MSD, AstraZeneca, GSK, Eisai, The National Cancer Control Programme Ireland (voluntary), and the ARC Cancer Support Centers (voluntary). Dr. Kyi reports grant support paid to the institution from Merus, Gritstone, and BMS; consulting fees from Scenic Immunology BV and OncLive; and meeting/travel support from the Conquer Cancer Foundation and Gritstone. Dr. Zamarin reports grant support paid to the institution from AstraZeneca, Roche, Plexxikon, and Synthekine; patents from Merck and Newcastle Disease Virus for Cancer Therapy; consulting fees from Memgen, Celldex, Agenus, Astellas, AstraZeneca, Crown Biosciences, Roche, GSK, Hookipa, ImmunOS, Kalivir, Synologic Therapeutics, Synthekine, Takeda, Targovax, Tessa Therapeutics, and Xencor; and stock options from Accurius Therapeutics, ImmunOS Therapeutics, and Calidi Biotherapeutics. Dr. Aghajanian reports grant support paid to the institution from Abbvie, Clovis, Genentech, and AstraZeneca; consulting fees from Eisai/Merck, Mersana Therapeutics, Roche/Genentech, Abbvie, AstraZeneca/Merck, and Repare Therapeutics; advisory board participation for Blueprint Medicine; and unpaid participation on the Board of Directors of the GOG Foundation and NRG Oncology. Dr. Billinson reports stock ownership in Abbvie and Johnson and Johnson. Dr. Makker reports unpaid board participation for Eisai, Merck, Clovis, Faeth, Duality, Morphyes, Karyopharm, Novartis, Lilly and Immunocore. The authors have no potential conflicts of interest to disclose.
References
- [1].Internation WCRF. Endometrial Cancer Statistics, cited 2022; Available from https://www.wcrf.org/cancer-trends/endometrial-cancer-statistics/2022. [Google Scholar]
- [2].Clarke MA, et al. , Racial and ethnic differences in hysterectomy-corrected uterine Corpus Cancer mortality by stage and histologic subtype, JAMA Oncol. 8 (6) (2022) 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Giaquinto AN, et al. , The changing landscape of gynecologic Cancer mortality in the United States, Obstet. Gynecol 139 (3) (2022) 440–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Islami F, et al. , Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States, CA Cancer J. Clin 68(1) (2018) 31–54. [DOI] [PubMed] [Google Scholar]
- [5].Miller DS, et al. , Carboplatin and paclitaxel for advanced endometrial Cancer: final overall survival and adverse event analysis of a phase III trial (NRG oncology/GOG0209), J. Clin. Oncol 38 (33) (2020) 3841–3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fleming GF, Second-line therapy for endometrial Cancer: the need for better options, J. Clin. Oncol 33 (31) (2015) 3535–3540. [DOI] [PubMed] [Google Scholar]
- [7].Makker V, et al. , Lenvatinib plus Pembrolizumab for advanced endometrial Cancer, N. Engl. J. Med 386 (2022) 437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cancer Genome Atlas Research N, et al. , Integrated genomic characterization of endometrial carcinoma, Nature 497 (7447) (2013) 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Oaknin A, et al. , Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: interim results from GARNET–a phase I, single-arm study, J. ImmunoTher. Cancer 10 (1) (2022), e003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Marabelle A, et al. , Efficacy of Pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient Cancer: results from the phase II KEYNOTE-158 study,J. Clin. Oncol 38 (1) (2020) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Soumerai TE, et al. , Clinical utility of prospective molecular characterization in advanced endometrial Cancer, Clin. Cancer Res 24 (23) (2018) 5939–5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ott PA, et al. , Safety and Antitumor Activity of Pembrolizumab in Advanced Programmed Death Ligand 1–Positive Endometrial Cancer: Results From the KEYNOTE-028 Study,J. Clin. Oncol 35 (22) (2017) 2535–2541. [DOI] [PubMed] [Google Scholar]
- [13].Oaknin ADL, Sullivan RJ, et al. , Preliminary safety, efficacy, and pharmacokinetic/pharmacodynamic characterization from GARNET, a phase I/II clinical trial of the anti–PD-1 monoclonal antibody, TSR-042, in patients with recurrent or advanced MSI-h and MSS endometrial cancer, The Society of Gynecologic Oncology (SGO)’s 50th Annual Meeting on Women’s Cancer, 2019. , Honolulu, Hawaii. [Google Scholar]
- [14].Konstantinopoulos PA, et al. , Phase II study of Avelumab in patients with mismatch repair deficient and mismatch repair proficient recurrent/persistent endometrial Cancer, J. Clin. Oncol 37 (30) (2019) 2786–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Antill YC, et al. , Activity of durvalumab in advanced endometrial cancer (AEC) according to mismatch repair (MMR) status: The phase II PHAEDRA trial (ANZGOG1601), J. Clin. Oncol 37 (15_suppl) (2019) p. 5501–5501. [Google Scholar]
- [16].Wolchok JD, et al. , Overall survival with combined Nivolumab and Ipilimumab in advanced melanoma, N. Engl. J. Med 377 (14) (2017) 1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Motzer RJ, et al. , Nivolumab plus Ipilimumab versus Sunitinib in advanced renal-cell carcinoma, N. Engl. J. Med 378 (14) (2018) 1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hellmann MD, et al. , Nivolumab plus Ipilimumab in advanced non-small-cell lung Cancer, N. Engl. J. Med 381 (21) (2019) 2020–2031. [DOI] [PubMed] [Google Scholar]
- [19].Abou-Alfa GK, et al. , Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA, J. Clin. Oncol 40 (4_suppl) (2022) 379. [Google Scholar]
- [20].Snyder A, et al. , Genetic basis for clinical response to CTLA-4 blockade in melanoma, N. Engl. J. Med 371 (23) (2014) 2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Eisenhauer EA, et al. , New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1), Eur. J. Cancer 45 (2) (2009) 228–247. [DOI] [PubMed] [Google Scholar]
- [22].Cheng DT, et al. , Memorial Sloan Kettering-integrated mutation profiling of actionable Cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology, J Mol Diagn 17 (3) (2015) 251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Middha S, et al. , Reliable Pan-Cancer Microsatellite Instability Assessment by Using Targeted Next-Generation Sequencing Data, 1, 2017. 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Talhouk A, et al. , Confirmation of ProMisE: a simple, genomics-based clinical classifier for endometrial cancer, Cancer 123 (5) (2017) 802–813. [DOI] [PubMed] [Google Scholar]
- [25].Murali R, et al. , Evolving roles of histologic evaluation and molecular/genomic profiling in the Management of Endometrial Cancer, J. Natl. Compr. Cancer Netw 16 (2) (2018) 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].R.-D. et al. , Molecular Classification of Endometrial Carcinomas: A Single-Institution Review, Society of Gynecologic Oncology, Arizona, 2022. [Google Scholar]
- [27].World Medical Association Declaration of Helsinki, Ethical principles for medical research involving human subjects, Jama 310 (20) (2013) 2191–2194. [DOI] [PubMed] [Google Scholar]
- [28].Administration, U.S.F.a.D, Regulations: Good Clinical Practice and Clinical Trials, cited 2022; Available from https://www.fda.gov/science-research/clinical-trials-and-human-subject-protection/regulations-good-clinical-practice-and-clinical-trials 2021. [Google Scholar]
- [29].Muggia FM, et al. , Phase II trial of the pegylated liposomal doxorubicin in previously treated metastatic endometrial cancer: a gynecologic oncology group study, J. Clin. Oncol 20 (9) (2002) 2360–2364. [DOI] [PubMed] [Google Scholar]
- [30].Dizon DS, et al. , Phase II trial of ixabepilone as second-line treatment in advanced endometrial cancer: gynecologic oncology group trial 129-P, J. Clin. Oncol 27 (19) (2009) 3104–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Garcia AA, et al. , A phase II evaluation of weekly docetaxel in the treatment of recurrent or persistent endometrial carcinoma: a study by the gynecologic oncology group, Gynecol. Oncol 111 (1) (2008) 22–26. [DOI] [PubMed] [Google Scholar]
- [32].Concin N, et al. , ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma, Int. J. Gynecol. Cancer 31 (1) (2021) 12–39. [DOI] [PubMed] [Google Scholar]
- [33].Henley SJ, et al. , Annual report to the nation on the status of cancer, part I: national cancer statistics, Cancer 126 (10) (2020) 2225–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fleming GF, et al. , Clinical activity, safety and biomarker results from a phase Ia study of atezolizumab (atezo) in advanced/recurrent endometrial cancer (rEC), J. Clin. Oncol 35 (15_suppl) (2017) 5585. [Google Scholar]
- [35].O’Malley DM, et al. , Pembrolizumab in patients with microsatellite instability-high advanced endometrial Cancer: results from the KEYNOTE-158 study, J. Clin. Oncol 40 (7) (2022) 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]