Abstract
Background
Drug–drug interactions (DDIs) can lead to medication-related harm, and the older population is at greatest risk. We conducted a systematic review and meta-analysis to estimate DDI prevalence and identify common DDIs in older community-dwelling adults.
Methods
PubMed and EMBASE were searched for observational studies published between 01/01/2010 and 10/05/2021 reporting DDI prevalence in community-dwelling individuals aged ≥ 65 years. Nursing home and inpatient hospital studies were excluded. Study quality was assessed using the Joanna Briggs Institute critical appraisal tool. Meta-analysis was performed using a random-effects model with logit transformation. Heterogeneity was evaluated using Cochran’s Q and I2. DDI prevalence and 95% confidence intervals (CIs) are presented. All analyses were performed in R (version 4.1.2).
Results
There were 5144 unique articles identified. Thirty-three studies involving 17,011,291 community-dwelling individuals aged ≥ 65 years met inclusion criteria. Thirty-one studies reported DDI prevalence at the study-participant level, estimates ranged from 0.8% to 90.6%. The pooled DDI prevalence was 28.8% (95% CI 19.3–40.7), with significant heterogeneity (p < 0.10; I2 = 100%; tau2 = 2.13) largely explained by the different DDI identification methods. Therefore, 26 studies were qualitatively synthesised and seven studies were eligible for separate meta-analyses. In a meta-analysis of three studies (N = 1122) using Micromedex®, pooled DDI prevalence was 57.8% (95% CI 52.2–63.2; I2 = 69.6%, p < 0.01). In a meta-analysis of two studies (N = 809,113) using Lexi-Interact®, pooled DDI prevalence was 30.3% (95% CI 30.2–30.4; I2 = 6.8%). In a meta-analysis of two studies (N = 947) using the 2015 American Geriatrics Society Beers criteria®, pooled DDI prevalence was 16.6% (95% CI 5.6–40.2; I2 = 97.5%, p < 0.01). Common DDIs frequently involved cardiovascular drugs, including ACE inhibitor-potassium-sparing diuretic; amiodarone-digoxin; and amiodarone-warfarin.
Conclusions
DDIs are prevalent among older community-dwelling individuals; however, the methodology used to estimate these events varies considerably. A standardised methodology is needed to allow meaningful measurement and comparison of DDI prevalence.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40266-022-01001-5.
Key Points
| Drug–drug interactions (DDIs) are prevalent among older community-dwelling individuals, with estimates that range from 0.8% to 90.6% across studies. |
| Approximately two-thirds of studies reporting DDI prevalence involve (potentially) clinically important DDIs, and drugs routinely prescribed in this older population are commonly implicated, including ACE inhibitor and potassium-sparing diuretic; amiodarone and digoxin; amiodarone and warfarin; beta-blocker and verapamil. |
| Significant statistical heterogeneity between studies, and the wide variability in DDI prevalence estimates, reflects the lack of consensus on the optimal approach to measuring DDIs in this population. |
| A standardised methodology to measure DDI prevalence in the older population is urgently needed. |
Introduction
Medication safety in the older population has been recognised as an important challenge facing global healthcare systems [1]. In 2017, the World Health Organization (WHO) launched its Third Global Patient Safety Challenge: Medication Without Harm, which aims to reduce severe avoidable medication-related harm by 50%, globally between 2017 and 2022 [1, 2]. A drug–drug interaction (DDI) is an example of a potentially avoidable cause of medication-related harm, and occurs when the effect of one drug is altered by the use of another drug [3]. The affected drug is commonly referred to as the object, and the affecting drug as the precipitant [4, 5]. The precipitant drug can increase or decrease the effect of an object drug by multiple mechanisms, including pharmacokinetic and pharmacodynamic mechanisms [4]. Pharmacokinetic interactions arise where the absorption, distribution, metabolism or excretion of an object drug is altered by a precipitant drug (e.g. digoxin toxicity caused by the use of clarithromycin) [6, 7]. Pharmacodynamic interactions occur when a precipitant drug alters the dose–response relationship of an object drug, resulting in a synergistic (equal) or antagonistic (opposing) effect (e.g. the synergistic interaction between aspirin and warfarin, increasing a patient’s bleeding risk) [6, 7].
Pharmacoepidemiological studies measuring DDI prevalence commonly refer to DDIs as “potential”, since it is difficult to precisely establish if a DDI has indeed occurred in the absence of corroborating clinical data. Clinically relevant (or significant/important) DDIs refer to those associated with an established or greatest risk of adverse outcomes [4, 8], and, in general, there is consensus that these are often predictable and largely avoidable causes of medication-related harm [9]. Polypharmacy (regular use of five or more medications) is an independent risk factor for potential DDI exposure [10, 11]. In addition, patients prescribed drugs that have a narrow therapeutic index (e.g. digoxin; lithium; warfarin; phenytoin) [12] and individuals who are more vulnerable because of disease (e.g. renal impairment) [13] are more likely to experience clinically important DDIs. The potential clinical impact of DDIs is, therefore, greatest in older populations due to polypharmacy as well as age-related physiological decline, including decreased renal and hepatic drug clearance [14, 15]; and previous research has reported DDIs to be implicated in adverse drug events in the older population [16–18], including a literature review that estimates approximately 4.8% of hospitalisations in older adults (aged ≥ 65 years) are due to DDIs, with cardiovascular drugs and non-steroidal anti-inflammatory drugs (NSAIDs) most often implicated [16]. The identification of DDIs in older community-dwelling populations, therefore, presents an opportunity to mitigate, often preventable, medication-related harm.
The prevalence of DDIs in older community-dwelling individuals has been studied by many researchers across different countries [19–22]. However, the different methods (e.g. Summary of medicinal Product Characteristics [SmPC], drug interaction databases and expert consensus) used to identify DDIs, as well as the different classifications (e.g. mild, moderate, severe and contraindicated) used to describe the clinical relevance of these events, make it challenging to understand the overall DDI prevalence in this population [13, 14]. In the current literature, systematic reviews examining the prevalence of potential sources of medication-related harm in the older community-dwelling population have largely focused on potentially inappropriate prescribing [23, 24] and adverse drug reactions (ADRs) [25–27]. In contrast, systematic reviews published to date which examine DDI prevalence among older patients have been limited to the hospital setting [28, 29]; have involved multiple settings [30]; or have focused on a specific drug class [31]. Consequently, the prevalence of DDIs among older community-dwelling individuals is unknown. Research in this area of pharmacoepidemiology is important to understand the nature and extent of DDI prevalence in this growing and vulnerable population, and also to inform the WHO’s patient safety agenda. The aim of this systematic review is to summarise the prevalence of DDIs in older community-dwelling adults, and to identify common DDIs in this population.
Methods
This study was conducted and reported using the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement and the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) reporting guidelines (Appendix 1 of the Electronic Supplementary Material [ESM]) [32, 33]. The protocol for this systematic review and meta-analysis was registered on PROSPERO on 27 November, 2020 (ID: CRD42020216686).
Search Strategy
An electronic database search was conducted in PubMed and EMBASE. Date of publication was limited to studies published in the past 11 years (1 January, 2010–10 May, 2021). This time period was chosen as findings would best reflect current prescribing practices in this older population. The search strategy was developed with assistance from a medical librarian. Key search terms included: “prevalence”, “aged” and “drug interactions” (see Appendix 2 of the ESM). Scopus was used for citation tracking and the literature search was supplemented by hand searching the reference lists of included studies for relevant articles meeting inclusion criteria.
Inclusion Criteria
Observational studies published in English reporting the prevalence of DDIs in older adults aged ≥ 65 years in the community setting (including primary care and outpatient settings) were included in this review. Studies that focused on a specific population in the community (e.g. cancer, HIV, epilepsy) were also included for separate subgroup analysis. To be included, studies had to use an objective method (e.g. British National Formulary [34], Micromedex® [35], Beers criteria® [36, 37]) to measure DDIs. Full details of the inclusion criteria are provided in Appendix 2 of the ESM.
Exclusion Criteria
We excluded studies that: focused exclusively on the population aged <65 years; did not report/measure DDI prevalence; only examined drug-disease/alcohol/food interactions and studies that included vitamins or non-allopathic medicines (e.g. herbal and complementary/alternative medicine) in their analysis; were conducted in inpatient hospital settings or nursing home/residential care settings; involved mixed settings (e.g. community dwelling and nursing home), unless DDI prevalence data were reported separately for the community-dwelling population of interest; did not clearly report the method used to identify DDIs; and reported DDI prevalence related to adverse health outcomes/adverse drug reactions. Studies where DDI prevalence was not reported separately from other prescribing criteria and conference proceedings/grey literature were also excluded.
Study Selection
Titles and abstracts were independently double screened (all authors) for eligibility using the agreed inclusion/exclusion criteria, differences were resolved by discussion. Studies were included for full-text review where there was any mention of “drug–drug interactions” in the abstract, including those reporting incidence data, since incidence is often confused with prevalence in epidemiology. In addition, as some explicit criteria for potentially inappropriate medication (PIM) use also include DDIs (e.g. the American Geriatrics Society [AGS] Beers criteria®), full-text review of studies using such PIM measures was undertaken. Full texts were reviewed for eligibility by J.H., and a second review was carried out independently (C.W., C.C. or K.B.). Disagreements between reviewers were resolved by discussion or consensus involving an independent third reviewer (C.C. or K.B.).
Data Extraction
A data extraction form was developed, based on the Joanna Briggs Institute (JBI) data extraction form for prevalence studies template [38]. This form was piloted by three reviewers (J.H., C.W. and C.C.); a copy of the form is included in Appendix 2 of the ESM. Data were extracted independently by J.H., and a 20% sample was extracted in duplicate by C.C. and K.B. for accuracy. Any discrepancies in data extraction were resolved by discussion. Where DDI prevalence data were not extractable for the population aged ≥ 65 years, the corresponding study author was contacted. If we received no reply within 3 weeks of initial contact, the study was excluded.
Quality Assessment
The quality of included studies was independently assessed by two reviewers (J.H. and C.C.) using the JBI critical appraisal tool for prevalence studies [39, 40]. This checklist includes nine criteria, and was specifically developed to assess the internal and external validity of prevalence data included in a systematic review (see Appendix 2 of the ESM). Disagreements between reviewers were resolved by consensus involving an independent third reviewer (K.B.).
Statistical Analysis
A meta-analysis of proportions was performed using a random-effects model with logit transformation and study participants as the unit of analysis. Statistical heterogeneity was assessed using Cochran’s Q (chi-squared statistic) and I2. A p-value <0.10 for the Cochran’s Q test or I2 >50% indicated heterogeneity between studies [41]. Between-study heterogeneity (τ2) was estimated using the maximum likelihood method [42]. To investigate potential sources of heterogeneity, graphic display of study heterogeneity (GOSH) diagnostics were conducted to detect outliers, influential cases, and distinct homogenous subgroups within the modelled data [43]. In addition, subgroup meta-analyses were performed by systematically examining pre-specified a priori study-level characteristics, including study design, setting, DDI classification, and DDI identification method. Sensitivity analyses were also undertaken to assess the effect of removing outliers, studies using non-common DDI identification methods and studies limited to specific patients cohorts (e.g. dementia) on the pooled DDI prevalence estimate. Ninety-five percent confidence intervals (CIs), estimated using the Logit method, and forest plots that summarise weighted proportions are presented. All analyses were performed using the metafor package (version 3.0.2) [44] in R statistical software (version 4.1.2) [45]. The random-effects meta-analysis models were fit using the rma.uni function of the metafor package.
Results
Selection of Studies
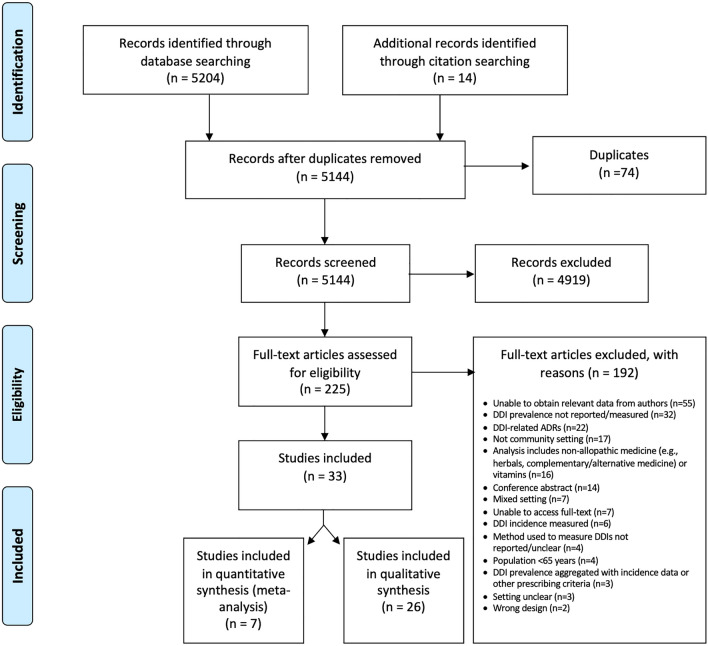
There were 5130 unique articles identified by the electronic database search. Full texts of 211 articles were reviewed, of which 28 studies met inclusion criteria [22, 46–72]. Citation tracking identified an additional 14 articles for full-text review, five of which were included in the final review, resulting in a total of 33 studies [20, 22, 46–76] involving 17,011,291 community-dwelling individuals aged ≥ 65 years (age range 65–103 years) across 17 countries for data extraction (Fig. 1).
Fig. 1.
Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow diagram of systematic literature search and study selection process for the prevalence of drug-drug interactions (DDIs) in older (aged ≥ 65 years) community-dwelling adults. ADRs adverse drug reactions
Characteristics of Included Studies
Table 1 presents the main characteristics of the 33 studies included in this systematic review. The majority of studies were conducted in Europe (n = 13) [20, 46, 49, 53, 54, 56, 61, 64, 69, 70, 74–76], ten were conducted in North America [47, 48, 51, 52, 57, 58, 60, 65, 67, 72], four in South America [59, 62, 63, 68], three in Asia [50, 66, 71], two in Australia [22, 55] and one in Africa [73]. Most studies (n = 23) used a cross-sectional design. Studies ranged in size from a small cross-sectional study of 175 community-dwelling individuals in Albania [53] to a large population-based cohort study of 14.32 million community-dwelling Medicare beneficiaries in the USA [65]. Twenty-four studies [22, 46–52, 55, 57–61, 64, 65, 67, 70–76] reported sex for 16,026,598 community-dwelling individuals aged ≥ 65 years, of whom 8,851,384 (55.2%) were female. Thirty-one studies [20, 22, 46, 48–60, 62–76] reported DDI prevalence estimates using study participants as the unit of analysis, one study [75] also reported DDI prevalence using the total number of prescriptions as the unit of analysis, and one study [64] also reported DDI prevalence using the total number of drugs as the unit of analysis. One study [61] only reported the total number of drug combinations potentially leading to serious DDIs, and one study [47] only reported the proportion of PIMs that were due to DDIs. Most studies (n = 23) measured DDI prevalence for all drugs dispensed/prescribed for study participants, and a limited number of studies measured DDI prevalence according to some defined dispensing or prescribing pattern. This included four studies [20, 55, 66, 76] which measured DDI prevalence for co-prescribed drugs; three studies [51, 57, 63] which measured DDI prevalence for concomitantly prescribed drugs; two studies [22, 56] which measured DDI prevalence for concurrently prescribed drugs; and one study [69] that measured DDI prevalence for both concomitantly and co-prescribed drugs. In general, co-prescribed drugs refers to the prescribing of one or more drug by the same prescriber on the same day [77]; while concomitant and concurrent prescribing are defined as drugs prescribed by one or more different prescribers, not necessarily on the same day [77].
Table 1.
Characteristics of included studies (n = 33)
| Study | Country | Design | Setting | Time period | Source of data | Age (years)£ | Sex (%F)◊ | Number of drugs∆ | Population subgroup | Method used to identify DDIs | Classification of DDIs π | Total N | N ≥ 65 years† | n ≥ 65 years with a DDI† | Prevalence estimate [95% CI] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abubakar et al. (2021) | Nigeria | Cross-sectional | Outpatient | Jun–Sep 2016 | Medical records | 71.1 (±6.1) | 50.8 | 5.4 (±2.3) | – | 2015 AGS Beers criteria® | (Potentially) clinically important | 244 | 244 | 79 | 32.4% [26.8–38.5]F |
| Bacic-Vrca et al. (2010) | Croatia | Cohort | Outpatient | Mar 2009 | Patient interview, pharmacist/physician record | 73 (65–95) | 70.6 | 5 (2–12) | Arterial hypertension | Lexi-interact® | (Potentially) clinically important | 265 | 265 | 240 | 90.6% [86.4–93.5]F |
| Bazargan et al. (2018) | USA | Cross-sectional | Community | Nov 2015–Feb 2017 | Patient interview, drug inventory method | 75.2 (±7) | 67 | 7.3 (±3.60) | Hypertension | 2015 AGS Beers criteria® | (Potentially) clinically important | 193 | 193 | NR≈ | – |
| Bogetti-Salazar et al. (2016) | Mexico | Cross-sectional | Outpatient | Jan 2007–Jan 2010 | Data from the QOL-AD study | 80.11 (±8.28) | 68.5 | 5.20 (±3.04) | Dementia | Micromedex® | All | 181 | 181 | 107 | 59.1% [51.8–66.0]F |
| Burato et al. (2021) | Italy | Cohort | Community | Jan–Jun 2018 | LHA administrative healthcare data | 77.0 (±0.9) | 56.9 | 16.1% used ≥ 5 prescription drugs | – | Delphi consensus | (Potentially) clinically important | 835,247 | 835,247 | 220,175 | 26.4% [26.3–26.5]F |
| Chen et al. (2020) | China | Cross-sectional | Outpatient | Oct 2018–Apr 2019 | Questionnaire | 42.92 (18–85) | 34.7 | 22.1% had co-medications¥ | HIV | University of Liverpool HIV Drug Interactions Checker | All | 1804 | 163¥ | 11¥ | 6.7% [3.8–11.8]F |
| Faught et al. (2018) | USA | Cohort | Community | 2008–2010 | Medicare claims data | NR | 61.6 | NR | Epilepsy | MultipleA | All | 36,912 | 36,912 | 14,396 | 39.0% [38.5–39.5]G |
| Guthrie et al. (2015) | Scotland | Cross-sectional | Community | 1995 and 2010 | GP prescribing data | 50.1 | 51.5 | 17.2% dispensed ≥ 10 drugs in 2010 | – | British National Formulary | (Potentially) clinically important | 311,811 | 73,522 | 25,071 | 34.1% [33.8–34.4]H |
| Hanlon et al. (2017) | USA | Cross-sectional | Community | 1997–1998 | Data from the Health ABC study | 73.6 (±2.9) | 51.5 | 1.73 (±2.0) ; 9.2% took ≥ 5 drugs | – | MultipleB | (Potentially) clinically important | 3055 | 3055 | 767 | 25.1% [23.6–26.7]F |
| Harasani et al. (2020) | Albania | Cross-sectional | Community | Mar–May 2019 | Medical records, patient interview | 73.5 (8) | 43.1 | NR* | – | 2019 AGS Beers criteria® | (Potentially) clinically important | 174 | 125¥ | 1¥ | 0.8% [0.1–5.5]F |
| Hermann et al. (2021) | Norway | Cross-sectional | Community | NR | Interview, visual inspection, medication list | 78 (±3) | 54 | 43% used ≥ 5 drugs | – | Micromedex® | (Potentially) clinically important | 233 | 197 | 107 | 54.3% [47.3–61.1]F |
| Jazbar et al. (2018) | Slovenia | Cohort | Outpatient | 2015 | Pharmacy claims data | NR | 56.8 | 7 (4–11) | – | Lexi-interact® | (Potentially) clinically important | 1,179,803 | 346,708 | 105,355 | 30.4 [30.2–30.5]F |
| Kerr et al. (2014) | Australia | Cohort | Community | Mar 2007–Nov 2009∑ | Data from the ‘Ageing in General Practice’ study | NR | 58.7 | 6.1 (±3.0) µ | CYP enzyme inhibitor and substrate drugs | Flockhart list of CYP450 DDIs | (Potentially) clinically important | 1045 | 1045 | 65 | 6.2% [4.9–7.9]H |
| Lopez-Picazo et al. (2010) | Spain | Cross-sectional | Community | Mar 2007 | Electronic medical record data (OMI-AP) | NR | 51 | NR* | – | Medications database (BOT) of the CGCOF in Spain | All | 430,525 | 64,579 | 18,405 | 28.5% [28.2–28.8]I |
| Lopez-Rodriguez et al. (2020) | Spain | Cross-sectional | Community | Dec 2016–Jan 2017 | Interview with patient's GP | 69.7 (±2.7) | 55.8 | 7.4 (±2.4) ; 17.9% prescribed ≥ 10 drugs | – | ChecktheMeds® | All | 593 | 589 | 373 | 63.3% [59.4–67.1]F |
| Matos et al. (2020) | USA | Cohort | Community | 2017 | Pharmacy claims data | 75.5 (±10.4) | 70.7 | NR | BPSD | Proprietary CDSS | (Potentially) clinically important | 1190 | 1071 | 725 | 67.7% [64.8–70.4]G |
| Naples et al. (2016) | USA | Cohort | Community | 1998–1999‡ | Data from the Health ABC study | 74.6 (±2.9) | 51.3 | 3.2 (±2.7) | 20-m gait speed recorded | MultipleC | (Potentially) clinically important | 2402 | 2402 | 251 | 10.4% [9.3–11.7]F |
| Nikolic et al. (2014) | Serbia | Cross-sectional | Outpatient | Nov 2011 | Electronic prescription database | NR | 57.8 | 4.66 (±2.10)¥ | – | Drug Interaction Facts® | (Potentially) clinically important | 4467 | 2022¥ | 755¥ | 37.3% [35.3–39.5]H |
| Novaes et al. (2017) | Brazil | Cross-sectional | Community | Oct 2014–Mar 2015 | Interview, questionnaire | 73.80 (±8.019) | 64.5 | 44.6% used ≥ 5 drugs | – | Medscape Drug Interactions Checker | All | 368 | 328¥ | 240¥ | 73.2% [68.1–77.7]F |
| Patel et al. (2018) | USA | Cross-sectional | Community | Oct–Nov 2015 | Patient interview | NR | 62.1 | 5.7 (±3.3) | – | 2015 AGS Beers criteria® | (Potentially) clinically important | 703 | 703 | 54 | 7.7% [5.9–9.9]F |
| Popović et al. (2014) | Croatia | Cohort | Outpatient | 2010 | Electronic database (Croatian Health Insurance Fund) | 77 | 63.2 | All n = 29,418 used ≥ 5 drugs | – | Mimica Matanović and Vlahović-Palčevskii DDI list | (Potentially) clinically important | 29,418 | 29,418 | NRΩ | – |
| Roughead et al. (2010) | Australia | Cross-sectional | Community | Jun–Nov 2005 | Veterans’ Affairs Pharmacy claims data | 78.1 (±10.8) | 45 | 9 (±6) | – | MultipleD | (Potentially) clinically important | 287,074 | 287,074 | 4211 | 1.5% [1.4–1.5]I |
| Santos et al. (2019) | Brazil | Cross-sectional | Community | Apr 2015–Feb 2016 | Pharmacy records | 70.2 (±7.8) | 61.3 | NR* | – | 2015 AGS Beers criteria®; Dumbreck et al. disease-specific DDI list | (Potentially) clinically important | 408 | 285¥ | 13 (Beers)¥ | 4.6% [2.7–7.7]F |
| 79 (Dumbreck)¥ | 27.7% [22.8–33.2]F | ||||||||||||||
| Secoli et al. (2010) | Brazil | Cross-sectional | Community | 2000 | Data from the SABE survey study | NR | 65.5 | NR* | – | Micromedex® | All | 1035 | 531 | 288 | 54.2% [50.0–58.4]G |
| Sell and Schaefer (2020) | Germany | Cross-sectional | Community | Apr 2015 | Brown bag medication review | 72.0 (±9.1) | 51.9 | 10.7 (±3.7) | – | PI-Doc® classification | Unclear | 1090 | 830 | 447¥ | 53.9% [50.5–57.2]F |
| Skaar et al. (2011) | USA | Cohort | Community | 2006 | Medicare Current Beneficiary Survey data | NR | 57 | 8.2 | Medicare beneficiaries with a dental visit | Malone et al., 2004 DDI list | (Potentially) clinically important | 14,361,198 | 14,361,198 | 490,874 | 3.4% [3.4–3.4]F |
| Song et al. (2019) | South Korea | Cross-sectional | Outpatient | 2014ø | National insurance claims data | 59 (±13.2) | 67.3 | 8.0 (±6.7) patients with polypharmacy~ | Cancer | MultipleE | (Potentially) clinically important | 118,258 | 41,697 | 4923 | 11.8% [11.5–12.1]H,# |
| Steinman et al. (2014) | USA | Cross-sectional | Outpatient | 2007 | National Veterans Affairs data linked with Medicare claims data | 75 | 2 | 5 (3–8) | – | Lexi-interact® | (Potentially) clinically important | 462,405 | 462,405 | 139,807 | 30.2 [30.1–30.4]F |
| Teixeira et al. (2012) | Brazil | Cross-sectional | Community | May–Dec 2010 | Electronic medical record data | 64.1 (±10.6) | 65.9 | NR* | – | Micromedex® | All | 827 | 394 | 253 | 64.2% [59.4–68.8]F |
| Tragni et al. (2013) | Italy | Cross-sectional | Community | Jan 2004–Aug 2005 | Pharmacy claims data | NR | 51.2 | NR* | – | Micromedex® | (Potentially) clinically important | 2,115,326 | 456,852 | 88,394 | 19.3% [19.2–19.5] H§ |
| Trevisan et al. (2019) | Italy | Cohort | Community | Feb 2002–Feb 2004 | GP databases and records | 76 (71–80) | 61.1 | 53.5% used ≥ 3 prescription drugs | Mild cognitive impairment | INTERcheck® | All | 342 | 342 | 154 | 45.0% [39.8–50.3]F |
| Truong et al. (2019) | Vietnam | Cross-sectional | Outpatient | Aug 2018 | Prescription database | 63.4 (±11.3) | 64.3 | 6.8 ± (2.3); 85.7% used ≥ 5 drugs¥ | Coronary artery diseases | Drugs.com Interactions Checker | (Potentially) clinically important | 683 | 314¥ | 62¥ | 19.7% [15.7–24.5]F |
| Yazdanshenas et al. (2016) | USA | Cross-sectional | Community | 2013 | Patient interview, drug inventory method | NR | 65 | NR | – | Drugs.com Interactions Checker | Unclear | 400 | 400 | 211 | 52.7% [47.8–57.6]F |
AGS American Geriatrics Society, BPSD Behavioural and Psychological Symptoms of Dementia and prescribed an atypical antipsychotic, CDSS clinical decision support system, CGCOF General Council of Official Colleges of Pharmacists, CYP cytochrome P450, F female, GP general practitioner, NR not reported, NR* not reported for the ≥ 65 years of age population, n numerator (number aged ≥ 65 years with a DDI), N denominator (sample size, aged ≥65 years)
£Age data for the full study population, presented as mean; mean (± standard deviation); mean (minimum-maximum); median (interquartile range); or median (minimum-maximum)
◊Data on sex are for the full study population and were extracted directly or estimated from the data reported in the published study
∆Data on the number of drugs used are reported for the population aged ≥65 years, and are presented as mean; mean (± standard deviation); median (minimum-maximum); or median (interquartile range), unless otherwise stated
∑Per ANZCTR registry (trial ID ACTRN12607000117415)
øDDI prevalence data for ≥65 years of age population reported in the published study for 2014 only
¥Data provided by corresponding author
µRefers to the cohort of study participants with a potential CYP DDI
~Definition of polypharmacy not provided in this study
πA full description of the DDI classification for each study is included in Appendix 6 of the ESM
†A full description of the numerator and denominator for each study is provided in Appendix 7 of the ESM
≈Almost 23% (43 out of 188 potentially inappropriate medications) of potentially inappropriate medications were due to drugs with potential DDIs
ΩThe total number of drug combinations potentially leading to serious DDIs was 33,321
#9.6% is reported in Table 6 of the published study; however, the corresponding denominator for this % does not reconcile with the data reported in Table 1, see Appendix 7 of the ESM
‡DDI prevalence data from year 2 of this study were extracted. Hanlon et al., 2017 used the same data source (Health ABC study), but the authors report data from year 1 of the study
AUS Food and Drug Administration-approved package insert as the primary source, supplemented with the literature; Medscape Drug Interactions Checker; as well as consulting lists of interactions from other proprietary services: Micromedex®; Clinical Pharmacology; and Lexicomp®
BLiterature and 2015 AGS Beers criteria®
C2015 AGS Beers criteria®, Mimica Matanovic and Vlahovic-Palcevski protocol DDI list, and other expert panel consensus explicit criteria from the literature
DVidal, British National Formulary, Drug Interaction Facts, and Micromedex® Drug-Reax
EDrug Interaction Facts®, Micromedex®, Lexi-interact®
FDispensing/prescribing pattern for DDI prevalence: all drugs dispensed/prescribed
GDispensing/prescribing pattern for DDI prevalence: concomitant
HDispensing/prescribing pattern for DDI prevalence: co-prescribed
IDispensing/prescribing pattern for DDI prevalence: concurrent
§Tragni et al., 2013 DDI prevalence for concomitantly dispensed/prescribed drugs: n = 120,921 (26.5% [26.3–26.6])
Quality Assessment
Eighteen studies [20, 22, 49, 52, 54–56, 58, 61, 64–70, 75, 76] were rated as being of high methodological quality, and 15 studies [46–48, 50, 51, 53, 57, 59, 60, 62, 63, 71–74] were judged to have moderate methodological quality. A full description of the JBI methodological quality assessment, and rating justification, for each of the 33 studies included in this review is provided in Appendices 3–5 of the ESM.
DDI Identification Method
The method used by individual studies to identify DDIs varied. Five studies [48, 63, 68, 69, 74] used the Micromedex® drug interaction database; three studies [47, 60, 73] used the 2015 AGS Beers criteria®; three studies [46, 54, 67] used the Lexi-Interact® drug interaction database; two studies [71, 72] used the drugs.com interaction checker; six studies [22, 51, 52, 58, 62, 66] used multiple methods (e.g. Roughead et al. [22] used Vidal, British National Formulary, Drug Interaction Facts and Micromedex®); and 14 studies [20, 49, 50, 53, 55–57, 59, 61, 64, 65, 70, 75, 76] used a single unique method (Table 1).
DDI Classification
Of the 33 studies included, 22 studies [20, 22, 46, 47, 49, 52–55, 57, 58, 60–62, 65–67, 69, 71, 73, 74, 76] measured the prevalence of DDIs, which were broadly classified as (potentially) clinically important; nine studies [48, 50, 51, 56, 59, 63, 68, 70, 75] measured the prevalence of any DDI (e.g. mild/moderate/severe/contraindicated), of which three studies [48, 51, 68] reported DDI prevalence by classification rating for the ≥ 65 years population; and in two studies [64, 72], the DDI classification was unclear. A full description of the DDI classification rating(s) used by each study included in this review is outlined in Appendix 6 of the ESM.
DDI Prevalence
A description of the numerator and denominator extracted and used to estimate DDI prevalence for each study is presented in Appendix 7 of the ESM. Across 31 studies using study participants as the unit of analysis, DDI prevalence estimates varied, ranging from 0.8% in Albania [53] to 90.6% in Croatia [46] (Table 1). A random-effects meta-analysis revealed considerable variability in the pooled DDI prevalence estimate (28.8% [95% CI 19.3–40.7]), and significant statistical heterogeneity between studies (df = 29, Q = 1317371.14; p < 0.10; I2 = 100%; tau2 = 2.13) [Appendix 8 of the ESM]. For this reason, a meta-analysis of the full data was not possible. Following extensive investigation of heterogeneity using GOSH diagnostics, as well as subgroup and sensitivity analyses (see Appendices 9–11 of the ESM), the heterogeneity was largely explained by the different DDI identification methods used across studies. Therefore, 26 studies were qualitatively synthesised and seven studies were deemed eligible for meta-analyses.
Qualitative Synthesis
Twenty-six studies were identified for qualitative synthesis, of which 14 studies [20, 22, 49, 52, 53, 56, 59, 61, 62, 64, 69, 72, 75, 76] measured DDI prevalence in the general older (aged ≥ 65 years) community-dwelling population, and 12 studies [46–48, 50, 51, 55, 57, 58, 65, 66, 70, 71] measured DDI prevalence for a specific patient subgroup of this population (Table 1). Of the 14 studies reporting DDI prevalence for the general older population, nine studies were conducted in Europe, where DDI prevalence estimates ranged from 0.8% in Albania to 63.3% in Spain (Table 1). Six of these European studies measured DDIs broadly classified as (potentially) clinically important, with DDI prevalence estimates that ranged from 0.8% to 37.3% (Table 1). A summary of all studies included in this systematic review which report DDI prevalence estimates by classification rating (e.g. mild, moderate, severe/contraindicated) for the ≥ 65 years of age population is provided in Appendix 12 of the ESM.
Meta-analysis
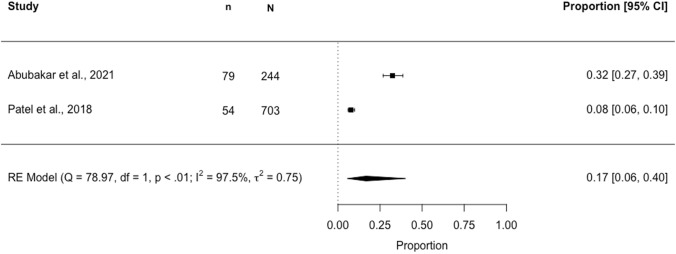
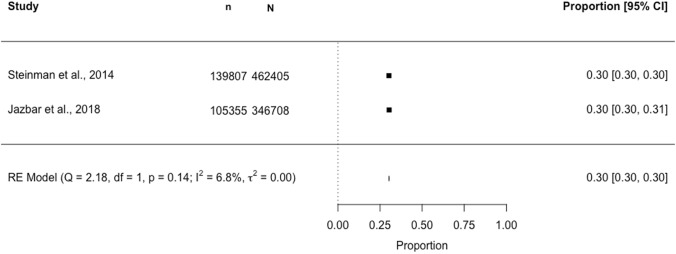
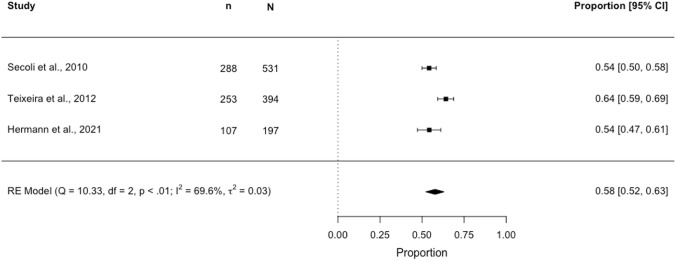
Seven studies were identified for the meta-analysis. Three separate meta-analyses estimating DDI prevalence across subgroups of studies using a common DDI identification method are presented in Figs. 2, 3 and 4. In a meta-analysis of two studies (N = 947) using the 2015 AGS Beers criteria®, the pooled DDI prevalence in older (≥ 65 years) community-dwelling individuals was estimated to be 16.6% (95% CI 5.6–40.2; I2 97.5%, p < 0.01) (Fig. 2). In a meta-analysis of two studies (N = 809,113) using Lexi-Interact®, the pooled DDI prevalence was 30.3% (95% CI 30.2–30.4; I2 6.8%, p = 0.14) (Fig. 3). In a meta-analysis of three studies (N = 1122) using Micromedex®, the pooled DDI prevalence was 57.8% (95% CI 52.2–63.2; I2 69.6%, p < 0.01) (Fig. 4).
Fig. 2.
Forest plot showing the proportion [95% confidence interval (CI)] of older (aged ≥ 65 years) community-dwelling individuals potentially exposed to a drug–drug interaction (DDI)*‡, identified using the 2015 American Geriatrics Society Beers criteria®. n, numerator (number aged ≥65 years with a DDI ); N, denominator (sample size, aged ≥ 65 years). *Denominator (N): total number of participants aged ≥ 65 years included in the study; ‡DDI classification: “Potentially Clinically Important Non-Anti-infective Drug–Drug Interactions That Should Be Avoided in Older Adults”.
Fig. 3.
Forest plot showing the proportion [95% confidence interval (CI)] of older (aged ≥ 65 years) community-dwelling individuals potentially exposed to a drug–drug interaction (DDI)*‡, identified using the Lexi-Interact® database. n, numerator (number aged ≥65 years with a DDI ); N, denominator (sample size, aged ≥ 65 years). *Denominator (N): study participants ≥ 65 years dispensed/prescribed two or more drugs (< 20% of the population in Steinman et al. used one to two medications); ‡DDI classification: clinically significant DDIs, classified as type D or X per Lexi-Interact®
Fig. 4.
Forest plot showing the proportion [95% confidence interval (CI)] of older (aged ≥ 65 years) community-dwelling individuals potentially exposed to a drug–drug interaction (DDI)*†‡, identified using the Micromedex® database. n, numerator (number aged ≥ 65 years with a DDI); N, denominator (sample size, aged ≥ 65 years). *Denominator (N): study participants aged ≥65 years dispensed/prescribed two or more drugs; ‡DDI classification: potentially clinically important DDIs, classified as moderate, major, high or contraindicated per Micromedex®; †mild DDIs were identified in < 10% of the overall study population aged ≥ 60 years for Secoli et al. and Teixeira et al.
Common DDIs Across Included Studies
Of the 33 studies included in this review, 15 studies [22, 46–49, 52, 53, 55, 58, 59, 61, 67, 70, 73, 76] reported data at the individual-drug or drug-class level for at least one DDI implicated in the overall DDI prevalence reported for the ≥65 years of age community-dwelling population (Appendix 13 of the ESM). DDIs were broadly classified as (potentially) clinically important in 14 studies, and in one study [70] the classification rating for the most common DDIs was unclear. Common DDIs reported across the 14 studies included: ACE inhibitor potassium-sparing diuretic (n = 6 studies [22, 52, 58, 61, 73, 76]); amiodarone-digoxin (n = 5 studies [22, 46, 52, 58, 61]); amiodarone-warfarin (n = 3 studies [46, 52, 55]); beta-blocker-verapamil (n = 2 studies [22, 61]); warfarin-NSAID (n = 2 studies [22, 52]); and ACE inhibitor-allopurinol (n = 2 studies [46, 67]). Appendix 14 of the ESM provides a summary of all common DDIs that were identified in at least two studies included in this review.
Discussion
To our knowledge, this is the first systematic review and meta-analysis on DDI prevalence in older (aged ≥ 65 years) community-dwelling adults. We identified 31 studies reporting DDI prevalence at the study-participant level. Most studies (n = 22) measured DDIs which were broadly classified as (potentially) clinically important. There was significant heterogeneity between studies when DDI prevalence estimates were pooled in a meta-analysis; and this was largely explained by the different DDI identification methods used by studies. When subgroup meta-analyses were conducted for studies using a common DDI identification method, there was a reduction in heterogeneity and variance within, but not between, subgroups. Moreover, there was a wide variation in the pooled DDI prevalence estimates across these subgroups (ranging from 16.6% in studies using the 2015 AGS Beers criteria®, to 30.3% in studies using Lexi-Interact®, to 57.8% in studies using Micromedex®), which could not unequivocally be attributed to clinical heterogeneity (e.g. polypharmacy). Indeed, DDI prevalence might also be expected to vary across different countries where different healthcare systems are in operation [21]; however, we found no clear trend in the data. This systematic review therefore highlights that DDI prevalence estimates vary depending on the identification method used. This review also identified several (potentially) clinically important DDIs, involving routinely prescribed drugs in this population, many of which were common across multiple studies, including: ACE inhibitor-potassium-sparing diuretic [22, 52, 58, 61, 73, 76]; amiodarone-digoxin [22, 46, 52, 58, 61]; amiodarone-warfarin [46, 52, 55]; beta-blocker-verapamil [22, 61]; warfarin-NSAID [22, 52]; and ACE inhibitor-allopurinol [46, 67]. These specific DDIs may confer severe and potentially life-threatening harm to the older patient, including hospitalisation for haemorrhage, as has been highlighted by previous studies [78–83].
The wide variation in DDI prevalence estimates identified by this systematic review is similar to a recent systematic review which reports DDI prevalence in hospitalised older patients (8.34–100%) [28]. The authors suggested the use of different DDI identification methods to be potentially responsible for this variation, but did not test this hypothesis. Another review by Sánchez-Fidalgo et al. [30] similarly reports wide variation in the prevalence of drug interactions in older patients with multimorbidity (25.1 to 100%); however, this review included both primary care and nursing home settings, and identified only a limited number of studies (n = 703) for title and abstract review. In another recent systematic review, Zheng et al. report an overall prevalence of 33% (95% CI 17.5–51.3; I2 = 99.7%, p < 0.0001) of general inpatients with at least one potential DDI during their hospital stay; however, only three of the 11 studies included in their meta-analysis used a common DDI identification method (Micromedex®) [29], and as we have shown, DDI prevalence estimates vary depending on the identification method used. The large variation and significant heterogeneity in the pooled DDI prevalence estimate reported by Zheng et al. is therefore not surprising, and further suggests that restricting a meta-analysis to studies using a common DDI identification method may provide more meaningful DDI prevalence estimates. Previous research has shown DDI prevalence to increase over time [20, 84]; however, such a trend is difficult to interpret when different methods are used to measure DDIs, as the present review highlights. The relatively high DDI prevalence reported by some studies included in this systematic review should be acknowledged, in particular since this was not unique to the nine studies which measured the prevalence of any DDI, as one would expect. The high DDI prevalence reported by some studies could be due to multiple prescribers [85], as most studies measured DDI prevalence for all drugs dispensed/prescribed. Further, previous research has found poor or limited awareness of clinically important DDIs among prescribers [86, 87], which may also explain the high prevalence of (potentially) clinically important DDIs reported by many studies included in this systematic review. However, the reasons underlying the high and variable DDI prevalence estimates across studies identified by this systematic review are likely more complex. Indeed, previous research has suggested that DDI prevalence estimates vary due to differences in patient populations, and the databases and information sources used to measure these events [13]. Our systematic review confirms these theories, and highlights the need for consensus on how to identify and measure DDIs in the older population.
Currently, DDIs for a specific medicine can be identified using the product’s SmPC, though this legal document tends to include all potential DDIs and generally provides non-specific recommendations [88], which is of limited utility in clinical practice. The AGS Beers criteria® are also used to identify DDIs, though these criteria include only a limited number of DDIs, largely reported at the drug-class level [36, 37], and therefore likely under-estimate true DDI prevalence. In addition, there are multiple DDI databases that are commonly used in both research and clinical practice, including: Micromedex®; Lexi-Interact®; the British National Formulary (electronic and paper); and Stockley’s, often referred to as the gold standard [89]. These compendia generally provide evidence-based guidance to manage any possible DDI; however, recommendations can vary across these databases (e.g. monitor vs avoid). Further, although US and European regulatory authorities require that relevant interaction studies be performed before a marketing authorisation for a medicine can be granted [90, 91], older adults are generally not included in these studies [90, 92]. Consequently, DDIs in the older population are often identified using post-marketing spontaneous pharmacovigilance surveillance methods [92], adding further complexity to the identification and assessment of DDIs in this population. In addition, there is currently no standardised taxonomy for the identification of DDIs (i.e. whether to measure DDIs at the individual-drug level or drug-class level, and which classification rating to use [i.e. mild/moderate/severe/contraindicated]). Further, with the approval of new medicines, which potentially may confer important interactions with other commonly used drugs, the validity of DDI lists in the current literature is therefore time varying and hence these lists need to be updated in line with new evidence. The use of a common DDI identification methodology instead of a static DDI list, which is vulnerable to becoming outdated, is one possible solution to manage this issue; indeed, this would also facilitate a meaningful comparison of DDI prevalence estimates across different studies and settings.
Strengths and Limitations
This is the first systematic review and meta-analysis to describe DDI prevalence in the older community-dwelling population. Our search strategy was comprehensive and identified a large number (n = 5144) of articles, published over the past 11 years, for review. In addition, we used rigorous systematic review methods to extract, appraise and report the data. Our study has some important limitations to acknowledge. Significant heterogeneity meant that it was not possible to estimate a meaningful overall DDI prevalence estimate for the older community-dwelling population. Due to the lack of standardised reporting of polypharmacy/medication burden across studies, it was not possible to fully investigate this potential source of clinical heterogeneity. Further, given the limited number of included studies that used a common identification method to measure DDI prevalence in this older population, the pooled DDI prevalence estimates we report should be interpreted with caution. Some studies used DDI identification methods with limited validity, and future research should address this limitation. Most of the studies included in this review did not include data on over-the-counter medications, hence our findings may underestimate the true DDI prevalence in this population. Additionally, conference proceedings and grey literature were not included.
Implications
This systematic review provides a greater understanding of the prevalence of DDIs in the older community-dwelling population over the past 11 years, and also offers an insight into some of the DDIs commonly reported for this population during this time period. Our findings clearly highlight the need for a standardised method to measure DDI prevalence, for meaningful comparison across studies. A single DDI identification methodology needs to be agreed and endorsed; or alternatively, a comprehensive list of DDIs, which is periodically updated (e.g. every 6 months) to reflect both current clinical practice and emerging evidence of clinically important DDIs, needs to be developed and maintained. Such a list could first be developed at a European level in collaboration with expert stakeholders (e.g. the European Medicine Agency’s Geriatric Expert Group) [93, 94]. This may help to address common issues in current clinical practice such as “alert-fatigue” [95]; and may also prompt the development of interventions to improve prescribing for this older population, including pharmacist-led medication review and reconciliation processes. As an initial starting point, and based on our overall findings and appraisal of the current literature, we have developed methodological reporting recommendations (Box 1) to encourage the standardised reporting of DDI prevalence data. Indeed, in the absence of a common DDI identification methodology, if studies measuring DDI prevalence can report baseline characteristics of their population and the specific DDIs identified in a standardised manner (as proposed in Box 1), then this would help to identify further common DDIs, which could then be assessed in health outcomes studies. This would facilitate the identification of a core set of common clinically important DDIs that could then be measured and monitored over time, with greater uniformity. More generally, in clinical practice, pharmacists and other expert healthcare professionals could develop a local list of known and clinically important DDIs specific to their patient group and setting—this would provide the opportunity to undertake routine quality improvement initiatives, such as a clinical audit, to monitor and improve prescribing habits, and ultimately to mitigate medication-related harm.
The overall high DDI prevalence identified by this review has important implications for clinicians, patients and health systems; in particular since this was not unique to the nine studies which measured the prevalence of any DDI, as one would expect. Drug–drug interactions are generally considered to be a predictable and avoidable cause of medication-related harm [9], and, globally, as the older population continues to grow, healthcare professionals caring for these individuals should be aware of the medications commonly implicated in clinically significant DDIs when prescribing, dispensing, and during medication review. In clinical practice, routine surveillance of prescriptions for the older population represents one DDI mitigation measure. However, healthcare professionals need to be cognisant of medications commonly implicated in known clinically important DDIs; indeed, a basic understanding of the mechanism of DDIs may also help prescribers to recognise common precipitant and object drugs, and thereby mitigate the risk of avoidable medication-related harm in this growing and vulnerable patient population. In general, further pharmacoepidemiological research is needed to monitor trends in DDI prevalence, as well as DDI-related health outcomes, and studies which are uniform in both methodology and reporting are needed globally to better understand the prevalence of DDIs in this older population.
Box 1: Recommendations for studies measuring drug–drug interaction (DDI) prevalence at the population level.
Studies measuring drug-drug interaction (DDI) prevalence should consider the following methodological and reporting recommendations:
- Methods
- Describe the DDI identification method used and the rationale for using this specific method;
- Describe the DDI classification rating used (i.e. all; mild; moderate; severe; or contraindicated);
- Identify the unit of analysis used: when measuring prevalence, the unit of analysis should be the individual study participant, i.e. for DDI prevalence:
*since the use of at least 2 distinct medications is a prerequisite for a potential DDI to occur.
- Declare the specific prescribing/dispensing pattern, i.e. whether the DDI prevalence estimates reported refer to DDIs involving any drugs prescribed/dispensed; co-prescribed; concurrently prescribed/dispensed; or drugs which were prescribed/dispensed on different days or within a given time interval (e.g. ±7 days). The prescribing/dispensing pattern should be clearly reported in the methods.
-
2.Results
-
2.
Present baseline characteristics for study participants, including: mean number of medications used and/or polypharmacy (use a standard definition, i.e. regular use of ≥5 drugs); co-morbidities; sex.
- Report DDI prevalence as a percentage with 95% confidence intervals.
-
i.As the use of at least 2 drugs is a limiting step in potential DDI exposure, to standardise the reporting of DDI prevalence we suggest that researchers report DDI prevalence among those using ≥2 distinct drugs.
-
ii.Report DDI prevalence for the number of study participants potentially exposed to at least one (≥1) DDI. Higher sets (e.g. ≥2, 3) can also be reported separately.
-
iii.The total number (and/or proportion) of DDIs and/or prescriptions with a DDI can also be reported; however, the DDI prevalence estimate should be expressed in terms of the total number of study participants using at least 2 distinct drugs.
-
iv.At a minimum, report the top 10 most prevalent DDIs, preferably at the individual drug level, not the drug class level—this will facilitate meaningful comparison of common DDIs across studies, and allow the most prevalent DDIs reported in the literature for a given population to be identified and discussed more concretely. The classification rating for specific individual-level DDIs should be clearly reported.
-
i.
Conclusions
Drug–drug interactions are prevalent among older community-dwelling individuals, and most are classified as (potentially) clinically important; however, the methodology used to estimate these events varies considerably. A standardised methodology is urgently needed to allow meaningful measurement and comparison of DDI prevalence in this growing and vulnerable population.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge and thank Paul Murphy (RCSI librarian) for his support in developing the search strategy. We also thank all authors who replied to our correspondence request for DDI prevalence data; in particular, we are grateful to the authors who provided the requested data that were not originally included in their published study. Finally, we wish to acknowledge the SPHeRE PhD programme.
Declarations
Funding
Open Access funding provided by the IReL Consortium. This research was supported by funding from the Health Research Board Research Leader Award (grant number RL-15-1579) and the Irish Research Council Government of Ireland Postgraduate Scholarship Programme Award to J.H. (grant number GOIPG/2021/1213). C.C. is supported by funding from the Health Research Board (SDAP-2021-020). The funding bodies had no part in the study design, the identification, analysis and collection of the data, or preparation of the manuscript for publication.
Conflicts of Interest/Competing Interests
The authors have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
All data generated or analysed during this study are included in this article (and its supplementary information files).
Code Availability
Not applicable.
Authors’ Contributions
JH, CC and KB were involved in the concept and design of the study. JH led on the analysis and interpretation of the results. All authors were involved in title and abstract screening. JH led on the full-text selection and all authors were involved in this process. JH and CC undertook the quality appraisal of the included studies, and KB acted as the third reviewer. JH prepared the first draft of the manuscript, CC and KB provided initial feedback. All authors read and approved the final manuscript.
References
- 1.The World Health Organization. WHO global patient safety challenge: medication without harm. 2017. http://apps.who.int/iris/bitstream/handle/10665/255263/WHO-HIS-SDS-2017.6-eng.pdf?ua=1&ua=1?sequence=1. Accessed 10 May 2021.
- 2.Donaldson LJ, Kelley ET, Dhingra-Kumar N, Kieny M-P, Sheikh A. Medication without harm: WHO's third global patient safety challenge. Lancet. 2017;389(10080):1680–1681. doi: 10.1016/S0140-6736(17)31047-4. [DOI] [PubMed] [Google Scholar]
- 3.Preston CL. Stockley's drug interactions: a source book of interactions, their mechanisms, clinical importance and management. 12. Pharmaceutical Press; 2019. [Google Scholar]
- 4.Aronson JK, Grahame-Smith DG. Clinical pharmacology: adverse drug interactions. Br Med J (Clin Res Ed) 1981;282(6260):288. doi: 10.1136/bmj.282.6260.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hennessy S, Leonard C, Gagne J, Flory J, Han X, Brensinger C, et al. Pharmacoepidemiologic methods for studying the health effects of drug–drug interactions. Clin Pharmacol Ther. 2016;99(1):92–100. doi: 10.1002/cpt.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas D. Clinical pharmacy education, practice and research: clinical pharmacy, drug information, pharmacovigilance, pharmacoeconomics and clinical research. Elsevier; 2018. [Google Scholar]
- 7.Katzung BG. Basic and clinical pharmacology. 14. McGraw-Hill Education; 2017. [Google Scholar]
- 8.Patel AM, Shariff S, Bailey DG, Juurlink DN, Gandhi S, Mamdani M, et al. Statin toxicity from macrolide antibiotic coprescription: a population-based cohort study. Ann Intern Med. 2013;158(12):869–876. doi: 10.7326/0003-4819-158-12-201306180-00004. [DOI] [PubMed] [Google Scholar]
- 9.Magro L, Moretti U, Leone R. Epidemiology and characteristics of adverse drug reactions caused by drug–drug interactions. Expert Opin Drug Saf. 2012;11(1):83–94. doi: 10.1517/14740338.2012.631910. [DOI] [PubMed] [Google Scholar]
- 10.Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57–65. doi: 10.1517/14740338.2013.827660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wastesson JW, Morin L, Tan EC, Johnell K. An update on the clinical consequences of polypharmacy in older adults: a narrative review. Expert Opin Drus Saf. 2018;17(12):1185–1196. doi: 10.1080/14740338.2018.1546841. [DOI] [PubMed] [Google Scholar]
- 12.Blix HS, Viktil KK, Moger TA, Reikvam A. Drugs with narrow therapeutic index as indicators in the risk management of hospitalised patients. Pharm Pract. 2010;8(1):50. doi: 10.4321/s1886-36552010000100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hines LE, Murphy JE. Potentially harmful drug–drug interactions in the elderly: a review. Am J Geriatr Pharmacother. 2011;9(6):364–377. doi: 10.1016/j.amjopharm.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Mallet L, Spinewine A, Huang A. The challenge of managing drug interactions in elderly people. Lancet. 2007;370(9582):185–191. doi: 10.1016/S0140-6736(07)61092-7. [DOI] [PubMed] [Google Scholar]
- 15.Delafuente JC. Understanding and preventing drug interactions in elderly patients. Crit Rev Oncol Hematol. 2003;48(2):133–143. doi: 10.1016/j.critrevonc.2003.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Becker ML, Kallewaard M, Caspers PW, Visser LE, Leufkens HG, Stricker BH. Hospitalisations and emergency department visits due to drug–drug interactions: a literature review. Pharmacoepidemiol Drug Saf. 2007;16(6):641–651. doi: 10.1002/pds.1351. [DOI] [PubMed] [Google Scholar]
- 17.Olivier P, Bertrand L, Tubery M, Lauque D, Montastruc J-L, Lapeyre-Mestre M. Hospitalizations because of adverse drug reactions in elderly patients admitted through the emergency department. Drugs Aging. 2009;26(6):475–482. doi: 10.2165/00002512-200926060-00004. [DOI] [PubMed] [Google Scholar]
- 18.Gurwitz JH, Field TS, Harrold LR, Rothschild J, Debellis K, Seger AC, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289(9):1107–1116. doi: 10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- 19.Hughes JE, Russo V, Walsh C, Menditto E, Bennett K, Cahir C. Prevalence and factors associated with potential drug-drug interactions in older community-dwelling adults: a prospective cohort study. Drugs Aging. 2021;38(11):1025–1037. doi: 10.1007/s40266-021-00898-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guthrie B, Makubate B, Hernandez-Santiago V, Dreischulte T. The rising tide of polypharmacy and drug-drug interactions: population database analysis 1995–2010. BMC Med. 2015;13(1):74. doi: 10.1186/s12916-015-0322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Björkman IK, Fastbom J, Schmidt IK, Bernsten CB, Group PCotEiER Drug–drug interactions in the elderly. Ann Pharmacother. 2002;36(11):1675–1681. doi: 10.1345/aph.1A484. [DOI] [PubMed] [Google Scholar]
- 22.Roughead EE, Kalisch LM, Barratt JD, Gilbert AL. Prevalence of potentially hazardous drug interactions amongst Australian veterans. Br J Clin Pharmacol. 2010;70(2):252–257. doi: 10.1111/j.1365-2125.2010.03694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tommelein E, Mehuys E, Petrovic M, Somers A, Colin P, Boussery K. Potentially inappropriate prescribing in community-dwelling older people across Europe: a systematic literature review. Eur J Clin Pharmacol. 2015;71(12):1415–1427. doi: 10.1007/s00228-015-1954-4. [DOI] [PubMed] [Google Scholar]
- 24.Opondo D, Eslami S, Visscher S, De Rooij SE, Verheij R, Korevaar JC, et al. Inappropriateness of medication prescriptions to elderly patients in the primary care setting: a systematic review. PLoS ONE. 2012;7(8):e43617. doi: 10.1371/journal.pone.0043617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alhawassi TM, Krass I, Bajorek BV, Pont LG. A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin Interv Aging. 2014;9:2079. doi: 10.2147/CIA.S71178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kongkaew C, Noyce PR, Ashcroft DM. Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Ann Pharmacother. 2008;42(7–8):1017–1025. doi: 10.1345/aph.1L037. [DOI] [PubMed] [Google Scholar]
- 27.Taché SV, Sönnichsen A, Ashcroft DM. Prevalence of adverse drug events in ambulatory care: a systematic review. Ann Pharmacother. 2011;45(7–8):977–989. doi: 10.1345/aph.1P627. [DOI] [PubMed] [Google Scholar]
- 28.de Oliveira LM, Diel JdAC, Nunes A, Dal Pizzol TdS. Prevalence of drug interactions in hospitalised elderly patients: a systematic review. Eur J Hosp Pharm. 2021;28(1):4–9. doi: 10.1136/ejhpharm-2019-002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng WY, Richardson L, Li L, Day R, Westbrook J, Baysari M. Drug-drug interactions and their harmful effects in hospitalised patients: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2018;74(1):15–27. doi: 10.1007/s00228-017-2357-5. [DOI] [PubMed] [Google Scholar]
- 30.Sánchez-Fidalgo S, Guzmán-Ramos MI, Galván-Banqueri M, Bernabeu-Wittel M, Santos-Ramos B. Prevalence of drug interactions in elderly patients with multimorbidity in primary care. Int J Clin Pharm. 2017;39(2):343–353. doi: 10.1007/s11096-017-0439-1. [DOI] [PubMed] [Google Scholar]
- 31.Thai M, Reeve E, Hilmer SN, Qi K, Pearson S-A, Gnjidic D. Prevalence of statin-drug interactions in older people: a systematic review. Eur J Clin Pharmacol. 2016;72(5):513–521. doi: 10.1007/s00228-016-2011-7. [DOI] [PubMed] [Google Scholar]
- 32.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 33.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 34.Joint Formulary Committee. British National Formulary. London: BMJ Group and Pharmaceutical Press. http://www.medicinescomplete.com. Accessed 10 May 2021.
- 35.IBM Micromedex®. Drug interaction checking (electronic version). Greenwood Village, Colorado, USA: IBM Watson Health. https://www.micromedexsolutions.com/. Accessed 10 May 2021.
- 36.Fick DM, Semla TP, Beizer J, Brandt N, Dombrowski R, DuBeau CE, American Geriatrics Society Beers Criteria Update Expert Panel et al. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227–2246. doi: 10.1111/jgs.13702. [DOI] [PubMed] [Google Scholar]
- 37.Fick DM, Semla TP, Steinman M, Beizer J, Brandt N, Dombrowski R, et al. American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674–694. doi: 10.1111/jgs.15767. [DOI] [PubMed] [Google Scholar]
- 38.Munn Z MS, Lisy K, Riitano D, Tufanaru C. Chapter 5: systematic reviews of prevalence and incidence. In: Aromataris E, Munn Z, editors. JBI manual for evidence synthesis. 2020. https://synthesismanual.jbi.global. Accessed 4 Oct 2021.
- 39.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13(3):147–153. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 40.Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014;3(3):123. doi: 10.15171/ijhpm.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pereira TV, Patsopoulos NA, Salanti G, Ioannidis JP. Critical interpretation of Cochran's Q test depends on power and prior assumptions about heterogeneity. Res Synth Methods. 2010;1(2):149–161. doi: 10.1002/jrsm.13. [DOI] [PubMed] [Google Scholar]
- 42.Viechtbauer W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J Educ Behav Stat. 2005;30(3):261–293. doi: 10.3102/10769986030003261. [DOI] [Google Scholar]
- 43.Viechtbauer W, Cheung MWL. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1(2):112–125. doi: 10.1002/jrsm.11. [DOI] [PubMed] [Google Scholar]
- 44.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 45.R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing. 2020. https://www.R-project.org/. Accessed 2 Jan 2023.
- 46.Bacic-Vrca V, Marusic S, Erdeljic V, Falamic S, Gojo-Tomic N, Rahelic D. The incidence of potential drug–drug interactions in elderly patients with arterial hypertension. Pharm World Sci. 2010;32(6):815–821. doi: 10.1007/s11096-010-9442-5. [DOI] [PubMed] [Google Scholar]
- 47.Bazargan M, Smith JL, King EO. Potentially inappropriate medication use among hypertensive older African-American adults. BMC Geriatr. 2018;18(1):238. doi: 10.1186/s12877-018-0926-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bogetti-Salazar M, González-González C, Juárez-Cedillo T, Sánchez-García S, Rosas-Carrasco O. Severe potential drug-drug interactions in older adults with dementia and associated factors. Clinics (Sao Paulo) 2016;71(1):17–21. doi: 10.6061/clinics/2016(01)04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burato S, Leonardi L, Antonazzo IC, Raschi E, Ajolfi C, Baraghini M, et al. Comparing the prevalence of polypharmacy and potential drug-drug interactions in nursing homes and in the community dwelling elderly of Emilia Romagna region. Front Pharmacol. 2021;11:624888. doi: 10.3389/fphar.2020.624888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen R, Chen J, Tang Q, Meng Z, Luo L, Zhang W, et al. Use of comedications and potential drug-drug interactions in people living with HIV in China. J Infect Chemother. 2020;26(7):722–728. doi: 10.1016/j.jiac.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 51.Faught E, Szaflarski JP, Richman J, Funkhouser E, Martin RC, Piper K, et al. Risk of pharmacokinetic interactions between antiepileptic and other drugs in older persons and factors associated with risk. Epilepsia. 2018;59(3):715–723. doi: 10.1111/epi.14010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanlon JT, Perera S, Newman AB, Thorpe JM, Donohue JM, Simonsick EM, et al. Potential drug-drug and drug-disease interactions in well-functioning community-dwelling older adults. J Clin Pharm Ther. 2017;42(2):228–233. doi: 10.1111/jcpt.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harasani K, Xhafaj D, Begolli A, Olvera-Porcel MC. Prevalence of potentially inappropriate prescriptions in primary care and correlates with mild cognitive impairment. Pharmacy Practice (Granada). 2020;18(3):2017. doi: 10.18549/PharmPract.2020.3.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jazbar J, Locatelli I, Horvat N, Kos M. Clinically relevant potential drug-drug interactions among outpatients: a nationwide database study. Res Social Adm Pharm. 2018;14(6):572–580. doi: 10.1016/j.sapharm.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 55.Kerr KP, Mate K, Magin PJ, Marley J, Stocks N, Disler P, et al. The prevalence of co-prescription of clinically relevant CYP enzyme inhibitor and substrate drugs in community-dwelling elderly Australians. J Clin Pharm Ther. 2014;39(4):383–389. doi: 10.1111/jcpt.12163. [DOI] [PubMed] [Google Scholar]
- 56.Lopez-Picazo JJ, Ruiz JC, Sanchez JF, Ariza A, Aguilera B, Lazaro D, et al. Prevalence and typology of potential drug interactions occurring in primary care patients. Eur J Gen Pract. 2010;16(2):92–99. doi: 10.3109/13814788.2010.481709. [DOI] [PubMed] [Google Scholar]
- 57.Matos A, Bain KT, Bankes DL, Furman A, Skalski B, Verzicco J, et al. Cytochrome P450 (CYP450) interactions involving atypical antipsychotics are common in community-dwelling older adults treated for behavioral and psychological symptoms of dementia. Pharmacy. 2020;8(2):63. doi: 10.3390/pharmacy8020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naples JG, Marcum ZA, Perera S, Newman AB, Greenspan SL, Gray SL, et al. Impact of drug–drug and drug–disease interactions on gait speed in community-dwelling older adults. Drugs Aging. 2016;33(6):411–418. doi: 10.1007/s40266-016-0373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Novaes PH, da Cruz DT, Lucchetti ALG, Leite ICG, Lucchetti G. The, "iatrogenic triad": polypharmacy, drug-drug interactions, and potentially inappropriate medications in older adults. Int J Clin Pharm. 2017;39(4):818–825. doi: 10.1007/s11096-017-0470-2. [DOI] [PubMed] [Google Scholar]
- 60.Patel R, Zhu L, Sohal D, Lenkova E, Koshki N, Woelfel J, et al. Use of 2015 Beers criteria medications by older medicare beneficiaries. Consult Pharm. 2018;33(1):48–54. doi: 10.4140/TCP.n.2018.48. [DOI] [PubMed] [Google Scholar]
- 61.Popović B, Quadranti NR, Matanović SM, Lisica ID, Ljubotina A, Duliba DP, et al. Potentially inappropriate prescribing in elderly outpatients in Croatia. Eur J Clin Pharmacol. 2014;70(6):737–744. doi: 10.1007/s00228-014-1667-0. [DOI] [PubMed] [Google Scholar]
- 62.Santos TOD, Nascimento M, Nascimento YA, Oliveira GCB, Martins UCM, Silva DFD, et al. Drug interactions among older adults followed up in a comprehensive medication management service at Primary Care. Einstein (Sao Paulo). 2019;17(4):eAO4725. doi: 10.31744/einstein_journal/2019AO4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Secoli S-R, Figueras A, Lebrao ML, de Lima FD, Santos JLF. Risk of potential drug-drug interactions among Brazilian elderly. Drugs Aging. 2010;27(9):759–770. doi: 10.2165/11538460-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 64.Sell R, Schaefer M. Prevalence and risk factors of drug-related problems identified in pharmacy-based medication reviews. Int J Clin Pharm. 2020;42(2):588–597. doi: 10.1007/s11096-020-00976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skaar DD, O'Connor H. Potentially serious drug-drug interactions among community-dwelling older adult dental patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(2):153–160. doi: 10.1016/j.tripleo.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 66.Song Y-K, Oh JM. Nationwide prevalence of potential drug-drug interactions associated with non-anticancer agents in patients on oral anticancer agents in South Korea. Support Care Cancer. 2020;28(8):3711–3720. doi: 10.1007/s00520-019-05221-1. [DOI] [PubMed] [Google Scholar]
- 67.Steinman MA, Miao Y, Boscardin WJ, Komaiko KD, Schwartz JB. Prescribing quality in older veterans: a multifocal approach. J Gen Intern Med. 2014;29(10):1379–1386. doi: 10.1007/s11606-014-2924-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Teixeira JJ, Crozatti MT, dos Santos CA, Romano-Lieber NS. Potential drug-drug interactions in prescriptions to patients over 45 years of age in primary care, southern Brazil. PLoS ONE. 2012;7(10):e47062. doi: 10.1371/journal.pone.0047062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tragni E, Casula M, Pieri V, Favato G, Marcobelli A, Trotta MG, et al. Prevalence of the prescription of potentially interacting drugs. PLoS ONE. 2013;8(10):e78827. doi: 10.1371/journal.pone.0078827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trevisan C, Limongi F, Siviero P, Noale M, Cignarella A, Manzato E, et al. Mild polypharmacy and MCI progression in older adults: the mediation effect of drug–drug interactions. Aging Clin Exp Res. 2021;33(1):49–56. doi: 10.1007/s40520-019-01420-2. [DOI] [PubMed] [Google Scholar]
- 71.Truong TT, Phan NK, Vo QV, Diep HG, Vuong HT, Le TV, et al. Drug-related problems in prescribing for coronary artery diseases in Vietnam: cross-sectional study. Trop Med Int Health. 2019;24(11):1335–1340. doi: 10.1111/tmi.13310. [DOI] [PubMed] [Google Scholar]
- 72.Yazdanshenas H, Bazargan M, Smith J, Martins D, Motahari H, Orum G. Pain treatment of underserved older African Americans. J Am Geriatr Soc. 2016;64(10):2116–2121. doi: 10.1111/jgs.14302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abubakar U, Tangiisuran B, Kolo M, Yamma AI, Hammad MA, Sulaiman SAS. Prevalence and predictors of potentially inappropriate medication use among ambulatory older adults in Northern Nigeria. Drugs Ther Perspect. 2021;37(2):94–99. doi: 10.1007/s40267-020-00800-3. [DOI] [Google Scholar]
- 74.Hermann M, Carstens N, Kvinge L, Fjell A, Wennersberg M, Folleso K, et al. Polypharmacy and potential drug–drug interactions in home-dwelling older people: a cross-sectional study. J Multidiscip Healthc. 2021;14:589. doi: 10.2147/JMDH.S297423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lopez-Rodriguez JA, Rogero-Blanco E, Aza-Pascual-Salcedo M, Lopez-Verde F, Pico-Soler V, Leiva-Fernandez F, et al. Potentially inappropriate prescriptions according to explicit and implicit criteria in patients with multimorbidity and polypharmacy. MULTIPAP: a cross-sectional study. PLoS ONE. 2020;15(8):e0237186. doi: 10.1371/journal.pone.0237186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nikolic B, Jankovic S, Stojanov O, Popovic J. Prevalence and predictors of potential drug-drug interactions. Open Med. 2014;9(2):348–356. doi: 10.2478/s11536-013-0272-4. [DOI] [Google Scholar]
- 77.Tobi H, Faber A, Van Den Berg PB, Drane JW, de Jong-van den Berg LT. Studying co-medication patterns: the impact of definitions. Pharmacoepidemiol Drug Saf. 2007;16(4):405–411. doi: 10.1002/pds.1304. [DOI] [PubMed] [Google Scholar]
- 78.Juurlink DN, Mamdani M, Kopp A, Laupacis A, Redelmeier DA. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289(13):1652–1658. doi: 10.1001/jama.289.13.1652. [DOI] [PubMed] [Google Scholar]
- 79.Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329(7456):15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leone R, Magro L, Moretti U, Cutroneo P, Moschini M, Motola D, et al. Identifying adverse drug reactions associated with drug-drug interactions: data mining of a spontaneous reporting database in Italy. Drug Saf. 2010;33(8):667–675. doi: 10.2165/11534400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 81.Lam J, Gomes T, Juurlink DN, Mamdani MM, Pullenayegum EM, Kearon C, et al. Hospitalization for hemorrhage among warfarin recipients prescribed amiodarone. Am J Cardiol. 2013;112(3):420. doi: 10.1016/j.amjcard.2013.03.051. [DOI] [PubMed] [Google Scholar]
- 82.Battistella M, Mamdami MM, Juurlink DN, Rabeneck L, Laupacis A. Risk of upper gastrointestinal hemorrhage in warfarin users treated with nonselective NSAIDs or COX-2 inhibitors. Arch Intern Med. 2005;165(2):189–192. doi: 10.1001/archinte.165.2.189. [DOI] [PubMed] [Google Scholar]
- 83.Saedder EA, Thomsen AH, Hasselstrøm JB, Jornil JR. Heart insufficiency after combination of verapamil and metoprolol: a fatal case report and literature review. Clin Case Rep. 2019;7(11):2042–2048. doi: 10.1002/ccr3.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Becker ML, Visser LE, van Gelder T, Hofman A, Stricker BHC. Increasing exposure to drug-drug interactions between 1992 and 2005 in people aged≥ 55 years. Drugs Aging. 2008;25(2):145–152. doi: 10.2165/00002512-200825020-00006. [DOI] [PubMed] [Google Scholar]
- 85.Andersson ML, Bottiger Y, Kockum H, Eiermann B. High prevalence of drug-drug interactions in primary health care is caused by prescriptions from other healthcare units. Basic Clin Pharmacol Toxicol. 2018;122(5):512–516. doi: 10.1111/bcpt.12939. [DOI] [PubMed] [Google Scholar]
- 86.Glassman PA, Simon B, Belperio P, Lanto A. Improving recognition of drug interactions: benefits and barriers to using automated drug alerts. Med Care. 2002;40:1161–1171. doi: 10.1097/00005650-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 87.Ko Y, Malone DC, Skrepnek GH, Armstrong EP, Murphy JE, Abarca J, et al. Prescribers’ knowledge of and sources of information for potential drug-drug interactions. Drug Saf. 2008;31(6):525–536. doi: 10.2165/00002018-200831060-00007. [DOI] [PubMed] [Google Scholar]
- 88.Bergk V, Haefeli WE, Gasse C, Brenner H, Martin-Facklam M. Information deficits in the summary of product characteristics preclude an optimal management of drug interactions: a comparison with evidence from the literature. Eur J Clin Pharmacol. 2005;61(5):327–335. doi: 10.1007/s00228-005-0943-4. [DOI] [PubMed] [Google Scholar]
- 89.Kheshti R, Aalipour M, Namazi S. A comparison of five common drug–drug interaction software programs regarding accuracy and comprehensiveness. J Res Pharm Pract. 2016;5(4):257. doi: 10.4103/2279-042X.192461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.European Medicines Agency. Investigation of drug interactions, guideline on the investigation of drug interactions: revision 1. 2015. https://www.ema.europa.eu/en/investigation-drug-interactions. Accessed 1 Jun 2021.
- 91.US Food and Drug Administration. Drug interactions: relevant regulatory guidance and policy documents. 2022. https://www.fda.gov/drugs/drug-interactions-labeling/drug-interactions-relevant-regulatory-guidance-and-policy-documents. Accessed 1 Jun 2021.
- 92.Thakrar BT, Grundschober SB, Doessegger L. Detecting signals of drug–drug interactions in a spontaneous reports database. Br J Clin Pharmacol. 2007;64(4):489–495. doi: 10.1111/j.1365-2125.2007.02900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.European Medicines Agency. Geriatric Expert Group. https://www.ema.europa.eu/en/committes/working-parties-other-groups/chmp/geriatric-expert-group. Accessed 1 Jun 2021.
- 94.European Medicines Agency. Medicines for older people. https://www.ema.europa.eu/en/human-regulatory/research-development/medicines-older-people. Accessed 1 Jun 2021.
- 95.Phansalkar S, Van der Sijs H, Tucker AD, Desai AA, Bell DS, Teich JM, et al. Drug–drug interactions that should be non-interruptive in order to reduce alert fatigue in electronic health records. J Am Med Inform Assoc. 2013;20(3):489–493. doi: 10.1136/amiajnl-2012-001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.