Abstract
Purpose
Physiotherapy interventions are prescribed as first-line treatment for people with sciatica; however, their effectiveness remains controversial. The purpose of this systematic review was to establish the short-, medium- and long-term effectiveness of physiotherapy interventions compared to control interventions for people with clinically diagnosed sciatica.
Methods
This systematic review was registered on PROSPERO CRD42018103900. Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL (EBSCO), Embase, PEDro, PubMed, Scopus and grey literature were searched from inception to January 2021 without language restrictions. Inclusion criteria were randomised controlled trials evaluating physiotherapy interventions compared to a control intervention in people with clinical or imaging diagnosis of sciatica. Primary outcome measures were pain and disability. Study selection and data extraction were performed by two independent reviewers with consensus reached by discussion or third-party arbitration if required. Risk of bias was assessed independently by two reviewers using the Cochrane Risk of Bias tool with third-party consensus if required. Meta-analyses and sensitivity analyses were performed with random effects models using Revman v5.4. Subgroup analyses were undertaken to examine the effectiveness of physiotherapy interventions compared to minimal (e.g. advice only) or substantial control interventions (e.g. surgery).
Results
Three thousand nine hundred and fifty eight records were identified, of which 18 trials were included, with a total number of 2699 participants. All trials had a high or unclear risk of bias. Meta-analysis of trials for the outcome of pain showed no difference in the short (SMD − 0.34 [95%CI − 1.05, 0.37] p = 0.34, I2 = 98%), medium (SMD 0.15 [95%CI − 0.09, 0.38], p = 0.22, I2 = 80%) or long term (SMD 0.09 [95%CI − 0.18, 0.36], p = 0.51, I2 = 82%). For disability there was no difference in the short (SMD − 0.00 [95%CI − 0.36, 0.35], p = 0.98, I2 = 92%, medium (SMD 0.25 [95%CI − 0.04, 0.55] p = 0.09, I2 = 87%), or long term (SMD 0.26 [95%CI − 0.16, 0.68] p = 0.22, I2 = 92%) between physiotherapy and control interventions. Subgroup analysis of studies comparing physiotherapy with minimal intervention favoured physiotherapy for pain at the long-term time points. Large confidence intervals and high heterogeneity indicate substantial uncertainly surrounding these estimates. Many trials evaluating physiotherapy intervention compared to substantial intervention did not use contemporary physiotherapy interventions.
Conclusion
Based on currently available, mostly high risk of bias and highly heterogeneous data, there is inadequate evidence to make clinical recommendations on the effectiveness of physiotherapy interventions for people with clinically diagnosed sciatica. Future studies should aim to reduce clinical heterogeneity and to use contemporary physiotherapy interventions.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00586-022-07356-y.
Keywords: Sciatica, Radicular pain, Lumbar radiculopathy, Physiotherapy, Systematic review
Introduction
‘Sciatica’ is a broad term describing spinally referred pain of neural origin that radiates into the leg. The reported prevalence of sciatica varies widely (1.2–43%) [1], probably due to different diagnostic criteria, reflecting a heterogeneous patient population. Sciatica is a significant burden to healthcare and the economy, as a neuropathic component in low back pain it is not only linked to poorer quality of life, but also increases the already high costs of back pain by a further 67% [2]. Although prognosis is good for most patients, up to 45% continue to have symptoms for 12 months or longer [3].
Physiotherapy interventions such as exercise, manual therapy and psychological therapy are recommended in clinical guidelines for people with sciatica [4]. However, the available systematic reviews examining the effectiveness of physiotherapy interventions are at least ten years old. For example, study selection in the most recent systematic review comparing surgery versus conservative care ended in 2009 [5]. Their results could not be meta-analysed due to poor reporting and clinical heterogeneity. Similarly, a network-meta-analysis concluded its search in 2009 [6], finding no support for the effectiveness of exercise or traction while manipulation may be beneficial. However, the latter was based on a single study only. Prior to this, reviews specifically focusing on conservative management of sciatica were published in 2010 [7] and 2007 [8] and were unable to make strong conclusions on the superiority of any treatment. More recent reviews published in 2015 and 2016 were limited to a subset of physiotherapy interventions (e.g. physical activity versus surgery [9] and exercise versus advice to stay active [10]). A recent review [11] looked at a range of physiotherapy interventions, however the review did not include a meta-analysis.
Of note, sciatica is a heterogeneous condition with no agreed diagnostic criteria [12]. Most reviews to date make no reference to the clinical diagnosis of included study participants rendering it unclear whether patients had confirmed nerve involvement. The objective of this systematic review was therefore to assess the up-to-date evidence on the effectiveness of physiotherapy interventions compared with control interventions in people with clinically diagnosed sciatica.
Methods
Registration
The protocol was prospectively registered on PROSPERO (CRD42018103900). We are reporting our findings according to the updated guidance for the PRISMA guidance [13].
Search strategy
We searched the following databases from inception to 29th January 2021: Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL (EBSCO), Embase, PEDro, PubMed and Scopus. We also searched grey literature including trial registries (OpenGrey and clinicaltrials.gov). The search strategy was developed in consultation with a medical librarian and included keywords relating to sciatica, physiotherapy and randomised controlled trials (Supplemental Table 1).
Study eligibility
Included studies were randomised controlled trials evaluating physiotherapy interventions compared to a control intervention in people with ‘sciatica’. Trials were eligible if study participants were diagnosed with spinally referred leg pain of neural origin. This diagnosis required at least one of the following: positive sensory, myotomal or reflex tests on neurological examination; positive neurodynamic test (e.g. straight leg raise, slump); imaging confirming spinal nerve compromise correlating with symptoms; presence of neuropathic pain determined with neuropathic pain questionnaires; electrodiagnostic testing or quantitative sensory testing suggesting nerve root involvement. Studies which either did not specify how the sciatica diagnosis was made or were simply using pain referral into the leg without other clinical tests confirming a neural component were excluded. No restrictions were made on sciatica symptom duration or intensity. Eligible trials must evaluate physiotherapy interventions such as exercise, manual therapy, physiotherapy-led education, or a combination of these. The control intervention needed to be a non-physiotherapy intervention (e.g. surgery, GP care, other non-physiotherapy care). The control intervention could also be placebo, sham or no intervention. No restrictions were made on language.
Trials that included participants with serious pathology (e.g. cancer, fracture, cauda equina), pregnant women or participants aged below 18 were excluded. Studies evaluating post-surgical physiotherapy were excluded. As recent reviews address the effectiveness of acupuncture for people with sciatica [14, 15], and acupuncture is not core physiotherapy practice in many countries, trials evaluating acupuncture were excluded.
Study selection
Two reviewers (LD, GJ) screened studies independently. In a first step, titles and abstracts were screened, followed by full texts. Discrepancies were resolved by discussion and arbitration by a third reviewer (AS) if required.
Quality assessment
Two reviewers (LD, LK) independently used the Cochrane Risk of Bias tool to assess study quality and risk of bias [16]. The tool was piloted on three excluded studies to test agreement of decision-making. Disagreements between reviewers were resolved by a third reviewer where required (GJ).
Data extraction
Two reviewers (LD, LK) independently extracted data using a standardised form; consensus was used to resolve any discrepancies. The following information was extracted: author, year, country, characteristics of participants (e.g. age, duration, severity of symptoms), diagnostic criteria, physiotherapy and control intervention (type, frequency and duration). Outcomes were extracted at baseline and follow-up time points. Primary outcomes of interest were pain (e.g. numerical pain rating scale) and disability (e.g. Oswestry disability index). Secondary outcomes were global perceived effect, quality of life, change in neurological function, psychological parameters, adverse events, and dropout rates. Means, standard deviations and sample sizes were extracted for each outcome. If alternative summary statistics were provided, we transformed the data using recommended calculations [17]. If available, outcomes were extracted for different time points, and grouped according to time after randomisation as: short term (< 3 months); medium-term (> 3 months but < 12 months) or long-term (≥ 12 months). If multiple terms were reported within one period, the outcome closest to 7 weeks, 6 months and 12 months was used. When more than one body part was used to assess pain (e.g. leg and back pain), the highest score at baseline was used to reflect patients’ dominant symptoms. When more than one outcome measure was used within a trial for a specific outcome of interest, the outcome measure described by the trial authors as their primary measure was used.
Data synthesis and analysis
If data were available for the same outcome measure from at least two trials, meta-analysis was performed using Revman v5.4. We calculated standardised mean differences (SMD) and 95% confidence intervals (CI). Random effects models with inverse variance weighting were used to account for the variability of included studies. Heterogeneity was calculated with I2 statistics and interpreted as follows: ‘might not be important’ (0–40%), ‘moderate’ (30–60%), ‘substantial ‘(50–90%), and ‘considerable’ (75–100%) [16]. We performed separate overall meta-analyses comparing physiotherapy interventions with control interventions for our primary outcomes of pain and disability.
We planned to perform a subgroup analysis according to type of physiotherapy interventions. However, this was impossible as interventions were too heterogeneous to pool. We performed a post hoc subgroup analysis comparing the effect of physiotherapy interventions according to the type of control intervention (minimal vs. substantial). Minimal intervention included advice/education only, GP care, or sham treatment. Substantial intervention included surgery, disc and epidural injections. Due to high risk of bias, we performed a post hoc sensitivity analysis, removing those studies where at least two parameters of risk of bias were rated as high. Results that could not be included in the meta-analysis were narratively described.
Results
Search
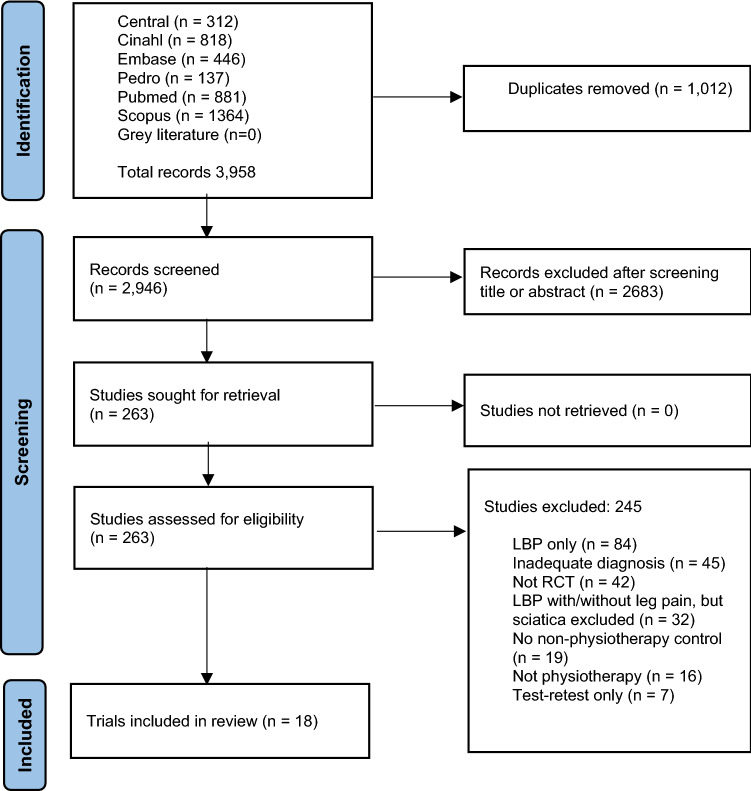
The electronic database searches returned 3958 records. Duplicates and studies deemed ineligible from titles/abstracts were removed, leaving 263 full-text articles. Of those, 245 were discarded as they did not meet the inclusion criteria. A total of 18 studies were included in this systematic review (Fig. 1) [18–35].
Fig. 1.
PRISMA flow diagram
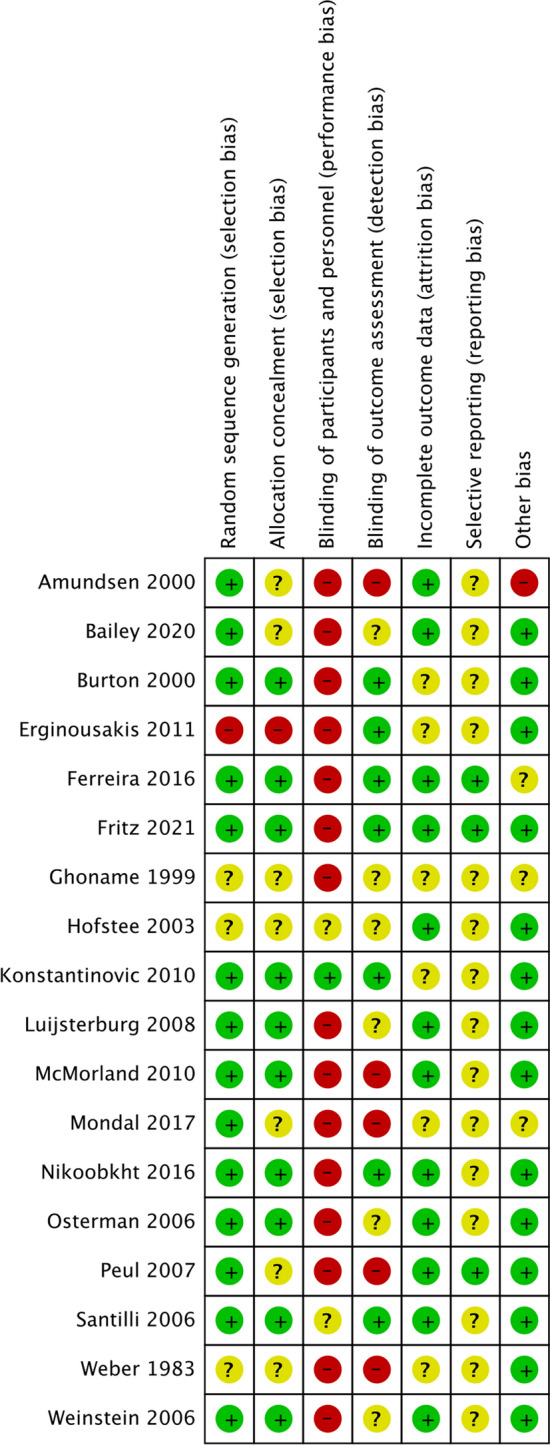
Risk of bias
Blinding of participants was understandably challenging to achieve in these trials, risk of performance bias was therefore high in 15 trials [18–20, 23, 25–35] and unclear in two trials [21, 24]. Detection bias was high or unclear in 11 [20, 21, 23, 25, 26, 29, 30, 32–35] of 18 studies (Fig. 2).
Fig. 2.
Risk of bias summary + low risk of bias? Unsure risk of bias—high risk of bias
Participants
Table 1 contains details of study characteristics. A total of 2699 participants were included, 1198 (44.4%) of them were female. According to data available from 13 trials [18–23, 26, 28, 30–33, 35] participants’ age ranged from a mean of 36.0 (SD 5.8) [28] to 48.38 (SD 6.39) years [30]. Baseline duration of sciatica was reported in eight trials, [18–22, 31–33], ranging from a mean of 1.8 (SD 1.3) weeks [21] to (median) 5.8 years (range 0.25–50) [18]. Pain severity at baseline was reported by 16 trials [18–24, 26–33, 35], ranging from a mean of 4.8 (SD 1.9) [19] to 8.0 (SD 1.8) [26] on an 11-point scale. The diagnostic criteria for sciatica used in the included studies are listed in Supplemental Table 2.
Table 1.
Characteristics of included studies
| Study Year Country |
Number of participants Age in years (SD) Pain duration (SD) Pain severity (SD) |
Physiotherapy intervention (group G1) | Control intervention (group G2) | Primary outcome measures and time points | Results G1* | Results G2* |
|---|---|---|---|---|---|---|
| Physiotherapy vs. minimal intervention (7 RCTs) | ||||||
|
Ferreira et al. [18] 2016 Brazil |
N = 60 Age: G1 43.9(14.5) G2 40.3(12.9) Duration: G1 5.8yrs G2 2.0yrs Severity: NPRS (back) G1 5.5 (2.3) G2 5.1 (2.5) NPRS (leg) G1 6.1 (1.6) G2 6.1 (1.9) |
Neurodynamic treatment. Passive or active movements. Education on nerve sensitisation. Grade III lumbar foramen opening mobilisations and neurodynamic sliders. Home exercise: one sliding and one tensioning technique. | Both groups advice to remain active, face-to-face. Advised to avoid prolonged rest, do not avoid daily-life activity, do not excessively brace muscles. Advised light activity and movement beneficial for pain. |
NPRS (leg) 4w ODI 4w |
Pain short 3.7(2.6) ODI short 20 (12) |
Pain short 6.1(2.4) ODI short 23 (12) |
|
Fritz et al. [19] 2021 USA |
N = 220 Age: G1 40.0(11.2) G2 37.9(11.2) Duration: (days)G1 35.8(25.6) G2 35.9(26.8) Severity: NPRS (back) G1 5.1(1.8) G2 4.8(1.9) NPRS (leg) G1 4.3(2.2) G2 3.8(2.2) |
Physical therapy within 3 days of assignment. Exercise and manual therapy in each session. Written directions and instructed to do assigned exercises at home. | Medication and imaging at discretion of primary care provider. Given copy of The Back Book, about favourable prognosis, and importance of remaining active. |
NPRS (back) 4w, 6m, 12m ODI 4w, 6m, 12m |
Pain short 2.4, (95%CI 2, 2.8) Pain med 2.6, (95%CI 2.2, 3) Pain long 2.3, (95%CI 1.9, 2.7) Disability short 19.9 (95%CI 17.2, 22.7) Disability med 14.5, (95%CI 11.6, 17.3) Disability long 14.4, (95%CI 11.5, 17.4) |
Pain short 3.9 (95%CI 3.5, 4.3) Pain med 3.3, (95%CI 2.9, 3.7) Pain long 3.3, (95%CI 2.9, 3.7) Disability short 28.1 (95%CI 25.4, 30.8) Disability med 19.8, (95%CI 17.0, 22.7) Disability long 19.2, (95%CI 16.3, 22.0) |
|
Ghoname et al. [20] 1999 USA |
N = 64 Age: G1 43(19) G2 43(19) Duration: (months) 21(9) Severity: NPRS (leg) G1 7 (1.9) G2 6.6 (1.9) |
Standard TENS therapy: 4 electrode pads in standardised pattern, stimulated at 4Hz, pulse duration 0.1s. Intensity adjusted to maximum tolerated without producing muscle contractions. | Sham-PENS: placement of 10 acupuncture-like needle probes in identical montage to PENS treatment. However, no electrical stimulation was applied to the probes. |
NPRS (leg) 3w VAS physical activity 3w |
Pain short 5.4 (1.9) Disability short 4.5 (1.7) |
Pain short 6.1 (1.9) Disability 5.5 (2.1) |
|
Hofstee et al. [21] 2003 The Netherlands |
N = 250 Age: G1 38(9.5) G2 38(9.5) Duration: (wks)G1 1.8(1.3) G2 1.9(1.2) Severity: VAS G1 60.9 (20.1) G2 65.5 (18.5) |
Physiotherapy (exercises, advice, hydrotherapy, home exercise programme). | Continuation of normal activities as much as possible (modify duration, intensity, and frequency according to pain). |
Pain VAS 2m, 6m QDS 2m, 6m |
Pain short 23.9 (IQR 20,60) Pain med 14.1 (IQR 29,70) Disability short 29.7 (IQR 8.5, 44) Disability med 21.4 (IQR 20,51) |
Pain short 23.4 (IQR 17,64) Pain med 12.9 (IQR 26,66) Disability short 31.1 (IQR 10, 42) Disability med 22 (IQR 18,52) |
|
Konstantinovic et al. [22] 2010 Serbia |
N = 364 Age: G1 43.5(7.7) G2 41.87 (8.37) Duration: < 4w Severity: (leg) G1 78.5(3.14) G2 74.7(6.05) |
Active low-level laser therapy behind involved spine segment using stationary skin-contact method. 5x weekly, total of 15 treatments, frequency 5000Hz, dose 3J/cm2; treatment time 150 seconds. | Placebo laser treatment applied in same manner as active device by identical device that was deactivated by member of Institute for Physics. |
VAS leg 3w ODI 3w |
Pain short median 34 (IQR 30.5; 38) Disability short median 20 (IQR 19;21) |
Pain short median 54 (IQR 50;56) Disability short median 22 (IQR 20;24) |
|
Luijsterburg et al. [23] 2008 The Netherlands |
N = 135 Age: G1 42 (10) G2 43 (12) Duration: (inclusion) < 6wks Severity: NRS G1 6.3(2.2) G2 6.3(2.2) |
Exercise therapy, advice, guidance: return to activity despite pain, type/content of exercise left to PT. Passive treatment not allowed. | GP care according to clinical guideline, information, advice and, if necessary, pain medication prescribed. |
NRS leg 6w, 12w, 12m RDQ 6w, 12w, 12m |
Pain short 3.3 (2.67) Pain med 2.4 (2.96) Pain long 1.9 (2.82) Disability short 10.6 (6.67) Disability med 8.2(7.11) Disability long 5.9(6.37) |
Pain short 3 (2.67) Pain med 2.6 (2.96) Pain long 2.6 (2.82) Disability short 8.8 (6.67) Disability med 6.9(7.11) Disability long 6.3(6.37) |
|
Santilli et al. [24] 2006 Italy |
N = 102 Age: (inclusion) 18 to 65 Duration: (inclusion) < 10d Severity: VAS G1 6.4(0.9) G2 6.4(0.8) |
Active manipulations according to protocol by chiropractor including soft tissue manipulations and rotational thrust away from greatest restriction. | Simulated manipulations, soft muscle pressing not specific patterns, not rapid thrusts. Chiropractors as G1. | Local pain reduction 90d, 180d; Radiating pain reduction 90d, 180d; Local pain-free 90d, 180d; Radiating pain-free 90d, 180d. |
Pain med (n) radiating pain reduction 48, % pain free 100 Pain long (n) radiating pain reduction 48, % pain free 100 |
Pain med (n) radiating pain reduction 39, % pain free 81 Pain long (n) radiating pain reduction 40, % pain free 83 |
| Physiotherapy vs. substantial intervention (11 RCTS) | ||||||
|
Amundsen et al. [25] 2000 Norway |
N = 31 Age: G1 83% 40-70; G2 84% 40-70 Duration not reported Severity: G1 28% mod, 72% severe G2 46% mod, 54% severe |
1-month inpatient stay, 3-point hyperextension thoracolumbar brace. Physiotherapy when home, walking and stabilising exercises, kyphotic position encouraged. | Partial/total laminectomy, medial facetectomy/discectomy and/or removal of osteophytes. 1–2 days post-op brace, physiotherapy as previously. | Subjective report 6m, 12m |
Pain med (n): No/light 2 (cross 5) Mod 5 (cross 4) Severe 1 (cross 1) Pain long (n): No/light 1 (cross 1); Mod 7 (cross 3); Severe 0 (cross 4) |
Pain med (n) No/light 2, Moderate 11, Severe 0 Pain long (n): No/light 5, Moderate 7, Severe 0 |
|
Bailey et al. [26] 2020 Canada |
N = 128 Age: G1 37.1(11.9) G2 38 (8.3) Duration: (inclusion) 4-12m Severity: VAS back G1 6.5(2.8) G2 6.7(2.6) VAS leg G1 8.0(1.8) G2 7.7(2.0) |
Education regarding activity and exercise, use of oral analgesics. Active physiotherapy provided at the discretion of PT. Optional epidural, 2nd/3rd injection at discretion of physician. | Microdiscectomy fellowship-trained spine surgeon open/minimal access approach, loupe/microscope assistance. |
VAS leg 6m, 12m ODI 6m, 12m |
Pain med 5.2 (0.4SE) Pain long 4.7 (0.4SE) Disability med 33.7(2.3SE) Disability long 34.7(2.4SE) |
Pain med 2.8 (0.4SE) Pain long 2.6 (0.4SE) Disability med 22.8 (2.3SE) Disability long 22.9 (2.3SE) |
|
Burton et al. [27] 2000 UK |
N = 40 Age: 41.9 (10.6) no reports per group Duration not reported Severity: 7 pt scale G1 3.79(1.62) G2 4.05(1.28) |
Soft tissue stretching of lumbar/buttock muscles, low-amplitude passive manoeuvres lumbar spine. Clinical discretion re: manipulation. Advice: continue normal activity, encouraged return work. | General anaesthetic, single injection of chymopapain into nucleus of disc and bupivacaine. Discharge following day to usual care of family doctor. |
7-point scale back pain 6w, 12m RDQ 6w, 12m |
Pain short 2.68 (1.6) Pain long 2.27 (1.53) Disability short 7.79 (6.65) Disability long 5.87 (5.96) |
Pain short 3.58 (0.97) Pain long 2.87 (1.36) Disability short 11 (5.69) Disability long 7.27 (6.65) |
|
Erginousakis et al. [28] 2011 Greece |
N = 62 Age: G1 36(5.8) G2 38(4.2) Duration not reported Severity: NVS G1 6.9(1.9) G2 7.4 (1.4) |
Conservative therapy including education, counselling, physical therapy, NSAIDs, muscle relaxants, analgesics. | Fluoroscopically guided percutaneous disc decompression. | NVS 3m, 12m |
Pain short 0.9 (2) Pain long 4 (3.4) |
Pain short 3.0 (2.4) Pain long 1.7 (2.4) |
|
McMorland et al. [29] 2010 Canada |
N = 40 Age: G1 42.4 G2 41.5 (SD unreported) Duration: (inclusion) > 3m Severity: McGill PRI(R) G1 28.7 (17.4) G2 32.5 (12.9) |
Spinal manipulative therapy at discretion of treating clinician, ice or heat, information, education, intro to rehab exercises. Core stability exercise, emphasis on technique. | Surgical microdiscectomy, hospital for 1-2 days. Analgesia for 10 days and advised to avoid heavy lifting, bending or twisting for 6-8 weeks. |
McGill PRI(R) 6w RMDQ 6w |
Pain short 21.7 (13.7) Disability short 9.5 (6.0) |
Pain short 18.4 (16.3) Disability short 9.4 (6.4) |
|
Mondal et al. [30] 2017 India |
N = 60 Age: G1 48.38 (6.39) G2 42.11 (8.58) Duration: > 3m Severity: (inclusion) > 5 NRS |
Spine extension exercises. | Single transforaminal epidural steroid injection with methylprednisolone acetate (20mg and 0.25% bupivacaine (total 2ml) and spine extension exercises. |
NRS 1m ODI 1m |
Pain short 5.03 (2.06) Disability short 56.94 (23.8) |
Pain short 3.11 (2.06) Disability short 34.79 (23.8) |
|
Nikoobakht et al. [31] 2016 Iran |
N = 177 Age: G1 38.0(9.0) G2 37.6(7.3) Duration: (m)G1 25.9(8.6) G2 18.6(12.0) Severity: VAS G1 7.4(1.5) G2 7.6(1.5) |
Bed rest, active physical therapy, education & counselling, home exercises, spinal manipulation, analgesics, muscle relaxants, NSAIDs & local injections. | Percutaneous disc decompression under moderate sedation. Graduated return to normal activity in the 2 wks following procedure. |
VAS 1m, 3m, 12m ODI 1m, 3m, 12m |
Pain short 6.94 (2.27) Pain med 6.6 (2.67) Pain long 6.14 (3.07) Disability short 38.75 (13.27) Disability med 36.76 (15.39) Disability long 35.29 (16.43) |
Pain short 5.83 (3.25) Pain med 5.36 (3.43) Pain long 4.68 (3.58) Disability short 28.50 (17.02) Disability med 19.87 (15.49) Disability long 10.84 (12.75) |
|
Osterman et al. [32] 2006 Finland |
N = 56 Age: G1 38(7); G2 37(7) Duration (d): G1 60(21); G2 77(32) Severity: VAS G1 57(21); G2 61(20) |
Encouraged early physical activity within pain limits, instruction on isometric exercises. | Microdiscectomy within 2 wks of randomisation. Analgesia per individual requirements. Isometric exercise pre and post-op. Active physiotherapy |
VAS leg 6w, 6m, 12m ODI 6w, 6m,12m |
Pain short 25(27) Pain med 18 (29) Pain long 9 (19) Disability short 22 (16) Disability med 12 (15) Disability long 11(14) |
Pain short 12(20) Pain med 9 (20) Pain long 6 (11) Disability short 16 (16) Disability med 8 (12) Disability long 10 (13) |
|
Peul et al. [33] 2007 The Netherlands |
N = 283 Age: G1 43.5(9.6) G2 41.7(9.9) Duration: (wks) G1 9.5(2.1) G2 9.4(2.4) Severity: VAS back G1 30.8(27.7) G2 33.8(29.6) VAS leg G1 64.4(21.2) G2 67.2(27.7) |
GPs provided prolonged conservative treatment. Informed favourable prognosis, website informed natural course of illness & expectation of recovery. Patients fearful of movement referred to physiotherapy. | Surgery within 2 weeks to remove symptomatic disc herniation. Rehabilitation at home by physiotherapists standardised exercise protocol. Advice to resume activity. |
VAS leg 8w, 6m, 12m RDQ 8w, 6m, 12m |
Pain short 27.9 (1.9SE) Pain med 14.5 (1.9SE) Pain long 11 (1.9SE) Disability short 9.2 (0.5SE) Disability med 4.8 (0.5SE) Disability long 3.7 (0.5SE) |
Pain short 10.2 (1.9SE) Pain med 8.4 (1.9SE) Pain long 11 (1.9SE) Disability short 6.1 (0.5SE) Disability med 4 (0.5SE) Disability long 3.3 (0.5SE) |
|
Weber et al. [34] 1983 Norway |
N = 126 Age: G1 41.7 G2 40 (SD not reported) Duration not reported Severity not reported |
Wk 1 strict bed rest, moderate isometric exercises, analgesics. Wk 2 partial bed rest, gradual increase in exercise. Group ‘back school’ continued. | Surgical extradural removal of herniated mass of cartilage, out of bed day 1 post-op and discharge home 7-9d post-op without further treatment. | Patient subjective report of improvement as good/fair/poor/bad 12m. | Long term (n): Good 16 (8 cross); Fair 24 (4 cross; Poor 9 (4 cross); Bad 0 (1 cross) | Long term(n): Good 39 (0 cross); Fair 15 (1 cross); Poor 5 (0 cross); Bad 0 |
|
Weinstein et al. [35] 2006 USA |
N = 501 Age: G1 43(11.3) G2 41.7(11.8) Duration: (inclusion) > 6wks Severity: SF-36 G1 26.7(17.4) G2 27.1(18.5) |
Usual care, at least active physical therapy, education/counselling, home exercise, NSAIDs if tolerated. Individualised treatment tracked prospectively. | Standard open discectomy with examination of the involved nerve root. General/local anaesthetic. Nerve root decompressed. |
SF-36 3m, 12m ODI 3m, 12m |
Pain med 27.6 (1.8SE) Pain long 36.9 (1.8SE) Disability med 25 (1.6SE) Disability long 18.9 (1.6SE) |
Pain med 30.5 (1.9SE) Pain long 39.7 (1.8SE) Disability med 21.5 (1.7SE) Disability long 16.9 (1.7SE) |
RCT randomised controlled trial; G group; SD standard deviation; SE standard error; NPRS numeric pain rating scale; ODI Oswestry disability scale; VAS visual analogue scale; QDS Quebec disability scale; GP general practitioner; RDQ Roland disability scale; NVS numeric visual scale; APS Aberdeen pain scale; McGill PRI(R) McGill pain rating index rank value; TENS transcutaneous electrical nerve stimulation; PENS percutaneous electrical nerve stimulation; PT physiotherapist/physical therapist; IQR interquartile range; CI confidence interval; med medium; cross crossover; m month; wk week; d days
*Data are reported as mean (SD) unless stated otherwise
Physiotherapy intervention
Physiotherapy interventions varied considerably in the components included which prevented the preplanned subgroup analyses according to type of physiotherapy. Eleven trials included exercise [18, 19, 21, 23, 25, 29–32, 34, 35]. Type of exercise was most often unspecified or was at the discretion of the treating physiotherapist. Four studies made specific reference to neurodynamic exercise, [18] core stability [29], extension exercises [30] and isometric exercise [32]. Eleven trials provided advice or education as part of the physiotherapy intervention [18, 21–23, 26–29, 32, 33, 35] with the most common advice to continue normal activity. Five studies used manual therapy or manipulations [19, 24, 27, 29, 31]. The frequency and duration of physiotherapy interventions were unreported in seven trials [23, 25, 29, 30, 33–35]. Where duration was reported, it ranged from 2 weeks [18] to 6 months [26]. Further details on physiotherapy interventions are available in Tables 1 and 2.
Table 2.
Components of physiotherapy interventions
| Study | Exercise | Advice/Education | Manual therapy | Home exercise | Oral analgesia/ neuropathic |
Frequency/duration of physiotherapy intervention | Additional interventions/ adjuncts |
|---|---|---|---|---|---|---|---|
| Physiotherapy vs. minimal intervention (7 RCTs) | |||||||
| Ferreira et al. [18] | √ | √ | √ | 4 treatment sessions over 2 weeks | |||
| Fritz et al. [19] | √ | √ | √ | 6-8x during 4 wks, 2x each wk during first 2 wks and 1-2x in wks 3&4. Home exercises every 4-5 hours days between sessions. | |||
| Ghoname et al. [20] | 30 mins 3x weekly for 3 weeks | TENS therapy, 4 x 2.5cm cutaneous pads at 4Hz, pulse duration 0.1s | |||||
| Hofstee et al. [21] | √ | √ | √ | Twice weekly, minimum 4 wks maximum 8 wks | Hydrotherapy | ||
| Konstantinovic et al. [22] | √ | √ | 5x weekly for a total of 15 treatments | Low level laser therapy, 5000 frequency, 100mW, 3J | |||
| Luijsterburg et al. [23] | √ | √ | Not reported | ||||
| Santilli et al. [24] | √ | 5 days per week for 30 days | |||||
| Physiotherapy vs. surgical (11 RCTS) | |||||||
| Amundsen et al. [25] | √ | Not reported | 3m inpatient stay, 3-point thoracolumbar hyperextension brace | ||||
| Bailey et al. [26] | √ | √ | Spinal specialist medications, education & assessment of response to treatment on 6-wk basis min of 6m | Active physiotherapy at discretion of physiotherapists (number unspecified). Optional epidural injection | |||
| Burton et al. [27] | √ | √ | 12 weeks maximum | Soft tissue stretching of lumbar and buttock muscles | |||
| Erginousakis et al. [28] | √ | √ | Mean duration 22 days (range 7–35 days) | ||||
| McMorland et al. [29] | √ | √ | √ | Not reported | Ice or heat | ||
| Mondal et al. [30] | √ | √ | Not reported | ||||
| Nikoobakht et al. [31] | √ | √ | √ | √ | 20 sessions, 12 weeks | Bed rest, local injections | |
| Osterman et al. [32] | √ | √ | 3 times (at follow-ups 6wk, 3m, 12m) | ||||
| Peul et al. [33] | √ | Not reported | Patients fearful of movement referred to physiotherapy (number unspecified) | ||||
| Weber et al. [34] | √ | Not reported | Strict bed rest week 1, partial bed rest week 2. Group lessons in ‘back school’ | ||||
| Weinstein et al. [35] | √ | √ | √ | √ | Not reported | ||
m month; wk week
Control intervention
Minimal intervention included advice to stay active [18] provision of a Back Book education booklet [19], bedrest or advice to continue normal activity [21], sham electrical nerve stimulation [20], sham laser therapy [22], GP care [23] or simulated manipulations [24]. Substantial interventions involved surgery such as microdiscectomy or discectomy [26, 29, 32–35], or decompression [25, 28, 31]. One study compared epidural injection with extension exercises [30] and one compared chemonucleolysis disc injection [27] with physiotherapy.
Reporting of outcomes
Fifteen studies reported pain as a continuous outcome [18–23, 26–33, 35]. The three remaining studies reported a categorical outcome [24, 25, 34]. Fourteen studies reported a measure of disability [18–23, 26, 27, 29–33, 35]. Secondary outcome measures were not always reported (Supplemental Table 3). One trial reported treatment adherence [18]. Adverse events were unreported in seven trials [20, 23–25, 28, 30, 34]. Of these, five [20, 23–25, 34] pre-date publication of Consort Guidelines [36] which includes reporting of adverse events. Supplemental Table 4 summarises details of the adverse events, which were less frequent with physiotherapy interventions than substantial control interventions. Dropout rates were unreported in three trials [20, 28, 29].
Overall meta-analysis on physiotherapy versus control intervention
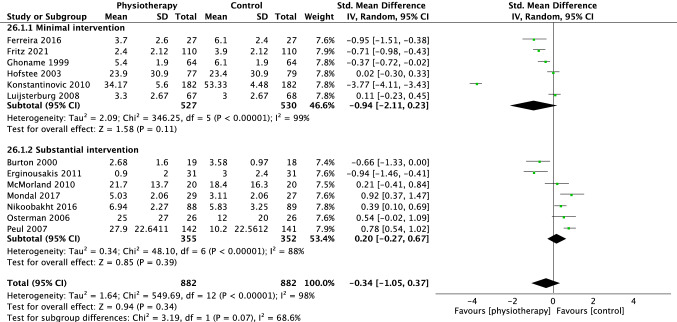
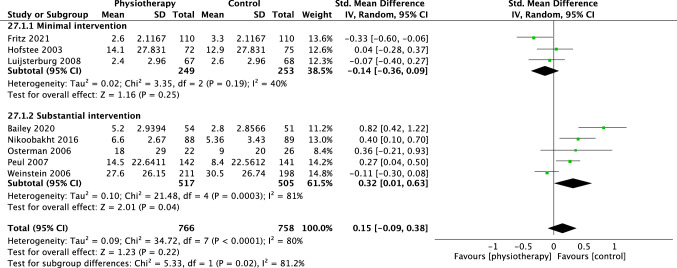
For pain, 13 trials were included in the overall meta-analysis comparing physiotherapy versus all control interventions at short term, eight trials at medium term and nine trials at long-term time points. There was no difference in effectiveness of physiotherapy versus control interventions at short term (SMD − 0.34 [95%CI − 1.05, 0.37] p = 0.34, I2 = 98%, Fig. 3), medium term (SMD 0.15 [95%CI − 0.09, 0.38], p = 0.22, I2 = 80%, Fig. 4) and long term (SMD 0.09 [95%CI − 0.18, 0.36], p = 0.51, I2 = 82% Fig. 5).
Fig. 3.
Forest plot pain short term (< 3 months)
Fig. 4.
Forest plot pain medium term (> 3 months < 6 months)
Fig. 5.
Forest plot pain long term (> or = 12 months)
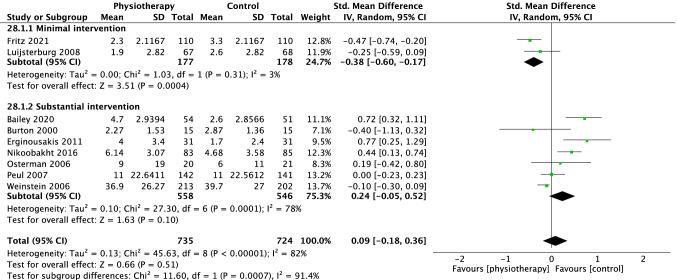
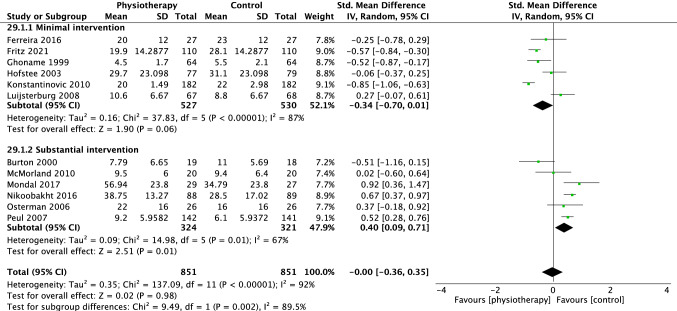
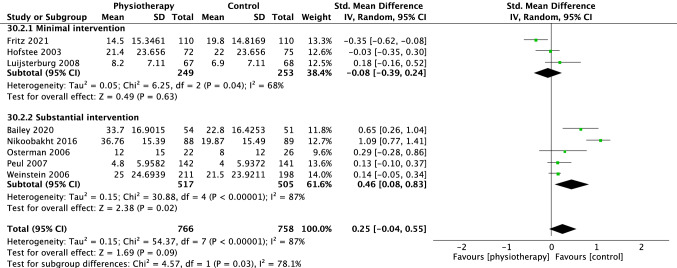
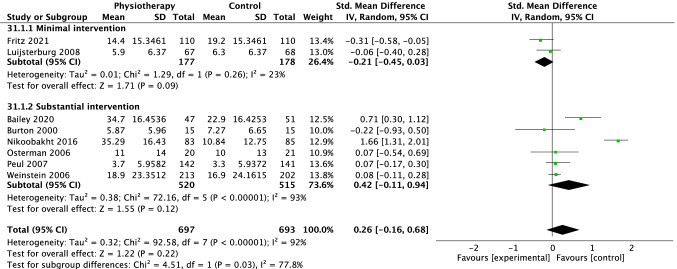
For disability, 12 trials were included in the overall meta-analysis at short term, eight trials at medium term and eight trials at long term. There was no difference in effectiveness of physiotherapy versus control interventions at short (SMD − 0.00 [95%CI − 0.36, 0.35], p = 0.98, I2 = 92%, Fig. 6), medium (SMD 0.25 [95%CI − 0.04, 0.55] p = 0.09, I2 = 87%, Fig. 7) and long term (SMD 0.26 [95%CI − 0.16, 0.68] p = 0.22, I2 = 92%, Fig. 8).
Fig. 6.
Forest plot disability short term (< 3 months)
Fig. 7.
Forest plot disability medium term (> 3 months < 6 months)
Fig. 8.
Forest plot disability long term (> or = 12 months)
Subgroup analysis on physiotherapy versus minimal intervention
For pain, six studies comparing physiotherapy with a minimal intervention were included in the subgroup analysis at short term, [18–23] three at medium [19, 21, 23] and two at long term [19, 23]. There were no group differences at short (SMD − 0.94 [95%CI − 2.11, 0.23] p = 0.11 I2 = 99%, Fig. 3) or medium-term (SMD − 0.14 [95% CI − 0.36, 0.09] p = 0.25, I2 = 40%, Fig. 4). However, there was a small effect (SMD − 0.38 [95% CI − 0.60, − 0.17, p = 0.0004, I2 = 3%], Fig. 5) in favour of physiotherapy interventions for pain reduction at the long-term time point.
One study [24] could not be meta-analysed due to insufficient data. Nonetheless, the results were broadly consistent with the meta-analysis. Santilli et al. [24] reported number of participants with reduction in radiating pain. At medium term, 48 participants (100%) of the physiotherapy group (spinal manipulation) reported reduction in radiating pain compared with 39 (81%) of those in the sham group. At long-term follow-up, 48 patients (100%) of the physiotherapy group continued to report reductions in radiating pain compared with 40 participants (83%) in the sham group.
For disability, six trials were meta-analysed comparing physiotherapy with minimal intervention at short term, [18–23] three at medium [19, 21, 23] and two trials at long term [19, 23]. No group differences were observed at short (SMD − 0.34 [95%CI − 0.70, − 0.01] p = 0.06, I2 = 87%, Fig. 6) medium, (SMD − 0.08 [95% CI − 0.39, 0.24] p = 0.63, I2 = 68%, Fig. 7) or long-term time points (SMD − 0.21 [95% CI − 0.45, 0.03] p = 0.09, I2 = 23%, Fig. 8). The Santilli [24] study did not report a measure of disability at any time point. Overall, these findings suggest that physiotherapy interventions are slightly more effective than minimal treatment for pain in the long term but not at short or medium term.
Subgroup analysis on physiotherapy versus substantial intervention
Eleven trials compared physiotherapy with substantial control intervention. Nine [26–33, 35] were included in the subgroup analysis for pain. There was no difference between physiotherapy and substantial intervention for the outcome of pain in the short (SMD 0.20 [95%CI − 0.27, 0.67] p = 0.39, I2 = 88%, Fig. 3) or long term (SMD 0.24 [95%CI − 0.05, 0.52], p = 0.10, I2 = 78%, Fig. 5). There was a small effect in favour of substantial intervention in the medium term (SMD 0.32 [95%CI 0.01, 0.63], p = 0.04, I2 = 81%, Fig. 4).
Two trials reported results that were not possible to incorporate in either meta-analysis [25, 34]. Amundsen [25] reported improvements in both the physiotherapy and surgical arms, however groups were not statistically compared. Weber [34] reported slightly higher rates of improvement in surgical compared to physiotherapy interventions at one year.
Seven trials were included in the meta-analysis for the outcome of disability [26, 27, 30–33, 35]. There was a small effect in favour of substantial interventions at short (SMD 0.40 [95%CI 0.09, 0.71] p = 0.01, I2 = 67%, Fig. 6) and medium term (SMD 0.46 [95%CI 0.08, 0.83], p = 0.02, I2 = 87%, Fig. 7) but no difference in the long term (SMD 0.42 [95%CI − 0.11, 0.94], p = 0.12, I2 = 93%, Fig. 8).
Sensitivity analysis
Four studies with high risk of bias in at least 2 parameters [28–30, 33] were removed from the meta-analysis. The sensitivity analyses revealed consistent results for all comparisons apart from the subgroup comparison of physiotherapy versus substantial control intervention (Supplemental Figs. 1–6). With the removal of high risk of bias studies, the effect on pain at medium term and on disability at short term favouring substantial interventions was no longer present (Supplemental Figs. 2 and 4).
Discussion
This systematic review, including 18 studies and 2699 participants with a clinical diagnosis of sciatica suggests that physiotherapy interventions are only better than minimal interventions in reducing pain at long-term time points. Physiotherapy interventions are less effective than substantial interventions (e.g. surgery) in reducing pain at medium term and disability at short- and medium-term time points. However, heterogeneity was considerable in most meta-analyses, and confidence intervals were large, indicating substantial uncertainly surrounding the precision of these estimates. The favourable results for substantial intervention for pain in medium term and disability in short term did not persist following sensitivity analyses removing studies with high risk of bias. The currently available literature therefore provides insufficient evidence to support strong recommendations for physiotherapy interventions in the treatment of people with sciatica.
This systematic review reflects a wider collective inability to show significant benefit of non-surgical treatments for people with sciatica. Pharmacological options fail to demonstrate effects beyond placebo [37], including non-steroidal anti-inflammatories [38], anti-convulsants [39], anti-depressants [40] or opioids [4, 41]. Epidural cortisone injections have small effect sizes and short-term benefits [42]. These findings are disappointing given the clear need for effective conservative interventions voiced by patients [43].
Apart from the possibility that physiotherapy is indeed not effective for patients with sciatica, there are multiple possible reasons for the lack of evidence. The physiotherapy interventions used in the 11 trials comparing physiotherapy with substantial interventions are not all considered contemporary in line with current clinical guidelines [4]. This is a reflection of a lack of recent physiotherapy trials, with only four of the 11 studies published in the last decade [26, 28, 30, 31]. Current clinical guidelines recommend group exercise and continuation of normal activities; however, bedrest was a component of the conservative treatment arm in two trials [28, 34]. The UK NICE Guidelines [4] find no evidence supporting the use of corsets or belts, but these were a core component in another trial [25] conducted before publication of these guidelines. The physiotherapy interventions are highly heterogeneous and remain unclear in several studies. The Bailey study [26] leaves physiotherapy interventions at the discretion of the treating clinician, and the Peul study [33] refers people to physiotherapy only if they are fearful of movement, leaving uncertainty about how many participants in those trials had active physiotherapy treatment. It could also be argued that patients deemed suitable for surgery are likely to represent a specific subgroup that may be less amenable to physiotherapeutic interventions (e.g. with intractable pain or neurological deficit). Indeed, two trials comparing physiotherapy interventions with surgery included patients who had already failed conservative treatment [28, 29], raising serious concerns that physiotherapy interventions could possibly succeed in such a population.
A further challenge to progress in treatment is the diagnosis of sciatica itself [44]. There is no agreed definition for sciatica, reflected in the wide range of definitions used in clinical trials [12], including our review. The broad term ‘sciatica’ comprises radiculopathy, radicular pain, or somatic referred pain. The differing patient populations bring clinical heterogeneity to most meta-analyses. Unfortunately, the high heterogeneity among studies reduces the confidence in our results. Together with previous systematic reviews with inconclusive findings, our results question the value of continuing to perform clinical trials in heterogeneous groups of patients. Although subgrouping according to risk stratification showed promise in the management of people with non-specific low back pain [45], this has failed in patients with sciatica [46]. Subgrouping using a mechanism-based approach shows promising signals in patients with neuropathic pain of different aetiologies [47], but has yet to be examined in sciatica.
The risk of bias analysis highlights areas of improvement for future trials. Performance bias is the area with the highest risk of bias. Although recent studies have shown that blinding of participants is possible [48], it is not easy to eradicate this bias where the intervention is a physical one such as surgery or physiotherapy. The main area that could easily be addressed is detection bias. Blinding outcome assessment would have reduced overall risk of bias in four studies.
Strengths and limitations
The main strength of this review was the strict inclusion criteria based on clinical diagnosis confirming spinally referred leg pain of neural origin. A consequence of the tight inclusion criteria is the exclusion of 45 studies due to inadequate information on diagnosis of sciatica. As a result, our data reflect outcomes in patients with true nerve involvement. Insufficient reporting and low number of studies prevented a subgroup analysis according to type of physiotherapy intervention. Future trials with physiotherapy intervention should adhere to the TIDieR framework to fully describe the complexity of the intervention [49].
Conclusion
In summary, in patients with clinically diagnosed sciatica, physiotherapy interventions trialed to date provide inadequate evidence to make specific recommendations on their effectiveness in reducing pain or disability. The lack of convincing evidence may be due to several factors including incomplete trial reporting, clinical, methodological, and statistical heterogeneity, and trials lacking high methodological quality. Rather than continuing to perform trials in the heterogeneous population of ‘sciatica’, future studies should focus on reducing clinical heterogeneity, using contemporary physiotherapy interventions and high methodological quality to hopefully end the roadblock of discovery on the most effective physiotherapy interventions for these patient populations.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to recognise the contribution of Neal Thurley, Outreach Librarian at the Bodleian Library, University of Oxford, in assisting with the search strategy for this systematic review. The authors would also like to thank Hubert van Griensven, Lecturer, University of Hertfordshire for assisting in translation from Dutch to English of one of the included studies.
Author contributions
All authors contributed to the study conception and design. LD performed literature search, LD and GJ reviewed articles for inclusion/exclusion with third-party arbitration by AS if required. Data collection was performed by LD and LAK and data analysis was performed by LD and AS. The first draft of the manuscript was written by LD and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The research and Lucy Dove were supported by the NIHR Biomedical Research Centre Oxford, based at Oxford University and Oxford University Hospitals NHS Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. ABS is supported by a Clinical Research Career Development Fellowship from the Wellcome Trust (222101/Z/20/Z). For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript arising from this submission.
Declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
This is a secondary analysis of study data and therefore ethical approval was not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Konstantinou K, Dunn KM. Sciatica: review of epidemiological studies and prevalence estimates. Spine (Phila Pa 1976) 2008;33:2464–2472. doi: 10.1097/BRS.0b013e318183a4a2. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt CO, Schweikert B, Wenig CM, Schmidt U, Gockel U, Freynhagen R, Tölle TR, Baron R, Kohlmann T. Modelling the prevalence and cost of back pain with neuropathic components in the general population. Eur J Pain (London, England) 2009;13:1030–1035. doi: 10.1016/j.ejpain.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Konstantinou K, Dunn KM, Ogollah R, Lewis M, Van Windt D, Hay EM, Team AS. Prognosis of sciatica and back-related leg pain in primary care: the ATLAS cohort. Spine J. 2018;18:1030–1040. doi: 10.1016/j.spinee.2017.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NICE NIoHaCE (2016) Low back pain and sciatica in over 16s: assessment and management [PubMed]
- 5.Lewis RA, Williams NH, Sutton AJ, Burton K, Din NU, Matar HE, Hendry M, Phillips CJ, Nafees S, Fitzsimmons D, Rickard I, Wilkinson C. Comparative clinical effectiveness of management strategies for sciatica: systematic review and network meta-analyses. Spine J. 2015;15:1461–1477. doi: 10.1016/j.spinee.2013.08.049. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs WC, van Tulder M, Arts M, Rubinstein SM, van Middelkoop M, Ostelo R, Verhagen A, Koes B, Peul WC. Surgery versus conservative management of sciatica due to a lumbar herniated disc: a systematic review. Eur Spine J. 2011;20:513–522. doi: 10.1007/s00586-010-1603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hahne AJ, Ford JJ, McMeeken JM. Conservative management of lumbar disc herniation with associated radiculopathy: a systematic review. Spine (Phila Pa 1976) 2010;35:E488–504. doi: 10.1097/BRS.0b013e3181cc3f56. [DOI] [PubMed] [Google Scholar]
- 8.Luijsterburg PA, Verhagen AP, Ostelo RW, van Os TA, Peul WC, Koes BW. Effectiveness of conservative treatments for the lumbosacral radicular syndrome: a systematic review. Eur Spine J. 2007;16:881–899. doi: 10.1007/s00586-007-0367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez M, Ferreira ML, Refshauge KM, Hartvigsen J, Silva IR, Maher CG, Koes BW, Ferreira PH. Surgery or physical activity in the management of sciatica: a systematic review and meta-analysis. Eur Spine J. 2016;25:3495–3512. doi: 10.1007/s00586-015-4148-y. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez M, Hartvigsen J, Ferreira ML, Refshauge KM, Machado AF, Lemes IR, Maher CG, Ferreira PH. Advice to Stay Active or Structured Exercise in the Management of Sciatica: A Systematic Review and Meta-analysis. Spine (Phila Pa 1976) 2015;40:1457–1466. doi: 10.1097/BRS.0000000000001036. [DOI] [PubMed] [Google Scholar]
- 11.Lee JH, Choi KH, Kang S, Kim DH, Kim DH, Kim BR, Kim W, Kim JH, Do KH, Do JG, Ryu JS, Min K, Bahk SG, Park YH, Bang HJ, Shin KH, Yang S, Yang HS, Yoo SD, Yoo JS, Yoon KJ, Yoon SJ, Lee GJ, Lee SY, Lee SC, Lee SY, Lee IS, Lee JS, Lee CH, Lim JY, Han JY, Han SH, Sung DH, Cho KH, Kim SY, Kim HJ, Ju W. Nonsurgical treatments for patients with radicular pain from lumbosacral disc herniation. Spine J. 2019;19:1478–1489. doi: 10.1016/j.spinee.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Lin CW, Verwoerd AJ, Maher CG, Verhagen AP, Pinto RZ, Luijsterburg PA, Hancock MJ. How is radiating leg pain defined in randomized controlled trials of conservative treatments in primary care? A systematic review. Eur J Pain (London, England) 2014;18:455–464. doi: 10.1002/j.1532-2149.2013.00384.x. [DOI] [PubMed] [Google Scholar]
- 13.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin Z, Liu X, Wu J, Zhai Y, Liu Z. Effectiveness of acupuncture for treating sciatica: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2015;2015:425108. doi: 10.1155/2015/425108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji M, Wang X, Chen M, Shen Y, Zhang X, Yang J. The Efficacy of Acupuncture for the treatment of sciatica: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2015;2015:192808. doi: 10.1155/2015/192808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods G, Cochrane Statistical Methods G. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira G, Stieven F, Araujo F, Wiebusch M, Rosa C, Plentz R, Silva M. Neurodynamic treatment did not improve pain and disability at two weeks in patients with chronic nerve-related leg pain: a randomised trial. J Physiother. 2016;62:197–202. doi: 10.1016/j.jphys.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Fritz JM, Lane E, McFadden M, Brennan G, Magel JS, Thackeray A, Minick K, Meier W, Greene T. Physical therapy referral from primary care for acute back pain with sciatica: a randomized controlled trial. Ann Intern Med. 2021;174:8–17. doi: 10.7326/m20-4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghoname E, White P, Ahmed H, Hamza M, Craig W, Noe C. Percutaneous electrical nerve stimulation: an alternative to TENS in the management of sciatica. Pain. 1999;83:193–199. doi: 10.1016/S0304-3959(99)00097-4. [DOI] [PubMed] [Google Scholar]
- 21.Hofstee DJ, Gijtenbeek JJM, Hoogland PH, Van Houwelingen JC, Kloet A, Lotters F, Tans JTJ. Bed rest and physiotherapy are of no added value in the management of acute lumbosacral radicular pain: A randomised clinical study. Ned Tijdschr Geneeskd. 2003;147:249–254. [Google Scholar]
- 22.Konstantinovic LM, Kanjuh ZM, Milovanovic AN, Cutovic MR, Djurovic AG, Savic VG, Dragin AS, Milovanovic ND. Acute low back pain with radiculopathy: a double-blind, randomized, placebo-controlled study. Photomed Laser Surg. 2010;28:553–560. doi: 10.1089/pho.2009.2576. [DOI] [PubMed] [Google Scholar]
- 23.Luijsterburg PA, Verhagen AP, Ostelo RW, van den Hoogen HJ, Peul WC, Avezaat CJ, Koes BW. Physical therapy plus general practitioners' care versus general practitioners' care alone for sciatica: a randomised clinical trial with a 12-month follow-up. Eur Spine J. 2008;17:509–517. doi: 10.1007/s00586-007-0569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santilli V, Beghi E, Finucci S. Chiropractic manipulation in the treatment of acute back pain and sciatica with disc protrusion: a randomized double-blind clinical trial of active and simulated spinal manipulations. Spine J. 2006;6:131–137. doi: 10.1016/j.spinee.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Amundsen T, Weber H, Nordal HJ, Magnaes B, Abdelnoor M, Lilleås F. Lumbar spinal stenosis: conservative or surgical management? A prospective 10-year study. Spine. 2000;25:1424–1436. doi: 10.1097/00007632-200006010-00016. [DOI] [PubMed] [Google Scholar]
- 26.Bailey CS, Rasoulinejad P, Taylor D, Sequeira K, Miller T, Watson J, Rosedale R, Bailey SI, Gurr KR, Siddiqi F, Glennie A, Urquhart JC. Surgery versus conservative care for persistent sciatica lasting 4 to 12 months. N Engl J Med. 2020;382:1093–1102. doi: 10.1056/NEJMoa1912658. [DOI] [PubMed] [Google Scholar]
- 27.Burton A, Tillotson K, Cleary J. Single-blind randomised controlled trial of chemonucleolysis and manipulation in the treatment of symptomatic lumbar disc herniation. Eur Spine J. 2000;9:202–207. doi: 10.1007/s005869900113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erginousakis D, Filippiadis D, Malagari A, Kostakos A, Brountzos E, Kelekis N, Kelekis A. Comparative prospective randomized study comparing conservative treatment and percutaneous disk decompression for treatment of intervertebral disk herniation. Radiology. 2011;260:487–493. doi: 10.1148/radiol.11101094. [DOI] [PubMed] [Google Scholar]
- 29.McMorland G, Suter E, Casha S, Du Plessis SJ, Hurlbert RJ. Manipulation or microdiskectomy for sciatica? A prospective randomized clinical study. J Manipulative Physiol Ther. 2010;33:576–584. doi: 10.1016/j.jmpt.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Mondal P, Goswami S, Basak S. Assessment of efficacy of transforaminal epidural steroid injection for management of low back pain with unilateral radiculopathy in industrial workers: A randomized control trial. J Clin Diagn Res. 2017;11:UC01–UC05. doi: 10.7860/JCDR/2017/26400.10765. [DOI] [Google Scholar]
- 31.Nikoobakht M, Yekanineajd MS, Pakpour AH, Gerszten PC, Kasch R. Plasma disc decompression compared to physiotherapy for symptomatic contained lumbar disc herniation: A prospective randomized controlled trial. Neurol Neurochir Pol. 2016;50:24–30. doi: 10.1016/j.pjnns.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Osterman H, Seitsalo S, Karppinen J, Malmivaara A. Effectiveness of microdiscectomy for lumbar disc herniation: a randomized controlled trial with 2 years of follow-up. Spine(Phila Pa 1976) 2006;31:2409–2414. doi: 10.1097/01.brs.0000239178.08796.52. [DOI] [PubMed] [Google Scholar]
- 33.Peul WC, van Houwelingen HC, van den Hout WB, Brand R, Eekhof JAH, Tans JTJ, Thomeer RTW, Koes BW. Surgery versus prolonged conservative treatment for sciatica. N Engl J Med. 2007;356:2245–2256. doi: 10.1056/NEJMoa064039. [DOI] [PubMed] [Google Scholar]
- 34.Weber H. Lumbar disc herniation: a controlled, prospective study with ten years of observation. Spine. 1983;8:131–140. doi: 10.1097/00007632-198303000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Weinstein JN, Tosteson TD, Lurie JD, Tosteson ANA, Hanscom B, Skinner JS, Abdu WA, Hilibrand AS, Boden SD, Deyo RA, Weinstein JN, Tosteson TD, Lurie JD, Tosteson ANA, Hanscom B, Skinner JS, Abdu WA, Hilibrand AS, Boden SD, Deyo RA. Surgical vs nonoperative treatment for lumbar disk herniation: the spine patient outcomes research trial (SPORT): a randomized trial. JAMA. 2006;296:2441–2450. doi: 10.1001/jama.296.20.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinto RZ, Maher CG, Ferreira ML, Hancock M, Oliveira VC, McLachlan AJ, Koes B, Ferreira PH. Epidural corticosteroid injections in the management of sciatica: a systematic review and meta-analysis. Ann Intern Med. 2012;157:865–877. doi: 10.7326/0003-4819-157-12-201212180-00564. [DOI] [PubMed] [Google Scholar]
- 38.Rasmussen-Barr E, Held U, Grooten WJ, Roelofs PD, Koes BW, Van Tulder MW, Wertli MM. Non-steroidal anti-inflammatory drugs for sciatica. Cochrane Database Syst Rev. 2016;10:CD012382. doi: 10.1002/14651858.CD012382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Enke O, New HA, New CH, Mathieson S, McLachlan AJ, Latimer J, Maher CG, Lin CC. Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ. 2018;190:E786–E793. doi: 10.1503/cmaj.171333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferreira GE, McLachlan AJ, Lin CC, Zadro JR, Abdel-Shaheed C, O'Keeffe M, Maher CG. Efficacy and safety of antidepressants for the treatment of back pain and osteoarthritis: systematic review and meta-analysis. BMJ. 2021;372:m4825. doi: 10.1136/bmj.m4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinto RZ, Maher CG, Ferreira ML, Ferreira PH, Hancock M, Oliveira VC, McLachlan AJ, Koes B. Drugs for relief of pain in patients with sciatica: systematic review and meta-analysis. BMJ. 2012;344:e497. doi: 10.1136/bmj.e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oliveira CB, Maher CG, Ferreira ML, Hancock MJ, Oliveira VC, McLachlan AJ, Koes BW, Ferreira PH, Cohen SP, Pinto RZ. Epidural corticosteroid injections for sciatica: an abridged Cochrane systematic review and meta-analysis. Spine. 2020;45:E1405–E1415. doi: 10.1097/BRS.0000000000003651. [DOI] [PubMed] [Google Scholar]
- 43.Ryan C, Roberts L. 'Life on hold': the lived experience of radicular symptoms. A qualitative, interpretative inquiry. Musculoskelet Sci Pract. 2019;39:51–57. doi: 10.1016/j.msksp.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Jesson T, Runge N, Schmid AB. Physiotherapy for people with painful peripheral neuropathies: a narrative review of its efficacy and safety. Pain Rep. 2020;5:e834. doi: 10.1097/PR9.0000000000000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hill JC, Whitehurst DG, Lewis M, Bryan S, Dunn KM, Foster NE, Konstantinou K, Main CJ, Mason E, Somerville S, Sowden G, Vohora K, Hay EM. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): a randomised controlled trial. Lancet. 2011;378(9802):1560–1571. doi: 10.1016/S0140-6736(11)60937-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konstantinou K, Lewis M, Dunn KM, Ogollah R, Artus M, Hill JC, Hughes G, Robinson M, Saunders B, Bartlam B, Kigozi J, Jowett S, Mallen CD, Hay EM, van der Windt DA, Foster NE. Stratified care versus usual care for management of patients presenting with sciatica in primary care (SCOPiC): a randomised controlled trial. Lancet Rheumatol. 2020;2:e401–e411. doi: 10.1016/s2665-9913(20)30099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baron R, Wasner G, Binder A. Chronic pain: genes, plasticity, and phenotypes. Lancet Neurol. 2012;11:19–21. doi: 10.1016/S1474-4422(11)70281-2. [DOI] [PubMed] [Google Scholar]
- 48.Beard DJ, Rees JL, Cook JA, Rombach I, Cooper C, Merritt N, Shirkey BA, Donovan JL, Gwilym S, Savulescu J, Moser J, Gray A, Jepson M, Tracey I, Judge A, Wartolowska K, Carr AJ. Arthroscopic subacromial decompression for subacromial shoulder pain (CSAW): a multicentre, pragmatic, parallel group, placebo-controlled, three-group, randomised surgical trial. Lancet (London, England) 2018;391:329–338. doi: 10.1016/S0140-6736(17)32457-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, Lamb SE, Dixon-Woods M, McCulloch P, Wyatt JC, Chan AW, Michie S. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. doi: 10.1136/bmj.g1687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.