Abstract
Background
While patients with diffuse low-grade glioma (LGG) often survive for years, there is a risk of tumor progression which may impact patients’ long-term health-related quality of life (HRQOL) and neurocognitive functioning (NCF). We present a follow-up of LGG patients and their informal caregivers (T3) who took part in our previous HRQOL investigations (T1, M = 7 and T2 M = 13 years after diagnosis).
Methods
Participants completed HRQOL (short form-36 health survey [SF-36]; EORTC-BN20), fatigue (Checklist Individual Strength [CIS]), and depression (Center for Epidemiological Studies-Depression [CES-D]) questionnaires and underwent NCF assessments. T3 scores were compared with matched controls. Changes over time (T1–T2–T3) on group and participant level were assessed. Where available, histology of the initial tumor was revised and immunohistochemical staining for IDH1 R132H mutant protein was performed.
Results
Thirty patients and nineteen caregivers participated. Of N = 11 with tissue available, 3 patients had confirmed diffuse LGG. At T3, patients (M = 26 years after diagnosis) had HRQOL and NCF similar to, or better than controls, yet 23.3% and 53.3% scored above the cut-off for depression (≥16 CES-D) and fatigue (≥35 CIS), respectively. Caregivers’ HRQOL was similar to controls but reported high rates of fatigue (63.2%). Over time, patients’ mental health improved (P < .05). Minimal detectable change in HRQOL over time was observed in individual patients (30% improvement; 23.3% decline; 20% both improvement and decline) with 23.3% remaining stable. NCF remained stable or improved in 82.8% of patients.
Conclusions
While HRQOL and NCF do not appear greatly impacted during long-term survivorship in LGG, depressive symptoms and fatigue are persistent.
Keywords: depression, fatigue, low-grade glioma, quality of life, survivorship
Key Points.
HRQOL, NCF, depression, and fatigue were assessed on average 7, 13, and 26 years after LGG diagnosis.
While HRQOL was mostly stable over time, psychological functioning appears least stable.
Elevated levels of depressive symptoms and fatigue were observed.
Importance of the Study.
Diffuse low-grade glioma (LGG) comes with the risk of tumor progression, which can impact patient and caregiver health-related quality of life. We present a follow-up of a unique cohort of long-term survivors of LGG and their caregivers, now on average 26 years after diagnosis. Our findings show persistent unmet needs in both patients and caregivers, and emphasize the importance of providing adequate psychosocial and supportive care throughout LGG survivorship.
Diffuse low-grade gliomas (LGG) are uncommon primary brain tumors with a relatively favorable prognosis.1 The specific tumor type, grade, and molecular profile (particularly 1p/19q codeletion, and IDH mutation) dictate treatment choices and outcome.2 Many patients continue to survive with stable disease for years before the tumor eventually dedifferentiates to a more malignant form.3,4 Maintaining the optimal health-related quality of life (HRQOL) during that stable disease phase is therefore essential.
Before confirmed diagnosis and during initial treatment, HRQOL of LGG patients is worse than normative data or healthy controls.5–7 Shortly after treatment, HRQOL limitations are typically mild in nature.6,8–10 After this initial period dominated by the diagnosis and treatment, often a prolonged period of stable disease follows. While remaining under the care of their treatment team, with regular clinical and radiological follow-up, HRQOL issues can remain or even emerge as patients aspire to return to normal life. Studies to date show mild HRQOL impediments during stable disease across domains (eg, physical [role] functioning, general health, social functioning, mental health, cognitive functioning, future uncertainty), below the level of healthy controls.11,12 Moreover, studies using objective measures of neurocognitive functioning (NCF) typically show a delayed onset of NCF deficits,13–17 with radiotherapy treatment affecting executive functioning, information processing, and attention.18
Patient functioning and HRQOL can have a wider impact on family members and friends, who frequently act as informal caregivers. High levels of burden, distress, and unmet needs are not uncommon among caregivers.19 While our previous study, comparing caregivers of stable LGG patients to those caring for patients with hematological malignancies, showed no great caregiver HRQOL impediments,20 this remains a relatively unexplored area. Few studies have reported on caregivers of LGG patients specifically, and fewer still during long-term survivorship.
The same holds true for patient-focused reports. During long-term survivorship, HRQOL studies in LGG are scarce as confirmed by a recent systematic review, which could only identify three studies looking at >10 years after diagnosis of grade II or III glioma.21 In general, emotional, social, and psychological functioning remain vulnerable throughout survivorship, with a clear dearth of longitudinal studies.21 Updates in histopathological and molecular diagnostics, placing greater emphasis on molecular markers that predict tumor behavior (eg, 1p/19q codeletion, IDH1/IDH2 mutation),22,23 complicate matters as long-term follow-up studies in LGG rarely have such molecular data available.
We have previously assessed HRQOL in 195 LGG patients on average 6 years after diagnosis,24 and their caregivers (N = 213).20 A follow-up study of patients whose disease remained stable (N = 65) was undertaken on average 12 years after diagnosis.25 We currently present a follow-up of HRQOL in this cohort, now on average 26 years after diagnosis. This very long-term follow-up means our participants have had an LGG diagnosis in the more distant past. While this may not completely overlap with present-day diagnostic criteria, these patients have lived with a diffuse LGG diagnosis for decades. Insights from this follow-up of the largest longitudinal HRQOL study in LGG can help inform patients, their families, and clinicians, and may serve as a benchmark for treatment trials evaluating interventions that can have very long-term effects.
Materials and Methods
Participants
Participants form a sub-sample of LGG patients recruited to our previous studies (N = 195 at T1, mean of 6 years after diagnosis; N = 65 at T2, mean of 12 years after diagnosis). Study design and methods have been described in detail elsewhere.18,24,25 Briefly, for the initial study, patients with clinically and radiologically stable, histologically confirmed World Health Organization (WHO) grade I or II glioma conform diagnostic criteria of the time, not receiving corticosteroid treatment, were recruited if proficient in Dutch. Those with persistent clinically and radiologically stable diseases were then recruited for a second assessment (T2). For the present study, (T3) regardless of disease status, we invited all patients to take part, but only those originally diagnosed with diffuse grade II glioma were included in this report. The treating physician, or general practitioner if patients had not seen the treating physician for over a year, invited patients and caregivers via letter. The institutional review boards of the participating centers approved the study, and all participants provided written informed consent.
Procedure
Following consent procedures, participants received a questionnaire pack by mail consisting of sociodemographic questions plus outcome measures specified below, to complete and return. Patient clinical data were obtained from medical records. To determine if patients would be classified as having diffuse LGG according to today’s diagnostic criteria, we aimed to retrieve the original tumor blocks from participating centers for central review of histopathological and molecular features (immunohistochemical staining for IDH1 R132H mutant protein). Neurocognitive data were collected by trained test assistants supervised by a board-certified neuropsychologist. Assessments were done at home or at the hospital to suit participant preference and took ~60 min.
Outcome measures
Repeated assessments (T1, T2, T3)
Medical outcomes study short form-36 health survey
26 The Dutch version of the short form-36 health survey (SF-36) was used to assess generic HRQOL. The 36-item instrument has 8 multi-item scales (physical functioning; physical role functioning; emotional role functioning; pain, vitality; social functioning; mental health; general health perceptions [score range 0–100]). The Physical Component Summary (PCS) and Mental Component Summary (MCS) are higher-order component scales with a general population mean of 50 and standard deviation (SD) of 10. Higher scores represent better functioning.
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–brain cancer module (BN20)
27 This 20-item questionnaire assesses patients’ disease-specific HRQOL (completed by patients only). It yields 4 multi-item scales (future uncertainty; visual disorders; motor dysfunctions; communication deficits) plus 7 single items (headaches; seizures; drowsiness; hair loss; itching skin; weakness in the legs; difficulties with bladder control [score range 1–4]). Raw scores were converted to scales ranging from 0 to 100, with higher scores indicating more symptoms.
Neurocognitive ability was assessed using a neuropsychological test battery identical to our previous investigations,15,18 consisting of the visual-verbal learning test, concept shifting test, memory comparison test, categorical word fluency, letter-digit modalities test, and Stroop color-word test.
Cross-sectional assessments (T3)
Center for Epidemiologic Studies Depression Scale
Depressive symptoms were assessed with the 20-item Center for Epidemiologic Studies Depression Scale (CES-D; 4-point scale).28 Higher scores indicate more depressive symptoms (range 0–60), with scores ≥16 indicating a high risk for clinical depression.
Checklist Individual Strength
Fatigue was assessed with the 20-item Checklist Individual Strength (CIS; 7-point Likert scale), yielding 4 subscales (fatigue severity; concentration problems; reduced motivation; reduced activity) and a total score with items scores.29 Higher scores indicate worse functioning on all scales. The standard cut-off for severe fatigue is ≥35 on the fatigue severity subscale.
Statistical Analysis
Data were analyzed using SPSS version 26.0 for Windows (SPSS, Chicago, IL). Questionnaires were scored along the respective manuals. For neurocognitive data, raw test scores were converted to z scores using the mean and SD of healthy controls matched for age, sex, and educational level.30 Six NCF domain scores were calculated: executive functioning, verbal memory, working memory, attention, information processing, and psychomotor functioning.31,32 Descriptive statistics were generated to describe the samples and scores on outcomes assessed at T3 (SF-36; EORTC BN20; NCF; CES-D; CIS). Family caregivers were not always the same individuals as those assessed at T1,20 hence these were treated as a new cross-sectional sample.
Mann-Whitney U tests were done to compare SF-36 scores of patients and caregivers at T3, with controls from the general population matched for age, sex, and educational level. Similarly, NCF domain scores were compared between patients and matched controls from the Maastricht Aging Study33 at T3 using Mann-Whitney U tests. To assess changes over time for LGG patients at the group level, Friedman tests were used for generic and disease-specific HRQOL (SF-36, BN20) and neurocognitive domains between T1, T2, and T3, with Kendall’s W values as an indicator of effect size. Minimal clinically important differences (MCIDs) were assessed, based on established cut-offs from comparable patient groups. For SF-36, the MCIDs for the component summaries were set at 3.0 points (PCS) and 4.6 points (MCS).34 For the scale scores, MCIDs are commonly <10 points so we set the MCID conservatively at 10 points.35 For BN20 scores MCIDs were set at 10 points for the scales and single-item scores.36 For NCF domains, group-level change >1 SD was considered clinically important.
To assess changes over time on the participant level, for questionnaires minimal detectable changes, defined as 1.96 × √2 × standard error of measurement (SEM),37 were calculated for the multi-item scales. Test-retest reliability scores and SDs necessary to calculate the SEM were derived from other studies performed in comparable patient populations,38–40 similar to our previous report.25 For NCF domains, an individual participant change over time of >1SD was considered equivalent to minimal detectable change. P < .05 was considered statistically significant. For descriptive purposes, individual participants’ demographic and clinical characteristics are presented alongside information on whether overall HRQOL remained stable, improved, declined, or whether both improvement and decline took place (T1–T3).
Results
Participants
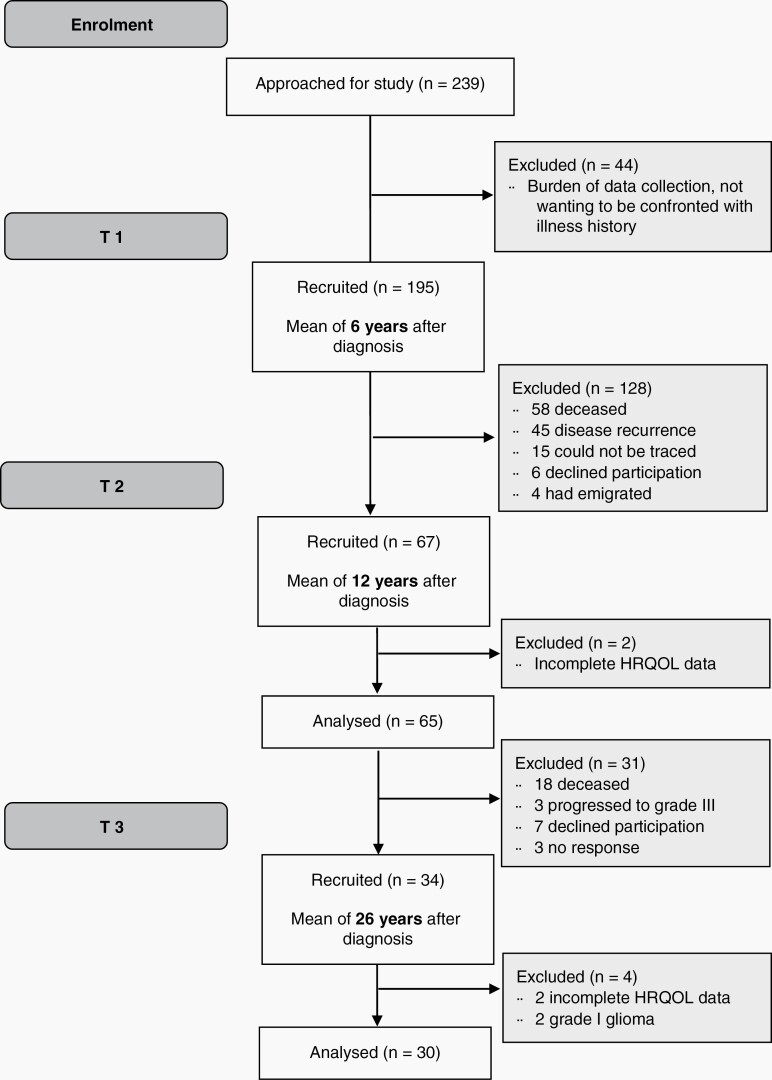
Data were collected between March 2017 and July 2019. Out of N = 65 patients who took part in T1 and T2, N = 34 (52%) consented to take part in T3. Of these, two patients were diagnosed with a grade I glioma and two lacked HRQOL data, thus N = 30 could be included in the present analysis (see Figure 1). Nineteen caregivers took part. All original tumor blocks were requested, but only from 11 patients, the blocks could be retrieved (37%). In an attempt to assign a pathological diagnosis following WHO 2016 criteria, a central review of the histology of these cases was performed as well as immunohistochemistry for IDH1 R132H mutant protein.2 Three cases indeed concerned diffuse LGG IDH-mutant, 2 with astrocytic features, and 1 with oligodendroglial feature. Other cases included diffuse LGG/astrocytoma IDH wild type (N = 1), pilocytic astrocytoma (N = 1), pilocytic astrocytoma or ganglioglioma (N = 1), dysembryoplastic neuroepithelial tumor (N = 2), glioma “not otherwise specified” IDH wild type (N = 3).
Fig. 1.
Participant flow diagram.
Those not taking part in T3 were older at the time of diagnosis (median 39.89 vs 23.32, P < .001), but comparable with respect to sex, tumor type, and treatment (extent of resection, radiotherapy) (all P = n.s.). At T1, T2, and T3 patients were on average 6.7 (SD = 3.5), 13.0 (SD = 3.7), and 26.0 (SD = 3.7) years after diagnosis, respectively. Most caregivers were men (58%, N = 11), spouses of patients (84%, N = 16), and their average age was M = 53.53 (SD = 10.21). Further characteristics of the study cohort over time are displayed in Table 1.
Table 1.
Participant Characteristics of the Cohort Over Time (T1, T2, T3)
| Full Cohort T1 (N = 195) |
First Follow-up T2 (N = 65) |
Current Follow-up T3 (N = 30) |
||
|---|---|---|---|---|
| Age in years | M (SD), range | 40.8 (11.6), 18–70 | 44.5 (12.1), 23–72 | 52.33 (11.52), 36–72 |
| Age at diagnosis | M (SD), range | 34.3 (12.5), 8–65 | 32.0 (13.6), 8–62 | 25.97 (12.44), 8–50 |
| Sex, N(%) | Male | 120 (61.5) | 36 (55.4) | 15 (50.0) |
| Female | 75 (38.5) | 29 (44.6) | 15 (50.0) | |
| Level of education, N(%) | Low | 58 (29.7) | 21 (32.3) | 5 (16.7) |
| Middle | 74 (37.9) | 22 (33.8) | 11 (36.7) | |
| High | 60 (30.8) | 22 (33/8) | 13 (43.3) | |
| Other | 3 (1.5) | 0 (0) | 1 (3.3) | |
| Marital status, N(%) | Single | 56 (28.7) | 17 (26.2) | 6 (20.0) |
| Married or living together | 124 (63.6) | 38 (58.5) | 20 (66.7) | |
| Divorced | 6 (3.1) | 7 (10.8) | 2 (6.7) | |
| Widowed | 6 (3.1) | 3 (4.6) | 2 (6.7) | |
| Missing | 3 (1.5) | 0 (0) | 0 (0) | |
| Tumor type, N(%) | Astrocytoma | 127 (65.1) | 47 (72.3) | 25 (83.3) |
| Oligoastrocytoma | 12 (6.2) | 7 (10.7) | 2 (6.7) | |
| Oligodendroglioma | 39 (20.0) | 11 (16.9) | 3 (10.0) | |
| Missing | 17 (8.7) | 0 (0) | 0 (0) | |
| WHO tumor grade, N(%) | I | 11 (5.6) | 7 (10.8) | 0 (0) |
| II | 163 (83.6) | 57 (87.7) | 30 (100) | |
| Missing | 21 (10.8) | 1 (1.5) | 0 (0) | |
| Neurosurgical intervention, N(%) | Biopsy | 71 (36.4) | 19 (29.2) | 7 (23.3) |
| Resection | 94 (48.2) | 41 (63.1) | 20 (66.7) | |
| Craniotomy, unspecified | 12 (6.2) | 5 (7.7) | 3 (10.0) | |
| Missing | 18 (9.2) | 0 (0) | 0 (0) | |
| Radiotherapy (ever), N (%) | Yes | 104 (53.3) | 32 (49.0) | 13 (43.3) |
| No | 91 (46.7) | 33 (51.0) | 17 (56.7) | |
| Recurrence (ever), N (%) | Yes | 19 (9.7) | 0 (0) | 6 (20.0) |
| No | 154 (79.0) | 65 (100) | 23 (76.7) | |
| Missing | 19 (9.7) | 0 (0) | 1 (3.3) |
HRQOL, NCF, depressive symptoms, and fatigue 26 years after diagnosis
Patients
Patients had better scores on the SF-36 subscales physical functioning (median 95.00 vs 85.00, P = .021) and bodily pain (median 84.00 vs 62.00, P = .022), as well as PCS scores (median 51.05 vs 46.68, P = 0.039), compared to matched controls. On group level, NCF scores did not differ from matched controls (P > .05). At T3, patients’ depressive symptoms (CES-D) were M = 10.04 (SD = 8.30; see Supplementary Table 1). Seven patients (23.3%) scored above the cut-off, indicating high risk for clinical depression. The total fatigue score was M = 82.63 (SD = 8.03), with 53.3% (N = 16) classed as severely fatigued (see Supplementary Table 1).
Caregivers
Caregivers’ HRQOL did not differ from matched controls (SF-36 scales and component summaries all P > .05). Caregivers’ depressive symptoms (CES-D) were M = 7.00 (SD = 6.01; see Supplementary Table 1), with 1 caregiver (5.3%) scoring above the cut-off indicating high risk for clinical depression. Caregivers’ total fatigue score was M = 83.78 (SD = 6.36), with 63.2% (N = 12) of caregivers classed as severely fatigued.
Patient Group-Level Change in HRQOL and NCF Over Time
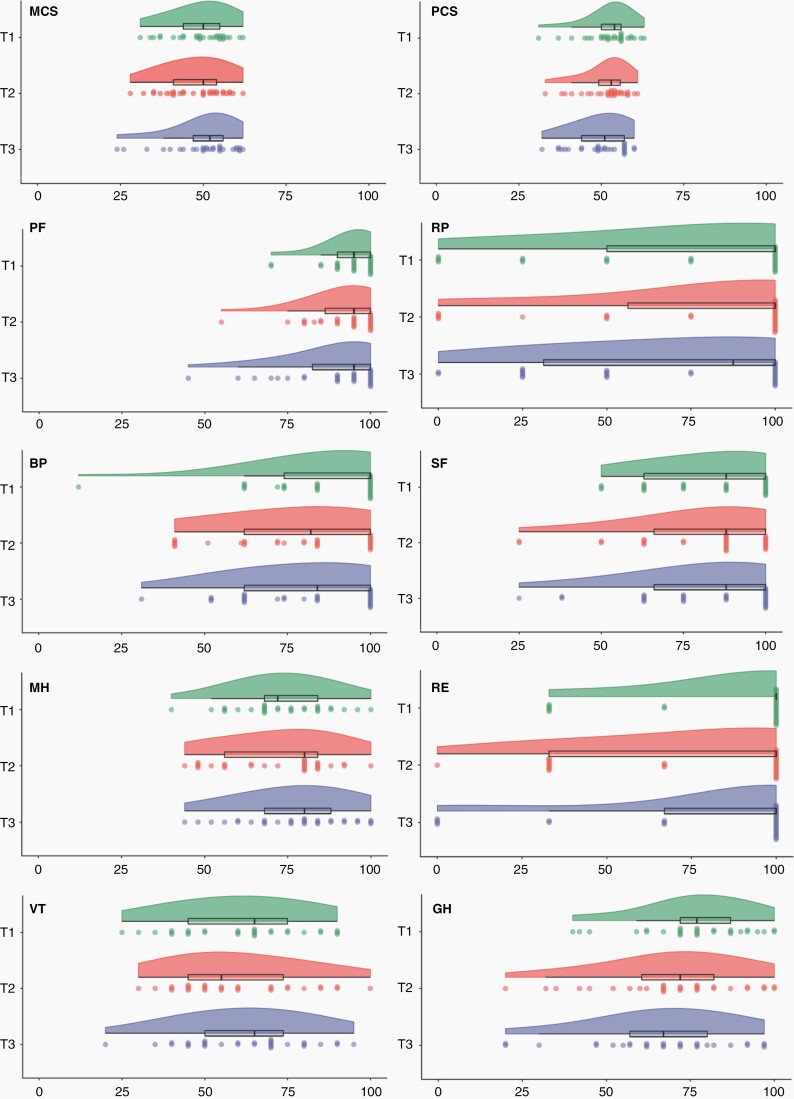
Analysis at the group level revealed a statistically significant, but not clinically relevant, improvement in mental health over time (P = .011, W = 0.155, see Figure 2). For MCS, a statistically significant change over time is seen with a dip at T2 (P = .039, W = .116; not clinically relevant). No other statistically significant differences were observed between T1, T2, and T3 for generic or disease-specific HRQOL (see Supplementary Figure 1). A clinically significant decline on emotional role functioning was observed from T1 to T2 (Mdiff = −11.49, SD = 40.11) which did not reach statistical significance. For NCF, a statistically significant, but not clinically relevant, improvement over time was observed on all domain scores (P < .05; Kendall’s W values ranging from 0.122 for executive functioning to 0.351 for verbal memory).
Fig. 2.
Generic health-related quality of life scale scores at T1, T2, and T3. Raincloud plots depict all individual patients’ scores with each “raindrop” representing a patient, as well as the boxplot and overall distribution of scores visualized as a “cloud”. MCS, Mental Component Summary; PCS, Physical Component Summary; PF, physical functioning; RP, role functioning physical; BP, bodily pain; SF, social functioning; MH, mental health; RE, role functioning emotional; VT, vitality; GH, general health perceptions.
Patient Individual-Level Change in HRQOL and NCF Over Time
From 7 to 13 years after diagnosis (T1 to T2)
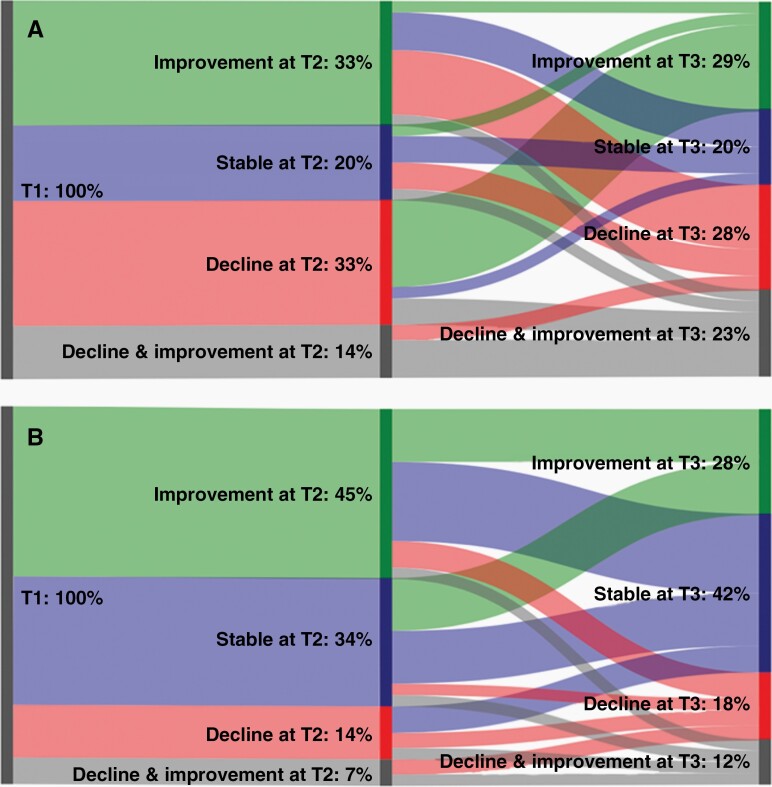
Nearly all patients (93.3%, N = 28) maintained a stable level of physical HRQOL as assessed with SF-36 PCS, with 3.3% (N = 1) experiencing decline or improvement, respectively. Detectable decline on the SF-36 MCS was observed in 16.7% (N = 5) of the sample, and improvement in 6.7% (N = 2), with the majority (76.7%, N = 23) maintaining a stable level of mental HRQOL. This pattern of results is largely similar on all other SF-36 scales and BN20 multi-item scales, see Supplementary Table 2. Between T1 and T2, 20.0% of LGG patients (N = 6) had stable HRQOL on all scales, 33.3% (N = 10) experienced decline on ≥1 scale, 33.3% (N = 10) experienced improvement on ≥1 scale, and 13.3% (N = 4) experienced both decline and improvement (see Figure 3A). For NCF, 34.5% (N = 10) maintained stable domain scores between T1 and T2; 44.8% (N = 13) experienced improvement on ≥1 domain, 13.8% (N = 4) experienced decline on ≥1 domain, and 6.9% (N = 2) experienced both decline and improvement (see Figure 3B).
Fig. 3.
Sankey diagram of patient overall change in HRQOL (A) and NCF (B) over time in percentages. HRQOL, health-related quality of life; NCF, neurocognitive functioning.
From 13 to 26 years after diagnosis (T2 to T3)
—Detectable decline on the SF-36 PCS was observed in 6.7% (N = 2), and improvement was seen in 3. 3% (N = 1), with the majority maintaining a stable physical HRQOL (86.7%, N = 26). Detectable decline on the SF-36 MCS was found in 10.0% (N = 3), and improvement in 13.3% (N = 4), with the majority maintaining a stable mental HRQOL (73.3%, N = 22). On other multi-item scales of SF-36 and BN20, a similar pattern was seen (Supplementary Table 2). From T2 to T3, 20.0% LGG patients (N = 6) had stable HRQOL on all scales, 26.7% (N = 8) experienced decline on ≥1 scale, 30.0% (N = 9) experienced improvement on ≥1 scale, and 23.3% (N = 7) experienced both decline and improvement (see Figure 3A). On NCF domains, 41.4% (N = 12) maintained stable scores, 31.0% (N = 9) experienced improvement, 13.8% (N = 4) experienced decline, and 13.8% (N = 4) experienced both decline and improvement (see Figure 3B).
From 7 to 26 years after diagnosis (T1 to T3)
Detectable decline on the SF36 PCS was seen in 6.7% (N = 2), the rest remained stable (86.7%, N = 26). Detectable decline on the SF-36 MCS was seen in 16.7% (N = 5), and improvement was observed in 10.0% (N = 3), with the majority maintaining a stable score (66.7%, N = 20). Changes on other multi-item scales of SF-36 and BN20 show a similar pattern (Supplementary Table 2). From T1 and T3, 26.7% of LGG patients (N = 8) had stable HRQOL on all scales, 23.3% (N = 7) experienced decline on ≥1 scale, 30.0% (N = 9) experienced improvement on ≥1 scale, and 20.0% (N=6) experienced both decline and improvement. Table 2 shows demographic and clinical characteristics of individual patients within these four groups. NCF domain scores were stable in 27.6% (N = 8), with 55.2% (N = 16) improving over time, 10.3% (N = 3) declining, and 6.9% (N = 2) having declining scores on some domains and improving scores on other domains.
Table 2.
Individual Participant Demographic and Clinical Characteristics Offset Against Change in overall HRQOL (T1–T3)
| Age Range | Years since Diagnosis | Sex | Original Diagnosis | Review Diagnosis | Treatment | Recurrence and Treatment | Epilepsy | Cognitive Functioning | Depression Above Cut-off | Fatigue Above Cut-off | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biopsy | Resection | RT | Seizures (ever) | AEDs (at T3) | |||||||||||
| Improving HRQOL | |||||||||||||||
| 1 | 60–65 | 35 | M | Astrocytoma grade II | N/a | N | Y | N | N/a | Y | N | Stable across domains | Missing | Missing | |
| 2 | 40-45 | 28 | M | Astrocytoma grade II | N/a | Y | N | N | N/a | Y | Y | Improvement in EF, PF, WM, IP, AT | N | Y | |
| 3 | 35–40 | 31 | F | Astrocytoma grade II | N/a | N | Y | N | 1 Recurrence. Surgical resection | N | N | Improvement in PF; VM, WM, AT | N | Y | |
| 4 | 40–45 | 27 | F | Astrocytoma grade II | DNT | Y | N | Y | N/a | N | N | Improvement in PF, AT | N | Y | |
| 5 | 60–65 | 26 | M | Oligoastrocytoma grade II | N/a | N | Y | N | N/a | Y | Y | Stable across domains | Missing | N | |
| 6 | 40–45 | 19 | F | Astrocytoma grade II | Other glioma IDH wild type, NOS | N | Y | N | N/a | Y | Y | Improvement in PF, IP | Missing | Missing | |
| 7 | 60–65 | 26 | F | Astrocytoma grade II | N/a | N | Y | N | N/a | Y | N | Stable across domains | Y | Y | |
| 8 | 65–70 | 21 | M | Astrocytoma grade II | Other glioma IDH wild type, NOS | N | Y | Y | N/a | N | N | Improvement in EF, WM | N | Y | |
| 9 | 55–60 | 26 | M | Astrocytoma grade II | No evidence of tumour tissue | N | Y | N | N/a | Y | N | Stable across domains | Missing | Missing | |
| Stable HRQOL | |||||||||||||||
| 1 | 55–60 | 25 | F | Astrocytoma grade II | Diffuse astrocytoma IDH mutated | N | Y | Y | N/a | N | N | Improvement on PF | N | Y | |
| 2 | 40–45 | 25 | F | Astrocytoma grade II | N/a | ? | ? | N | N/a | Y | N | Improvement on VM | Y | N | |
| 3 | 65–70 | 23 | M | Astrocytoma grade II | N/a | N | Y | N | N/a | Missing | Missing | Missing | N | Y | |
| 4 | 65–70 | 31 | M | Astrocytoma grade II | N/a | N | Y | N | N/a | Y | Y | Stable across domains | N | Y | |
| 5 | 40–45 | 32 | F | Astrocytoma grade II | N/a | Y | N | Y | N/a | Y | N | Improvement on WM | Y | Y | |
| 6 | 45–50 | 26 | F | Astrocytoma grade II | N/a | N | Y | Y | 1 Recurrence. Radiotherapy | Y | N | Improvement on VM | N | N | |
| 7 | 35–40 | 23 | F | Astrocytoma grade II | N/a | N | Y | Y | N/a | N | N | Improvement on VM, IP | N | Y | |
| 8 | 70–75 | 22 | F | Oligodendroglioma grade II | Diffuse LGG, IDH mutated | N | Y | Y | 2 Recurrences. First time re-resection, second PCV chemotherapy. | N | N | Decline in IP | Missing | Missing | |
| Declining HRQOL | |||||||||||||||
| 1 | 40–45 | 29 | M | Astrocytoma grade II | N/a | Y | N | Y | N/a | N | N | Improvement in PF, WM | N | N | |
| 2 | 40–45 | 28 | F | Astrocytoma grade II | N/a | N | Y | N | N/a | N | N | Improvement in PF, VM | N | N | |
| 3 | 50–55 | 22 | F | Astrocytoma grade II | Diffuse astrocytoma IDH mutated | Y | N | N | 1 Recurrence. Surgery, radiotherapy and chemotherapy | Y | Y | Decline in IP | Y | Y | |
| 4 | 55–60 | 24 | M | Oligodendroglioma grade II | Other glioma IDH wild type, NOS | N | Y | N | N/a | Y | N | Improvement on WM | N | Y | |
| 5 | 60-65 | 33 | M | Astrocytoma grade II | N/a | N | Y | Y | 2 Recurrences. Two re-resections | Y | Y | Stable across domains | Missing | Missing | |
| 6 | 60-65 | 29 | M | Oligoastrocytoma grade II | N/a | ? | ? | Y | Missing | Y | Y | Decline in PF; improvement in VM | Y | Y | |
| 7 | 70-75 | 23 | F | Oligodendroglioma grade II | DNT | Y | N | N | N/a | Y | Y | Improvement on VM | N | Y | |
| Both declining and improving HRQOL | |||||||||||||||
| 1 | 40-45 | 26 | M | Astrocytoma grade II | N/a | N | Y | N | N/a | Y | N | Improvement in PF, WM | N | Y | |
| 2 | 50-55 | 23 | M | Astrocytoma grade II | Pilocytic astrocytoma or ganglioglioma | N | Y | N | N/a | Y | Y | Stable across domains | Y | N | |
| 3 | 40-45 | 28 | M | Astrocytoma grade II | N/a | N | Y | N | N/a | Y | N | Improvement in WM, IP, AT | N | N | |
| 4 | 40-45 | 27 | F | Astrocytoma grade II | N/a | Y | N | Y | N/a | Y | N | Decline in EF | Y | Y | |
| 5 | 45-50 | 26 | F | Astrocytoma grade II | N/a | N | Y | N | N/a | Y | N | Improvement in PF, IP, AT | Missing | Missing | |
| 6 | 35-40 | 29 | M | Astrocytoma grade II | Pilocytic astrocytoma | ? | ? | Y | 1 Recurrence. Surgery and radiotherapy | N | N | Improvement in PF; decline in AT | N | N |
AED, antiepileptic drug; AT, attention; EF, executive functioning; IP, information processing; LGG, low-grade glioma; NOS, not otherwise specified; PF, psychomotor functioning; VM, verbal memory; WM, working memory.
Discussion
We present the longest follow-up of adult patients diagnosed with LGG ever reported. In this prospective follow-up study, we found that LGG patients’ HRQOL was better or equal to the HRQOL of matched controls. HRQOL remained stable or improved over time in the majority of patients, assessed at an average of 7, 13, and 26 years after diagnosis. Those experiencing a decline in HRQOL at one-time point, may not experience further decline or even improve as time progresses. This is reassuring for LGG patients, their families, and their treatment team.
However, 23.3% of patients scored above the cut-off for high risk of clinical depression. This is a higher depression prevalence than seen in the general population (17.3%), as measured with self-report instruments.41 Fatigue was also highly prevalent with 53.3% of LGG patients being severely fatigued. Similarly, caregivers had comparable HRQOL scores to controls, and while depressive symptoms were not common (5.3% or N = 1), fatigue was prevalent with 63.2% of caregivers classed as severely fatigued. Fatigue rates among both patients and caregivers in our study are higher than commonly found in cancer patient populations (29%–51%).42,43
High risk of depression is a known issue in the glioma patient population,44 and our study indicates that this remains a problem in a subgroup of patients living with the diagnosis for decades. This may be intrinsically linked to illness uncertainty which is known to affect psychological functioning of cancer survivors.45,46 Evidence for effectiveness of interventions to treat depressive symptoms and fatigue in patients with brain tumors and their caregivers is still largely lacking,47,48 highlighting a persistent, unmet need.
At patient group level, the subscale for mental health showed statistically significant, but not clinically relevant, improvement over time. This is roughly reflected in the percentages of patients who showed detectable improvement on this scale (between 10% and 30% depending on the time points compared). Emotional role functioning showed a clinically relevant decline over time, which did not reach statistical significance. This can be in part because of the larger intra-individual score range in combination with the small sample size, making it harder to detect statistically significant group-level change. Still, the proportion of patients experiencing decline is similar to those for mental health improvement: between 10% and 20% depending on the time points compared. This discrepancy in findings for the mental health and emotional role functioning scales, which can be expected to correlate, could indicate that psychological functioning is least stable over time. In part, this could explain why there are no clear links between high scores on depressive symptoms and fatigue and HRQOL in our descriptive analysis of individual participants in Table 2.
Owing to our modest sample size, we could not formally analyze potential predictors of change in HRQOL and NCF over time, or depressive symptoms or fatigue. In descriptive analyses, those experiencing both improvement on some HRQOL scales and decline on others appear to be younger than those who remain stable or only experience improvement or decline. No clear pattern in high risk for depression or fatigue can be observed in relation to HRQOL or treatments received. The impact of the tumor type itself is particularly difficult to determine, as histopathology could be reviewed in only 11 participants (37% of the sample), with a confirmed WHO 2016 diagnosis of diffuse LGG in just 3 patients. It is therefore important to emphasize that these unique long-term survivors represent patients living with a diagnosis of LGG, made according to the then current histopathological approach which only partly overlaps with today’s “histomolecular” diagnosis of adult-type diffuse gliomas.
Study limitations include the small sample size, and uncertainty surrounding histopathological and molecular diagnosis of participants whose material could not be retrieved. This study, however, reflects the natural clinical course of a group of patients initially confronted with the diagnosis and prognosis of diffuse LGG, who ended up at the more favorable tail of the survival curve. The high proportion of grade I lesions (in available pathological specimens) in the current cohort of long-term survivors reflects the inherent differences in prognosis between tumor grades. Still, 3 out of 11 patients had a grade II, IDH-mutant astrocytoma which is associated with a severely shortened life expectancy. Our study indicates pathology review of original tumor blocks is valuable in long-term survivors in future efforts. There is an opportunity to learn more from long-term survivors for whom LGG diagnosis holds up according to present-day molecular stratification. Moreover, patients and caregivers might benefit from pathology review as it is likely that the ever-present possibility of tumor recurrence and/or dedifferentiation affects patients’ and caregivers’ general perspective and attitudes. Another limitation is the serial nature of our assessments, which led to a wide range in time since diagnosis at each time point—with some patients being farther from diagnosis at the first or second assessment than some at the second or third assessment. Future studies on long-term survival should include data on hormonal functioning, as this could be linked to, for example, mood and fatigue; as well as other indicators of everyday life functioning such as employment status. Yet, this is the longest follow-up of patients diagnosed with a rare central nervous system tumor ever performed, and provides valuable insight into the very long-term HRQOL of people diagnosed with LGG and their family members.
In conclusion, on the group level, HRQOL was mostly stable over time in long-term survivors with grade II glioma assessed at an average of 7, 13, and 26 years after diagnosis. Patients’ psychological functioning appears least stable over time, with elevated levels of depressive symptoms and fatigue even decades after diagnosis. High rates of fatigue were also observed in informal caregivers. This reflects persistent unmet needs in both patients and caregivers and emphasizes the importance of providing adequate psychosocial and supportive care throughout LGG survivorship.
Supplementary Material
Acknowledgments
Patients for this side study were recruited from Amsterdam UMC location Vrije Universiteit Amsterdam, Amsterdam, the Netherlands; Amsterdam UMC location Universiteit van Amsterdam, Amsterdam, the Netherlands; Haaglanden Medical Centre, Location Westeinde, The Hague, the Netherlands; HagaZiekenhuis, The Hague, the Netherlands; University Medical Center Utrecht, Utrecht, the Netherlands; University Medical Center Groningen, Groningen, the Netherlands; Radboud University Nijmegen Medical Center, Nijmegen, the Netherlands; Medisch Spectrum Twente, Enschede, the Netherlands; ElisabethTweeSteden Hospital (ETZ), Tilburg, the Netherlands; Alrijne Hospital, Leiden, the Netherlands; Catharina Hospital, Eindhoven, the Netherlands; Zuyderland Medical Center, Heerlen, the Netherlands; Medical Center Leeuwarden, Leeuwarden, the Netherlands; Isala, Zwolle, the Netherlands; Wilhelmina Hospital Assen, Assen, the Netherlands; VieCuri Medical Centre, Venlo, the Netherlands.
Contributor Information
Florien W Boele, Leeds Institute of Medical Research at St James’s, St James’s University Hospital, University of Leeds, Leeds, UK; Leeds Institute of Health Sciences, Faculty of Medicine and Health, University of Leeds, Leeds, UK.
Patricia W M den Otter, Department of Medical Psychology, Cancer Center Amsterdam, Brain Tumor Center Amsterdam, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, the Netherlands.
Jaap C Reijneveld, Department of Neurology, SEIN, Heemstede, the Netherlands; Department of Neurology, Cancer Center Amsterdam, Brain Tumor Center Amsterdam, Amsterdam UMC, Vrije Universiteit Amsterdam, the Netherlands.
Philip C de Witt Hamer, Department of Neurosurgery, Cancer Center Amsterdam, Brain Tumor Center Amsterdam, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, the Netherlands.
Hinke F van Thuijl, Department of Neurology, SEIN, Heemstede, the Netherlands; Department of Neurology, Cancer Center Amsterdam, Brain Tumor Center Amsterdam, Amsterdam UMC, Vrije Universiteit Amsterdam, the Netherlands.
Linda M C Lorenz, Department of Medical Psychology, Cancer Center Amsterdam, Brain Tumor Center Amsterdam, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, the Netherlands.
Pieter Wesseling, Department of Pathology, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, the Netherlands; Laboratory for Childhood Cancer Pathology, Princess Máxima Center for Pediatric Oncology, Utrecht, the Netherlands.
Frank J Lagerwaard, Department of Radiation Oncology, Cancer Center Amsterdam, Brain Tumor Center Amsterdam, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, the Netherlands.
Martin J B Taphoorn, Department of Neurology, Leiden University Medical Center, Leiden, the Netherlands; Department of Neurology, Haaglanden Medical Center, The Hague, the Netherlands.
Mathilde C M Kouwenhoven, Department of Neurology, Cancer Center Amsterdam, Brain Tumor Center Amsterdam, Amsterdam UMC, Vrije Universiteit Amsterdam, the Netherlands.
Tom J Snijders, UMC Utrecht Brain Center, Department of Neurology and Neurosurgery, University Medical Center Utrecht, Utrecht, the Netherlands.
Linda Douw, Department of Anatomy and Neurosciences, Amsterdam Neuroscience, Cancer Center Amsterdam, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, the Netherlands.
Martin Klein, Department of Medical Psychology, Cancer Center Amsterdam, Brain Tumor Center Amsterdam, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, the Netherlands.
Funding. None.
Conflict of Interest. None reported.
Authorship
Conceptualization: F.W.B.; J.C.R.; P.C.d.W.H.; H.F.v.T.; P.W.; F.J.L.; M.J.B.T.; M.C.M.K.; T.J.S.; L.D.; M.K.
Methodology: F.W.B.; J.C.R.; P.C.d.W.H.; H.F.v.T.; P.W.; F.J.L.; M.J.B.T.; M.C.M.K.; T.J.S.; L.D.; M.K.
Investigation: F.W.B.; P.W.M.d.O.; L.M.C.L.; J.C.R.; P.C.d.W.H.; H.F.v.T.; P.W.; F.J.L.; M.J.B.T.; M.C.M.K.; T.J.S.; L.D.; M.K.
Data curation: F.W.B.; H.F.v.T.; P.W.; P.W.M.d.O.; L.M.C.L.; M.K.
Formal analysis: F.W.B.; P.W.M.d.O.
Writing (original draft): F.W.B.
Writing (review and editing): F.W.B.; P.W.M.d.O.; L.M.C.L.; J.C.R.; P.C.d.W.H.; H.F.v.T.; P.W.; F.J.L.; M.J.B.T.; M.C.M.K.; T.J.S.; L.D.; M.K.
Resources: J.C.R.; P.C.d.W.H.; F.J.L.; M.J.B.T.; M.C.M.K.; T.J.S.; M.K.
References
- 1. Ostrom QT, Patil N, Cioffi G, et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro-oncology. 2020;22(Supp_1):iv1–iv96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis DN, Perry A, Reifenberger G, et al. . The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 3. Olson JD, Riedel E, DeAngelis LM. Long-term outcome of low-grade oligodendroglioma and mixed glioma. Neurology. 2000;54(7):1442–1448. [DOI] [PubMed] [Google Scholar]
- 4. Pignatti F, van den Bent M, Curran D, et al. . Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20(8):2076–2084. [DOI] [PubMed] [Google Scholar]
- 5. Reijneveld J, Sitskoorn M, Klein M, Nuyen J, Taphoorn M. Cognitive status and quality of life in patients with suspected versus proven low-grade gliomas. Neurology. 2001;56(5):618–623. [DOI] [PubMed] [Google Scholar]
- 6. Liu R, Solheim K, Polley M-Y, et al. . Quality of life in low-grade glioma patients receiving temozolomide. Neuro-oncology. 2009;11(1):59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reijneveld JC, Taphoorn MJ, Coens C, et al. . Health-related quality of life in patients with high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016;17(11):1533–1542. [DOI] [PubMed] [Google Scholar]
- 8. Brada M, Viviers L, Abson C, et al. . Phase II study of primary temozolomide chemotherapy in patients with WHO grade II gliomas. Ann Oncol. 2003;14(12):1715–1721. [DOI] [PubMed] [Google Scholar]
- 9. Pace A, Vidiri A, Galie E, et al. . Temozolomide chemotherapy for progressive low-grade glioma: clinical benefits and radiological response. Ann Oncol. 2003;14(12):1722–1726. [DOI] [PubMed] [Google Scholar]
- 10. Yavas C, Zorlu F, Ozyigit G, et al. . Prospective assessment of health-related quality of life in patients with low-grade glioma. Support Care Cancer. 2012;20(8):1859–1868. [DOI] [PubMed] [Google Scholar]
- 11. Fountain DM, Allen D, Joannides AJ, et al. . Reporting of patient-reported health-related quality of life in adults with diffuse low-grade glioma: a systematic review. Neuro-Oncology. 2016;18(11):1475–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lawrie TA, Gillespie D, Dowswell T, et al. . Long-term neurocognitive and other side effects of radiotherapy, with or without chemotherapy, for glioma. Cochrane Database Syst Rev. 2019;(8):CD013047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown PD, Buckner JC, O’Fallon JR, et al. . Effects of radiotherapy on cognitive function in patients with low-grade glioma measured by the Folstein Mini-Mental State Examination. J Clin Oncol. 2003;21(13):2519–2524. [DOI] [PubMed] [Google Scholar]
- 14. Surma-aho O, Niemelä M, Vilkki J, et al. . Adverse long-term effects of brain radiotherapy in adult low-grade glioma patients. Neurology. 2001;56(10):1285–1290. [DOI] [PubMed] [Google Scholar]
- 15. Klein M, Heimans JJ, Aaronson NK, et al. . Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet. 2002;360(9343):1361–1368. [DOI] [PubMed] [Google Scholar]
- 16. Laack NN, Brown PD, Ivnik RJ, et al. . Cognitive function after radiotherapy for supratentorial low-grade glioma: a North Central Cancer Treatment Group prospective study. Int J Radiat Oncol Biol Phys. 2005;63(4):1175–1183. [DOI] [PubMed] [Google Scholar]
- 17. Taphoorn M, Schiphorst AK, Snoek F, et al. . Cognitive functions and quality of life in patients with low-grade gliomas: the impact of radiotherapy. Ann Neurol. 1994;36(1):48–54. [DOI] [PubMed] [Google Scholar]
- 18. Douw L, Klein M, Fagel SS, et al. . Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810–818. [DOI] [PubMed] [Google Scholar]
- 19. Chen D, Zhu J, Xu Q, et al. . The role of informal caregivers for patients with glioma: a systematic review and meta-synthesis of qualitative studies. Ann Transl Med. 2021;9(12):1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boele FW, Heimans JJ, Aaronson NK, et al. . Health-related quality of life of significant others of patients with malignant CNS versus non-CNS tumors: a comparative study. J Neurooncol. 2013;115(1):87–94. [DOI] [PubMed] [Google Scholar]
- 21. Frances S, Velikova G, Klein M, et al. . Long-term impact of adult WHO grade II or III gliomas on health-related quality of life: a systematic review. Neuro-oncol Pract. 2022;9(1):3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Louis DN, Perry A, Wesseling P, et al. . The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro-Oncology. 2021;23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weller M, van den Bent M, Preusser M, et al. . EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18(3):170–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aaronson NK, Taphoorn MJ, Heimans JJ, et al. . Compromised health-related quality of life in patients with low-grade glioma. J Clin Oncol. 2011;29(33):4430–4435. [DOI] [PubMed] [Google Scholar]
- 25. Boele FW, Douw L, Reijneveld JC, et al. . Health-related quality of life in stable, long-term survivors of low-grade glioma. J Clin Oncol. 2015;33(9):1023–1029. [DOI] [PubMed] [Google Scholar]
- 26. Aaronson NK, Muller M, Cohen PD, et al. . Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol. 1998;51(11):1055–1068. [DOI] [PubMed] [Google Scholar]
- 27. Taphoorn MJ, Claassens L, Aaronson NK, et al. . An international validation study of the EORTC brain cancer module (EORTC QLQ-BN20) for assessing health-related quality of life and symptoms in brain cancer patients. Eur J Cancer. 2010;46(6):1033–1040. [DOI] [PubMed] [Google Scholar]
- 28. Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 29. Worm-Smeitink M, Gielissen M, Bloot L, et al. . The assessment of fatigue: Psychometric qualities and norms for the Checklist individual strength. J Psychosom Res. 2017;98:40–46. [DOI] [PubMed] [Google Scholar]
- 30. Jolles J, van Boxtel MP, Ponds RW, Metsemakers JF, Houx PJ. The Maastricht aging study (MAAS). The longitudinal perspective of cognitive aging. Tijdschr Gerontol Geriatr. 1998;29(3):120–129. [PubMed] [Google Scholar]
- 31. Klein M, Engelberts NHJ, van der Ploeg HM, et al. . Epilepsy in low-grade gliomas: The impact on cognitive function and quality of life. Ann Neurol. 2003;54(4):514–520. [DOI] [PubMed] [Google Scholar]
- 32. Bosma H, van Boxtel MPJ, Ponds RW, Houx PJ, Jolles J. Pesticide exposure and risk of mild cognitive dysfunction. Lancet. 2000;356(9233):912–913. [DOI] [PubMed] [Google Scholar]
- 33. Jolles J, Houx P, Van Boxtel M, Ponds RWHM.. The Maastricht Aging Study. Maastricht, the Netherlands: Neuropsych Publishers; 1995:192. [Google Scholar]
- 34. Edelvik A, Taft C, Ekstedt G, Malmgren K. Health-related quality of life and emotional well-being after epilepsy surgery: a prospective, controlled, long-term follow-up. Epilepsia. 2017;58(10):1706–1715. [DOI] [PubMed] [Google Scholar]
- 35. Bjorner JB, Wallenstein GV, Martin MC, et al. . Interpreting score differences in the SF-36 Vitality scale: using clinical conditions and functional outcomes to define the minimally important difference. Curr Med Res Opin. 2007;23(4):731–739. [DOI] [PubMed] [Google Scholar]
- 36. Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–144. [DOI] [PubMed] [Google Scholar]
- 37. De Vet HC, Terwee CB, Mokkink LB, Knol DL.. Measurement in Medicine: A Practical Guide. Cambridge: Cambridge University Press; 2011. [Google Scholar]
- 38. Dikmen S, Machamer J, Miller B, Doctor J, Temkin N. Functional status examination: a new instrument for assessing outcome in traumatic brain injury. J Neurotrauma. 2001;18(2):127–140. [DOI] [PubMed] [Google Scholar]
- 39. Osoba D, Aaronson N, Muller M, et al. . The development and psychometric validation of a brain cancer quality-of-life questionnaire for use in combination with general cancer-specific questionnaires. Qual Life Res. 1996;5(1):139–150. [DOI] [PubMed] [Google Scholar]
- 40. Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism. Phys Ther. 2008;88(6):733–746. [DOI] [PubMed] [Google Scholar]
- 41. Lim GY, Tam WW, Lu Y, et al. . Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci Rep. 2018;8(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Husson O, Mols F, van de Poll-Franse L, et al. . Variation in fatigue among 6011 (long-term) cancer survivors and a normative population: a study from the population-based PROFILES registry. Support Care Cancer. 2015;23(7):2165–2174. [DOI] [PubMed] [Google Scholar]
- 43. Jones JM, Olson K, Catton P, et al. . Cancer-related fatigue and associated disability in post-treatment cancer survivors. J Cancer Surviv. 2016;10(1):51–61. [DOI] [PubMed] [Google Scholar]
- 44. Rooney AG, McNamara S, Mackinnon M, et al. . Frequency, clinical associations, and longitudinal course of major depressive disorder in adults with cerebral glioma. J Clin Oncol. 2011;29(32):4307–4312. [DOI] [PubMed] [Google Scholar]
- 45. Hall DL, Mishel MH, Germino BB. Living with cancer-related uncertainty: associations with fatigue, insomnia, and affect in younger breast cancer survivors. Support Care Cancer. 2014;22(9):2489–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vivar CG, Canga N, Canga AD, Arantzamendi M. The psychosocial impact of recurrence on cancer survivors and family members: a narrative review. J Adv Nurs. 2009;65(4):724–736. [DOI] [PubMed] [Google Scholar]
- 47. Beevers Z, Hussain S, Boele FW, Rooney AG. Pharmacological treatment of depression in people with a primary brain tumour. Cochrane Database Syst Rev. 2020;(7):CD006932–CD006932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Day J, Yust-Katz S, Cachia D, et al. . Interventions for the management of fatigue in adults with a primary brain tumour. Cochrane Database Syst Rev. 2016;(4):CD011376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.