Abstract
Background
Global incidence for brain tumors varies substantially without explanation. Studies correlating radon exposure and incidence are inconclusive. Particulate pollution has been linked to increased tumor incidence. Particulates may disrupt the blood-brain barrier allowing intracranial exposure to oncogenic radon. We investigated the relationship between exposure to residential radon, particulate pollution, and brain tumor incidence in the United States (US).
Methods
County-level median radon testing results and annual air quality index values were obtained and divided into tertiles. Counties without both values were excluded. Four groups of counties were generated: high particulate/high radon (high/high), high/low, low/high, and low/low. Using incidence data from the Central Brain Tumor Registry of the US (provided by CDC’s National Program of Cancer Registries and NCI’s SEER), annual age-adjusted incidence rates (AAAIRs) by group were generated by behavior. Incidence rate ratios were calculated to examine for significant differences (α = .05). Poisson regression accounting for possible confounders was conducted.
Results
Counties with available data included 83% of the US population. High/high exposure was significantly associated with increased AAAIR of all non-malignant tumors (up to 26% higher, including most meningiomas) even after accounting for potential confounders. An increased AAAIR was noted for all malignant tumors (up to 10% higher), including glioblastoma, but was negated after accounting for demographic/socioeconomic differences.
Conclusions
We present the first report suggesting increased non-malignant brain tumor incidence in regions with high particulate and radon exposure. These findings provide insight into unexplained variation in tumor incidence. Future studies are needed to validate these findings in other populations.
Keywords: air pollution, brain tumors, incidence, meningioma, radon
Key Points.
Brain tumor incidence may rise in regions with high air pollution and radon levels.
In high exposure areas, an up to 26% increase in non-malignant tumors was seen.
These findings may provide insight into unexplained variation in tumor burden.
Importance of the Study.
Global incidence for brain tumors varies substantially without explanation. Studies correlating radon exposure and incidence are inconclusive, but particulate pollution has been linked to increased tumor incidence. Particulates may disrupt the blood-brain barrier allowing intracranial exposure to oncogenic radon. We investigated the relationship between exposure to residential radon, particulate pollution, and brain tumor incidence in the United States (US). In an epidemiologic study covering 83% of the US population, areas with high radon and high air pollution were significantly associated with an increased age-adjusted incidence of all non-malignant (up to 26% higher), including most meningiomas, with respect to regions with low pollution and/or radon exposures (all P < .001). Differences persisted even after accounting for key demographic/socioeconomic characteristics. This analysis represents the first report suggesting increased brain tumor incidence in regions with high particulate and high radon exposure. These findings may provide insight into unexplained variation in tumor incidence.
The incidence rate of non-malignant and malignant brain tumors varies widely, with some of the highest observed rates seen in the industrialized regions of Australia, Europe, and North America.1 Although prior therapeutic ionizing radiation exposure is a known risk factor,2 the cause of brain tumors remains unknown for the majority of patients, and the driving force behind this variable global disease burden is poorly understood.1 Radon is a gaseous element produced by decay of uranium and other radioactive elements native to the Earth’s crust. After rising from the soil and rocks, radon gas can enter commercial and residential buildings.3,4 Radon exposure is the predominant source of naturally occurring ionizing radiation worldwide and is implicated in the pathogenesis of extracranial malignancies including lung cancer.3,4 Concerningly, residential radon exposure may be increasing in North America.5 Thus, there is interest in determining whether radon is also responsible for the pathogenesis of benign and malignant intracranial tumors. However, studies addressing whether there is an association between increased background radon levels and brain tumor incidence are conflicting, with some population-based reports showing increased tumor incidence.6
Air particulate pollution contains numerous carcinogens produced by many modern technologies including combustion engines and industrial activity.7 In recent decades, while particulate air pollution has somewhat improved in some regions including Europe and North America, it has greatly increased worldwide primarily due to worsening pollution in Asia.8 A correlation between increased air pollution and incidence of brain tumors has been described7 raising the possibility that the conflicting data regarding the relationship between radon exposure and brain tumor incidence may result from unaccounted radon activation effects by particulate pollution. One potential mechanism for such an effect is that disruption of the blood–brain barrier (BBB) by high particulate pollution may be required for radon to access the cranium and express oncogenic potential.7 Chronic exposure to air pollution has been linked to increased neuroinflammation, oxidative stress, microglial activation, dysregulation of protein metabolism, alteration of the BBB, and cell death resulting in neurotoxicity and CNS dysfunction.9,10 The interaction between radon levels, particulate pollution, and brain tumor incidence has not been previously analyzed. We hypothesized that regions in the United States (US) with elevated background levels of radon and high particulate pollution would demonstrate an increased incidence of non-malignant and malignant brain tumors.
Methods
Study Design
The study was deemed exempt by the appropriate Institutional Review Board. County-level, annual air quality index (AQI) values (the percentage of unhealthy or worse days per year) were obtained from the Environmental Protection Agency (EPA), averaged from 2000 to 2010, and divided into tertiles (low, normal, and high) (Supplementary Figure 1). Tertiles were used so that counties could be divided into low and high exposures with removal of counties with median values. EPA data from 2000 to 2010 was used due to assumed latency between exposure and development of brain tumors. While data quantifying the latency period between exposure to radon and/or air pollution and brain tumor development is limited, the literature suggests that iatrogenic radiation-induced secondary brain tumors tend to develop as early as within 2.5 years after exposure and well within the first decade.11 Values were averaged to try to capture long term exposures. Annual average values, rather than cumulative values over the entire examined decade, were used to categorize counties by air pollution for consistency with annualized age-adjusted outcome measures.
Per the EPA, the AQI was derived by first identifying the highest concentration of ozone, particulate matter, carbon monoxide, sulfur dioxide, and/or nitrogen dioxide captured by any monitor within a single county over a 1-to-24-h period, depending on the pollutant.12 The maximum measured value for each pollutant was then converted to an index value that corresponded to air of quality good, moderate, unhealthy for sensitive groups, unhealthy, very unhealthy, or hazardous. The county’s AQI was then defined to be the highest index for any given pollutant; for example, if the maximum measured ozone value corresponded to good air quality, but the maximum measured value of carbon monoxide was unhealthy, then the county was considered to have an unhealthy AQI for that measuring period. In summary, high levels of particulate pollution signified inferior air quality. This metric was used, rather than levels of a single pollutant, for greater inclusivity in the absence of strong data connecting any one pollutant to brain tumor incidence.
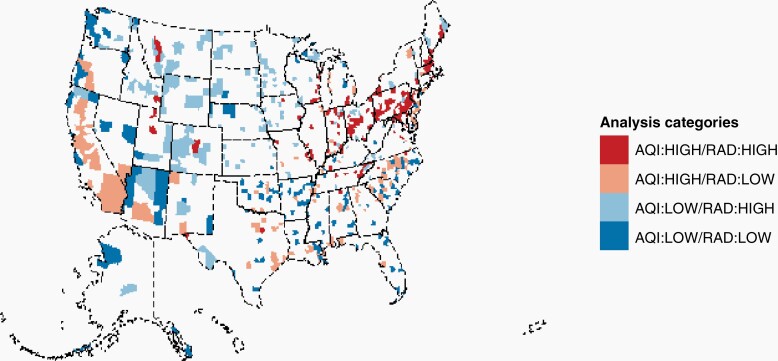
Summary county-level median radon testing results were obtained from AirChek and divided into tertiles (low, normal, and high) (Supplementary Figure 2). AirChek aggregates all radon levels (including from both residential and commercial buildings) collected by their own agency, the EPA, and state and county health departments.13 Counties without AQI or radon data were excluded. These data were superimposed to generate four groups of counties: those that were high particulate/high radon (high/high), high particulate/low radon (high/low), low particulate/high radon (low/high), and low particulate/low radon (low/low) (Figure 1).
Fig. 1.
Categorization of counties by level of pollution and radon exposure. AQI, air quality index; rad, radon exposure.
Statistical Analysis
Using incidence data from the Central Brain Tumor Registry of the United States (CBTRUS),14,15 which covers 100% of the US from 2006 to 2017, overall annual age-adjusted incidence rates (AAAIR), by AQI/radon level, for malignant and non-malignant tumors of the central nervous system (CNS) were generated overall, as well as by behavior and histologies. CBTRUS data were provided by the Centers for Disease Control and Prevention (CDC)’s National Program of Cancer Registries and the National Cancer Institute (NCI)’s Surveillance, Epidemiology, and End Results Program (SEER). AAAIR and 95% confidence intervals (95% CI) were estimated per 100,000 population-based on 5-year age groups and were standardized to the 2000 US standard population using SEER*Stat 8.3.9.16 Confidence intervals were calculated using the method described by Tiwari et al.17 Incidence rates, by AQI/radon level, for each histology included in the CBTRUS were also calculated. Tumors were classified using ICD-O-3 histology, behavior, and topography codes as per CBTRUS,14 and were considered to be malignant when they were assigned an ICD-O-3 behavior code of/3, while non-malignant tumors were assigned a behavior code of/0 or/1. Thus, non-malignant tumors were defined as those with a benign or uncertain ICD-O-3 behavior code. Histologies were classified using the 2007 World Health Organization Classification of Tumors of the Central Nervous System.
Incidence rates were also generated by age groups (0–14, 15–39, and ≥ 40 years), sex, race and ethnicity (Hispanic, Black non-Hispanic, and White non-Hispanic), county population-density (metropolitan versus nonmetropolitan, as defined by the US Department of Agriculture Rural-Urban Continuum Codes), and tumor location (parietal/occipital/cerebellar versus frontal/temporal/brainstem versus spinal). Event numbers for other races were too low to be analyzable (less than 16 cases within a given county). Incidence rate ratios (IRR) were used to compare event rates by AQI/radon level, using the formulas described by Fay et al.18 to calculate P-values with statistical significance evaluated at the α = .05 significance level.
Poisson regression in R 4.1.319 was used to identify significant associations with county-level incidence rates and AQI/radon level, after adjustment for urban/rural continuum, estimated county socioeconomic status (SES), and proportion of county that is white non-Hispanic as per prior published analyses.20 Counties with < 10 cases were excluded.
Results
Overall, 3,015 and 1,292 counties had available radon level and AQI data, respectively, of which 1,264 counties had both available and were included in analyses (Supplementary Table 1). Missing radon and/or AQI data was likely related to low population-density in affected counties, as the analyzable counties with complete information cover over 83% of the total US population. Radon levels in counties included in the low tertile ranged from 0.0 to 2.3 pCi/L, while levels ranged from 4.4 to 111 pCi/L in counties in the high radon tertile. Per the EPA, while there is no safe level of radon exposure, homes should be fixed if the radio level reaches 4 pCi/L21 confirming that exposures in the high tertile were unacceptably elevated. The percentage of days with unhealthy air quality in the counties included in the low AQI tertile was 0% (acceptable level), while the range was 1–23% for counties in the high AQI group which exceeded acceptable levels. As expected, the highest rates of brain tumor incidence were noted in the population over the age of 40 years, and non-malignant brain tumors were more prevalent than malignant tumors. Detailed information by histology is listed in Supplemental Table 2.
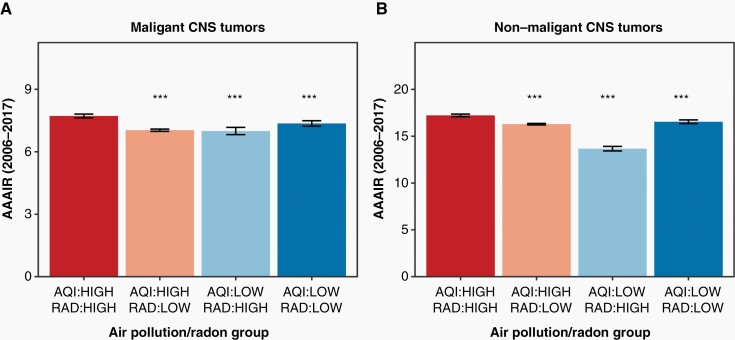
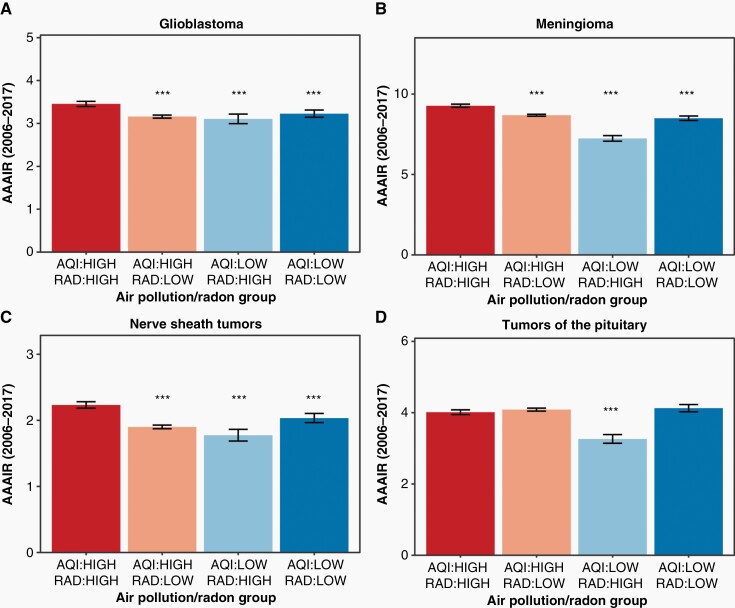
The AAAIRs for all malignant tumors overall were 7.72 (95% confidence interval [CI] 7.62–7.81), 7.04 (95% CI 6.98–7.09), 6.99 (95% CI 6.82–7.16), and 7.36 (95% CI 7.23–7.49) per 100,000 population for high/high, high/low, low/high, and low/low counties, respectively (Figure 2A). These rates corresponded to a significantly increased incidence of all malignant tumors in high/high counties with respect to high/low, low/high, and low/low counties (all P of IRRs < 0.001, Table 1). This difference translated to an incidence in high/high counties as much as 10% higher than in regions with inferior air quality or elevated radon levels. There was no significant difference in IRR between high/low, low/high, and low/low regions. The AAAIRs for glioblastoma (GBM), a highly aggressive malignant brain tumor, were 3.45 (95% CI 3.40–3.51), 3.16 (95% CI 3.12–3.19), 3.10 (95% CI 2.99–3.22), and 3.23 (95% CI 3.14–3.31) per 100,000 population for high/high, high/low, low/high, and low/low counties, respectively (Figure 3A). These rates corresponded to a significantly increased incidence of GBMs in high/high counties with respect to high/low, low/high, and low/low counties (all P of IRRs < 0.001, Table 1).
Fig. 2.
Relationship between air pollution, radon exposure, and tumor incidence. Malignant (A) and non-malignant (B) tumors. ***Denotes a significant difference with respect to the high/high group with a P < .001. Data provided by Centers for Disease Control’s National Program of Cancer Registries and National Cancer Institute’s Surveillance, Epidemiology and End Results Program, November 2019 submissions. AAAIR, average annual age-adjusted incidence rate per 100,000 population with 95% confidence interval, AQI, air quality index; CNS, central nervous system; rad, radon.
Table 1.
Incidence rates by behavior, histology, air pollution, and radon level
| Histology | Air pollution/radongroup | Number | AAAIR (95%CI) | IRR (95% CI) | P value |
|---|---|---|---|---|---|
| All malignant tumors | AQI:HIGH/RAD:HIGH | 28061 | 7.72 (7.62–7.81) | 1.00 | – |
| AQI:HIGH/RAD:LOW | 66409 | 7.04 (6.98–7.09) | 0.91 (0.90–0.92) | <.001 | |
| AQI:LOW/RAD:HIGH | 6568 | 6.99 (6.82–7.16) | 0.91 (0.88–0.93) | <.001 | |
| AQI:LOW/RAD:LOW | 12564 | 7.36 (7.23–7.49) | 0.95 (0.93–0.97) | <.001 | |
| All non-malignant tumors | AQI:HIGH/RAD:HIGH | 63165 | 17.22 (17.09–17.36) | 1.00 | – |
| AQI:HIGH/RAD:LOW | 153856 | 16.29 (16.20–16.37) | 0.95 (0.94–0.95) | <.001 | |
| AQI:LOW/RAD:HIGH | 12724 | 13.63 (13.39–13.88) | 0.79 (0.78–0.81) | <.001 | |
| AQI:LOW/RAD:LOW | 28277 | 16.54 (16.35–16.74) | 0.96 (0.95–0.97) | <.001 | |
| Glioblastoma | AQI:HIGH/RAD:HIGH | 13313 | 3.45 (3.40–3.51) | 1.00 | – |
| AQI:HIGH/RAD:LOW | 30592 | 3.16 (3.12–3.19) | 0.91 (0.90–0.93) | <.001 | |
| AQI:LOW/RAD:HIGH | 3104 | 3.10 (2.99–3.22) | 0.90 (0.86–0.94) | <.001 | |
| AQI:LOW/RAD:LOW | 5989 | 3.23 (3.14–3.31) | 0.93 (0.91–0.96) | <.001 | |
| All meningioma | AQI:HIGH/RAD:HIGH | 34969 | 9.27 (9.17–9.37) | 1.00 | – |
| AQI:HIGH/RAD:LOW | 82390 | 8.68 (8.62–8.74) | 0.94 (0.92–0.95) | <.001 | |
| AQI:LOW/RAD:HIGH | 6972 | 7.23 (7.06–7.41) | 0.78 (0.76–0.80) | <.001 | |
| AQI:LOW/RAD:LOW | 15214 | 8.49 (8.36–8.63) | 0.92 (0.90–0.93) | <.001 |
Data provided by Centers for Disease Control’s National Program of Cancer Registries and National Cancer Institute’s Surveillance, Epidemiology and End Results Program, November 2019 submissions.
AAAIR, average annual age-adjusted incidence rates; AQI, air quality index; CI, confidence interval; IRR, incidence rate ratios; rad, radon.
Fig. 3.
Pollution, radon exposure, and incidence rate of various tumors. Glioblastoma (A), meningioma (B), nerve sheath tumors (C), and tumors of the pituitary (D). ***Denotes a significant difference with respect to the high/high group with a P < .001. Data provided by Centers for Disease Control’s National Program of Cancer Registries and National Cancer Institute’s Surveillance, Epidemiology and End Results Program, November 2019 submissions. AAAIR, average annual age-adjusted incidence rate per 100,000 population with 95% CI; AQI, air quality index; rad, radon.
The pattern of increased malignant tumor incidence in high/high areas tended to persist even after stratifying urban versus rural residence, age, or sex (Supplemental Tables 3, 4 and 5). However, when stratifying by race, differences in incidence between high/high and other regions largely lost their significance (Supplementary Table 6). The White Non-Hispanic population had the highest overall AAAIRs. On Poisson regression accounting for these variables, the differences in GBM and all malignant tumor incidence in areas with high radon and high air pollution were eliminated (Table 2).
Table 2.
Results from Poisson regression
| Histology | Air pollution/radon group | Unadjusted | Adjusteda | ||
|---|---|---|---|---|---|
| Effect estimate (SE) | P-value | Effect estimate (SE) | P-value | ||
| All malignant tumors | AQI:HIGH/RAD:HIGH | 1 | Ref | 1 | Ref |
| AQI:HIGH/RAD:LOW | -0.06 (0.05) | .1986 | 0.03 (0.06) | .5857 | |
| AQI:LOW/RAD:HIGH | -0.02 (0.05) | .7507 | -0.00 (0.05) | .9371 | |
| AQI:LOW/RAD:LOW | -0.06 (0.05) | .2280 | 0.02 (0.06) | .7751 | |
| All-non malignant tumors | AQI:HIGH/RAD:HIGH | 1 | Ref | 1 | Ref |
| AQI:HIGH/RAD:LOW | -0.04 (0.03) | .1951 | -0.08 (0.04) | .0429 | |
| AQI:LOW/RAD:HIGH | -0.09 (0.03) | .0083 | -0.06 (0.04) | .1137 | |
| AQI:LOW/RAD:LOW | -0.14 (0.04) | <.0001 | -0.12 (0.04) | .0039 | |
| Glioblastoma | AQI:HIGH/RAD:HIGH | 1 | Ref | 1 | Ref |
| AQI:HIGH/RAD:LOW | -0.07 (0.08) | .3568 | 0.06 (0.09) | .4735 | |
| AQI:LOW/RAD:HIGH | -0.01 (0.08) | .9395 | 0.01 (0.08) | .9410 | |
| AQI:LOW/RAD:LOW | -0.06 (0.08) | .4670 | 0.06 (0.09) | .5263 | |
| All meningioma | AQI:HIGH/RAD:HIGH | 1 | Ref | 1 | Ref |
| AQI:HIGH/RAD:LOW | -0.07 (0.05) | .1130 | -0.10 (0.05) | .0618 | |
| AQI:LOW/RAD:HIGH | -0.08 (0.05) | .1135 | -0.05 (0.05) | .3206 | |
| AQI:LOW/RAD:LOW | -0.19 (0.05) | .0001 | -0.17 (0.06) | .0033 | |
aAdjusted for county SES, whether county is urban or rural, and proportion of county population that is Non-Hispanic White.
The AAAIRs for all non-malignant tumors were 17.22 (95% CI 17.09–17.36), 16.29 (95% CI 16.20–16.37), 13.63 (95% CI 13.39–13.88), and 16.54 (95% CI 16.35–16.74) per 100,000 population for high/high, high/low, low/high, and low/low counties, respectively (Figure 2B). These rates corresponded to a significantly increased incidence of all non-malignant tumors in high/high counties with respect to high/low, low/high, and low/low counties (all P of IRRs < 0.001, Table 1). This difference translated to an IRR as much as 26% higher for non-malignant tumors in regions with inferior air quality or elevated radon levels. While there was no significant difference in IRR between high/low and low/low regions, there was a significantly lower IRR in low/high regions with respect to high/low and low/low areas. Generally, the pattern of increased non-malignant tumor incidence in high/high areas persisted even after stratifying by race, sex, age, or urban versus rural residence (Supplemental Tables 3–6). On Poisson regression accounting for these variables, the differences in non-malignant tumor incidence in areas with high radon and air pollution largely persisted (Table 2).
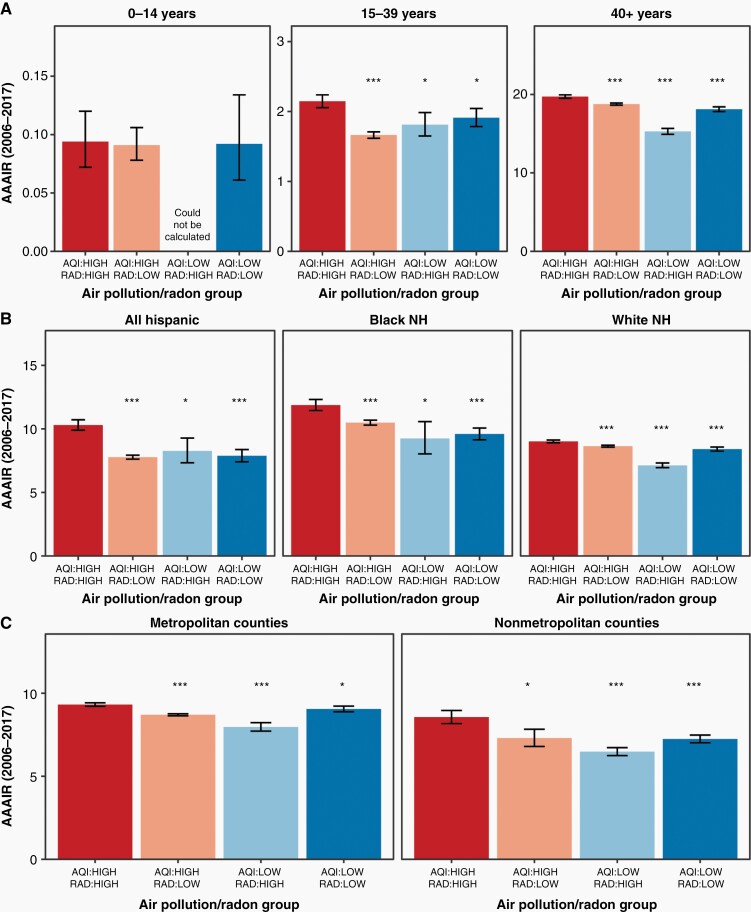
Additionally, the AAAIRs for meningiomas, which are typically non-malignant, were 9.27 (95% CI 9.17–9.37), 8.68 (95% CI 8.62–8.74), 7.23 (95% CI 7.06–7.41), and 8.49 (95% CI 8.36–8.63) per 100,000 population for high/high, high/low, low/high, and low/low counties, respectively (Figure 3B). These rates corresponded to a significantly increased incidence of meningiomas in high/high counties with respect to high/low, low/high, and low/low counties (all P of IRRs < 0.001, Table 1). Differences persisted between groups irrespective of stratification by demographic/socioeconomic characteristics (Figure 4 and Table 2). A similar increase in AAAIR in high/high regions with respect to other areas were observed for nerve sheath tumors (P < .001, Figure 3C), which are predominately non-malignant. For pituitary tumors, which are predominately non-malignant, there was a significant increase in AAAIR in high/high counties relative to low/high counties (P < .001) and a trend towards significantly increased AAAIR in high/high counties versus high/low (P = .0727) and low/low regions (P = .0692) (Figure 3D).
Figure 4.
Pollution, radon exposure, and meningioma incidence, stratified by demographic features. *Denotes a significant difference with respect to the high/high group with 0.001 < P < .05. ***Denotes a significant difference with respect to the high/high group with a P < .001. Data provided by Centers for Disease Control’s National Program of Cancer Registries and National Cancer Institute’s Surveillance, Epidemiology and End Results Program, November 2019 submissions. AAAIR, average annual age-adjusted incidence rate per 100,000 population with 95% CI; AQI, air quality index; NH, non-Hispanic; rad, radon.
For many histologies, the relationship between elevated AAAIR and high/high exposure was somewhat stronger for frontal/temporal/brainstem tumors than for tumors of the parietal/occipital/cerebellar lobes (Supplementary Table 7). However, for GBM, the relationship between elevated AQI and radon exposure was strong irrespective of tumor location.
Discussion
Prior therapeutic ionizing radiation exposure is a known risk factor for brain tumors,2 but in the majority of patients, risk factors have not been identified, and widely variable worldwide incidence rates remain unexplained.1 This analysis represents the first report associating increased non-malignant brain tumor incidence with the combined effects of elevations in county-level air particulate pollution and radon exposure after prior works assessing these variables in isolation produced conflicting conclusions. The IRRs for non-malignant CNS tumors in high/high areas were as much as 26% higher than in lower exposure areas, respectively. As of the 2010 US Census, regions identified as high/high have a population of 26 million Americans. Treatment and/or disease progression of non-malignant tumors can still be associated with substantial morbidity. Thus, if verified in another population, these results would be highly concerning. Although for malignant tumors, differences in incidence between regions with varying levels of radon and air pollution exposure disappeared when accounting for demographic variables, increased incidence of non-malignant tumors with high/high exposure largely persisted irrespective of sex, race and ethnicity, and other examined factors.
The leading source of naturally occurring ionizing radiation worldwide, exposure to radon gas has been linked to an 8–21% excess relative risk of developing lung cancer and 15% increase in lung cancer specific mortality per 100 Bq/m2 of radon in Europe and North America.3,22–25 These findings have prompted interest in evaluating the relationship between increased background radon levels and brain tumor incidence. However, prior works were inconclusive.6 Several European reports have proposed an association between residential radon exposure and increased incidence of brain tumors,26,27 and a US-based study found that increasing radon concentration in the water supply correlated with increased incidence of central nervous system (CNS) tumors.28 However, larger population-based studies from the US found no significant relationship between radon exposure and increased brain tumor incidence.4,29 Studies evaluating the risk of occupational radon exposure in miners were mixed6; two French studies found a positive association between radon exposure and CNS tumors30,31 while other European and American studies did not.32–37 Clarifying the poorly understood mechanisms behind brain tumorigenesis is a priority because of the high burden of morbidity and mortality associated with brain tumors.1
Some of the highest observed incidence rates of brain tumors are seen in industrialized countries in Australia, Europe, and North America.1 Air pollution is a known risk factor for lung cancer7; an 8–14% increase in lung cancer incidence per 10 µg/m3 of fine particulate air pollution has been noted.38,39 20–45% increases in lung cancer incidence with high nitrogen oxide exposure have also been reported.40,41 These findings may have informed efforts to evaluate whether there was a relationship between increased air pollution and incidence of other tumors including brain tumors. A Danish cohort study found an IRR of 2.28 per 100 µg/m3 of residential nitrogen oxides from traffic-related air pollution.7 Thus, one possibility is that conflicting data regarding the association between radon exposure and brain tumor incidence may result from an unaccounted effect of particulate air pollution. While inhalation of radon or air particulate is sufficient for lung cancer tumorigenesis, disruption of the blood–brain barrier, particularly the cribriform plate, by high particulate pollution may be required for oncogenic radon to penetrate the brain parenchyma7 and create the 4–26% increase in AAAIR of non-malignant tumors in high/high regions versus regions with lower exposures in our study. Our analysis of AAAIR by tumor location may be consistent with this hypothesis, as for many examined histologies, the relationship between elevated AAAIR and high/high exposure was moderately stronger for frontal/temporal/brainstem tumors than for tumors of the parietal/occipital/cerebellar lobes. Observed inconsistencies in this relationship may stem from challenges associated with registry coding of subsite information such as classification of tumors of overlapping regions.
Unlike our analysis, a Danish study found an adjusted IRR for brain tumor formation of 1.96 with each 100 Bq/m2 of radon but concluded that traffic-related air pollution levels did not modify this effect.27 However, the smaller size of that study may have obscured the interactive effects of radon and particulate pollution observed in our much larger population. One strength of our methodology is that areas of high radon and high pollution exposure do not perfectly overlap (Supplementary Figures 1 and 2), which allowed comparisons between regions that were high in both exposures and regions that were low with respect to one examined variable. Across all non-malignant brain tumors, we found a significantly increased AAAIR in counties of high air pollution and radon exposure with respect to counties that had a low levels of one or both factors. Interestingly, in our analysis, while a BBB opening mechanism is consistent with the decreased incidence of tumors with low particulate/high radon exposure with respect to high/high exposure areas, for unclear reasons, these counties had substantially lower incidence rates than low/low regions. The mechanism behind this finding is unclear and warrants further investigation.
After adjusting for confounders, the explanation for a significant relationship between radon, air pollution, and non-malignant (but not malignant) tumors is unclear. Perhaps, after excluding counties in the middle tertiles for radon level and AQI, the sample size for malignant tumors, which have a much lower prevalence than non-malignant tumors, was insufficient to detect a statistically significant difference in AAAIR between counties of varying exposure level. Alternatively, meningiomas comprise the bulk of non-malignant tumors. Some clinical or histopathological feature may render these tumors more likely to form after radon and pollution exposure. An unidentified confounding variable could drive the observed increased incidence in non-malignant tumors with increased exposures. Thus, although we controlled for key demographic variables, this analysis should be considered a hypothesis generating study.
Epidemiologic studies have certain limitations. AQI and radon data was not available for regions smaller than counties in either geography or population, and some counties may contain a mix of urban, suburban, and/or rural areas with variable rates of air pollution and radon exposure at a more individual or neighborhood level. Variables that are difficult to assess with this methodology, such as geographic disparities in access to health care resources or levels of pesticide/chemical exposures, are potential unaccounted for confounders. Histology is determined from patient records based on the prevailing WHO criteria at the time of diagnosis and without central pathology review. Because the CBTRUS does not include survival outcomes, the prognostic implications of living in a high exposure area are not evaluable. Additionally, AQI and/or radon data was not available for every county resulting in omission of 17% of the US population from this analysis. Detailed information about these counties was not available. However, the omitted counties were likely lower population areas with lower levels of population-density related particulate pollution, although it is possible that non-traffic sources of pollution such as industrial exposures may have been present. Furthermore, the latency period between exposures and brain tumor development is not well established and could be longer than anticipated. However, the number of counties for which particulate air pollution may be radically different in 2000 versus 1990 is likely negligible; legislation responsible for improvements in air quality in the US (such as the Clean Air Act of 1963) were passed and had their primary effect many decades prior to 2000.8 Thus, this potential source of inaccuracy is likely outweighed by the improved quality of modern quantification of exposure levels. Lastly, at this time, it is unknown whether observed relationships can be extrapolated to metropolitan areas abroad with much higher levels of air pollution than the US.
In conclusion, we present the first report associating increased non-malignant brain tumor incidence (up to 26% higher) with the combined effects of elevated air particulate pollution and radon exposure after prior efforts to evaluate these factors in isolation were inconclusive. While differences in incidence of malignant brain tumors between regions with high/high and other exposure levels were negated after controlling for demographic variables, increased incidence of non-malignant tumors persisted. Thus, this analysis represents perhaps the largest study seeking to explain the substantial but poorly understood variation in geographic incidence rates for non-malignant brain tumors. Future studies in other populations are needed to validate these findings.
Supplementary Material
Acknowledgments
The Central Brain Tumor Registry of the United States (CBTRUS) data were provided through an agreement with the Centers for Disease Control’s National Program of Cancer Registries. In addition, CBTRUS used data from the research data files of the National Cancer Institute (NCI)’s Surveillance, Epidemiology, and End Results Program. CBTRUS acknowledges and appreciates these contributions to this report and to cancer surveillance in general. The services of Jill Barnholtz-Sloan, Ph.D. and Gino Cioffi, M.P.H. from the NCI Department of Cancer Epidemiology and Genetics were provided at no cost to the project according to the employment rules of the NCI. Contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control or the NCI.
Contributor Information
Joshua D Palmer, Department of Radiation Oncology at the Arthur G. James Cancer Hospital and Richard J. Solove Research Institute, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio, USA.
Rahul N Prasad, Department of Radiation Oncology at the Arthur G. James Cancer Hospital and Richard J. Solove Research Institute, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio, USA.
Gino Cioffi, Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA; Trans Divisional Research Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland, USA.
Carol Kruchtko, Department of Radiation Oncology, University Hospitals Seidman Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio, USA.
Nicholas G Zaorsky, Department of Radiation Oncology, University Hospitals Seidman Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio, USA.
Daniel M Trifiletti, Department of Radiation Oncology, Mayo Clinic Florida, Jacksonville, Florida, USA.
Vinai Gondi, Brain and Spine Tumor Center, Northwestern Medicine Cancer Center and Proton Center, Warrensville, Illinois, USA.
Paul D Brown, Department of Radiation Oncology, Mayo Clinic, Rochester, Minnesota, USA.
Haley K Perlow, Department of Radiation Oncology at the Arthur G. James Cancer Hospital and Richard J. Solove Research Institute, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio, USA.
Mark V Mishra, Department of Radiation Oncology, University of Maryland, Baltimore, Maryland, USA.
Arnab Chakravarti, Department of Radiation Oncology at the Arthur G. James Cancer Hospital and Richard J. Solove Research Institute, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio, USA.
Jill S Barnholtz-Sloan, Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA; Trans Divisional Research Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland, USA; Center for Biomedical Informatics and Information Technology (CBIIT), National Cancer Institute, Bethesda, Maryland, USA.
Quinn T Ostrom, Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA; Department of Neurosurgery, Duke University School of Medicine, Durham, North Carolina, USA; The Preston Robert Tisch Brain Tumor Center, Duke University School of Medicine, Durham, North Carolina, USA; Duke Cancer Institute, Duke University Medical Center, Durham, North Carolina, USA.
Funding
Funding for the Central Brain Tumor Registry of the United States was provided by the Centers for Disease Control and Prevention (CDC) under Contract No. 75D30119C06056, the American Brain Tumor Association, The Sontag Foundation, Novocure, the Musella Foundation, National Brain Tumor Society, the Pediatric Brain Tumor Foundation, the Uncle Kory Foundation, the Zelda Dorin Tetenbaum Memorial Fund, as well as private and in-kind donations. Contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC or the National Cancer Institute.
Conflict of interest statement: None. JDP discloses Honoraria from Huron Consulting Group and research support from Varian Medical Systems and from Kroger outside the submitted work. NGZ is supported by National Institutes of Health Grant LRP 1 L30 CA231572-01, and the American Cancer Society’s Tri State CEOs Against Cancer Clinician Scientist Development Grant, CSDG-20-013-01-CCE outside the submitted work. PDB discloses honoraria from UpToDate outside the submitted work. VG discloses honoraria from UpToDate and is supported by National Institute of Health R42 Small Business Technology Transfer grant through Immunochem Therapeutics. All other authors declare no conflicts of interests in relation to the work presented in this manuscript.
Authorship Statement. Conceptualization—JDP, GC, CK, JSB, QTO. Data curation—JDP, RNP, QTO. Formal analysis—JDP, RNP, QTO. Funding acquisition—GC, CK, JSB, QTO. Investigation—JDP, RNP, QTO. Methodology—JDP, RNP, GC, CK, JSB, QTO. Project administration—JDP, GC, CK, JSB, QTO. Resources—JDP, GC, CK, JSB, QTO. Software—QTO. Supervision—JDP, QTO. Validation—JDP, RNP, QTO. Visualization—JDP, RNP, QTO. Writing—original draft—RNP. Writing—review and editing—JDP, RNP, GC, CK, NGZ, DMT, VG, PDB, HKP, MM, AC, JSB, QTO. All authors were involved in the writing of the manuscript at draft and any revision stages, and have read and approved the final version.
References
- 1. Barnholtz-Sloan JS, Ostrom QT, Cote D. Epidemiology of brain tumors. Neurol Clin. 2018;36(3):395–419. [DOI] [PubMed] [Google Scholar]
- 2. Braganza MZ, Kitahara CM, Berrington de González A, et al. Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro Oncol. 2012;14(11):1316–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Turner MC, Krewski D, Chen Y, et al. Radon and lung cancer in the American Cancer Society cohort. Cancer Epidemiol Biomarkers Prev. 2011;20(3):438–448. [DOI] [PubMed] [Google Scholar]
- 4. Turner MC, Krewski D, Chen Y, et al. Radon and nonrespiratory mortality in the American Cancer Society cohort. Am J Epidemiol. 2012;176(9):808–814. [DOI] [PubMed] [Google Scholar]
- 5. Stanley FKT, Irvine JL, Jacques WR, et al. Radon exposure is rising steadily within the modern North American residential environment, and is increasingly uniform across seasons. Sci Rep. 2019;9(1):18472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruano-Ravina A, Dacosta-Urbieta A, Barros-Dios JM, Kelsey KT. Radon exposure and tumors of the central nervous system. Gac Sanit. 2018;32(6):567–575. [DOI] [PubMed] [Google Scholar]
- 7. Raaschou-Nielsen O, Andersen ZJ, Hvidberg M, et al. Air pollution from traffic and cancer incidence: a Danish cohort study. Environ Health. 2011;10:67. https://ehjournal.biomedcentral.com/track/pdf/10.1186/1476-069X-10-67.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shaddick G, Thomas ML, Mudu P, Ruggeri G, Gumy S. Half the world’s population are exposed to increasing air pollution. Npj Clim Atmos Sci. 2020;3(1):1–5. [Google Scholar]
- 9. Block ML, Calderón-Garcidueñas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32(9):506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jankowska-Kieltyka M, Roman A, Nalepa I. The air we breathe: air pollution as a prevalent proinflammatory stimulus contributing to neurodegeneration. Front Cell Neurosci. 2021. https://www.frontiersin.org/articles/10.3389/fncel.2021.647643/full. Accessed May 31, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paulino AC, Mai WY, Chintagumpala M, Taher A, Teh BS. Radiation-induced malignant gliomas: is there a role for reirradiation? Int J Radiat Oncol Biol Phys. 2008;71(5):1381–1387. [DOI] [PubMed] [Google Scholar]
- 12. US EPA O. How is the AQI calculated? Published October 1, 2021. 2022. https://www.epa.gov/outdoor-air-quality-data/how-aqi-calculated. Accessed February 15, 2022.
- 13. Radon Analysis. Radon.com. 2022. https://www.radon.com/radon_analysis/. Accessed February 15, 2022.
- 14. Ostrom QT, Patil N, Cioffi G, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro Oncol. 2020;22(12 Suppl 2):iv1–iv96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. CDC National Program of Cancer Registries and NCI Surveillance, Epidemiology and End Results Incidence Data, 2019 submission (2000–2017). Central Brain Tumor Registry of the United States SEER*Stat Database. Published online 2020.
- 16. SEER*Stat Software. SEER. 2021. https://seer.cancer.gov/seerstat/index.html. Accessed April 7, 2021.
- 17. Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15(6):547–569. [DOI] [PubMed] [Google Scholar]
- 18. Fay MP, Tiwari RC, Feuer EJ, Zou Z. Estimating average annual percent change for disease rates without assuming constant change. Biometrics. 2006;62(3):847–854. [DOI] [PubMed] [Google Scholar]
- 19. R: The R Project for Statistical Computing. Accessed April 5, 2022. https://www.r-project.org/. Accessed April 5, 2022.
- 20. Cote DJ, Ostrom QT, Gittleman H, et al. Glioma incidence and survival variations by county-level socioeconomic measures. Cancer. 2019;125(19):3390–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. US EPA O. What is EPA’s action level for radon and what does it mean? Published February 19, 2019. https://www.epa.gov/radon/what-epas-action-level-radon-and-what-does-it-mean. Accessed May 31, 2022.
- 22. Darby S, Hill D, Auvinen A, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ. 2005;330(7485):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Darby S, Hill D, Deo H, et al. Residential radon and lung cancer—detailed results of a collaborative analysis of individual data on 7148 persons with lung cancer and 14,208 persons without lung cancer from 13 epidemiologic studies in Europe. Scand J Work Environ Health. 2006;32(Suppl 1):1–83. [PubMed] [Google Scholar]
- 24. Krewski D, Lubin JH, Zielinski JM, et al. A combined analysis of North American case-control studies of residential radon and lung cancer. J Toxicol Environ Health A. 2006;69(7):533–597. [DOI] [PubMed] [Google Scholar]
- 25. Krewski D, Lubin JH, Zielinski JM, et al. Residential radon and risk of lung cancer: a combined analysis of 7 North American case-control studies. Epidemiology. 2005;16(2):137–145. [DOI] [PubMed] [Google Scholar]
- 26. Ruano-Ravina A, Aragonés N, Kelsey KT, et al. Residential radon exposure and brain cancer: an ecological study in a radon prone area (Galicia, Spain). Sci Rep. 2017;7(1):3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bräuner EV, Andersen ZJ, Andersen CE, et al. Residential radon and brain tumour incidence in a Danish Cohort. PLoS One. 2013;8(9):e74435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hess CT, Weiffenbach CV, Norton SA. Environmental radon and cancer correlations in Maine. Health Phys. 1983;45(2):339–348. [DOI] [PubMed] [Google Scholar]
- 29. Monastero RN, Meliker J. Incidence of brain and spinal cord cancer and county-level radon levels in New Jersey, Wisconsin, Minnesota, Pennsylvania, and Iowa, USA. Environ Geochem Health. 2020;42(2):389–395. [DOI] [PubMed] [Google Scholar]
- 30. Tirmarche M, Raphalen A, Allin F, Chameaud J, Bredon P. Mortality of a cohort of French uranium miners exposed to relatively low radon concentrations. Br J Cancer. 1993;67(5):1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vacquier B, Rage E, Leuraud K, et al. The influence of multiple types of occupational exposure to radon, gamma rays and long-lived radionuclides on mortality risk in the French “post-55” sub-cohort of uranium miners: 1956–1999. Radiat Res. 2011;176(6):796–806. [DOI] [PubMed] [Google Scholar]
- 32. Darby SC, Radford EP, Whitley E. Radon exposure and cancers other than lung cancer in Swedish iron miners. Environ Health Perspect. 1995;103(SUPPL. 2):45–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Darby SC, Whitely E, Howe GR, et al. Radon and cancers other than lung cancer in underground miners: a collaborative analysis of 11 studies. J Natl Cancer Inst. 1995;87(5):378–384. [DOI] [PubMed] [Google Scholar]
- 34. Vacquier B, Caer S, Rogel A, et al. Mortality risk in the French cohort of uranium miners: Extended follow-up 1946–1999. Occup Environ Med. 2008;65(9):597–604. [DOI] [PubMed] [Google Scholar]
- 35. Kreuzer M, Walsh L, Schnelzer M, Tschense A, Grosche B. Radon and risk of extrapulmonary cancers: results of the German uranium miners’ cohort study, 1960–2003. Br J Cancer. 2008;99(11):1946–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kreuzer M, Grosche B, Schnelzer M, et al. Radon and risk of death from cancer and cardiovascular diseases in the German uranium miners cohort study: follow-up 1946–2003. Radiat Environ Biophys. 2010;49(2):177–185. [DOI] [PubMed] [Google Scholar]
- 37. Schubauer-Berigan MK, Daniels RD, Pinkerton LE. Radon exposure and mortality among white and American Indian uranium miners: an update of the Colorado Plateau cohort. Am J Epidemiol. 2009;169(6):718–730. [DOI] [PubMed] [Google Scholar]
- 38. Pope CA, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287(9):1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vineis P, Hoek G, Krzyzanowski M, et al. Air pollution and risk of lung cancer in a prospective study in Europe. Int J Cancer. 2006;119(1):169–174. [DOI] [PubMed] [Google Scholar]
- 40. Nyberg F, Gustavsson P, Järup L, et al. Urban air pollution and lung cancer in Stockholm. Epidemiology. 2000;11(5): 487–495. [DOI] [PubMed] [Google Scholar]
- 41. Raaschou-Nielsen O, Bak H, Sørensen M, et al. Air pollution from traffic and risk for lung cancer in three Danish cohorts. Cancer Epidemiol Biomark Prev. 2010;19(5):1284–1291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.