Abstract
Background
Approximately 50% of newly diagnosed glioblastomas (GBMs) harbor epidermal growth factor receptor gene amplification (EGFR-amp). Preclinical and early-phase clinical data suggested efficacy of depatuxizumab mafodotin (depatux-m), an antibody–drug conjugate comprised of a monoclonal antibody that binds activated EGFR (overexpressed wild-type and EGFRvIII-mutant) linked to a microtubule-inhibitor toxin in EGFR-amp GBMs.
Methods
In this phase III trial, adults with centrally confirmed, EGFR-amp newly diagnosed GBM were randomized 1:1 to radiotherapy, temozolomide, and depatux-m/placebo. Corneal epitheliopathy was treated with a combination of protocol-specified prophylactic and supportive measures. There was 85% power to detect a hazard ratio (HR) ≤0.75 for overall survival (OS) at a 2.5% 1-sided significance level (ie traditional two-sided p ≤ 0.05) by log-rank testing.
Results
There were 639 randomized patients (median age 60, range 22–84; 62% men). Prespecified interim analysis found no improvement in OS for depatux-m over placebo (median 18.9 vs. 18.7 months, HR 1.02, 95% CI 0.82–1.26, 1-sided p = 0.63). Progression-free survival was longer for depatux-m than placebo (median 8.0 vs. 6.3 months; HR 0.84, 95% confidence interval [CI] 0.70–1.01, p = 0.029), particularly among those with EGFRvIII-mutant (median 8.3 vs. 5.9 months, HR 0.72, 95% CI 0.56–0.93, 1-sided p = 0.002) or MGMT unmethylated (HR 0.77, 95% CI 0.61–0.97; 1-sided p = 0.012) tumors but without an OS improvement. Corneal epitheliopathy occurred in 94% of depatux-m-treated patients (61% grade 3–4), causing 12% to discontinue.
Conclusions
Interim analysis demonstrated no OS benefit for depatux-m in treating EGFR-amp newly diagnosed GBM. No new important safety risks were identified.
Keywords: antibody drug conjugate, EGFR, depatuxizumab mafodotin, glioblastoma, phase III
Key Points.
Approximately 50% of newly diagnosed glioblastomas (GBMs) harbor EGFR-amplification (EGFR-amp).
The antibody–drug conjugate depatuxizumab mafodotin binds activated EGFR.
Depatuxizumab mafodotin did not improve overall survival in EGFR-amp newly diagnosed GBM.
Importance of the Study.
In this phase III clinical trial, there was no improvement in survival from treatment with the EGFR-directed antibody–drug conjugate depatuxizumab mafodotin (depatuix-m) over placebo in addition to standard chemoradiotherapy. Progression-free survival was longer among patients randomized to depatux-m, particularly in EGFRvIII-mutant cases. Corneal epitheliopathy occurred in most depatux-m-treated patients causing a small minority to discontinue.
Glioblastoma (GBM) is the most common primary brain tumor in adults. Prognosis is poor; new approaches are needed. Focal epidermal growth factor receptor (EGFR) gene amplification on chromosome 7 (EGFR-amp) has long been observed1 in approximately 50% of GBMs (although geographic differences exist).2 EGFR variant 3 (EGFRvIII) mutation, a tumor-specific deletion of exons 2-7, is constitutively active and observed in approximately 50% of EGFR-amp GBMs (~25% overall).3 Several EGFR/EGFRvIII-directed therapeutic approaches have been used, including receptor tyrosine kinase inhibitors (RTKIs),4 antibodies,5–7 and vaccines.8 Despite TKI success in molecularly selected non-small lung cancers9 and with antibodies in other solid tumors,10 these approaches have been disappointing for GBM.4
Depatuxizumab (depatux, formerly ABT-806) is a humanized recombinant monoclonal antibody originally generated against EGFRvIII in mice,11 although it also binds to wild-type EGFR when present at high levels.12 The epitope becomes accessible to the antibody when EGFR is activated, either by ligand for wild-type receptor or constitutive mutation (eg EGFRvIII).12,13 The antibody–drug conjugate (ADC) depatuxizumab mafodotin (depatux-m, formerly ABT-414, Figure S1) links depatux to a microtubule cytotoxic payload, monomethyl auristatin F (MMAF, mafodotin).14,15 Following binding to activated EGFR, the antibody and linked payload are endocytosed and degraded in acidic endocytic compartments, releasing the toxin causing cell death.16 This direct cytotoxic effect of the ADC, therefore, does not rely on inhibition of EGFR signaling and does not cause rash, diarrhea, or other toxicities typical of RTKIs or monoclonal antibodies that bind to unamplified wild-type receptor in normal organs.5 Although GBMs do not respond to the unconjugated antibody (depatux),5 depatux-m is effective against EGFR-amp and EGFRvIII harboring GBM cell lines and animal models, both alone and combined with radiotherapy (RT) and temozolomide.15 In addition, ADCs have superior efficacy to unconjugated monoclonal antibodies in other solid tumors, with several under investigation in many cancers and conditions17 including GBM.18
Therefore, we previously conducted a phase I trial of depatux-m and identified a recommended dose for use alone or in combination with RT and/or temozolomide. Radiographic responses were observed, mainly EGFR-amp disease.19–22 Corneal epitheliopathy (CE, previously termed ocular side effects or keratopathy)23 was very common but typically reversible. Of note, another mafodotin-containing biologic was US FDA approved for myeloma despite a similarly high frequency of CE.24
The encouraging preclinical15 and early-phase clinical data formed the basis of two large international randomized trials. The open-label phase II European Organisation for the Research and Treatment of Cancer (EORTC) trial 1410 (AbbVie M14-483, INTELLANCE-2, NCT02343406) accrued patients with EGFR-amp-recurrent GBM. In the primary analysis (median follow-up 15.0 months), results trended toward longer overall survival (OS) following treatment with depatux-m in combination with temozolomide compared to control of lomustine or temozolomide (hazard ratio [HR] 0.71; 95% confidence interval [CI] 0.50–1.02; log-rank p = 0.06). In the exploratory follow-up analysis (median follow-up 28.7 months), the HR was 0.66 for the comparison of the combination arm versus control (95% CI 0.4–0.93, log-rank p = 0.017).25 Concurrently, we conducted a randomized, double-blind placebo-controlled phase III trial of depatux-m for newly diagnosed EGFR-amp GBM as an academic-industry collaboration between the Radiation Therapy Oncology Group Foundation (RTOG-F 3508) and AbbVie (M13-813, INTELLANCE-1, NCT02573324) and report results here.
METHODS
Eligibility
Patients were ≥18 years old and had Karnofsky Performance Status ≥70, an RT and chemotherapy naïve unifocal GBM harboring EGFR-amp, and end-organ function. Laser-assisted in situ keratomileusis (LASIK) within the prior year, cataract surgery within the prior 3 months, and other contraindication to ocular corticosteroids required as supportive care for CE (below) were exclusionary. All subjects provided written informed consent prior to any study-specific procedures, and the study was approved by the Institutional Review Board of each participating institution. Detailed criteria are available in the protocol (Supplementary Material).
Biomarkers
Biomarkers (Table S1) and histology (GBM by World Health Organization 2016 criteria,26 KA) were confirmed centrally before randomization as described previously: EGFR-amp by Fluorescence in Situ Hybridization,2 O6-methylguanine-DNA-methyltransferase (MGMT) by methylation-specific polymerase chain reaction (PCR),19 and EGFRvIII mRNA by reverse-transcription-PCR.19Isocitrate dehydrogenase (IDH) mutation is typically mutually exclusive with EGFR-amp and was not assessed.26
Treatment
Up to 7 weeks following diagnostic surgery, eligible subjects were randomly assigned 1:1 to RT, temozolomide, and either depatux-m or placebo in a stratified (below) double-blind manner. RT was planned using a postoperative contrast-enhanced baseline brain MRI to a total dose of 60 Gy in 30 fractions (or 59.4 Gy in 33 fractions) over approximately 6 weeks. A planning MRI (repeated if necessary) was obtained ≤4 weeks postoperatively and ≤3 weeks before RT. Either a sequential boost to the contrast-enhanced region of the target as per standard RTOG approach or single-phase technique as per the EORTC approach were permitted.
Temozolomide was dosed at 75 mg/m2 daily during RT followed by 6 adjuvant cycles of 150–200 mg/m2 on days 1–5/2827 with up to 12 adjuvant cycles allowed. Depatux-m was dosed at 2.0 mg/kg during RT, then 1.25 mg/kg thereafter on days 1 and 15/2819,21 and allowed to continue until disease progression. Postprogression treatment was at the discretion of the treating investigator except cross-over from placebo to depatux-m was disallowed.
Supportive care
Prophylactic ocular corticosteroids were mandatory with each dose of depatux-m/placebo to reduce the potential for CE as described previously.28 Additional ocular supportive care measures (eg lubricating eye drops, therapeutic bandage contact lenses, punctal plugs, and/or antibiotic drops, etc.) were recommended for both symptomatic relief of CE (eg photophobia, blurry vision, and/or other eye discomforts) and to reduce side effect–driven interruptions or reductions of depatux-m dosing.
Pneumocystis jirovecii pneumonia prophylaxis during chemoradiotherapy27 and antiemetic prophylaxis before temozolomide were recommended. Growth factor and transfusion support were permitted for cytopenias other than to induce eligibility or affect temozolomide cycle length or dose. Systemic corticosteroids and anticonvulsants were allowed without restriction.
Follow-up
In addition to serial ophthalmologic examinations, patients underwent routine physical, neurologic, bone marrow, serum chemistry, and hepatic function evaluations at baseline, before every cycle, and more frequently as clinically indicated. Dose interruptions and reductions of depatux-m/placebo were permitted for treatment-related CTCAE grade 2–3 and required for grade 4 ocular adverse events (such as corneal perforation or acuity ≤20/200). Up to 3 consecutive depatux-m/placebo dose reductions during chemoradiotherapy (by −0.5 mg/kg each) and up to 4 during adjuvant treatment (by −0.25 mg/kg each) were permitted for treatment-related toxicities. Re-escalations were permitted only for improved CE and serum chemistry abnormalities but not for other adverse events. Temozolomide adjustments were allowed per local prescribing regulations.
Baseline contrast-enhanced brain MRI scans, neurocognitive function (NCF) tests, and patient-reported outcomes (PROs) were required before chemoradiotherapy and serially before odd-numbered adjuvant cycles (1, 3, 5, etc.) of temozolomide and depatux-m/placebo, and then every 8 weeks thereafter. Progression as a study endpoint was assessed centrally and retrospectively using Response-Assessment in Neuro-Oncology (RANO) criteria,29 but treatment decisions were made using local interpretation in real time with continuation encouraged in equivocal scenarios.
Results of NCF testing and PROs were also performed at the time of locally determined progression, although scoring and results were not used in treatment decision-making; rather, NCF results and PROs were verified and associations evaluated centrally. The M.D. Anderson Symptom Inventory—Brain Tumor (MDASI-BT) questionnaire is a validated PRO instrument used to assess the severity of brain tumor–related symptoms and its impact on daily function. It consists of 22 symptom items and 6 interference items, each rated from 0 (best) to 10 (worse).30,31 The symptom severity score and symptom interference score are the average of the symptom and interference items, respectively.32–34 The Hopkins Verbal Learning Test—Revised (HVLT-R)35 is a sensitive, highly standardized, validated neurocognitive test to assess change in verbal episodic learning and memory over time. There are 6 alternate forms to limit practice effects. The Total Recall score was chosen a priori as a secondary endpoint and is the sum of the total number of words recalled across 3 trials.
Study design
In order to balance known and potential prognostic factors between arms, randomization (using permuted block36 sizes of 4 that was generated by the AbbVie Data and Statistical Sciences department) was stratified by Region of world, Radiation Therapy Oncology Group—Recursive Partitioning Analysis (RTOG-RPA) class (which incorporates age, performance status, extent of resection, and neurological function, Table 1 legend),37MGMT promoter methylation status, and EGFRvIII mutation (as a mechanistically predictive biomarker for enhanced depatux binding to tumor cells). All randomized subjects were included in the intent-to-treat analysis.
Table 1.
Patient characteristics among randomized patients, n (%)
| Baseline Characteristics: randomized patients (n = 639) | Placebo (n = 316) | Depatux-m (n = 323) |
|---|---|---|
| Age, years | ||
| Median | 60 | 59 |
| Range | 29–82 | 22–84 |
| Gender, n (%) | ||
| Male | 188 (59) | 206 (64) |
| Female | 128 (41) | 117 (36) |
| Histology, (central review) n (%) | ||
| Glioblastoma | 311 (98) | 319 (99) |
| Gliosarcoma | 1 (<1) | 3 (1) |
| Other | 1 (<1) | 1 (<1) |
| Missing | 3 (1) | 0 (0) |
| Karnofsky Performance Status (KPS), n (%) | ||
| 70 | 38 (12) | 44 (14) |
| 80 | 80 (25) | 76 (23) |
| 90–100 | 198 (63) | 203 (63) |
| Extent of resection (EOR), n (%) | ||
| Gross total resection | 181 (57) | 185 (57) |
| Partial/subtotal resection | 122 (39) | 128 (40) |
| Biopsy | 10 (3) | 10 (3) |
| Missing | 3 (1) | 0 (0) |
| Impairment of Neurologic Function (INF), n (%) | ||
| > minor | 25 (8) | 27 (8) |
| ≤ minor | 288 (91) | 296 (92) |
| Missing | 3 (1) | 0 (0) |
| a Radiation Therapy Oncology Group—Recursive Partitioning Analysis (RTOG-RPA) Prognostic Class, n (%) | ||
| III | 46 (14) | 51 (16) |
| IV | 233 (74) | 236 (73) |
| V | 37 (12) | 36 (11) |
| HVLT-R | ||
| Total Recall, mean (Standard Deviation) | −1.5 (2.2) | −1.4 (1.9) |
| a Region of World, n (%) | ||
| Other | 214 (68) | 216 (67) |
| USA/Canada | 102 (32) | 107 (33) |
| a MGMT, n (%) | ||
| Methylated | 117 (37) | 118 (37) |
| Unmethylated | 199 (63) | 205 (63) |
| a EGFRvIII, n (%) | ||
| Mutated | 168 (53) | 164 (51) |
| Other | 148 (47) | 159 (49) |
aStratification factor.
RTOG-RPA Class definitions
•III: Age < 50, KPS ≥ 90
•IV: Age < 50, KPS < 90; OR Age ≥ 50, KPS ≥ 70, EOR > biopsy, INF ≤ minor
•V: Age ≥ 50, KPS ≥ 70, EOR > biopsy, INF > minor; OR Age ≥ 50, KPS ≥ 70, EOR = Biopsy
Originally, a phase II/III trial was planned but accrual to phase II rapidly outpaced both the planned Progression-Free survival (PFS) analysis and phase III accrual goals, despite the stringent requirements for central pathology review and biomarker testing (Figure S2). In addition, early results from the concurrently conducted INTELLANCE-2 trial in recurrent GBM suggested depatux-m in combination with temozolomide improved OS relative to control.25 Therefore, the trial design was amended as a phase III with OS as the primary endpoint, but prespecified an interim analysis for futility (or overwhelming superiority, below).
Median OS with placebo was estimated as 16 months and hypothesized to improve to 21.3 months with depatux-m. With 441 deaths among 640 randomized patients, we had 85% power to detect a ≥25% reduction in risk of death (HR ≤0.75) at a 2.5% 1-sided level of significance (ie traditional 2-sided p ≤ 0.05). Anticipating delayed treatment effect, a Fleming Harrington version of weighted log-rank test with parameters ρ = 0 and γ = 0.2 was used. Thus, at least 66% of information, due to increased weighting for later events, would be accumulated at the interim analysis and resulted in testing the futility bound at HR >0.9 or the efficacy bound (for superiority) at a 1-sided significance level of 0.0058.38,39 Secondary and exploratory endpoints included PFS, molecular subgroup analyses, NCF, and PROs.
PFS was defined as the interval from randomization to first of either progressive disease (by blinded independent central review per RANO criteria) or death from any cause, and OS to death from any cause. Subjects not experiencing progression or death were censored. NCF and PRO were analyzed using deterioration-free survival (DFS), with deterioration defined using the reliable change index criterion for the HVLT-R Total Recall (i.e., as a reduction of 5 points as compared to baseline)40 and a decrease of 1 point as compared to baseline for the MDASI-BT symptom severity and symptom interference scores. DFS was defined as the interval from randomization to the first occurrence of deterioration or death from any cause. Subjects not experiencing an event were censored.
Time-to-event (PFS, OS, and DFS) analyses were performed using the Kaplan–Meier method with HRs and 95% CIs estimated using Cox proportional hazards regression models adjusting for stratification factors. An Independent Data Monitoring Committee, managed by RTOG-F, reviewed unblinded data and interim results.
Importantly, hierarchical testing was used for all secondary and exploratory analyses (Table S2) to reduce the potential for falsely identifying a significant difference when conducting multiple comparisons.41 In this manner, subsequent differences in outcome between arms could only be considered statistically significant (regardless of the HR or p), if the prior analysis in the hierarchy were significant (2-sided p ≤ 0.05). However, we report the preplanned secondary and exploratory analyses descriptively to understand the trial outcomes thoroughly. Details of the statistical collaboration between AbbVie and RTOG Foundation can be found in Supplementary Materials.
RESULTS
Accrual
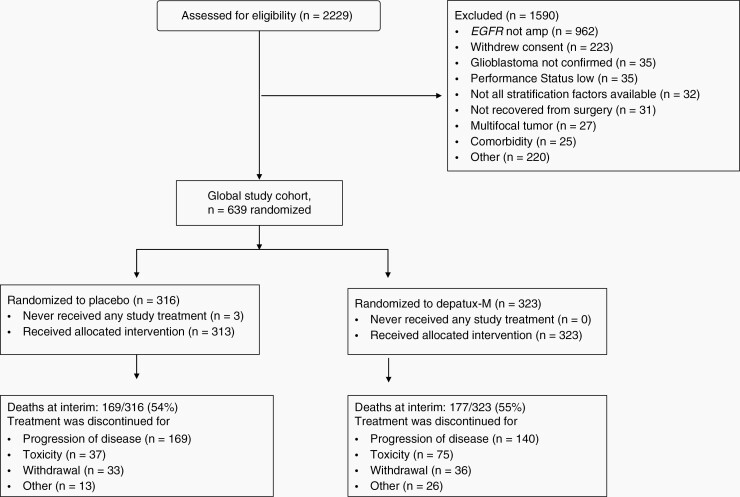
Accrual occurred at 190 sites in 26 countries from September 11, 2015, to March 31, 2018 (Figures 1 and S2; Table S3). Among 2229 screened, lack of EGFR-amp by central analysis was the most common reason for ineligibility (61% of excluded cases). Central histology review nearly always (98%) confirmed a GBM diagnosis. The pace of accrual exceeded projections (Figure S2). As a consequence, the phase II/III design was converted to a phase III trial as outlined above.
Fig. 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram of RTOG-F 3508/AbbVie M13-813 (INTELLANCE-1) at the time of preplanned interim analysis.
Median age was 60 years (range 22–84), 62% (394/639) of randomized subjects were men, and 13% (81) were ≥70 years old. Baseline characteristics of the study population were similar (53% EGFRvIII-mutant, 37% methylated MGMT) to those of other newly diagnosed GBM trials and reports.3,42,43 Arms were well balanced (Table 1).
Safety
The most common adverse events were ocular (grouped under the general term of CE, Table S4) consistent with prior reports.19–22,25 For example, CE of any grade occurred in 94% of subjects randomized to depatux-m, although was surprisingly reported in 36% on the placebo arm. Grade 3 CE (vision decline to worse than 20/40 but better than 20/200, or limiting self-care activities of daily living) was reported in 55%, and grade 4 perforation or blindness with acuity 20/200 or worse in 5% of patients randomized to depatux-m (Table 2). Corneal epitheliopathy of all grades was managed by a combination of both prophylactic and supportive measures and by dose interruptions or delays (44%), although complete discontinuation of protocol therapy was required infrequently (12% in the depatux-m arm, 0% in the placebo arm). Thrombocytopenia was also more commonly observed among patients randomized to depatux-m than placebo (61% any grade with 14% each grade 3 and 4 vs. 36% any grade with 6% each grade 3 and 4).
Table 2.
Grade 3 and 4 adverse events reported in at least 5% of patients
| Placebo (n=313) n (%) | Depatux-m (n=323) n (%) | |||
|---|---|---|---|---|
| Adverse event | Grade 3 | Grade 4 | Grade 3 | Grade 4 |
| Any | 135 (43.1) | 47 (15.0) | 191(59.1) | 69(21.4) |
| Corneal epitheliopathy (CE)a | 2 (0.6) | 0 | 179 (55.4) | 16 (5.0) |
| Thrombocytopenia | 20 (6.4) | 18 (5.8) | 44(13.6) | 46 (14.2) |
| Gamma-glutamyltransferase increased | 2 (0.6) | 1 (0.3) | 33(10.2) | 2 (0.6) |
| Lymphopenia | 37 (11.8) | 4 (1.3) | 23 (7.1) | 4 (1.2) |
| Seizure | 16 (5.4) | 4 (1.3) | 16 (5.0) | 2 (0.6) |
| Alanine aminotransferase increased | 5 (1.6) | 0 | 17 (5.3) | 0 |
| Neutropenia | 15 (4.8) | 10 (3.2) | 9 (2.8) | 6 (1.9) |
aIncludes keratopathy, vision blurred, photophobia, dry eye, eye pain, keratitis, and punctate keratitis.
Survival
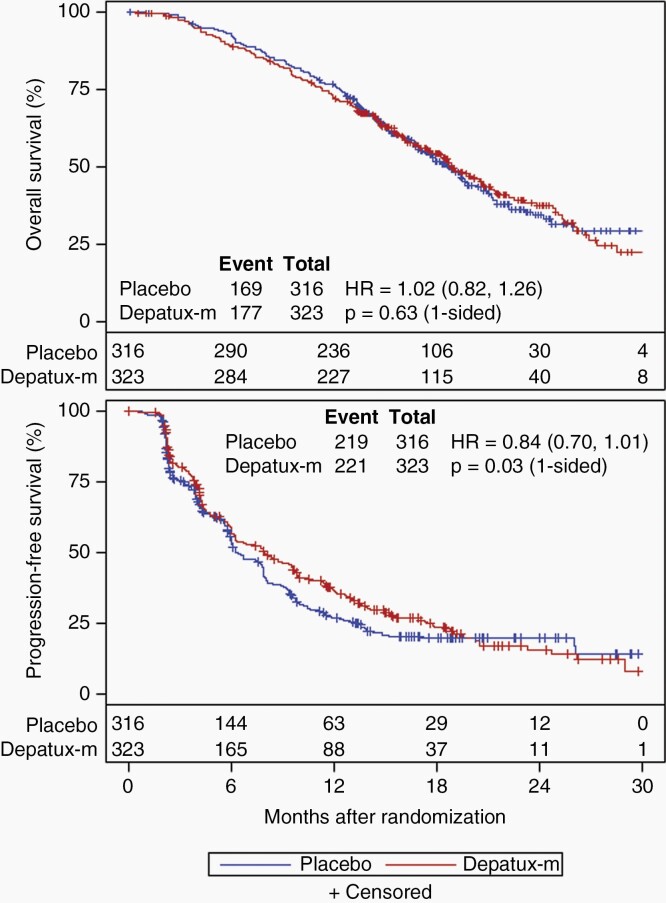
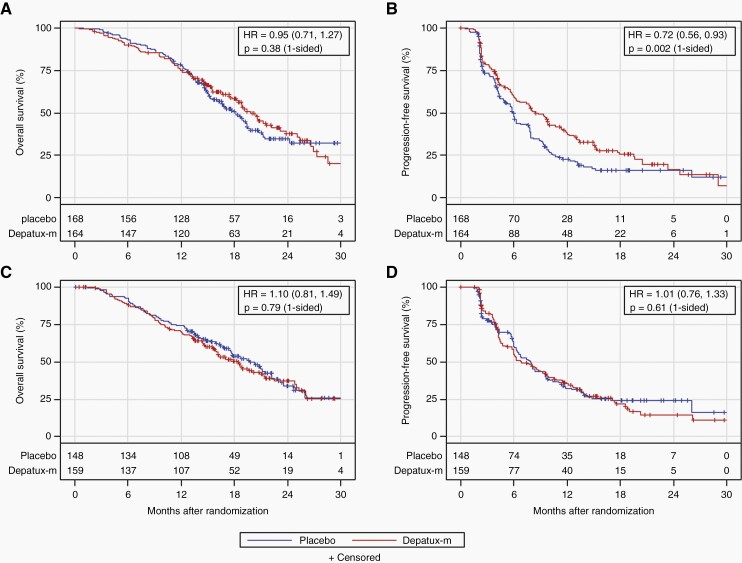
The preplanned interim analysis was conducted in May 2019 after 346 deaths among all randomized patients (>332 required). At that time, slightly more than 50% of patients in each arm died (169/316 placebo, 177/323 depatux-m), and nearly 70% in each arm had progressed by central review (219/316 placebo, 221/323 depatux-m). After median follow-up of 18.1 months among 293 surviving patients, there was no OS improvement for depatux-m over placebo (median 18.9 months for depatux-m vs. 18.7 months for placebo, HR 1.02, 95% CI 0.82–1.26, 1-sided log-rank p = 0.63; Figure 2). As the primary analysis for OS failed to demonstrate a significant difference between arms, subsequent endpoint analyses were exploratory (Table S2). PFS (centrally determined) was longer following depatux-m than placebo (median 8.0 months vs. 6.3 months; HR 0.84, 95% CI 0.70–1.01; 1-sided p = 0.029; Figure 2), driven at least in part by the EGFRvIII-mutant subgroup (median 8.3 vs. 5.9 months, HR 0.72, 95% CI 0.56–0.93, 1-sided p = 0.002; Figure 3). By contrast, among those without EGFRvIII-mutant disease, there was no difference in PFS between arms (median 6.9 months for depatux-m vs. 7.9 months for placebo, HR 1.01, 95% CI 0.76–1.33, p=0.61 one-sided, Figure 3).
Fig. 2.
Overall and progression-free survival. Overall (a) and progression-free survival (b) (by central review) curves by treatment arm among all randomized patients (intent-to-treat). The number of patients at risk over time is shown below the curves. HR, Hazard ratio (with 95% confidence intervals).
Fig. 3.
Overall and progression-free survival by EGFRvIII mutation. Overall (a, c) and progression-free survival (b, d; by central review) by treatment arm for patients with (a, b) or without (c, d) EGFRvIII mutation on an intent-to treat basis. The number of patients at risk over time is shown below the curves. HR, Hazard ratio (with 95% confidence intervals).
There was no improvement in OS by treatment for any subgroup, although, as above, the study was not powered to detect a statistically significant difference (Figures S3–S4; Tables S5–S14).
Finally, to explore EGFRvIII for prognostic importance regardless of treatment, we analyzed survival by mutational status among patients randomized to placebo to eliminate potential confounding by treatment with depatux-m (Figure S5). PFS was longer among cases without (n = 148) than with (n = 168) documented EGFRvIII (median PFS 7.9 months vs. 5.9 months, HR 0.74, 95% CI 0.57–0.97, p = 0.03) but without a difference in OS (HR 0.95, 95% CI 0.70–1.29, p = 0.76) in this post hoc, underpowered, univariate analysis.
NCF and PROs
The compliance for the HVLT-R and MDASI-BT was similar: ≥93% at baseline, >80% at adjuvant week 1, ≥70% at adjuvant week 9, ≥58% at adjuvant week 17, ≥51% at adjuvant week 25, and ≥47% at adjuvant week 33 (Table S15). There were no differences between treatment arms with respect to baseline HVLT-R Total Recall and MDASI-BT scores. There was no between arm difference in DFS for HVLT-R Total Recall, symptom severity, or symptom interference (HR 1.14, 95% CI 0.92–1.40, p = 0.81; HR 1.33, 95% CI 1.09–1.63, p = 0.99; HR 1.19, 95% CI 0.97–1.45, p = 0.94, respectively; Figures S6 and S7).
Discussion
In this phase III trial, survival was not improved by depatux-m for people with newly diagnosed EGFR-amp GBM; the study was stopped early and unblinded for futility. PFS (centrally determined) was longer with depatux-m than placebo, particularly in the EGFRvIII-mutant subgroup. No DFS differences between arms in verbal learning, symptoms, or symptom interference were observed. No new important safety risks from depatux-m were identified with reversible CE (which were also reported in the placebo arm) and thrombocytopenia observed most commonly. Patients on active treatment were permitted to continue after unblinding and re-consent.
There are several potential explanations for the negative result. Most importantly, despite encouraging preclinical and early-phase clinical data, it is possible that depatux-m is simply ineffective for treating GBM, notwithstanding any potential enrichment strategy. Other potential biologic explanations include the possibility that depatux-m effectively killed off EGFR-amp (and particularly EGFRvIII-mutant) tumor cells, lengthening PFS, but resistant clones emerged and voided any OS benefit, a hypothesis supported by results from patient-derived xenografts44; we also previously demonstrated that EGFR-amp was preferentially lost in GBMs following treatment with depatux-m among longitudinally sampled tumor tissue on an intra-patient basis.45 Preclinical work from others also supports emerging clones as a mechanism of acquired resistance. Also, our focus on EGFR-amp for eligibility may have inadequately enriched the study population for benefit, particularly as gene amplification correlated only imperfectly with response in our prior studies. A better strategy may have been to power the study for, or restrict eligibility to, the EGFR-vIII mutant subgroup or set a lower bound on the minimum number of patients with other potentially depatux-sensitizing EGFR mutations46,47 Our observation that PFS was shorter among EGFRvIII-mutant cases randomized to placebo further supports our impression that improved PFS with depatux-m in this subgroup was not spurious. We also previously described payload-sensitizing mutations,48 and penetration of depatux-m into large tumors may be limited,49 although neither of these biomarkers were screening criteria. Finally, limited penetration of the blood–brain barrier by depatux may also impede efficacy against intracranial tumors, particularly in the nonenhancing part of the tumor; this is a critically important lesson for future studies of large molecules.44
Finally, other ADCs are being investigated for GBM and other solid tumors.50 Higher affinity antibodies conjugated to cell-permeant payloads (permitting bystander killing of adjacent tumor cells) with different safety profiles may result in different outcomes.
In retrospect, it may have been prudent to complete the originally planned phase II study, suspending accrual and deferring phase III until analyses were complete. This is an important consideration for future studies with a phase II/III design.
Supplementary Material
Acknowledgments
AbbVie and RTOG Foundation participated in the design, study conduct, analysis, and interpretation of the data, as well as the writing, review, and approval of the manuscript. The study chair (ABL) drafted the initial manuscript, which was subsequently reviewed and approved by all coauthors, RTOG Foundation headquarters, and AbbVie before submission. All authors were involved in the data gathering, analysis, review, interpretation, and manuscript preparation and approved the decision to submit the final report for publication. We thank all the patients and their families, the participating site investigators and staff, and input from Andrew Scott, Timothy Cloughesy, and Martin van den Bent. Trials like this require a large team of highly dedicated professionals and was only made possible by academic-industry collaborators from both RTOG Foundation headquarters and AbbVie. This trial required a large team of highly dedicated and professional academic-industry collaborators from both RTOG Foundation headquarters and AbbVie to whom the authors are indebted.
Trial registration: Clinicaltrials.gov identifier: NCT02573324
Contributor Information
Andrew B Lassman, Division of Neuro-Oncology, Department of Neurology, Columbia University Vagelos College of Physicians and Surgeons and New York-Presbyterian Hospital, New York, New York, USA; Herbert Irving Comprehensive Cancer Center, New York, New York, USA.
Stephanie L Pugh, RTOG Foundation Statistics and Data Management Center, American College of Radiology, Philadelphia, Pennsylvania.
Tony J C Wang, Department of Radiation Oncology (in Neurological Surgery), Columbia University Vagelos College of Physicians and Surgeons and New York-Presbyterian Hospital, New York, New York, USA; Herbert Irving Comprehensive Cancer Center, New York, New York, USA.
Kenneth Aldape, Center for Cancer Research, National Cancer Institute, Bethesda, Maryland, USA.
Hui K Gan, Cancer Therapies and Biology Group, Centre of Research Excellence in Brain Tumours, Olivia Newton-John Cancer Wellness and Research Centre, Austin Hospital, Heidelberg, Melbourne, Australia; La Trobe University School of Cancer Medicine, Heidelberg, Victoria, Australia; Department of Medicine, University of Melbourne, Heidelberg, Victoria, Australia.
Matthias Preusser, Department of Medicine I, Division of Oncology, Medical University of Vienna, Vienna, Austria.
Michael A Vogelbaum, Department of Neuro-Oncology, Moffitt Cancer Center, Tampa, Florida, USA.
Erik P Sulman, Department of Radiation Oncology, New York University, Grossman School of Medicine, New York, New York, USA; Laura and Isaac Perlmutter Cancer Center, NYU Langone Health, New York, New York, USA.
Minhee Won, RTOG Foundation Statistics and Data Management Center, American College of Radiology, Philadelphia, Pennsylvania.
Peixin Zhang, RTOG Foundation Statistics and Data Management Center, American College of Radiology, Philadelphia, Pennsylvania.
Golnaz Moazami, Department of Ophthalmology, Columbia University Vagelos College of Physicians and Surgeons and New York-Presbyterian Hospital, New York, New York, USA.
Marian S Macsai, NorthShore University HealthSystem, Department of Ophthalmology, University of Chicago Pritzker School of Medicine, Evanston, Illinois, USA.
Mark R Gilbert, Neuro-Oncology Branch, National Cancer Institute, Bethesda, Maryland, USA.
Earle E Bain, Abbvie, Inc., North Chicago, Illinois, USA.
Vincent Blot, Abbvie, Inc., North Chicago, Illinois, USA.
Peter J Ansell, Abbvie, Inc., North Chicago, Illinois, USA.
Suvajit Samanta, Abbvie, Inc., North Chicago, Illinois, USA.
Madan G Kundu, Abbvie, Inc., North Chicago, Illinois, USA.
Terri S Armstrong, Neuro-Oncology Branch, National Cancer Institute, Bethesda, MD, USA.
Jeffrey S Wefel, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Clemens Seidel, University Hospital Leipzig, Leipzig, Germany.
Filip Y de Vos, University Medical Center Utrecht, Cancer Center, Utrecht, The Netherlands.
Sigmund Hsu, Department of Neurosurgery, University of Texas Health Sciences Center, McGovern School of Medicine, Houston, Texas, USA.
Andrés F Cardona, Foundation for Clinical and Applied Cancer Research-FICMAC/Clinical and Translational Oncology Group, Brain Tumor Section, Bogotá, Colombia.
Giuseppe Lombardi, Department of Oncology, Oncology 1, Veneto Institute of Oncology IOV-IRCCS, Padua, Italy.
Dmitry Bentsion, Sverdlovsk Regional Oncology Center, Ekaterinburg, Russia.
Richard A Peterson, Metro Minnesota NCORP, Saint Paul, Minnesota, USA.
Craig Gedye, Calvary Mater Newcastle, Waratah, New South Wales, Australia.
Véronique Bourg, Department of Neurology, Côte d’Azur University, Nice, France.
Antje Wick, Heidelberg University Medical Center, Heidelberg, Germany.
Walter J Curran, Winship Cancer Institute, Emory University, Atlanta, Georgia, USA.
Minesh P Mehta, Miami Cancer Institute, Baptist Hospital, Miami, Florida, USA.
Funding
AbbVie sponsored and provided funding and drug supply for this study which was conducted in partnership with the Radiation Therapy Oncology Group Foundation (RTOG-F). Andrew B. Lassman was also supported in part by the William Rhodes and Louise Tilzer-Rhodes Center for Glioblastoma at NewYork–Presbyterian; the Michael Weiner Glioblastoma Research Into Treatment Fund; and grants from the National Cancer Institute [grant numbers P30CA013696, UG1CA189960]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute/National Institutes of Health.
Conflict of interest statement
Drs Aldape, Armstrong, Bentsion, Bourg, Curran, Gilbert, Kundu, Macsai, Peterson and Wick have nothing to disclose. Drs Ansell, Bain, Blot report Employee and stockholder of AbbVie. Dr Cardona reports Grants or contracts from Merck Sharp & Dohme, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, Foundation Medicine, Roche Diagnostics, Termo Fisher, Broad Institute, Amgen, Flatiron Health, Teva Pharma, Rochem Biocare, Bayer, INQBox and The Foundation for Clinical and Applied Cancer Research – FICMAC, Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from EISAI, Merck Serono, Jannsen Pharmaceutical, Merck Sharp & Dohme, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, Pfizer, Novartis, Celldex Therapeutics, Foundation Medicine, Eli Lilly, Guardant Health, Illumina, and Foundation for Clinical and Applied Cancer Research – FICMAC, Payment for expert testimony from Merck Sharp & Dohme, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, Pfizer, Novartis, Foundation Medicine, Guardant Health, Illumina, and Foundation for Clinical and Applied Cancer Research – FICMAC, Support for attending meetings and/or travel from Merck Serono, Merck Sharp & Dohme, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, Pfizer, Novartis, Celldex Therapeutics, Foundation Medicine, Eli Lilly, and Foundation for Clinical and Applied Cancer Research – FICMAC, Participation on a Data Safety Monitoring Board or Advisory Board with Roche, Merck Sharp & Dohme, Receipt of equipment, materials, drugs, medical writing, gifts or other services from Roche, Roche diagnostics, Rochem Biocare. Dr de Vos reports grants or contracts from BMS, Roche/Genentech, STOPBraintumors.org foundation, Novartis, Agios (all sponsor driven studies), Participation on a Data Safety Monitoring Board or Advisory Board with Chloroquine trial in GBM patients, Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from Dutch Society for Neuro-Oncology and Dutch Society for Medical Oncology. Dr Gan reports Payment or honoraria for lectures, presentations, speakers’ bureaus, for Merck Serono, Receipt of equipment, materials, drugs, medical writing, gifts or other services- Material for lab research under MTA. Dr Gedye reports Normal clinical trial research agreement with institution (RTOG and Abbvie), Consulting and advisory boards with Merck EMD Serono, MSD, BMS, Astellas, Pfizer, Astra (All fees are paid direct and in full to independent 3rd party not-for-profit; no funds accepted personally) travel/conference support in lieu of public hospital NSW Health TESL contract entitlements from MSD, Astellas, BMS. Dr Hsu reports Institutional Research, Neuro-Oncology Advisory Board for AbbVie, royalties from Moleculin, Scientific Advisory Board for CNS Pharmaceuticals, Patent for Moleculin licensed from MD Anderson Cancer Center, Participation on a Data Safety Monitoring Board or Advisory Board from CNS Pharmaceuticals AbbVie, Options from CNS Pharmaceuticals. Dr Lassman reports support for the present manuscript from AbbVie and RTOG Foundation (to institution), Grants or contracts from Karyopharm, Novocure, QED, Forma, Bayer, Orbus, Agios, Kadmon, VBI, Beigene, Oncoceutics, Pfizer, Genentech/Roche, Millennium, Celldex, Novartis, AeternaZentaris, BMS (to institution), Consulting fees and use of equipment from Bioclinica as an expert blinded independent reviewer of clinical and imaging data for a BMS-sponsored trial, Sapience and Italian Foundation for Cancer Research, Payment or honoraria for lectures, presentations, educational events , or in-kind medical writing support from AbbVie, Physician’s Education Resource, MJH Life Sciences, Abbott Molecular, QED, VBI, Karyopharm, Novartis, Pfizer, Support for attending meetings and/or travel from Abbott Molecular, AbbVie, Agios, American Society of Clinical Oncology, FDA, Global Coalition for Adaptive Research, Karyopharm, QED, Forma, Bayer, Orbus, NW Biotherapeutics, Oncocuetics, MJH Life Sciences, National Brain Tumor Society, Society for Neuro-Oncology, NRG Oncology Foundation, Participation on a Data Safety Monitoring Board or Advisory Board from Karyopharm, Novocure, QED, Forma, Bayer, Orbus, NW Biotherapeutics, Agios, AbbVie, Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from Society for Neuro-Oncology – unpaid and Global Coalition for Adaptive Research – unpaid, Receipt of equipment, materials, drugs, medical writing support, gifts or other services Karyopharm, Novocure, QED, Forma, Bayer, Orbus, Agios, Kadmon, VBI, Beigene, Oncoceutics, Pfizer, Genentech/Roche, Millennium, Celldex, Novartis, AeternaZentaris, BMS (to institution). Dr Lombardi reports Grants or contracts from AbbVie, Bayer SpA, Orbus Therapeutics, BrainFarm, Novartis. Dr Mehta reports Consulting fees from Karyopharm, Tocagen, Sapience, Astra Zeneca, Payment or honoraria for lectures, presentations, speakers’ bureaus from Zap, Participation on a Data Safety Monitoring Board or Advisory Board from Mevion, Leadership or fiduciary role in other board, society, committee or advocacy group, from NRG Oncology, Stock or stock options from Chimerix, Board of Directors for Oncoceutics. Dr Moazami reports Chair of Society of Practitioners-unpaid, General investment in a broad set of companies, primarily through mutual funds or ETF’s. Dr Preusser reports that the following for-profit companies have supported clinical trials and contracted research conducted by MP with payments made to his institution: Böhringer Ingelheim, Bristol-Myers, Squibb, Roche, Daiichi, Sankyo, Merck Sharp & Dome, Novocure, GlaxoSmithKline, AbbVie, has received honoraria for lectures, consultation or advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, Tocagen, Adastra, Gan & Lee Pharmaceuticals, received honoraria for lectures, consultation or advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, Tocagen, Adastra, Gan & Lee Pharmaceuticals, EANO President, EORTC Brain Tumor Group Chair. Dr Pugh reports Salary support paid to my institution from AbbVie. Dr Samanta reports working in the Depatux-M program as stat lead; I received my salary and stock from Abbvie. Dr Seidel reports Payment or honoraria for lectures, presentations, speakers’ bureaus, from HRA Pharma, medac, Support for attending meetings and/or travel from AbbVie, Participation on a Data Safety Monitoring Board or Advisory Board from AbbVie, Bristol-Myers Squibb, Roche. Dr Sulman reports Grants or contracts from Novocure (to institution), Consulting fees from Novocure and Merck, Payment or honoraria for lectures, presentations, speakers’ bureaus from Zai Lab, Support for attending meetings and/or travel from Novocure and Zai Lab, Board member Society for Neuro-oncology. Dr Vogelbaum reports Research grant/contract to my institution from Tocagen, Celgene, Medicenna, Oncosynergy, Infuseon, Royalty rights to my inventions assigned to Cleveland Clinic and licensed to Infuseon Therapeutics, Inc. (wholly owned by the Cleveland Clinic), Consulting fees for Advisory board meeting from Cellinta, Celgene, Patents planned, issued or pending for Cleveland Clinic and Moffitt for Devices for drug delivery to the brain for treatment of brain tumors, Duke Brain Tumor Center DSMB. Dr Wang reports Honoraria, Travel Support and research funding to my institution from AbbVie, Research funding to my institution from RTOG Foundation, Grants or contracts Research funding to my institution from Varian, Research funding (drug funding) Genentech, Royalties or licenses from Wolters Kluwer, Consulting fees from Cancer Panels, Payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Elekta, Rutgers, University of Iowa, Support for attending meetings and/or travel from AbbVie, Elekta, Novocure, Participation on a Data Safety Monitoring Board or Advisory Board for Novocure, Stock or stock options from Doximity. Dr Wefel reports Consulting fees from Novocure, Vanquish Oncology, Roche, Angiochem, Bayer, Juno (to institution) GT Medical Technology (to self), Participation on a Data Safety Monitoring Board or Advisory Board for Bayer. Ms Won reports Salary support paid to my institution from AbbVie. Dr Zhang reports Employee of Jazz Pharmaceuticals since December 2018, Stock or stock options Jazz Pharmaceuticals
Author contributions
Conception and design: P.J.A., T.S.A., E.E.B., Vi.B., W.J.C., H.K.G., M.R.G., M.G.K., A.B.L., M.P.M., R.A.P., S.S., E.P.S., M.A.V., T.J.C.W., J.S.W., A.W., P.Z. Collection and assembly of data: K.A., P.J.A., D.B., Vé.B, A.F.C, F.Y. de V., H.K.G., C.G., S.H., A.B.L., G.L., M.S.M., G.M., S.S., C.S., E.P.S., M.A.V., J.S.W., A.W. Data analysis and interpretation: PP.J.A., T.S.A., E.E.B., Vi.B., A.F.C., W.J.C., H.K.G., M.G.K., A.B.L., M.P.M., M.P., S.L.P., S.S., E.P.S., M.A.V., J.S.W., M.W. Manuscript writing: all authors. Final approval of manuscript: all authors.
Data sharing statement
This study was sponsored by AbbVie. AbbVie and the authors are committed to responsible data sharing regarding clinical trial participation. This includes access to anonymized, individual, and trial-level data (analysis datasets), as well as other information (eg protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html
References
- 1. Libermann TA, Nusbaum HR, Razon N, et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature 1985;313(5998):144–147. [DOI] [PubMed] [Google Scholar]
- 2. Lassman AB, Roberts-Rapp L, Sokolova I, et al. Comparison of biomarker assays for EGFR: implications for precision medicine in patients with Glioblastoma. Clin Cancer Res. 2019;25(11):3259–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gan HK, Cvrljevic AN, Johns TG. The epidermal growth factor receptor variant III (EGFRvIII): where wild things are altered. FEBS J. 2013;280(21):5350–5370. [DOI] [PubMed] [Google Scholar]
- 4. Lee A, Arasaratnam M, Chan DLH, et al. Anti-epidermal growth factor receptor therapy for glioblastoma in adults. Cochrane Database Syst Rev. 2020;5:CD013238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cleary JM, Reardon DA, Azad N, et al. A phase 1 study of ABT-806 in subjects with advanced solid tumors. Invest New Drugs. 2015;33(3):671–678. [DOI] [PubMed] [Google Scholar]
- 6. Neyns B, Sadones J, Joosens E, et al. Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann Oncol. 2009;20(9):1596–1603. [DOI] [PubMed] [Google Scholar]
- 7. Crombet T, Torres O, Rodriguez V, et al. Phase I clinical evaluation of a neutralizing monoclonal antibody against epidermal growth factor receptor in advanced brain tumor patients: preliminary study. Hybridoma 2001;20(2):131–136. [DOI] [PubMed] [Google Scholar]
- 8. Weller M, Butowski N, Tran DD, et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373–1385. [DOI] [PubMed] [Google Scholar]
- 9. Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101(36):13306–13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. London M, Gallo E. Epidermal growth factor receptor (EGFR) involvement in epithelial-derived cancers and its current antibody-based immunotherapies. Cell Biol Int. 2020;44(6):1267–1282. [DOI] [PubMed] [Google Scholar]
- 11. Jungbluth AA, Stockert E, Huang HJ, et al. A monoclonal antibody recognizing human cancers with amplification/overexpression of the human epidermal growth factor receptor. Proc Natl Acad Sci USA. 2003;100(2):639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gan HK, Burgess AW, Clayton AH, Scott AM. Targeting of a conformationally exposed, tumor-specific epitope of EGFR as a strategy for cancer therapy. Cancer Res. 2012;72(12):2924–2930. [DOI] [PubMed] [Google Scholar]
- 13. Scott AM, Lee FT, Tebbutt N, et al. A phase I clinical trial with monoclonal antibody ch806 targeting transitional state and mutant epidermal growth factor receptors. Proc Natl Acad Sci USA. 2007;104(10):4071–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Doronina SO, Bovee TD, Meyer DW, et al. Novel peptide linkers for highly potent antibody-auristatin conjugate. Bioconjug Chem. 2008;19(10):1960–1963. [DOI] [PubMed] [Google Scholar]
- 15. Phillips AC, Boghaert ER, Vaidya KS, et al. ABT-414, an antibody-drug conjugate targeting a tumor-selective EGFR epitope. Mol Cancer Ther. 2016;15(4):661–669. [DOI] [PubMed] [Google Scholar]
- 16. Perera RM, Zoncu R, Johns TG, et al. Internalization, intracellular trafficking, and biodistribution of monoclonal antibody 806: a novel anti-epidermal growth factor receptor antibody. Neoplasia 2007;9(12):1099–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Birrer MJ, Moore KN, Betella I, Bates RC. Antibody-drug conjugate-based therapeutics: state of the science. J Natl Cancer Inst. 2019;111(6):538–549. [DOI] [PubMed] [Google Scholar]
- 18. Gan HK, van den Bent M, Lassman AB, Reardon DA, Scott AM. Antibody-drug conjugates in glioblastoma therapy: the right drugs to the right cells. Nat Rev Clin Oncol. 2017;14(11):695–707. [DOI] [PubMed] [Google Scholar]
- 19. Reardon DA, Lassman AB, van den Bent M, et al. Efficacy and safety results of ABT-414 in combination with radiation and temozolomide in newly diagnosed glioblastoma. Neuro-Oncology 2017;19(7):965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lassman AB, Van Den Bent MJ, Gan HK, et al. Efficacy analysis of ABT-414 with or without temozolomide (TMZ) in patients (pts) with EGFR-amplified, recurrent glioblastoma (rGBM) from a multicenter, international phase I clinical trial. J Clin Oncol. 2017;35(15_suppl):2003–2003. [Google Scholar]
- 21. Gan HK, Reardon DA, Lassman AB, et al. Safety, pharmacokinetics, and antitumor response of depatuxizumab mafodotin as monotherapy or in combination with temozolomide in patients with glioblastoma. Neuro-Oncology 2018;20(6):838–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van den Bent M, Gan HK, Lassman AB, et al. Efficacy of depatuxizumab mafodotin (ABT-414) monotherapy in patients with EGFR-amplified, recurrent glioblastoma: results from a multi-center, international study. Cancer Chemother Pharmacol. 2017;80(6):1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parrozzani R, Lombardi G, Midena E, et al. Corneal side effects induced by EGFR-inhibitor antibody-drug conjugate ABT-414 in patients with recurrent glioblastoma: a prospective clinical and confocal microscopy study. Ther Adv Med Oncol. 2020;12:1758835920907543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. FDA. Food and Drug Administration granted accelerated approval to belantamab mafodotin-blmf for multiple myeloma. 2020. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-granted-accelerated-approval-belantamab-mafodotin-blmf-multiple-myeloma. Accessed September 01, 2020.
- 25. van den Bent M, Eoli M, Sepulveda JM, et al. INTELLANCE 2/EORTC 1410 randomized phase II study of Depatux-M alone and with temozolomide vs temozolomide or lomustine in recurrent EGFR amplified glioblastoma. Neuro-Oncology 2020;22(5):684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 27. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 28. Lassman AB, van den Bent MJ, Gan HK, et al. Safety and efficacy of depatuxizumab mafodotin + temozolomide in patients with EGFR-amplified, recurrent glioblastoma: results from an international phase I multicenter trial. Neuro-Oncology 2019;21(1):106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 30. Armstrong TS, Mendoza T, Gning I, et al. Validation of the M.D. Anderson Symptom Inventory Brain Tumor Module (MDASI-BT). J Neurooncol. 2006;80(1):27–35. [DOI] [PubMed] [Google Scholar]
- 31. Armstrong TS, Vera-Bolanos E, Gning I, et al. The impact of symptom interference using the MD Anderson Symptom Inventory-Brain Tumor Module (MDASI-BT) on prediction of recurrence in primary brain tumor patients. Cancer 2011;117(14):3222–3228. [DOI] [PubMed] [Google Scholar]
- 32. Armstrong TS, Wefel JS, Wang M, et al. Net clinical benefit analysis of radiation therapy oncology group 0525: a phase III trial comparing conventional adjuvant temozolomide with dose-intensive temozolomide in patients with newly diagnosed glioblastoma. J Clin Oncol. 2013;31(32):4076–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brown PD, Gondi V, Pugh S, et al. Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: phase III trial NRG oncology CC001. J Clin Oncol. 2020;38(10):1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22(1):157–165. [DOI] [PubMed] [Google Scholar]
- 35. Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins verbal learning test – revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12(1):43–55. [Google Scholar]
- 36. Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27(7-8):365–375. [DOI] [PubMed] [Google Scholar]
- 37. Li J, Wang M, Won M, et al. Validation and simplification of the Radiation Therapy Oncology Group recursive partitioning analysis classification for glioblastoma. Int J Radiat Oncol Biol Phys. 2011;81(3):623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kundu MG. Comments on “Properties of the weighted log-rank test in the design of confirmatory studies with delayed effects” by Jose Jimenez, Viktoriya Stalbovskaya, and Byron Jones. Pharm Stat. 18:287-303, 2019, DOI: 10.1002/pst.1923. Pharm Stat. 2020;19(5):733–735. [DOI] [PubMed] [Google Scholar]
- 39. Hasegawa T. Group sequential monitoring based on the weighted log-rank test statistic with the Fleming-Harrington class of weights in cancer vaccine studies. Pharm Stat. 2016;15(5):412–419. [DOI] [PubMed] [Google Scholar]
- 40. Wefel JS, Cloughesy T, Zazzali JL, et al. Neurocognitive function in patients with recurrent glioblastoma treated with bevacizumab. Neuro-Oncology. 2011;13(6):660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Glimm E, Maurer W, Bretz F. Hierarchical testing of multiple endpoints in group-sequential trials. Stat Med. 2010;29(2):219–228. [DOI] [PubMed] [Google Scholar]
- 42. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marin BM, Porath KA, Jain S, et al. Heterogeneous delivery across the blood-brain barrier limits the efficacy of an EGFR-targeting antibody drug conjugate in glioblastoma. Neuro-Oncology. 2021;23(12):2042–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ahluwalia M, Narita Y, Muragaki Y, et al. OS1.2 Stability of EGFR amplification in glioblastoma is differentially impacted based on therapeutic pressure. Neuro-Oncology 2018;20(Suppl_3):iii217–iii217. [Google Scholar]
- 46. Orellana L, Thorne AH, Lema R, et al. Oncogenic mutations at the EGFR ectodomain structurally converge to remove a steric hindrance on a kinase-coupled cryptic epitope. Proc Natl Acad Sci USA. 2019;116(20):10009–10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hoogstrate Y, Vallentgoed W, Kros JM, et al. EGFR mutations are associated with response to depatux-m in combination with temozolomide and result in a receptor that is hypersensitive to ligand. Neurooncol Adv. 2020;2(1):vdz051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lassman AB, Roberts-Rapp L, He L, et al. P01.071 Genomic profiling identifies tubulin mutations that may predict response to depatuxizumab mafodotin in patients with glioblastoma. Neuro-Oncology 2018;20(Suppl_3):iii246–iii246. [Google Scholar]
- 49. Gan HK, Parakh S, Lassman AB, et al. Tumor volumes as a predictor of response to the anti-EGFR antibody drug conjugate depatuxizumab mafadotin. Neurooncol Adv. 2021;3(1):vdab102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Phillips AC, Boghaert ER, Vaidya KS, et al. Characterization of ABBV-221, a tumor-selective EGFR-targeting antibody drug conjugate. Mol Cancer Ther. 2018;17(4):795–805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.