Abstract
Rationale:
Vaccines remain central to the management of COVID-19 pandemic, including the need for repeat doses of vaccines to boost immunity. There has been an accumulating case count of glomerulopathies temporally associated with COVID-19 vaccination. This case series presents 4 patients who developed double-positive anti–glomerular basement membrane antibody (anti-GBM) and myeloperoxidase (MPO) antineutrophil cytoplasmic autoantibody (ANCA)-associated glomerulonephritis after COVID-19 mRNA vaccination. This report contributes to our collective knowledge about the pathophysiology and clinical outcomes associated with this rare complication.
Presenting Concerns of the Patient:
Four patients developed nephritic syndrome within 1 to 6 weeks after receiving a COVID-19 mRNA vaccine (3 post Pfizer-BioNTech and 1 post Moderna vaccination). Three of the 4 patients also had hemoptysis.
Diagnosis:
Three of the 4 patients had double-positive serology, whereas the fourth patient had renal biopsy findings consistent with double-positive disease, although anti-GBM serology was negative. All patients had renal biopsy findings consistent with double-positive anti-GBM and ANCA-associated glomerulonephritis.
Interventions:
All 4 patients were treated with pulse steroids, cyclophosphamide, and plasmapheresis.
Outcomes:
Of the 4 patients, 1 demonstrated complete remission, 2 remained dialysis-dependent, and the fourth is deceased. Of the 2 patients who received repeat vaccination with COVID-19 mRNA vaccine, 1 patient had second serologic flare of anti-GBM in response to the vaccine.
Novel Findings:
This case series reinforces growing evidence that COVID-19 mRNA vaccine-induced glomerulonephritis is a rare but real phenomenon. Dual ANCA and anti-GBM nephritis can present after the first dose of COVID-19 mRNA vaccine or after several administrations of the vaccine. We are the first to report cases of double-positive MPO ANCA and anti-GBM nephritis after Pfizer-BioNTech vaccination. To our knowledge, we are also the first to report outcomes of repeat COVID-19 vaccination in patients with de novo flare of ANCA and anti-GBM nephritis temporally associated with COVID-19 vaccination.
Keywords: mRNA vaccine, Pfizer BioNTech, Moderna, COVID-19, anti-GBM disease, ANCA disease, double-positive, kidney histopathology
Introduction
As of April 2022, upward of 11.29 billion doses of coronavirus disease 2019 (COVID-19) vaccine have been administered worldwide, with real-world data confirming vaccine efficacy in preventing severe manifestations of the disease.1,2 With the global mass vaccination campaign, rare side effects that could not adequately be captured by the vaccine trials emerged. There is a growing number of case reports describing both relapsed and de novo cases of glomerulonephritis (GN) in association with the mRNA vaccines and, more rarely, in association with the adenoviral vector vaccine and the vaccine based on inactivated virus.3 We add to the existing literature 4 cases of vaccine-associated double-positive anti–glomerular basement membrane antibody (anti-GBM) and myeloperoxidase antineutrophil cytoplasmic autoantibody (MPO ANCA)-associated nephritis. Three of the cases were double serology positive, with 1 case demonstrating only ANCA-positive serology with histologic evidence of double-positive disease. The 3 Pfizer-BioNTech-associated cases presented with pulmonary renal syndrome and the Moderna-associated case with renal limited disease. To our knowledge, these are the first cases to be reported of double-positive anti-GBM and MPO ANCA nephritis post COVID-19 Pfizer-BioNTech vaccination, although 2 similar cases after inactivated virus vaccine (BBV152/Covaxin) and mRNA vaccine (Moderna) were recently reported.4,5
Presenting Concerns and Diagnostic Findings
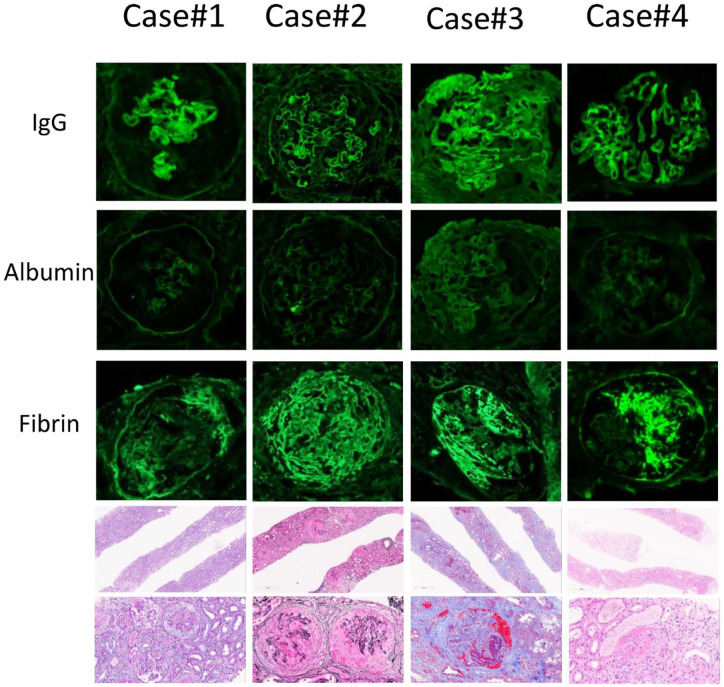
We report 4 cases of de novo double-positive MPO ANCA and anti-GBM nephritis, 3 occurring after Pfizer-BioNTech vaccinations and 1 occurring after Moderna vaccination. All 3 cases post Pfizer BioNTech vaccination occurred after the first dose of vaccine. The case post Moderna vaccination occurred after the third dose of the vaccine. All 3 Pfizer-BioNTech-vaccinated patients described evolving symptoms within the first 3 weeks after vaccination, and all presented to hospital in a delayed fashion with pulmonary renal syndrome. The Moderna-vaccinated patient had grumbling flu-like symptoms that started 6 weeks after vaccination. She presented to the hospital 2 weeks later with severe nephritic syndrome requiring hemodialysis, but she did not have any evidence of pulmonary involvement. All 4 cases had normal baseline renal function, negative COVID-19 nucleic acid amplification tests, and inclusive serologic work-up, all of which were noncontributory. Further case details on 4 four patients can be found in Table 1. Salient morphologic findings of all 4 biopsies are shown in Figure 1, and Table 2 summarizes major biopsy findings.
Table 1.
Characteristics of Patients Presenting with Dual ANCA and Anti-GBM Glomerulonephritis.
| Demographic information | Comorbidities | Vaccine dose | Presentation | Timing of symptom onset/hospital admission | Presenting creatinine | Positive seromarkers | Induction immunosuppression | Relapse after second dose | Renal trajectory | |
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 63F, white | 1. Mild asthma 2. Macular degeneration 3. Bilateral cataract excision 2012 |
All Pfizer: First dose April 22, 2021 Second dose November 8, 2021 Third dose April 22, 2022 |
Pulmonary renal syndrome | Symptoms: Days after receiving first dose Hospital presentation: 3.5 wk |
627 µmol/L | CRP 36.5 mg/L Anti-GBM 35 EU (N 0-7) MPO ANCA 54 U (N 0-3.5) |
Pulse steroids Cyclophosphamide Plasmapheresis |
No serologic or clinical flare post second or third dose. | Follow up period: 11 mo Presented without indications for dialysis. Complete remission Last Cr: 117 µmol/L |
| Case 2 | 51M, Indigenous | 1. Polysubstance use disorder: cocaine and alcohol 2. Hypertension 3. Heart failure with reduced ejection fraction (toxic cardiomyopathy?) |
All Pfizer: First dose February 27, 2021 Second dose June 10 2021 |
Pulmonary renal syndrome | Symptoms: 2-3 wk post first vaccine dose Hospital presentation: 8.5 wk post first dose |
2782 µmol/L | CRP 158.7 mg/L Anti-GBM >680 EU (N 0-7) MPO ANCA 105 U (N 0-3.5) Cardiolipin Ab: IgG 17 GPL U/mL (N 0-10) |
Pulse steroids Cyclophosphamide Plasmapheresis |
Serologic relapse post second dose (anti-GBM level 8.2 EU 6 days before, 310 on next bloodwork 38 days later) | Follow-up period: 11 mo Presented with indications for dialysis. Remains dialysis-dependent |
| Case 3 | 62M, Hispanic | 1. Remote cholecystectomy 2. Diverticulosis 3. Vitiligo 4. Ex-smoker, quit 1990s |
Pfizer: First dose June 4 2021 |
Pulmonary renal syndrome | Symptoms: 3-4 wk after first dose. Hospital presentation: 8.5 wk |
648 µmol/L | CRP 72.7 mg/L Anti-GBM 0.2 U (N 0.0-0.9) MPO ANCA 145.4 U (N 0.0-0.9) |
Pulse steroids Cyclophosphamide Plasmapheresis |
Not offered second dose | Patient deceased on October 2021, dialysis-dependent at time of death |
| Case 4 | 70F, white | 1. Hypothyroidism 2. Non-ST-elevation myocardial infarction with drug-eluting stent to left anterior descending artery 10 days post second dose of Moderna 3. Hypertension 4. Dyslipidemia 5. Type 2 diabetes mellitus 6. Remote smoker, quit at age 25 |
All Moderna: First dose April 16, 2021 Second dose June 24, 2021 Third dose December 7, 2021 |
Renal Limited | Symptoms: 6 wk after third dose. Hospital presentation: 8 wk |
601 µmol/L | CRP 204.4 mg/L Anti-GBM 64 U (N 0.0-7.0) MPO ANCA >134 U (N 0.0-3.5) |
Pulse steroids Cyclophosphamide Plasmapheresis |
N/A | Exhibiting renal recovery. On hemodialysis twice-weekly, with no inter-dialytic weight gain, and a decline in prehemodialysis creatinine to a most recent value of 311 µmol/L. |
Note. Please see separate attachment for Figure 1. MPO = myeloperoxidase; ANCA = antineutrophil cytoplasmic autoantibody; N/A = not applicable; CRP = C-reactive protein; EU = enzyme-linked immunosorbent assay (ELISA) units; GPL = IgG phospholipid units.
Figure 1.
Salient morphologic findings in diagnostic renal biopsies of 4 cases.
Note. By immunofluorescence, there is at least moderate linear staining of capillary walls for IgG while albumin staining is negative (cases 1, 2, and 4) or dull/weak (case 3). Fibrin/fibrinogen staining highlights crescents (cases 1, 2, and 3) or segmental necrosis (case 4). Light microscopy shows adequate specimens (upper row) with representative glomeruli on higher magnification demonstrating necrosis / cellular crescents highlighted by Periodic acid-Schiff stain (case 1), Jones methenamine silver stain (case 2), Masson’s trichrome stain (case 3), and hematoxylin and eosin stain (case 4). The latter also highlights 2 RBC casts next to the glomerulus involved by necrosis.
Table 2.
Summary of Biopsy Findings.
| Light microscopy | Immunofluorescence | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total glomeruli | Active lesions,a (%) | % global glomerulosclerosis | % glomerular scarringb | ATIN | % IFTA | Extraglomerular vasculitis | IgGc | Albuminc | |
| Case 1 | 54 | 50 | 24 | 14 | Severe | Mild (15) | No | 2+ | 0 |
| Case 2 | 28 | 92 | 4 | 4 | Severe | Minimal | No | 2+ | 0 |
| Case 3 | 21 | 66 | 30 | 4 | Absent | Mild (20) | No | 2+d | 1+ |
| Case 4 | 21 | 60 | 5 | 5 | Moderate | Minimal | No | 2+ | 0 |
Note. ATIN = acute tubulointerstitial nephritis; IFTA = interstitial fibrosis and tubular atrophy.
Active lesions defined by segmental necrosis, cellular crescents, or early fibro-cellular crescents with more than 25% cellular component in the crescents.
Glomerular scarring defined by fibrous or fibro-cellular crescent with less than 25% cellular component and involving less than 80% of the glomerulus, or presence of synechiae between the glomerular tufts and the Bowman capsule.
Positive staining defined as linear capillary wall staining with intensity scored as: 0 (negative), 1+ (mild), 2+ (moderate), 3+ (strong).
No features associated with diabetic nephropathy were seen on light microscopy to explain linear IgG staining.
Treatment, Follow-up, and Outcomes
All patients were treated with pulse methylprednisolone, cyclophosphamide, and plasmapheresis. Clinical courses varied widely. One patient recovered significant renal function. This patient received a repeat dose of COVID-19 Pfizer BioNTech vaccine 7 months after the first dose while on maintenance azathioprine and prednisone, and did not have another flare with repeat vaccination. A subsequent third dose of COVID-19 Pfizer BioNTech was similarly well-tolerated without any flare. Her MPO ANCA titers and anti-GBM titers were both in the normal range at the time of repeat vaccinations and remained normal after revaccinations. The second patient remained dialysis-dependent throughout. He received repeat dose of COVID-19 Pfizer BioNTech vaccine and subsequently developed a serological flare of anti-GBM but did not have a recurrence of pulmonary hemorrhage. Although the patient was not consistently taking the oral cyclophosphamide prescribed to him, his anti-GBM titer was only 8.2 EU (N 0.0-7.0 EU) 6 days before the vaccination and had increased to 310 EU (N 0.0-7.0 EU) when it was rechecked a month later. The MPO ANCA was not checked immediately prior to repeat vaccination but was 6.3 U (N 0.0-3.5 U) 1 month prior to vaccination and 5.4 U (N 0.0-3.5) a month after repeat vaccination. The third patient died due to evolving multiorgan failure and was never offered repeat COVID-19 vaccination. In the fourth case, the follow-up period is short but the patient, while still dialysis-dependent, is demonstrating convincing evidence of renal recovery.
Discussion
Over the past 2 years, there has been an accumulating case count of both ANCA-associated GN6 -10 and anti-GBM11 -14 nephritis temporally associated with COVID-19 vaccination. Whether these represent de novo cases or pre-existing subclinical disease that is unmasked after vaccination remains unclear. A recent case-based review identified 25 cases of ANCA-associated pauci-immune GN after COVID-19 vaccination, with the majority (18/25) secondary to mRNA vaccines. Most cases were de novo presentations (22/25), with 21 cases demonstrating crescentic GN on biopsy. Notably, after a short follow-up period, treatment response was noted to be favorable, with only 4 of 25 patients remaining dialysis-dependent.15 Of the vaccine-associated anti-GBM cases reported to date, 3 of 4 have not shown response to therapy, with the fourth case’s outcome not reported.11 -14 Previously, the clinical course of dual-positive ANCA and anti-GBM nephritis has been shown to be variable, suggesting that there may be multiple phenotypes of double-positive disease.16 In the largest retrospective study to date including 37 double-positive patients, a more favorable renal response to induction immunosuppression was noted compared with single-positive anti-GBM patients.17 Conversely, a prior 27 patient retrospective study showed infrequent renal recovery, more in keeping with the single-positive anti-GBM phenotype.18
To date, 3 cases of double-positive ANCA and anti-GBM nephritis post COVID-19 vaccination have been reported. The paucity of reported cases likely reflects the low incidence of double-positive disease in general, with an estimated 0.47 cases per million people per year.19 Moreover, serology-negative anti-GBM disease with linear IgG staining is also a rare phenomenon only occurring in 2% to 3% of all anti-GBM diseases.20 Three of the cases reported here are unique in that they are the first reported cases of double-positive anti-GBM and MPO ANCA nephritis post Pfizer BioNTech vaccine. The double-positive cases that have been reported to date include 2 cases after whole virion inactivated vaccine (BBV152/Covaxin), with one case of anti-GBM and MPO ANCA and the other of anti-GBM and c-ANCA (no serotype specified),4 and 1 case of anti-GBM and MPO ANCA nephritis after the second dose of Moderna vaccine.5 The renal trajectory of the latter case is not reported, but the 2 cases post BBV152/Covaxin vaccine demonstrated favorable renal response following a similar induction immunosuppression regimen to our 4 patients. The follow-up duration for both of these patients is less than 2 months.4 In reviewing our patients’ clinical trajectories, we note that all presented with crescentic disease. Two of the 4 patients (cases 1 and 4) demonstrated a favorable response to therapy, 1 remains dialysis-dependent at 11 months with no evidence of renal recovery (case 2), and 1 is deceased due to multiorgan failure (case 3). Their renal outcomes appear associated with time to presentation to hospital post vaccine administration and percentage of crescents on biopsy at the time of presentation. Overall, the clinical and histological presentation of vaccine-associated double-positive anti-GBM and ANCA cases does not appear to differ significantly from nonvaccine double-positive cases with the possible exception that the relative frequency of pulmonary renal syndrome was higher in our patient sample of vaccine-associated cases than would be expected in nonvaccine cases.17
Interestingly, all 3 post Pfizer-BioNTech vaccination cases presented with pulmonary-renal syndrome, whereas our case post Moderna presented with renal limited disease, in keeping with the previously reported case of anti-GBM and MPO ANCA nephritis post Moderna.5 In our case, symptom onset occurred 6 weeks after the third dose of Moderna, instead of 2 weeks after the second dose as reported in the previous case.5
To date, all cases of dual anti-GBM and ANCA nephritis post COVID-19 vaccination with a reported serotype have been MPO-positive. Notably, the majority of all previously reported dual anti-GBM and ANCA nephritis cases are also MPO-positive.18 We postulate that in these 4 double-positive anti-GBM and MPO ANCA nephritis cases, the mRNA vaccines may have induced an MPO ANCA vasculitis. One possible mechanism is via an increase in cell-surface MPO levels due to the proinflammatory stimulation mediated by the mRNA containing lipid nanoparticles, which function as an adjuvant.21 This can result in a subsequent loss of tolerance to self-antigens due to autoreactivity in the context of hyperactivation/bystander activation of the immune system. Alternatively, the production of COVID-19 spike protein following vaccination could itself provoke an immune response. There is at least 1 case reported of dual anti-GBM and ANCA nephritis following COVID-19 infection.22 There are also at least 20 cases reported so far of ANCA nephritis following COVID-19 infection23 and evidence that the frequency of anti-GBM increased with the advent of the COVID-19 pandemic prior to the distribution of COVID-19 vaccines.24
Once glomerular or pulmonary capillary endothelial damage occurs, this exposes basement membrane antigens to this same immunogenic environment, allowing for epitope spreading to occur. Epitope spreading is a process whereby tissue damage during an immune response from one epitope can trigger a secondary immune response against new epitopes. One recent study suggests that proteases released by neutrophils activated by ANCA digest collagen type IV to expose GBM epitopes.25 Following this, CD11c+ macrophages specific to ANCA-associated vasculitis are responsible for antigen presentation of the GBM epitopes to T cells, resulting in the production of anti-GBM antibodies. Moreover, in an in vitro study using human cells, Kubala et al26 demonstrated that MPO binds to collagen IV in the extracellular matrix in a dose-dependent fashion. It is possible that this direct interaction between MPO and type IV collagen plays a role in the increased frequency of dual-positive ANCA and anti-GBM cases in patients who are MPO-positive.
The patient from case 2 had known cocaine use. We cannot completely rule out cocaine contamination with levamisole as a contributing factor to the patient’s immunogenicity and clinical presentation with ANCA disease. However, the fact that the patient had 2 serological flares that were both temporally associated with COVID-19 vaccination is an unlikely coincidence and suggests that COVID-19 vaccination was the main trigger for the patient’s presentation.
In addition to positive ANCA and anti-GBM serologies, the patient in case 2 also had a weakly positive anti-cardiolipin (aCL) IgG antibody level (Table 1). However, the patient did not have any clinical features of antiphospholipid antibody syndrome (APS). Furthermore, the patient’s antinuclear antibody, complement levels, lupus anticoagulant, aCL IgM antibody, and beta-2 glycoprotein were all normal, and there were no features to suggest APS nephropathy on kidney biopsy. It remains to be seen whether weak aCL antibody positivity could also be related to COVID-19 vaccination or part of the epitope spreading cascade in our patient. Recent research around this phenomenon is conflicting, with some studies reporting a link between COVID-19 vaccination and development of antiphospholipid antibodies,27 and others reporting no link between vaccination and antiphospholipid antibody production.28 The patient in case 2 did not have any clinical consequences associated with positive aCL titers. This is consistent with the study that showed that although aCL levels are higher in Pfizer-vaccinated patients, these positive levels are not clinically pathogenic.27
We note that the patient in case 3 had linear IgG staining on renal biopsy but consistently normal anti-GBM levels. It is possible that he may have developed a higher anti-GBM level with time had he not received treatment with plasmapheresis. Alternatively, anti-GBM serology might be negative because it targeted an epitope on the collagen IV molecule that was not detected by conventional assays.20 Another possibility is that negative anti-GBM serology represents a feature of COVID-19 vaccination–induced dual anti-GBM and ANCA-associated nephritis. Of the 3 previously reported cases of dual anti-GBM and ANCA-associated nephritis post COVID vaccination, there is already one other reported case with linear IgG staining on renal biopsy but negative anti-GBM serology shortly after receiving the BBV152/Covaxin vaccine.4 This observation may support the concept that development of anti-GBM antibodies occurs after the triggering of MPO vasculitis by COVID-19 vaccines. The latter suggests that, depending on the timing of diagnosis, COVID-19 vaccine–associated dual anti-GBM and ANCA disease might have negative anti-GBM serology,4,5 further supporting the important role of biopsy in these patients.
As health organizations around the world continue to recommend further booster doses of mRNA COVID-19 vaccines, there are a rising number of patients with glomerulopathies that are temporally associated with a prior COVID-19 vaccination. However, there remains a lack of evidence on how to safely proceed with repeat mRNA COVID-19 vaccinations in these patients, especially in the case of severely life-threatening glomerulopathies such as ANCA and anti-GBM nephritis. In this case series, 1 patient was successfully revaccinated without relapse, and another developed a second serologic flare around the time of revaccination. The patient who did not flare with revaccination was in full clinical and serologic remission of her disease at the time of revaccination and was on stable doses of azathioprine and prednisone. On the other hand, the patient who had a serologic flare associated with revaccination was inconsistent with taking immunosuppressive medications, and both his ANCA and anti-GBM titers were slightly above the upper limit of normal before revaccination. However, despite a serologic flare, he did not have recurrence of clinical symptoms such as pulmonary hemorrhage. Although it is impossible to draw firm conclusions from only 2 patients, these findings do suggest that the risk of severe flare from repeat vaccination is low, especially if patients have stable disease and are on appropriate immunosuppressive therapy at the time of vaccination. Our findings are consistent with recent studies showing that in patients with pre-existing ANCA vasculitis, rates of post-vaccination relapse are low.29,30 One study found only a 1% to 2% absolute increase in 30-day relapse risk for ANCA-associated glomerulonephritis after vaccination and also reported that when relapses did occur, they were usually mild and did not require changes in immunosuppression.30 However, these studies did not specifically include patients with a prior history of vaccine-associated disease flare. Larger observational studies and prospective studies will be required in the future to gain a better understanding of the optimal management of COVID-19 revaccination in patients who have already shown a predisposition to vaccine-associated GN.
Concerted mass vaccination efforts are critical to addressing the current pandemic. However, like all effective treatments, there are potential adverse effects. We report here a rare but serious potential complication of the highly immunogenic COVID-19 mRNA vaccinations: inciting of double-positive MPO ANCA and anti-GBM renal disease, with variable serology. Improved understanding of the physiology of this occurrence and identification of individuals at risk should be the focus of future research. Our intention for this report is to highlight this possibility so that clinicians and local public health officers can be aware of symptoms post vaccine that would warrant expedited medical attention. Our small case series demonstrated a significant variation in clinical outcome, which was likely affected by the timing of presentation to medical attention after symptom onset. By providing early education surrounding symptoms of concern, we suggest that this awareness may be a potential opportunity to modify patients’ clinical trajectories. As need for repeated doses of COVID-19 mRNA vaccine becomes a fixture of our new reality, we also hope to shed some light on some possible outcomes associated with repeat doses of COVID-19 mRNA vaccines in these patients.
Footnotes
Ethics Approval and Consent to Participate: The study protocol was approved by the institutional review boards at the University of British Columbia, Providence Health Care, Vancouver Coastal Health, and Vancouver Island Health Authority (Research Ethics Board Application #H22-00327). This process included obtaining written consent from all living participants.
Consent for Publication: All authors have reviewed the manuscript and consented to publication.
Availability of Data and Materials: Request can be made to the corresponding author for access to the data and materials included.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Considerations: The study protocol was approved by the institutional review boards at the University of British Columbia, Providence Health Care, Vancouver Coastal Health, and Vancouver Island Health Authority (Research Ethics Board Application #H22-00327).
ORCID iDs: Julie Anne Ting  https://orcid.org/0000-0002-8204-3148
https://orcid.org/0000-0002-8204-3148
Elena-Bianca Barbir  https://orcid.org/0000-0001-8146-0268
https://orcid.org/0000-0001-8146-0268
Maziar Riazy  https://orcid.org/0000-0002-2190-0687
https://orcid.org/0000-0002-2190-0687
References
- 1. Ledford H. Six months of COVID vaccines: what 1.7 billion doses have taught scientists. Nature. 2021;594(7862):164-167. [DOI] [PubMed] [Google Scholar]
- 2. Ritchie H, Ortiz-Ospina E, Beltekian D, et al. Sweden: coronavirus pandemic. Our World Data. Date unknown. https://ourworldindata.org/coronavirus/country/sweden. Accessed January 24, 2023.
- 3. Chan ATP, Tang SCW. De novo and relapsing glomerulonephritis after COVID-19 vaccination: how much do we know? Nephrology (Carlton). 2022;27(1):5-6. [DOI] [PubMed] [Google Scholar]
- 4. Prema J, Muthukumaran A, Haridas N, et al. Two cases of double-positive antineutrophil cytoplasmic autoantibody and antiglomerular basement membrane disease after BBV152/Covaxin vaccination. Kidney Int Rep. 2021;6(12):3090-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gupta RK, Ellis BK. Concurrent antiglomerular basement membrane nephritis and antineutrophil cytoplasmic autoantibody–mediated glomerulonephritis after second dose of SARS-CoV-2 mRNA vaccination. Kidney Int Rep. 2022;7(1):127-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sekar A, Campbell R, Tabbara J, et al. ANCA glomerulonephritis after the Moderna COVID-19 vaccination. Kidney Int. 2021;100(2):473-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anderegg MA, Liu M, Saganas C, et al. De novo vasculitis after mRNA-1273 (Moderna) vaccination. Kidney Int. 2021;100(2):474-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dube GK, Benvenuto LJ, Batal I. Antineutrophil cytoplasmic autoantibody–associated glomerulonephritis following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int Rep. 2021;6(12):3087-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Obata S, Hidaka S, Yamano M, et al. MPO-ANCA-associated vasculitis after the Pfizer/BioNTech SARS-CoV-2 vaccination. Clin Kidney J. 2022;15(2):357-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Villa M, Díaz-Crespo F, Pérez de, José A, et al. A case of ANCA-associated vasculitis after AZD1222 (Oxford-AstraZeneca) SARS-CoV-2 vaccination: casualty or causality? Kidney Int. 2021;100:937-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tan HZ, Tan RY, Choo JCJ, et al. Is COVID-19 vaccination unmasking glomerulonephritis? Kidney Int. 2021;100(2):469-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sacker A, Kung V, Andeen N. Anti-GBM nephritis with mesangial IgA deposits after SARS-CoV-2 mRNA vaccination. Kidney Int. 2021;100(2):471-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klomjit N, Alexander MP, Fervenza FC, et al. COVID-19 vaccination and glomerulonephritis. Kidney Int Reports. 2021;6:2969-2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nagai K, Iwase M, Ueda A. A case of anti-GBM nephritis following centipede bites and COVID-19 vaccination. CEN Case Reports. 2021;11:166-170. doi: 10.1007/s13730-021-00646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prabhahar A, Naidu GSRSNK, Chauhan P, et al. ANCA-associated vasculitis following ChAdOx1 nCoV19 vaccination: case-based review. Rheumatol Int. 2022;42(4):749-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Canney M, Little MA. ANCA in anti-GBM disease: moving beyond a one-dimensional clinical phenotype. Kidney Int. 2017;92(3):544-546. [DOI] [PubMed] [Google Scholar]
- 17. McAdoo SP, Tanna A, Hrušková Z, et al. Patients double-seropositive for ANCA and anti-GBM antibodies have varied renal survival, frequency of relapse, and outcomes compared to single-seropositive patients. Kidney Int. 2017;92(3):693-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levy JB, Hammad T, Coulthart A, et al. Clinical features and outcome of patients with both ANCA and anti-GBM antibodies. Kidney Int. 2004;66(4):1535-1540. [DOI] [PubMed] [Google Scholar]
- 19. De Zoysa J, Taylor D, Thein H, et al. Incidence and features of dual anti-GBM-positive and ANCA-positive patients. Nephrology (Carlton). 2011;16(8):725-729. [DOI] [PubMed] [Google Scholar]
- 20. Salama AD, Dougan T, Levy JB, et al. Goodpasture’s disease in the absence of circulating anti-glomerular basement membrane antibodies as detected by standard techniques. Am J Kidney Dis. 2002;39(6):1162-1167. [DOI] [PubMed] [Google Scholar]
- 21. Ndeupen S, Qin Z, Jacobsen S, et al. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience. 2021;24(12):103479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sebastian R, Arunachalam J, Rajendran M. Temporal clustering of antiglomerular basement membrane disease in COVID-19 pandemic: a case series. Int J Nephrol Renovasc Dis. 2021;14:393-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ozcan S, Sonmez O, Karaca C, et al. ANCA-associated vasculitis flare might be provoked by COVID-19 infection: a case report and a review of the literature. Clin Kidney J. 2022;15:1987-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Prendecki M, Clarke C, Cairns T, et al. Anti-glomerular basement membrane disease during the COVID-19 pandemic. Kidney Int. 2020;98:780-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nishibata Y, Nonokawa M, Tamura Y, et al. Possible implication of intermolecular epitope spreading in the production of anti-glomerular basement membrane antibody in anti-neutrophil cytoplasmic antibody-associated vasculitis. Clin Exp Rheumatol. 2022;40(4):691-704. [DOI] [PubMed] [Google Scholar]
- 26. Kubala L, Kolářová H, Víteček J, et al. The potentiation of myeloperoxidase activity by the glycosaminoglycan-dependent binding of myeloperoxidase to proteins of the extracellular matrix. Biochim Biophys Acta. 2013;1830(10):4524-4536. [DOI] [PubMed] [Google Scholar]
- 27. Krashias G, Pafiti A, Deeba E, et al. SARS CoV-2 vaccination induces antibodies against cardiolipin. BMC Res Notes. 2022;15:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Borghi MO, Bombaci M, Bodio C, et al. Anti-phospholipid antibodies and coronavirus disease 2019: vaccination does not trigger early autoantibody production in healthcare workers. Front Immunol. 2022;13:930074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Machado PM, Lawson-Tovey S, Strangfeld A, et al. Safety of vaccination against SARS-CoV-2 in people with rheumatic and musculoskeletal diseases: results from the EULAR Coronavirus Vaccine (COVAX) physician-reported registry. Ann Rheum Dis. 2022;81:695-709. [DOI] [PubMed] [Google Scholar]
- 30. Canney M, Atiquzzaman M, Cunningham AM, et al. A population-based analysis of the risk of glomerular disease relapse after COVID-19 vaccination. J Am Soc Nephrol. 2022;33(12):2247-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]