Abstract
Due to the high global consumption of eggs, eggshell has become as one of the top domestic, agricultural and industrial wastes. This study determined eggshell characteristics after boiling at 95 °C and steaming at 121 °C, with additional heat treatments using hot air at 200 °C, microwaving at 900 W and infrared at 1050 W. Boiling in water for 60 min inhibited spoilage and pathogenic microorganisms that was the equivalent of steaming at 121 °C for 15 min. Scanning electron microscopy revealed that heat treatments on dried eggshell power modified the pore size and the accumulation of particles on the powder surface. From the X-ray diffraction pattern, all eggshell powder samples presented a peak at 29.40° demonstrating a crystallographic lattice of calcium carbonate with crystallinity in the range 90.20–91.05%. The calcium releasability of the control sample was 205.17–208.40 mg/L. Further treatment using hot air for 10–20 min increased the calcium releasability of the boiled and steamed eggshell powders to 219.95–225.50 and 230.35–305.20 mg/L, respectively while the microwave treatment for 2 min increased the calcium releasability of the boiled and steamed eggshell powders to 230.85 and 244.60 mg/L, respectively. The infrared treatment did not improve the calcium releasability of the sterilized eggshell powders. Up to 2% eggshell powder could be added to the dog biscuit dough. The fortified calcium biscuits contained 507.12 mg calcium/100 g of biscuit, while the Ca-to-P ratio was 1.94:1, which is within the recommended range for dog food.
Keywords: Egg, Microwave, Biscuits, Calcium, Pet food
1. Introduction
The egg is one of the good protein sources for humans that can be consumed directly or as an ingredient in many recipes. Global egg consumption is approximately 1012 eggs per year [1]. Normally, only the egg yolk and egg white are used, with the eggshell left as waste, which has been used in fertilizers (27%), feeds (21%), other limited applications (16%) and dumped as waste (26%) [2]. The weight of eggshell is about 5–6 g per egg, of which 85–95% is CaCO3 [3]. Brun et al. [4] reported 2.07 g of Ca per one eggshell and the bioavailability of the Ca was approximately 45%. Thus, it could be used as an alternative source of calcium.
However, eggshell is contaminated; consequently, its initial microbial load might be too high for further applications. Pretreatment by conventional heating, such as boiling, drying and steaming, should be carried out before using the eggshell for its rich ingredient of calcium. Drying at 100 °C for 3 h could increase the CaCO3 content in the eggshell from 79.3% to 99.2%. However, the CaCO3 was reduced to 0.4% after calcine treatment at 900 °C for 3 h, due to the change in composition into 3 forms of calcium: CaO (63.8%), Ca(OH)2 (24.9%) and C2H2CaO5 (10.9%) [3].
In addition to conventional heating, microwave heating has been recently applied in industrial applications, due to its ability to produce volumetric heating. A microwave is an electromagnetic wave generating heat in the materials by dipole rotation and ionization, causing the temperature of the treated materials to increase rapidly. Thus, microwaving can save processing time and avoid the casehardening problem of conventional heating. Infrared, which is a form of electromagnetic waves, generates heat by the vibrating movement of the dipolar molecules at frequencies in the range 60,000–150,000 MHz. Both microwave and infrared heating have been proposed to industry to improve heating processes. Due to their different heating mechanisms, several studies reported some variation in the structure, composition and properties in the heated materials, compared with conventionally heated materials.
After heat treatment, eggshell can be ground to obtain eggshell powder. Recently, eggshell powder has been used for the development of food and non-food products, including as a calcium supplement in human and animal foods, as fertilizer for plants and as a polymer filler [5], or heavy metal absorbent for reducing contamination in the environment [6]. Therefore, eggshell powder could be added to bread, sausages, pizza, spaghetti, or stew to improve the calcium content in human foods [4]. Fekadu et al. [7] reported that the amount of bioavailable calcium in eggshell power was 34.4–39.6%, following the addition of 4.5–9.0% eggshell powder in injera (a type of flat bread).
As mentioned above, eggshell has potential for use in many applications. The effect of heat generated from microwave and infrared heating on the modification of eggshell powder has not been widely studied. In addition, for a bio-circular economy, the potential of eggshell powder to be reused as a calcium source and included in pet foods has not been widely determined. Therefore, this study aimed to compare the effects of conventional heating, microwave heating and infrared heating on the structure and composition of eggshell. The findings should be useful in the development of eggshell powder for calcium fortification in pet food.
2. Material and methods
2.1. Materials
Chicken eggshell was collected from demonstration chicken laying farms at Kasetsart University, Bangkok, Thailand. After transportation to a laboratory at the Faculty of Agro-Industry on the Kasetsart University Bangkok campus, the eggshell sample was washed in tap water with an eggshell-to-water ratio of 3:1 at ambient temperature (30–35 °C) and prepared for experimental use within 24 h.
3. Methods
3.1. Preparation of eggshell
The washed eggshell (150 g per treatment) was subjected to 3 treatments: the control (no treatment), sterilization at 121 °C for 15 min using an autoclave (Hirayama; HA-300MD; Japan) and heating in water at 95 °C for 60 min using a water bath (JY; HH-S4; China). The ratio of eggshell-to-water was 1:10. All eggshell was determined for microbiological quality using the AOAC method [8] for total plate count and the level of yeast and mold, Escherichia coli and Salmonella sp.
3.2. Modification of eggshell powder
The eggshell from sterilization at 121 °C for 15 min and then heating in water at 95 °C for 60 min was dried in hot-air drier at 70 °C for 3 h before passing through a grinder (XA; LX-10A; China) and sifting through mesh no.100 (Endecotts; ASTM E11; UK) to obtain eggshell powder. Then, the obtained eggshell powder was subjected to 4 treatment methods: the control (no further heating); conventional heating using hot air at 200 °C for 10, 20 or 30 min; microwave heating at 900 W for 1, 2 or 3 min; and infrared heating at 1050 W for 10, 20 or 30 min. The particle size of the obtained eggshell powder was 150 μm.
3.3. Determination of eggshell powder characteristics
3.3.1. Determination of moisture content
The modified eggshell powder was determined for the moisture content using an oven method at 105 °C [8].
3.3.2. Determination of surface structure
Micrographs of the modified eggshell powder were determined using the modified methods [3,9]. The dried powder was coated with gold using sputter coater (Quorum; SC7620; UK). The micrograph was analyzed using a scanning electron microscope (FEI; FEG Quanta 450; USA) at a magnification of 20,000 ×.
3.3.3. Determination of X-ray diffraction pattern
The ratios of calcium in the forms of magnesium calcium carbonate (CaCO3Mg), calciupm carbonate (CaCO3), calcium oxide (CaO), calcium hydroxide (Ca(OH)2), calcium oxalate hydrate (C2H5CaO5) and calcium chloride (CaCl2) in the eggshell powder were determined using X-ray diffractometry (Bruker; Bruker D8 Advance; Germany) with Cu-Kα (Cu Kα = 1.5406 Å), using the method of Huang et al. [9]. The test conditions were set to 40 kV and 40 mA. The eggshell powder was scanned through the 2Ө range of 10–150° with a step size of 0.02° and a sampling interval of 0.5 s per step. In a material whose structure includes a mixture of crystalline and amorphous areas, the crystallinity is normally defined as a percentage of the crystalline structure in the total area. Thus, the degree of crystallinity of the sample was calculated using Eqn. (1):
| (1) |
where: Ac is the crystalline area on the X-ray diffractogram and Aa is the amorphous area on the X-ray diffractogram.
3.3.4. Determination of calcium releasability
The amount of calcium released was determined using atomic absorption spectroscopy. Eggshell powder (3 g) was mixed with 15 mL of HCl (0.1 mol/L) and NaOH 0.1 (mol/L) at pH 2. Then, the mixture was centrifuged at 4000×g and 25 °C for 30 min before passing through a Whatman No.4 filter paper. All samples were diluted 50 times using DI water. Then, 5 mL of each sample was taken to analyze calcium release using a Flame-AAS unit (PerkinElmer; AA 400; USA). Absorbance at a wavelength of 422.67 nm was determined and used for estimation of the calcium concentration [9].
3.4. Application of eggshell powder in biscuits
Wheat flour (100 g), margarine (20 g), palm oil (10 g), sugar (10 g), salt (0.5 g), dried chicken liver (18 g) and water (25 g) were combined in a mixer (Kitchen Aid; 5K5SS; USA) to make dog biscuit dough (modified from Refs. [10,11]). Due to its highest Ca releasability, the modified eggshell powder (2, 4 or 6 g/100 g flour) prepared from boiling at 95 °C and a further microwave treatment for 2 min was used for calcium fortification in the dog biscuits. The biscuit dough was kneaded and formed into a bone shape with 3 mm thickness before baking in a hot-air oven (DH4B-B; Linkrich Machinery Development Co., Ltd.; China) at 160 °C for 30 min.
Dog biscuits with and without calcium fortification from eggshell powder were prepared to determine their composition (moisture, protein, fat, crude fiber and ash contents) using AOAC methods [8]. Carbohydrate was calculated by subtracting the moisture, protein, fat, crude fiber and ash contents from 100. The calcium and phosphorous contents in the biscuits were determined using the in-house methods WI-TMC-19 and WI-TMC-133, respectively, based on [12].
Although color does not matter for dog preference, it is one of the attributes affecting the perception of the purchaser [13], often the pet owner who decides which dog biscuits to buy. Thus, the color (L*, a* and b*) of the biscuits was determined using a spectrophotometer (Minolta CM-3500d; Konica Minolta Inc., Japan), under a standard illuminant (D65) and with a standard observer angle (10°). The hardness of biscuits was measured using a texture analyzer (TA-XT plus; Stable Micro System; UK) set with 3-point bending rig (HDP/3 PB) and 5 mm distance. The pre-test, test and post-test speeds were set at 1, 3 and 10 mm/s, respectively [14]. The hardness of commercial dog biscuits (10.89–34.38 N) was used as a reference for the development of Ca-fortified dog biscuits.
3.5. Statistical analysis
All experiments were conducted with 2 replications. Experimental data were analyzed using ANOVA in the statistical package SPSS® version 12.0 (SPSS (Thailand) Co., Ltd.; Bangkok, Thailand). Duncan's multiple range test was used to carry out multiple comparisons of mean values and significance was tested at P < 0.05.
4. Results and discussion
4.1. Inhibition of microorganisms in farm eggshell
The fresh eggshell had a total plate count of 3.3 ± 0.2 × 107 cfu/g and a yeast and mold count of 2.5 ± 0.3 × 103. Pathogenic bacteria (Salmonella sp. and Escherichia coli) were also detected (Table 1). Salmonella spp. is the major pathogen regarding eggshell contamination, while Escherichia coli is recognized as a minor pathogenic microorganism causing foodborne diseases [15]. After sterilizing at 121 °C for 15 min, the total plate count and the level of yeast and mold were less than 10 cfu/g estimated (est.), Salmonella sp. was not detected in the 25 g sample and Escherichia coli was less than 3 MPN/g. By decreasing the temperature to the pasteurizing level which is below 100 °C [16], the processing time should be extended. In this study, by pasteurizing at 95 °C, at least 15 min was required to inhibit Salmonella sp. and Escherichia coli. However, the total plate count was still higher than for the sterilized sample. Further heating at 95 °C to 60 min, produced inhibition performance equivalent to that of the sterilizing conditions. Therefore, sterilization using steam at 121 °C for 15 min and pasteurization at 95 °C for 60 min were selected to prepare eggshell powder for further modification.
Table 1.
Thermal inhibition of microorganisms in eggshell.
| Eggshell sample | Total plate count (CFU/g) | Yeast and mold (CFU/g) | Salmonella sp. (per 25 g) | Escherichia coli (MPN/g) |
|---|---|---|---|---|
| Fresh | 3.3 ± 0.2 × 107 | 2.5 ± 0.3 × 103 | Detected | 1100 |
| Sterilized at 121 °C for 15 min | <10 est. | <10 est. | Not detected | <3 |
| Pasteurized at 95 °C for 15 min | 90 est. | <10 est. | Not detected | <3 |
| Pasteurized at 95 °C for 30 min | 20 est. | <10 est. | Not detected | <3 |
| Pasteurized at 95 °C for 45 min | 10 est. | <10 est. | Not detected | <3 |
| Pasteurized at 95 °C for 60 min | <10 est. | <10 est. | Not detected | <3 |
Note: est. = estimated, because the number of microbial counts was less than 25 CFU per plate for each dilution.
4.2. Characteristics of eggshell powder
According to moisture content analysis (Table 2), initially the eggshell powder contained 0.488–0.538% moisture content, which coincided with previous studies [[17], [18], [19]] that reported moisture content in eggshell of approximately 0.5%. The microbial inhibition conditions did not have a significant impact on the moisture content. Continuing with hot-air drying, microwave drying or infrared drying caused a reduction in the moisture content to 0.165–0.482%, possibly due to the increased temperature from the further heat treatment of the eggshell samples. Generally, temperatures above 100 °C up to 300 °C are associated with moisture loss and the oxidation of some organic matter. Further increasing the temperature to 480–580 °C could enhance the release of structural OH groups, due to the de-hydroxylation of clay phyllosilicates, resulting in potential additional weight loss of approximately 3.4% [20].
Table 2.
Moisture content of pasteurized and sterilized eggshell powder after further heat treatments.
| Treatment conditions | Time (min) | Moisture content (%) |
|
|---|---|---|---|
| Pasteurized at 95 °C for 60 min | Sterilized at 121 °C for 15 min | ||
| No further heating (control) | – | 0.538 ± 0.091a | 0.488 ± 0.127a |
| Hot air treatment at 200 °C | 10 | 0.406 ± 0.069abc | 0.242 ± 0.140b |
| 20 | 0.314 ± 0.124bc | 0.492 ± 0.175a | |
| 30 | 0.264 ± 0.082c | 0.329 ± 0.065ab | |
| Infrared treatment at 1050 W | 10 | 0.365 ± 0.018abc | 0.327 ± 0.092ab |
| 20 | 0.401 ± 0.027abc | 0.236 ± 0.036b | |
| 30 | 0.482 ± 0.176ab | 0.165 ± 0.023b | |
| Microwave treatment at 900 W | 1 | 0.423 ± 0.089abc | 0.250 ± 0.125b |
| 2 | 0.416 ± 0.039abc | 0.216 ± 0.071b | |
| 3 | 0.436 ± 0.066abc | 0.213 ± 0.063b | |
a–c Values (mean ± standard deviation) in same column with different lowercase letters are significantly (P ≤ 0.05) different.
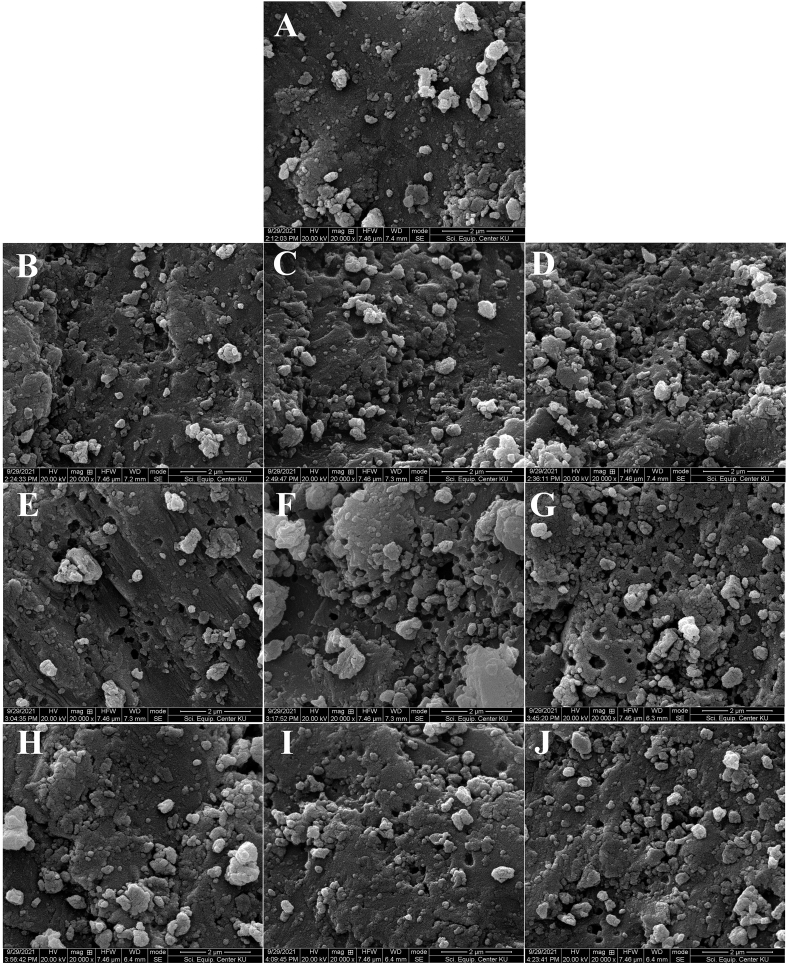
The SEM images indicated that steaming at high temperature under high pressure resulted in a rough surface with some round-shaped particles on the surface. Heating in water at pasteurizing temperature produced a smoother surface with some irregular-shaped particles observed randomly on the surface because the hot water caused the better movement of particles than the steam [3]. Further heat treatment caused some change in the surface structure of the eggshell powder, with the particle size on the surface decreasing, compared to the original size before the further heat treatment.
For the eggshell powder cleaned at pasteurizing temperature (Fig. 1A), further hot-air treatment at 200 °C for 10 min changed the structure from smooth to rough, with a porous surface with small pores. The particles on the surfaces were rounder (Fig. 1B). Continuing the hot-air treatment to 20–30 min caused expansion of the pore size together with producing smooth and agglomerated particles on the surface (Fig. 1C and D). Similarly, in the case of further treatment using infrared heating (Fig. 1E–G) for 20–30 min, the surface became rough and porous with a bigger pore size and the formation of agglomerated particles. Further treatment by microwave heating for 1–2 min enhanced the porosity of the surface structure, with fine particles randomly appearing on the surface (Fig. 1H–I). Increasing the microwave treatment time to 3 min expanded the pore size and agglomerates formed randomly on the surface (Fig. 1J).
Fig. 1.
SEM of eggshell powder from pasteurization and various thermal treatments: No further treatment (A), Hot air treatment at 200 °C for 10 min (B), 20 min (C), 30 min (D), Infrared treatment at 1090 W for 10 min (E), 20 min (F), 30 min (G), and Microwave treatment at 900 W for 1 min (H), 2 min (I), 3 min (J).
For eggshell powder cleaned at sterilizing temperature (Fig. 2A), further hot-air treatment increased the porosity of the surface and produced rounder particles on the surface. The formation of agglomerates on the surface increased when treatment time increased from 10 to 20 to 30 min (Fig. 2B–D). In contrast, further infrared heating for 10 min produced rough and irregular-shaped particles on the surface (Fig. 2E), while increasing the infrared treatment time to 20–30 min enhanced the formation of the porous structure and agglomeration on the surface (Fig. 2F–G). Further treatment by microwave heating for 1 min produced irregular particles on the surface (Fig. 2H). By further increasing the microwave treatment time to 2–3 min, the particles became rounder and finer, while the surface structure was more porous (Fig. 2I–J).
Fig. 2.
SEM of eggshell powder from sterilization and various thermal treatments: No further treatment (A), Hot air treatment at 200 °C for 10 min (B), 20 min (C), 30 min (D), Infrared treatment at 1090 W for 10 min (E), 20 min (F), 30 min (G), and Microwave treatment at 900 W for 1 min (H), 2 min (I), 3 min (J).
4.3. Calcium type and ratio in eggshell powder
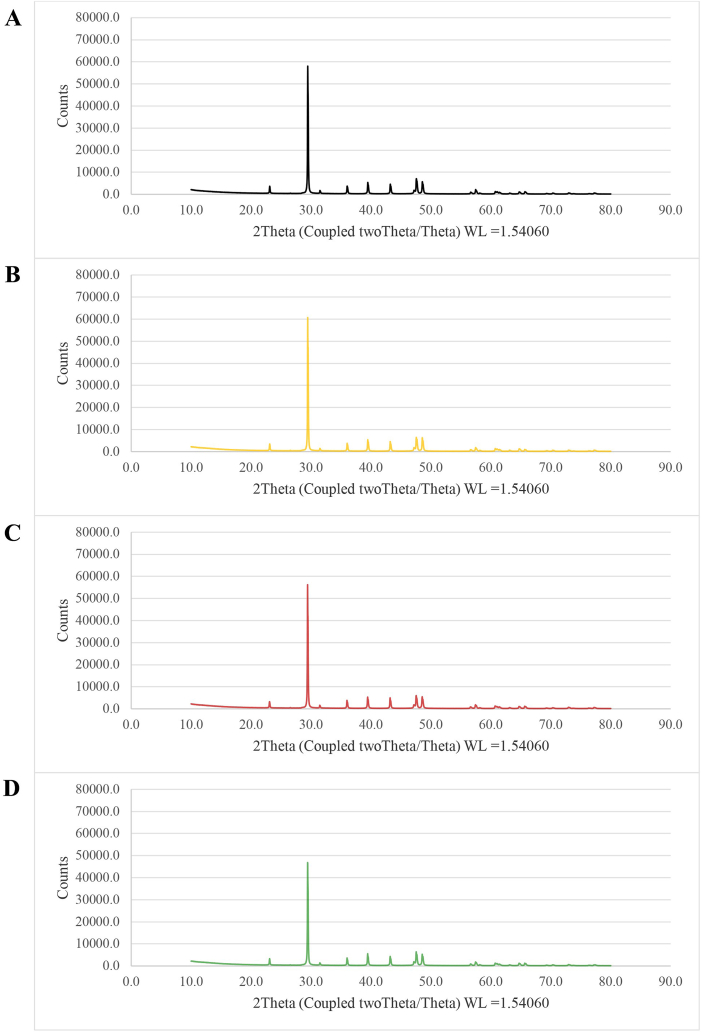
According to the X-ray diffraction pattern, the diffractogram of all eggshell samples presented only one crystalline phase (calcite with a peak at 29.4°), confirming the crystallographic lattice of calcium carbonate (CaCO3) in the eggshell powder (Fig. 3A–D and Fig. 4A–D). This was consistent with reports by Refs. [6,9]. Calcium carbonate is a useful substance as a supplement in feed and as a filler in paper, rubber and plastics [21]. However, the crystallographic lattice of magnesium calcium carbonate (CaCO3Mg), calcium oxide (CaO), calcium hydroxide (Ca (OH)2), calcium oxalate hydrate (C2H5CaO5) and calcium chloride (CaCl2) was not found in the eggshell powder. This indicated that the further heat treatment using hot-air (Figs. 3B and 4B), infrared (Figs. 3C and 4C) or microwaves (Figs. 3D and 4D) did not affect the crystallinity of the calcium in the eggshell powder (Figs. 3A and 4A).
Fig. 3.
X-ray diffraction patterns of eggshell powder from pasteurization and various thermal treatments: No further treatment (A), Hot air treatment at 200 °C for 20 min (B), Infrared treatment at 1090 W for 20 min (C) and Microwave treatment at 900 W for 2 min (D).
Fig. 4.
X-ray diffraction patterns of eggshell powder from sterilization and various thermal treatments: No further treatment (A), Hot air treatment at 200 °C for 20 min (B), Infrared treatment at 1090 W for 20 min (C) and Microwave treatment at 900 W for 2 min (D).
In the current study, the crystallinity of the calcium carbonate in all samples was approximately 90% (Table 3), which was slightly less than in another study that reported 94% [22]. Eggshell powder from pasteurization contained 90.50–91.05% calcium carbonate, while that from the sterilization contained 90.20–90.80% calcium carbonate, regardless of the further treatment conditions (P > 0.05). Microwave treatment applied to the pasteurized eggshell tended to slightly increase the crystallinity of the calcium carbonate. However, the reverse impact was observed when the microwave treatment was applied to the sterilized eggshell that had been in a high temperature process for a long time because the large amount of heat applied to the eggshell had impacted the dried surface through moisture loss, organic matter loss, de-hydroxylation of clay phyllosilicates and degradation of calcium carbonate molecules [3,23,24]. Temperatures in the range 100–300 °C caused water loss and oxidation of some organic matter, but did not cause de-hydroxylation of clay phyllosilicates, release of the structural OH groups or decomposition of CaCO3 [24].
Table 3.
Crystallinity of calcium carbonate in pasteurized and sterilized eggshell powder after further heat treatment.
|
Initial treatment of eggshell sample |
Additional treatment conditions | Time (min) | Crystallinity after all treatments (%) |
|---|---|---|---|
| Pasteurized at 95 °C for 60 min | No further heating (control) | – | 90.95 ± 0.35ab |
| Hot air drying at 200 °C | 20 | 90.50 ± 0.00ab | |
| Infrared drying at 1050 W | 20 | 90.65 ± 0.07ab | |
| Microwave at 900 W | 2 | 91.05 ± 0.21a | |
| Sterilized at 121 °C for 15 min | No further heating (Control) | – | 90.70 ± 0.57ab |
| Hot air drying at 200 °C | 20 | 90.65 ± 0.35ab | |
| Infrared drying at 1050 W | 20 | 90.80 ± 0.42ab | |
| Microwave at 900 W | 2 | 90.20 ± 0.14b |
a–b Values (mean ± standard deviation) with different lowercase letters are significantly (P ≤ 0.05) different).
4.4. Calcium releasability
The atomic absorption spectroscopy indicated that the cleaned eggshell powder released calcium at 205.17–208.40 mg/L (Table 4). Cleaning by steaming at sterilizing temperature caused a higher releasability of calcium than by cleaning using hot water at pasteurizing temperature. Further treatment caused both positive and negative effects on calcium releasability.
Table 4.
Calcium releasability of eggshell power from various treatment conditions.
| Treatment conditions | Time (min) | Calcium releasability (mg/L) |
|
|---|---|---|---|
| Pasteurized at 95 °C for 60 min | Sterilized at 121 °C for 15 min | ||
| No further heating (control) | – | 205.17 ± 0.38f | 208.40 ± 2.17f |
| Hot-air drying at 200 °C | 10 | 219.95 ± 0.17c | 230.35 ± 1.74d |
| 20 | 225.50 ± 3.02b | 305.20 ± 2.07a | |
| 30 | 208.75 ± 0.78e | 249.10 ± 1.80b | |
| Infrared drying at 1050 W | 10 | 225.35 ± 2.82b | 199.60 ± 1.58h |
| 20 | 151.25 ± 1.90h | 154.40 ± 0.56j | |
| 30 | 212.35 ± 1.54d | 158.35 ± 0.68i | |
| Microwave drying at 900 W | 1 | 204.25 ± 1.27e | 204.80 ± 0.60g |
| 2 | 230.85 ± 0.74a | 244.60 ± 1.28c | |
| 3 | 190.40 ± 0.85g | 226.65 ± 0.61e | |
a–j Values (mean ± standard deviation) in same column with different lowercase letters are significantly (P ≤ 0.05) different.
In the case of the pasteurized eggshell, further treatment using hot air at 200 °C for 10–20 min improved the releasability of calcium from 205.17 to 219.95–225.50 mg/L. However, if the treatment time were too long, the reverse effect on calcium releasability would occur, although this was still better than no treatment (control) (P ≤ 0.05). The degree of improvement could be increased by microwave treatment. Microwave treatment for 2 min increased calcium releasability to 230.85 mg/L. Extending the microwave treatment time to 3 min caused a significant reduction of calcium releasability to 190.40 ± 0.85 mg/L. Infrared treatment did not produce better performance than the microwave treatment. To be equivalent to the hot-air treatment for 20 min, infrared heating should be only carried out for 10 min. Any time longer than that would reduce the calcium releasability of the eggshell power to151.25 mg/L which was the lowest releasability found in this study.
Further treatment of the sterilized eggshell using hot air at 200 °C for 10–20 min continually improved the releasability of calcium from 208.40 to 230.35–305.20 mg/L. Similar to the pasteurized eggshell, the hot-air treatment for 30 min would reduce the calcium releasability, but the performance was still better than no treatment. In addition, the microwave treatment for 2 min had the best positive effect on calcium releasability, similar to its application in the pasteurized sample. However, the infrared treatment had a the negative effect on the calcium releasability of the sterilized eggshell powder, with the calcium releasability after the infrared treatment in the range 154.40–199.60 mg/L.
Variation of calcium releasability could have been due to variations in the pore, particle size and agglomeration on the powder surface as affected by the heat treatment condition [9,25].
4.5. Dog biscuits with calcium fortification from eggshell
Considering the physical qualities (Table 5), the biscuits appeared light brown with lightness (L*) in the range 63.40–65.33, redness (a*) in the range 7.01–7.94 and yellowness (b*) in the range 23.26–24.44. The addition of the eggshell powder significantly reduced redness and yellowness, due to the white color of the eggshell powder (L*, a* and b*values of 89.75, 0.13 and 7.07, respectively). In addition, the use of eggshell powder with 4–6 g/100 g flour significantly increased the hardness of the biscuits. To avoid any change in texture, addition of eggshell powder in the dog biscuits should not be more than 2 g/100 g flour.
Table 5.
Color and texture of biscuits with and without addition of eggshell powder.
| Attribute | Biscuits +0 g eggshell powder/100 g flour | Biscuits +2 g eggshell powder/100 g flour | Biscuits +4 g eggshell powder/100 g flour | Biscuits +6 g eggshell powder/100 g flour |
|---|---|---|---|---|
| L* | 63.63 ± 0.11c | 65.33 ± 0.27a | 64.82 ± 0.16b | 63.40 ± 0.32c |
| a* | 7.94 ± 0.07a | 7.01 ± 0.19d | 7.40 ± 0.04b | 7.22 ± 0.04c |
| b* | 24.44 ± 0.19a | 23.96 ± 0.38b | 23.76 ± 0.06b | 23.26 ± 0.13c |
| Hardness (N) | 9.42 ± 1.16b | 8.51 ± 1.02b | 13.15 ± 0.53a | 13.53 ± 4.44a |
a–d Values (mean ± standard deviation) in same row with different lowercase letters are significantly (P ≤ 0.05) different.
All biscuits had contents of 0.32–0.36% moisture, 22.78–24.08% protein, 19.04–21.01% fat, 0.60–0.81% fiber and 52.28–53.45% carbohydrate (Table 6). The addition of eggshell in the dough did not affect these major components in the biscuits (P > 0.05). However, it increased calcium from 21.95 (0.02%) to 585.61–1783.50 mg/100 g of biscuits (0.59–1.78%) when eggshell was added at 2–6 g/100 g flour was added. The amount of calcium was in the recommended range of 0.50–2.50% for dog food according to the European Pet Food Industry Federation [26]. Consequently, the ash content increased from 1.32% to 2.57–5.20%; however, it was still less than the maximum allowance in pet food of 10% [27].
Table 6.
Composition of biscuits with and without addition of eggshell powder.
| Composition | Biscuits +0 g eggshell powder/100 g flour | Biscuits +2 g eggshell powder/100 g flour | Biscuits +4 g eggshell powder/100 g flour | Biscuits +6 g eggshell powder/100 g flour |
|---|---|---|---|---|
| Moisture content (%) | 0.34 ± 0.01a | 0.36 ± 0.02a | 0.34 ± 0.02a | 0.32 ± 0.04a |
| Protein content (%) | 24.08 ± 0.50a | 22.78 ± 1.10a | 23.56 ± 0.24a | 22.89 ± 0.87a |
| Ash content (%) | 1.32 ± 0.01d | 2.57 ± 0.03c | 3.81 ± 0.14b | 5.20 ± 0.08a |
| Fiber content (%) | 0.78 ± 0.08a | 0.81 ± 0.03a | 0.66 ± 0.16a | 0.60 ± 0.16a |
| Fat content (%) | 21.01 ± 0.56a | 20.39 ± 0.41a | 19.39 ± 0.23b | 19.04 ± 0.05b |
| Carbohydrate (%) | 52.81 ± 1.08a | 53.45 ± 0.98a | 52.60 ± 0.15a | 52.28 ± 1.01a |
| Calcium (mg/100 g) | 21.95 ± 0.15d | 585.61 ± 3.22c | 1011.00 ± 2.83b | 1783.50 ± 9.19a |
| Phosphorous (mg/100 g) | 138.83 ± 0.53a | 131.39 ± 2.28a | 161.31 ± 1.21b | 126.78 ± 1.53a |
a–b Values (mean ± standard deviation) in same row with different lowercase letters are significantly (P ≤ 0.05) different.
Additionally, all biscuits contained phosphorous in the range 126.78–161.31 mg/100 g. The Ca-to-P ratio in the control sample was 0.158:1 which was within the recommended range (not more than 2:1). However, the addition of 2, 4 or 6 g eggshell powder per 100 g flour caused increases in the Ca-to-P ratios of 4.46:1, 6.27:1 and 14.07:1, respectively, which are too high for dog food and might cause a negative effect on nutrient balance in dogs. Therefore, the amount of dried chicken liver (a good source of phosphorous) in the dough was increased from 18.0 g to 27.5 g per 100 g flour. As a result, the phosphorous content of biscuits increased to 261.17 mg, while calcium content was 507.12 mg/100 g of biscuits. Thus, the Ca-to-P ratio was 1.94:1 and so remained in the recommended range for dog food. In addition, the increased amount of dried chicken liver could improve protein content in biscuits. Therefore, the dog biscuits with eggshell powder at 2 g/100 g flour had contents of 32.73% protein, 20.16% fat, 44.05% carbohydrate, 2.76% ash, 0.31% fiber and 0.48% moisture. Their lightness, redness and yellowness values were 55.79, 8.42 and 20.72, respectively. The hardness of the biscuits was 11.15 N.
5. Conclusion
Eggshell powder could be used as an alternative source of calcium supplement in pet food. To ensure microbiological safety, eggshell obtained directly from a farm should be cleaned by either heating in water at 95 °C for 60 min or steaming at 121 °C for 15 min to reduce the levels of total plate count and yeast and mold to less than 10 cfu/g est. Salmonella sp. was not detected in the 25 g samples and Escherichia coli was less than 3 MPN/g. The powder form was obtained after drying and grinding the eggshell. Further treatment by either hot air at 200 °C for 20 min or microwave heating at 900 W for 2 min could improve calcium releasability, compared to the untreated samples. However, none of the heat treatments changed calcium crystallinity in the eggshell powder. The major calcium component in the non-treated and treated eggshell powder was calcium carbonate with approximately 90% crystallinity. To fortify calcium in dog biscuits, eggshell powder could be added up to 2% to produce biscuits containing calcium at 507.12 mg/100 g of biscuits, while the calcium-to-phosphorous ratio was still less than 2:1.
Author contribution statement
Therdthai, N.: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Soontrunnarudrungsri, A.: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Khotchai, W.: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
Dr Nantawan Therdthai was supported by National Research Council of Thailand and Ministry of Higher Education, Science, Research and Innovation (year 2021) [(วช.อว.(อ)(กทบ.1)/154/2564)].
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no competing interests.
References
- 1.Mittal A., Teotia M., Soni R.K., Mittal J. Applications of egg shell and egg shell membrane as adsorbents: a review. J. Mol. Liq. 2016;223:376–387. [Google Scholar]
- 2.Tizo M.S., Blanco L.A.V., Cagas A.C.Q., Dela Cruz B.R.B., Encoy J.C., Gunting J.V., Arazo R.O., Mabayo V.I.F. Efficiency of calcium carbonate from eggshells as an adsorbent for cadmium removal in aqueous solution. Sustain. Environ. Res. 2018;28:326–332. [Google Scholar]
- 3.Awogbemi O., Inambao F., Onuh E.I. Modification and characterization of chicken eggshell for possible catalytic applications. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e05283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brun L.R., Lupo M., Delorenzi D.A., Di Loreto V.E., Rigalli A. Chicken eggshell as suitable calcium source at home. Int. J. Food Sci. Nutr. 2013;64(6):740–743. doi: 10.3109/09637486.2013.787399. [DOI] [PubMed] [Google Scholar]
- 5.McGauran T., Dunne N., Smytha B.M., Cunningham E. Incorporation of poultry eggshell and litter ash as high loading polymer fillers in polypropylene. Composites Part C. 2020;3 [Google Scholar]
- 6.Harripersadth C., Musongea P., Isa Y.M., Morales M.C., Sayago A. The application of eggshells and sugarcane bagasse as potential biomaterials in the removal of heavy metals from aqueous solutions. S. Afr. J. Chem. Eng. 2020;34:142–150. [Google Scholar]
- 7.Fekadu T., Cassano A., Angos I., Mate J.I. Effect of fortification with eggshell powder on injera quality. LWTâ“Food Sci. Technol. 2022;158 [Google Scholar]
- 8.Association of Official Analytical Chemists (AOAC) seventeenth ed. AOAC International; Gaithersburg, MD: 2000. Official Methods of Analysis of AOAC International. [Google Scholar]
- 9.Huang X., Dong K., Liu L., Luo X., Yang R., Song H., Li S., Huang O. Physicochemical and structural characteristics of nano eggshell calcium prepared by wet ball milling. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2020;131 [Google Scholar]
- 10.Aly A.A., El-Deeb F.E., Abdelazeem A.A., Hameed A.M., Abdulaziz Alfi A., Alessa H., Alrefaei A.F. Addition of whole barley flour as a partial substitute of wheat flour to enhance the nutritional value of biscuits. Arab. J. Chem. 2021;14(5) [Google Scholar]
- 11.Hedhili A., Lubbers S., Bou-Maroun E., Griffon F., Akinyemi B.E., Husson F., Valentin D. Moringa Oleifera supplemented biscuits: nutritional values and consumer segmentation. South Afr. J. Bot. 2021;138:406–414. doi: 10.1016/j.sajb.2021.01.017. [DOI] [Google Scholar]
- 12.Association of Official Analytical Chemists (AOAC) twenty-first ed. AOAC International; Gaithersburg, MD: 2019. Official Methods of Analysis of AOAC International. [Google Scholar]
- 13.Sucapane D., Roux C., Sobol K. Exploring how product descriptors and packaging colors impact consumers' perceptions of plant-based meat alternative products. Appetite. 2021;167 doi: 10.1016/j.appet.2021.105590. [DOI] [PubMed] [Google Scholar]
- 14.Santos M.K.R., Baptista L.M.S., Hauptli L., Lima A.L.F., Netto D.P., Dahlke F., Moraes P.O. Development of baked biscuits containing propolis and pomegranate for oral health in dogs. Anim. Feed Sci. Technol. 2021:280. doi: 10.1016/j.anifeedsci.2021.115056. [DOI] [Google Scholar]
- 15.Suwannarach N., Kaewyana C., Yodmeeklin A., Kumla J., Matsui K., Lumyong S. Evaluation of Muscodor cinnamomi as an egg biofumigant for the reduction of microorganisms on eggshell surfaces and its effect on egg quality. Int. J. Food Microbiol. 2017;244:52–61. doi: 10.1016/j.ijfoodmicro.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 16.Fellows P.J. second ed. Woodhead Publishing Limited and CRC Press LLC; England: 2002. Food Processing Technology: Principles and Practice; p. 575p. [Google Scholar]
- 17.Ajala E., Eletta O., Ajala M., Oyeniyi S. Characterization and evaluation of chicken eggshell for use as a bio-resource. Arid Zone J. Eng. Technol. Environ. 2018;14:26–40. [Google Scholar]
- 18.Ray S., Barman A.K., Roy P.K., Singh B.K. Chicken eggshell powder as dietary calcium source in chocolate cakes. Pharma Innov. 2017;6:1–4. [Google Scholar]
- 19.Waheed M., Yousaf M., Shehzad A., Inam-Ur-Raheem M., Khan M.K.I., Khan M.R., Aadil R.M. Channeling eggshell waste to valuable and utilizable products: a comprehensive review. Trends Food Sci. Technol. 2020;106:78–90. [Google Scholar]
- 20.GaĺanArboledas R.J., Aĺvarez de Diego J., Dondi M., Bueno S. Energy, environmental and technical assessment for the incorporation of EAF stainless steel slag in ceramic building materials. J. Clean. Prod. 2017;142:1778–1788. [Google Scholar]
- 21.Mattila H.-P., Zevenhoven R. In: Aresta M., van Eldik R., editors. Vol. 66. CO2 Chemistry. Academic Press is an imprint of Elsevier; 2014. Chapter 10 - production of precipitated calcium carbonate from steel converter slag and other calcium-containing industrial wastes and residues; pp. 347–384. (Advances in Inorganic Chemistry). [Google Scholar]
- 22.Murakami F.S., Rodrigues P.O., Campos C.M.T., Silva M.A.S. Physicochemical study of CaCO3 from egg shells. Campinas. 2007;27:658–662. [Google Scholar]
- 23.Onwubu S.C., Vahed A., Singh S., Kanny K.M. Reducing the surface roughness of dental acrylic resins by using an eggshell abrasive material. J. Prosthet. Dent. 2017;117:310–314. doi: 10.1016/j.prosdent.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 24.Vilarinho I.S., Filippi E., Seabra M.P. Development of eco-ceramic wall tiles with bio-CaCO3 from eggshells waste. Open Ceramics. 2022;9 [Google Scholar]
- 25.Jeong M.S., Cho H.S., Park S.J., Song K.S., Ahn K.S., Cho M.H., Kim J.S. Physico-chemical characterization-based safety evaluation of nanocalcium. Food Chem. Toxicol. 2013;62:308–317. doi: 10.1016/j.fct.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 26.European Pet Food Industry Federation (FEDIAF) FEDIAF; 2021. Nutritional Guidelines for Complete and Complementary Pet Food for Cats and Dogs.https://www.fediaf.org/self-regulation/nutrition.html [Google Scholar]
- 27.Miller A. What is crude ash in dog food? 2022. https://www.purepetfood.com/help/what-is-crude-ash-in-dog-food Last access on 31/5/2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.