Abstract
Background:
Intrathecal immunoglobulin-G synthesis is a hallmark of multiple sclerosis (MS), which can be detected by oligoclonal IgG bands (OCB) or by κ-free light chains (κ-FLC) in cerebrospinal fluid.
Objective:
To perform a systematic review and meta-analysis to evaluate whether κ-FLC index has similar diagnostic value to identify patients with clinically isolated syndrome (CIS) or MS compared to OCB, and to determine κ-FLC index cut-off.
Methods:
PubMed was searched for studies that assessed diagnostic sensitivity and specificity of κ-FLC index and OCB to discriminate CIS/MS patients from control subjects. Two reviewers following preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines performed study eligibility assessment and data extraction. Findings from studies were analyzed with bivariate mixed models.
Results:
A total of 32 studies were included in the meta-analysis to evaluate diagnostic value of κ-FLC index. Sensitivity and specificity ranged from 52% to 100% (weighted average: 88%) and 69% to 100% (89%) for κ-FLC index and from 37% to 100% (85%) and 74% to 100% (92%) for OCB. Mean difference of sensitivity and specificity between κ-FLC index and OCB was 2 and −4 percentage points. Diagnostic accuracy determined by mixed models revealed no significant difference between κ-FLC index and OCB. A discriminatory cut-off for κ-FLC index was determined at 6.1.
Conclusion:
The findings indicate that κ-FLC index has similar diagnostic accuracy in MS as OCB.
Keywords: Cerebrospinal fluid, kappa free light chains, multiple sclerosis, clinically isolated syndrome, diagnosis, biomarker, index, intrathecal fraction, meta-analysis, systematic review
Introduction
Cerebrospinal fluid (CSF) analysis is of high importance in the diagnostic work-up of patients with suspected multiple sclerosis (MS). 1 Evidence of intrathecal immunoglobulin G (IgG) synthesis in the CSF, although not specific for MS, substitutes for dissemination in time according to current diagnostic criteria 2 and increases diagnostic certainty in the appropriate clinical setting. 3 Currently, the gold standard to prove intrathecal IgG synthesis is the detection of CSF-restricted oligoclonal IgG bands (OCB). 4
In the last decade, κ-free light chains (κ-FLC) in the CSF have emerged as new biomarker in MS. κ-FLC are secreted by B cells along with intact immunoglobulins and accumulate in the CSF in case of chronic intrathecal inflammation. 5 In contrast to OCB, determination of κ-FLC has considerable advantages. First, κ-FLC are measured by nephelometry or turbidimetry, which are easy, reliable, labor-saving, and cost-effective methods. Second, the determination of κ-FLC returns a metric and rater-independent result.6,7
Most studies used the κ-FLC index to prove an intrathecal synthesis and showed its high diagnostic accuracy to discriminate patients with MS from other neurological diseases.8 –12 However, a strong consensus on the role of κ-FLC as biomarker in MS is still lacking. This might be due to heterogeneity between published studies ranging from different patient populations included, different assays used, to the different κ-FLC measures (e.g. κ-FLC index versus absolute CSF κ-FLC concentration) and cut-off values applied.
Therefore, we aimed to compare the diagnostic value of κ-FLC index to OCB in patients with clinically isolated syndrome (CIS) and MS and to identify an appropriate cut-off for κ-FLC index. Furthermore, we aimed to elucidate differences to other κ-FLC measures.
Methods
This study followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) reporting guideline. 13
Search strategy
A comprehensive search of the electronic database PUBMED was performed on 1 February 2022. The search included the following terms: “free light chain” and “multiple sclerosis.” “Multiple sclerosis” was searched as a MeSH Term and keyword, “free light chain” was searched as keyword. The publication date was restricted from 1 January 2000 (prior to that date there were no studies on κ-FLC in CSF as determined by nephelometry or turbidimetry) to 1 February 2022. Only original articles in English were included. Two authors (HH and FD) independently conducted the literature search, that is, screened titles and abstracts of identified articles after removing duplicates, then independently assessed the full text of potentially relevant articles for inclusion and exclusion criteria. Discrepancies between the two authors were discussed and resolved.
Selection criteria
Studies were included if they were original articles investigating the diagnostic value of κ-FLC index, the percentage intrathecal κ-FLC fraction (IFκ-FLC), CSF κ-FLC concentration or κ-FLC quotient (Qκ-FLC) in patients with CIS or MS compared to any healthy or disease control. Definition and calculation of κ-FLC index, IFκ-FLC or Qκ-FLC are provided in the supplemental material.
Patients of any age were included, with no restrictions on MS disease course, disease duration, disability, comorbidities or treatment. Diagnosis of CIS or MS should be stated with referring to the established diagnostic criteria.2,14 –16 Only studies using immunonephelometry or immunoturbidimetry to determine κ-FLC concentrations in paired CSF and serum/plasma samples, or in the CSF only were included. When patient populations overlapped in several articles, only the one with the most complete information was included. Studies could be retrospective or prospective.
Data extraction
Data extraction forms were created. Data were extracted from selected articles independently in duplicate (HH and FD). Disagreements were resolved by consensus and if needed with another author (JW).
The following data were extracted: the first author, publication year, number of patients per disease group (i.e. CIS or MS, control group), type of samples collected (CSF, serum, or plasma), method used for κ-FLC detection (principal method (nephelometry, turbidimetry), assay kit (Freelite, N Latex), and the platform, as appropriate), diagnosis and used diagnostic criteria of CIS/ MS patients, allocation of controls to one of predefined control groups (non-inflammatory neurological disease control (NINDC), inflammatory neurological disease control (INDC), peripheral inflammatory neurological disease control (PINDC), symptomatic control (SC), healthy control (HC), 17 and non-neurological disease control (NNDC)), corticosteroid treatment prior to sample collection in CIS/ MS patients, disease-modifying treatment at the time of sample collection in CIS/MS patients, number of positive OCB test results (pattern II or pattern III) 4 in the CIS/MS patients, number of negative OCB test results in the control subjects, number of positive test results for κ-FLC index, IFκ-FLC, CSF κ-FLC concentration or Qκ-FLC in the CIS/MS patients, number of negative test results for κ-FLC index, IFκ-FLC, CSF κ-FLC concentration or Qκ-FLC in the control subjects, the applied cut-off values to define test positivity. If the number of positively or negatively tested patients and controls, respectively, was not available, the reported diagnostic sensitivity and specificity were used to back-calculate this number.
Statistical analysis
Studies with data available of diagnostic sensitivity and specificity of κ-FLC index, IFκ-FLC, CSF κ-FLC concentration or Qκ-FLC to discriminate CIS or MS patients from controls were included in the quantitative meta-analysis.
Both sensitivity and specificity of each κ-FLC measure were compared to sensitivity and specificity of OCB used within the same study thereby holding the within study conditions for both parameters constant (e.g. characteristics of CIS/MS patients and control subjects, administration of prior immune treatment). Findings are presented in forest plots separately for sensitivity and specificity. The magnitude of heterogeneity was assessed by Higgins/Thompson’s I2, which is an estimate of the variability across studies based on heterogeneity rather than chance. I2 ranges from 0% to 100%; and low, moderate, and high heterogeneity are indicated by I2 values below 25%, 50%, and 75%, respectively. 18
To consider simultaneously within-study variation, between-study variation and the degree of correlation between sensitivity and specificity because of the chosen cut-off point, a bivariate mixed model was employed. 19 Using REML (restricted maximum likelihood) for estimation, the estimates of sensitivity and specificity and their 95% elliptical confidence interval (CI) were used to compare the accuracy of each κ-FLC measure with OCB. To ensure the validity of our meta-analysis, we did an outlier diagnostic. 20 The estimated bivariate distribution was used to show summary receiver operating curves (sROC). The findings were checked for robustness by splitting the studies according to their different patients and control groups and performing the corresponding sub-analyses.
A power analysis was conducted 21 to investigate whether sample size was sufficient to interpret statistically non-significant findings. A significance level of 5% and the number of studies included in the meta-analysis were used. A large between-study heterogeneity was assumed. A difference in sensitivity and specificity of 5 percentage points (pp) was regarded as substantial.
Cut-off values for the discrimination between CIS/MS patients and control subjects were determined for the κ-FLC index and the CSF κ-FLC concentration. Bivariate confidence intervals of sensitivity and specificity for each of these two κ-FLC measure were computed at the 99% confidence levels. The weighted average over all cut-offs from the studies in this confidence interval was calculated. The weighting was based on the sample size of the studies.
A two-sided significance level of 5% was considered statistically significant. R software 22 and the package mada 23 were used for all analyses.
Results
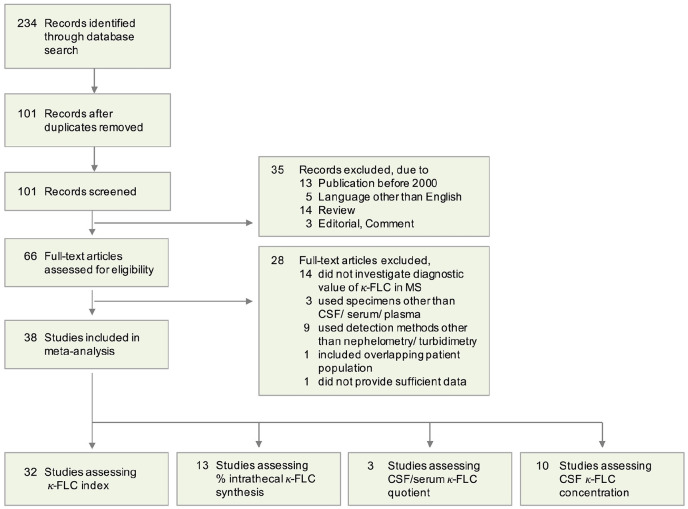
The search strategy identified 234 references (Figure 1). After removing duplicate records, 101 references were screened for potential relevance through titles and abstracts. This process yielded 66 potentially eligible studies that underwent full-text eligibility review. Of these, 38 studies were included in the systematic review.8 –12,24 –57 Thirty-two studies addressed the diagnostic value of κ-FLC index, 13 studies of IFκ-FLC, 10 studies of CSF κ-FLC concentration and 3 studies of Qκ-FLC; 15 studies addressed more than one of these parameters.
Figure 1.
PRISMA flow diagram of study identification, screening, eligibility review, and selection for this systematic review and meta-analysis.
PRISMA: preferred reporting items for systematic reviews and meta-analyses; CSF: cerebrospinal fluid; FLC: free light chain; MS: multiple sclerosis.
κ-FLC index versus OCB
A total of 32 studies addressed the diagnostic accuracy of κ-FLC index including 3322 patients with CIS/ MS and 5849 controls. All studies reported significantly elevated κ-FLC index in CIS/ MS patients compared to controls. Diagnostic sensitivity and specificity ranged from 52% to 100% (weighted average: 88%) and 69% to 100% (89%) for κ-FLC index and from 37% to 100% (85%) and 74% to 100% (92%) for OCB.
Studies differed with regard to demographics, clinical characteristics, and laboratory methods. While 22 studies included distinct cohorts of MS patients and 8 studies included patients with CIS, 8 studies analyzed mixed cohorts comprising both patients with CIS and MS. Twenty-four (75%) of 32 studies applied either the 2010 or 2017 revised McDonald criteria in CIS/ MS patients, 3 studies used earlier diagnostic criteria, and 5 studies did not specify the applied criteria. Nephelometry was applied in 22 (69%) studies and turbidimetry in 9 (28%) studies; 16 (50%) studies used the Freelite assay, and 15 studies (47%) the N Latex assay. One study (3%) applied different type of platform and assay in the patient and control group. Cut-off values of the κ-FLC index denoting test positivity ranged from 2.4 to 20.0. For further details on each study characteristics, we refer to Supplemental Table S1.
First, we performed power analysis for a bivariate mixed model to ensure a valid interpretation for not statistically significant differences. For that, we used a significance level of 5%, a sample size of 32 studies, the studies within variance, assumed a large between heterogeneity and chose a 5 pp difference in sensitivity or specificity between κ-FLC index and OCB as important to detect (e.g. OCB 90% and κ-FLC index 85%). Therewith, we obtained a power of 98.7% for sensitivity and 99.9% for specificity.
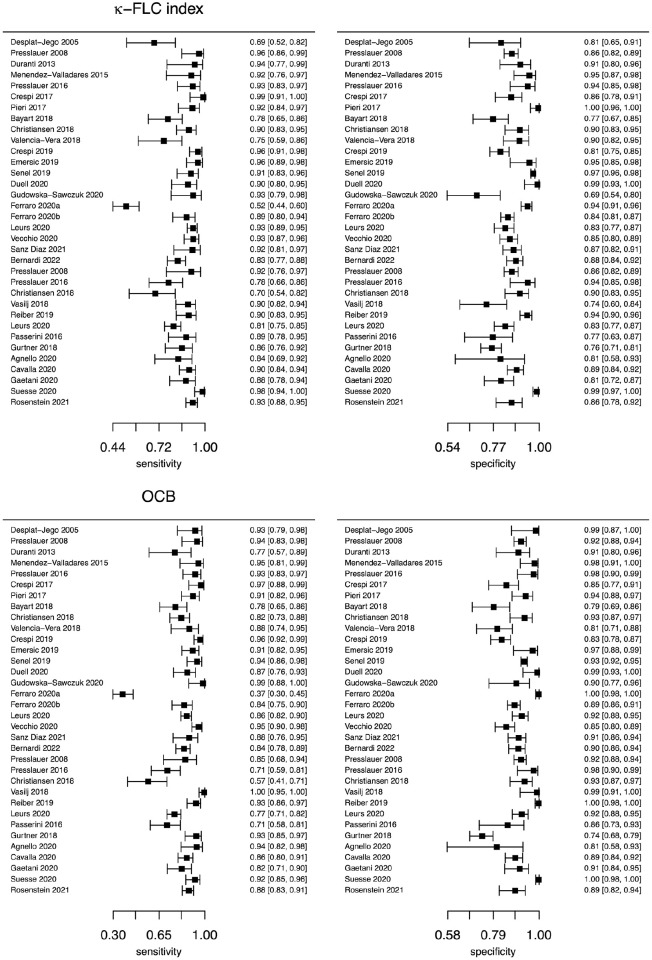
Forest plots were used to visualize sensitivities and specificities and to get an overview of between-study heterogeneity. They showed mostly overlapping confidence intervals and revealed low to moderate between-study heterogeneity (I2 = 29.5%; (95% CI): 0, 55.0%; Figure 2).
Figure 2.
Forest plot of studies comparing the diagnostic accuracy of κ-FLC index and OCB.
In the left column, forest plot of sensitivities for the studies included in the meta-analysis are shown for κ-FLC index (above) and OCB (below); in the right column forest plot of specificities for the studies included in the meta-analysis for κ-FLC index (above) and OCB (below) are provided. Confidence intervals are computed at a 95% confidence level.
FLC: free light chain; OCB: oligoclonal band.
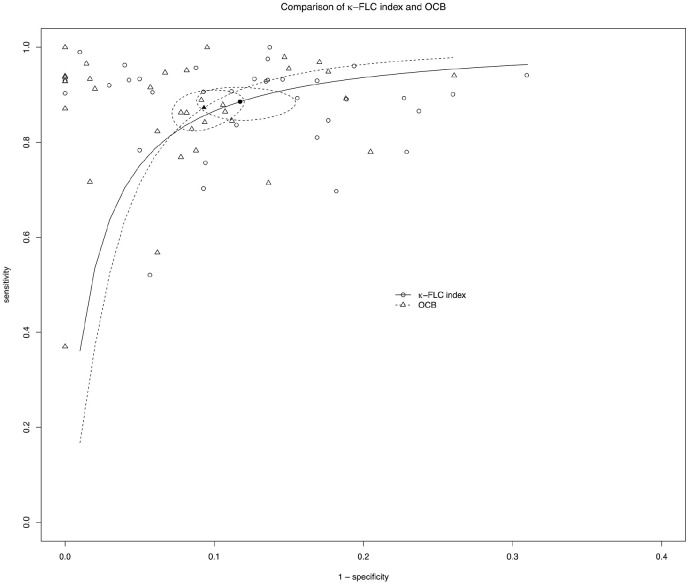
Mean difference of diagnostic sensitivity between κ-FLC index and OCB was 2 pp and −4 pp of specificity. The estimated bivariate mixed model assessed no statistically significant difference between κ-FLC index and OCB for the accuracy to discriminate CIS and MS patients from controls (Figure 3, Supplemental Table S5). In addition, we evaluated a possible impact of the type of assay on the diagnostic sensitivity and specificity of κ-FLC index and observed a statistically significant lower sensitivity with the Freelite assay (p < 0.001, Supplemental Table S6). Further analysis comparing the accuracy of κ-FLC index and OCB controlling for the type of assay and excluding mixed cohorts of CIS/ MS patients (Supplemental Table S7) showed that in the group of Freelite assay not only sensitivity of κ-FLC index was lower, but also of OCB. This implies that not the type of assay, but another confounding factor is responsible for this observation. Indeed, studies using the Freelite assay included more frequently patients with CIS (5 of 13 studies), while studies using the N Latex assay were done with MS patients mainly (8 of 10 studies). The bivariate model analyzing the diagnostic accuracy of κ-FLC index and OCB controlling for the type of disease (CIS vs MS) confirmed that patients with CIS showed a lower sensitivity than patients with MS for the κ-FLC index, but also for OCB (Supplemental Table S8).
Figure 3.
Comparison of the diagnostic accuracy of κ-FLC index with OCB to identify CIS/MS patients
Bivariate summary estimates of sensitivity and specificity for κ-FLC index with OCB and the corresponding 95% confidence ellipse around these mean values are shown as well as the original data of the meta-analysis together with the corresponding sROC curves.
FLC: free light chain; OCB: oligoclonal band; sROC: summary receiver operating curve; CIS: clinically isolated syndrome; MS: multiple sclerosis.
In analogy, we investigated the possible impact of the platform (nephelometry or turbidimetry) on the diagnostic sensitivity and specificity of κ-FLC index and observed at first a statistically significantly lower sensitivity for turbidimetry (Supplemental Table S9). In the subgroup turbidimetry as well as in the subgroup nephelometry, the sensitivity of κ-FLC index and OCB did not significantly differ; thus, a potential impact of the platform could be excluded (Supplemental Table S10).
To further investigate the impact of different patient (MS, CIS, mixed CIS/MS) and control groups (non-inflammatory diseases, inflammatory and/or non-inflammatory diseases), subgroup analyses were performed. This robustness check revealed consistent findings for all subgroups (Supplemental Figures S1 and S2).
A cut-off for κ-FLC index at 6.1 was determined to discriminate CIS/MS patients from controls (Supplemental Figure S3).
Intrathecal κ-FLC fraction versus OCB
The diagnostic accuracy of IFκ-FLC was addressed by 13 studies including 1428 CIS/MS patients and 3299 controls. All studies reported significantly elevated IFκ-FLC in patients with CIS/MS compared to controls. IFκ-FLC showed a diagnostic sensitivity ranging from 66% to 100% (weighted average: 93%) and a specificity from 53% to 100% (84%). In comparison, OCB had a diagnostic sensitivity of 57%–97% (89%) and a specificity of 74%–100% (91%).
Study characteristics concerning demographics, clinical variables, and laboratory methods are detailed in Supplemental Table S2. A total of nine studies included MS patients, three studies CIS patients, and four studies analyzed mixed cohorts comprising both CIS and MS patients. Eleven (85%) of 13 studies applied either the 2010 or 2017 revised McDonald criteria in CIS/MS patients. Nephelometry was used in 11 (85%) studies, turbidimetry in one (8%) study. Four (31%) studies used the Freelite assay, while eight studies (62%) the N Latex assay. One study applied different type of platform and assay in the patient and control group. Studies applied different formulae for the definition of the cut-off (i.e. the Qlim κ-FLC): six (46%) studies applied the formula by Reiber et al., 46 five by Presslauer et al., 58 and one study by Senel et al. 11
Forest plots of sensitivity and specificity are shown in Supplemental Figure S4 and revealed a high between-study homogeneity (I2 = 5.9%; (95% CI): 0, 57.8%).
Diagnostic sensitivity between IFκ-FLC and OCB differed on average by 4 pp and specificity by −8 pp. The diagnostic accuracy as determined by the mixed model revealed no difference between IFκ-FLC and OCB to discriminate CIS and MS patients from controls (Supplemental Figure S5). We also considered different formulae (Presslauer versus Reiber formula) in the model, but did not find evidence for an impact on diagnostic sensitivity and specificity. However, the calculated power for the model was smaller than 80% due to the small number of studies.
CSF κ-FLC concentration versus OCB
A total of 10 studies addressed the value of CSF κ-FLC including 901 patients with CIS/MS and 2251 controls. All studies reported significantly elevated CSF κ-FLC concentration in patients with MS compared to controls. CSF κ-FLC concentration showed a diagnostic sensitivity ranging from 66% to 96% (weighted average: 84%) and a specificity from 70% to 100% (87%). In comparison, OCB had a diagnostic sensitivity of 57% to 100% (86%) and a specificity of 72% to 100% (88%).
Seven studies included distinct groups of MS patients, three studies patients with CIS, while three studies analyzed mixed cohorts. In all studies, either the 2010 or 2017 revised McDonald criteria were applied for CIS/MS patients. Nephelometry was used in eight (80%) studies and turbidimetry in the remaining two (20%) studies; half of the studies used the Freelite assay, whereas the other half the N Latex assay. Cut-off values for the CSF κ-FLC concentration test positivity ranged from 0.3 to 7.1 mg/L. Detailed study characteristics are shown in Supplemental Table S3.
Forest plots of sensitivity and specificity are provided in Supplemental Figure S6. They show a low to moderate between-study heterogeneity (I2 = 28.7%; (95% CI): 0, 63.2%).
Mean difference of diagnostic sensitivity between CSF κ-FLC index and OCB was 0 pp and of specificity −3 pp. Diagnostic accuracy between CSF κ-FLC concentration and OCB to discriminate CIS/MS patients from controls was similar (Supplemental Figure S7). A cut-off for CSF κ-FLC concentration of 0.96 mg/L to discriminate CIS/MS patients from controls was observed (Supplemental Figure S8). However, the calculated power was smaller than 80% due to the small number of studies.
κ-FLC quotient versus OCB
Two studies including MS patients and one study with a cohort of CIS patients investigated the diagnostic accuracy of Qκ-FLC. These studies included a total of 256 CIS/MS patients and 1249 controls. Study characteristics are given in Supplemental Table S4. Overall, sensitivity of Qκ-FLC ranged from 92% to 94% (weighted average: 93%) and specificity from 73% to 96% (95%), while OCB showed a sensitivity of 91% to 100% (96%) and a specificity of 93% to 100% (94%) in these studies. Mean difference of diagnostic sensitivity between Qκ-FLC index and OCB was 3 pp and of specificity −8 pp. Forest plots of sensitivity and specificity are shown in Supplemental Figure S9.
Comparisons between different κ-FLC measures
Studies that applied different κ-FLC measures on the same patient cohort were eligible: 7 studies compared κ-FLC index with CSF κ-FLC concentration, 4 studies IFκ-FLC with CSF κ-FLC concentration, and 11 studies compared κ-FLC index with IFκ-FLC. Diagnostic accuracy between all three κ-FLC measures was similar; however, the statistical power for the comparison with the most employed studies was already less than 80% (Supplemental Figure S10).
Discussion
This systematic review and meta-analysis provides evidence that the determination of intrathecal κ-FLC shows a high diagnostic accuracy to discriminate patients with CIS and MS from other neurological diseases. All approaches to capture intrathecal κ-FLC—including the κ-FLC index, the IFκ-FLC, the Qκ-FLC, and the absolute CSF κ-FLC concentration—showed comparable performance, which was equal to OCB testing. With high statistical power of 99%, significant evidence exists just for κ-FLC index with 32 studies performed on approximately 3300 CIS/MS patients and 5800 control subjects.
κ-FLC in the CSF—similar to immunoglobulins or other proteins—originate either from blood by diffusion across the blood–CSF barrier, or are produced within the intrathecal compartment under pathological conditions. 59 Conceptually, it seems necessary to determine the locally synthesized κ-FLC fraction separate from the blood-derived fraction (as it is also done for IgG) to prove intrathecal B cell activity. Therefore, majority of studies used the κ-FLC index8 –12,24 –45,47 –49,51,52 or the IFκ-FLC.9 –11,34 –37,39,42,50,52 –54 Both approaches consider the albumin quotient (Qalb) which is an established marker of the blood–CSF-barrier function 60 and correct for the absolute serum κ-FLC concentration. Few studies used the Qκ-FLC.11,33,35,45 Other authors determined the absolute CSF κ-FLC concentrations only.10,30,35,37,39,45,51,55 –57 As the intrathecal κ-FLC fraction is greater than 80% in most CIS/MS patients,9,46 one might argue that the contribution of blood-derived κ-FLC to the total CSF κ-FLC concentration is negligible in cases with intrathecal synthesis. In this meta-analysis, we did not find a statistically significant difference in the diagnostic performance between both κ-FLC index and IFκ-FLC compared to CSF κ-FLC concentration. However, the statistical power was below 80% and, thus, insufficient to interpret the not-statistically-significant results with a small enough Type II error. This means that superiority of κ-FLC index over CSF κ-FLC concentration (or even vice versa) cannot be excluded. A recent study further elaborated this research question, separated patients into low and high CSF κ-FLC categories (based on median values) and observed that CSF κ-FLC concentration, Qκ-FLC and κ-FLC index showed similar diagnostic performance in the high category, but not in the low category with inferiority of CSF κ-FLC and to some extent also of Qκ-FLC. 61 Thus, the impact of serum κ-FLC and Qalb is indeed negligible in patients with high intrathecal κ-FLC synthesis, but probably not in patients with only low or modest intrathecal κ-FLC production. This might be of importance in CIS patients who showed lower diagnostic sensitivity and lower amount of intrathecal κ-FLC. 9 Another very recent large multicenter study including more than 1600 patients also reported that κ-FLC index and IFκ-FLC performed slightly better than absolute CSF κ-FLC concentration. 62 Further studies are required to compare the different κ-FLC measures in patients with varying degree of intrathecal B cell activity and varying blood–CSF-barrier function.
Different cut-off values for κ-FLC index, for CSF κ-FLC concentration, as well as different formulae defining the Qlim κ-FLC (Presslauer et al., 58 Reiber et al., 46 Senel et al. 11 ) for calculating the IFκ-FLC have been published. In general, different cut-off values might apply depending on the clinical question, for example, to provide an upper reference limit determined in a (non-inflammatory) control population 17 or to differentiate MS from other INDC. Furthermore, cut-off values might vary whether the main aim is to increase diagnostic sensitivity or specificity. 63 Here, we observed a discriminatory cut-off for κ-FLC index at 6.1 to differentiate CIS/MS patients from controls, as well as at 0.96 mg/L for CSF κ-FLC concentration. Even though the cut-off for κ-FLC index8,9,12,30 as well as for the CSF κ-FLC concentration30,55 is in line with those identified by several large—partly multicenter—studies, we have to clearly state that this analysis was exploratory. Comparison of studies applying different non-linear formulae11,46,58 did not reveal a difference, but the power for this analysis was low due to the small number of studies. So far, there is one study that compared the performance of all three formulae within an independent cohort reporting a diagnostic sensitivity ranging from 96% to 98% in MS patients and 40% to 44% in CIS patients. 36
At this point, it has to be stated that studies dealt differently with samples in case of non-detectable CSF κ-FLC concentrations. Some studies used the lower detection limit, while others set these samples to “zero” or even omitted these samples from the statistical analysis. For the absolute CSF κ-FLC concentration, samples treated as “zero” or set to the lower detection limit still means that these samples are in the lower concentration range. Hence, determination of cut-off values is not affected, and the clinical interpretation is clear (i.e. no intrathecal synthesis) as the lower detection limit (e.g. 0.3 mg/L) is by far lower than the cut-off (in this meta-analysis 0.96 mg/L). However, κ-FLC index values depend also on serum κ-FLC concentration and Qalb, so that different handling of non-detectable CSF κ-FLC concentration might indeed lead to considerably varying index values, which might then affect cut-off values.
Studies that validate the herein observed cut-offs in a multicenter setting are needed. These studies should consider different handling in case of non-detectable CSF κ-FLC values, and the potential impact of different assays and platforms as well. We did not find a statistically significant impact of assay and platform on κ-FLC index, in line with a recent large multicentre study that also did not observe any impact of the platform on κ-FLC index. 62 Using the ratio of the CSF and serum κ-FLC concentration (for calculating the index) might be less prone to laboratory variations. The potentially different susceptibility of κ-FLC index and absolute CSF κ-FLC concentrations to laboratory variation should be further addressed. Altogether, for calculation of κ-FLC index (or IFκ-FLC) in case of CSF κ-FLC concentrations below detection limit, we suggest setting the value to the detection limit rather than to zero to avoid larger variation across laboratories.
Diagnostic sensitivities and specificities as reported by different studies showed a certain variability not only for κ-FLC measures, but also for OCB. This arises from a certain heterogeneity of included patients between studies. It is evident that sensitivity differs whether CIS patients or MS patients are included. 64 Specificity is lowered when patients with inflammatory neurological disease (IND) were included into the control group. κ-FLC in the CSF are—similar to CSF-restricted OCB—a sign of intrathecal inflammation and thus can support the diagnosis of MS, but they are not specific for MS. The spectrum of diseases which show an intrathecal κ-FLC synthesis is probably similar to that with CSF-restricted OCB. κ-FLC synthesis reflects IgG synthesis, but might be present also in case of intrathecal IgA or IgM synthesis. Studies on the frequency of intrathecal κ-FLC synthesis in neurological diseases other than MS are still rare. Apart from a mixture of different IND as part of control populations, dedicated disease-specific studies exists only for a few entities, for example, neuroborreliosis.65,66 For this meta-analysis, we applied a model considering not only between-study variation, but also within-study variation and used only studies using both κ-FLC measures and OCB. Therefore, potential sources of bias were reduced and allowed a reliable comparison of the above-mentioned parameters. Furthermore, robustness of findings was checked by subgroup analyses (different patient groups (CIS, MS, mixed cohorts), different control groups (non-inflammatory and inflammatory/non-inflammatory), and different assays (Freelite, N Latex) and platforms (nephelometry, turbidimetry)).
There are some limitations of the meta-analysis. The statistical power for κ-FLC measures apart from the κ-FLC index was low, so that firm conclusions on the similar diagnostic performance of IFκ-FLC, Qκ-FLC, CSF κ-FLC concentration, and OCB cannot yet be drawn. Most of the studies did not report how their cut-off values were obtained. This might have an impact on our estimated cut-off values for κ-FLC index and CSF κ-FLC concentration (as discussed above). Another limitation is that the analytic performance of OCB detection probably differed between studies, as different methods were used, for example, commercial versus in-house assays; and interpretation of results is rater-dependent. 67 It cannot be excluded that OCB would have shown better performance if tested only in few, specialized laboratories. However, it has to be clearly stated that one of the clear advantages of κ-FLC is the reliable and rater-independent determination, which should overcome technical difficulties and finally allow a widespread use.
In conclusion, it seems reasonable to consider intrathecal κ-FLC synthesis equally to CSF-restricted OCB, both reaching a diagnostic sensitivity and specificity of approximately 90% without significant differences when meta-analyzed. Statistically sufficient power for the comparisons exists only for κ-FLC index. The potential of κ-FLC in the CSF as new biomarker in MS was clearly demonstrated. Due to considerable methodological advantages as a fast, time- and labor-saving, rater-independent and reliable method, intrathecal κ-FLC synthesis might serve as alternative tool to measure intrathecal immunoglobulin synthesis. A detailed review of the advantages and limitations of κ-FLC and OCB, respectively, and consensus recommendations for implementation of κ-FLC in clinical routine are given elsewhere. 68 In future, κ-FLC might be used as a screening test and in certain constellations OCB as a confirmation test, for example, in case of borderline κ-FLC results, as already implemented by some clinical laboratories. 55 Since the best algorithm to determine intrathecal κ-FLC synthesis has to be established and universal cut-off values for different platforms remain to be confirmed, the combination of both tests—intrathecal κ-FLC and OCB—might be the best option at this moment.
Supplemental Material
Supplemental material, sj-pdf-1-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental Material
Supplemental material, sj-pdf-10-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental Material
Supplemental material, sj-pdf-11-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental Material
Supplemental material, sj-pdf-12-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental Material
Supplemental material, sj-pdf-13-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental Material
Supplemental material, sj-pdf-14-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental Material
Supplemental material, sj-pdf-15-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental Material
Supplemental material, sj-pdf-16-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental Material
Supplemental material, sj-pdf-17-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental Material
Supplemental material, sj-pdf-18-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental Material
Supplemental material, sj-pdf-19-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental Material
Supplemental material, sj-pdf-2-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental Material
Supplemental material, sj-pdf-3-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental Material
Supplemental material, sj-pdf-4-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental Material
Supplemental material, sj-pdf-5-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental Material
Supplemental material, sj-pdf-6-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental Material
Supplemental material, sj-pdf-7-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental Material
Supplemental material, sj-pdf-8-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental Material
Supplemental material, sj-pdf-9-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Footnotes
Authors’ Contributions: H.H. has contributed in conception and design of the study, acquisition of data, analysis and interpretation of data, and drafting the manuscript. J.W. has contributed in analysis and interpretation of data and revision of the manuscript for intellectual content. G.A., S.G., B.K., M.K., R.S., C.T., H.T., L.M.V., M.A.V.W., and H.Z. have contributed in revision of the manuscript for intellectual content. K.B. has contributed in acquisition of data and revision of the manuscript for intellectual content. F.D. has contributed in conception and design of the study, acquisition of data, analysis and interpretation of data, and revision of the manuscript for intellectual content.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: HH has participated in meetings sponsored by, received speaker honoraria or travel funding from Bayer, Biogen, Celgene, Merck, Novartis, Sanofi-Genzyme, Siemens, Teva, and received honoraria for acting as consultant for Biogen, Celgene, Novartis and Teva. GA has received speaking honoraria and compensation for consulting services or participation in advisory boards from Sanofi, Merck, Roche and Horizon Therapeutics; travel funding from Novartis, Roche and ECTRIMS; is the editor for Europe of Multiple Sclerosis Journal—Experimental, Translational and Clinical; and is a member of the International Women in Multiple Sclerosis (iWiMS) network executive committee. KB has participated in meetings sponsored by, received speaking honoraria or travel funding from Roche, Biogen, Sanofi, and Teva. SG has received speaker honoraria and has been a member of scientific boards for Biogen Idec, Genzyme, Novartis, and Merck and received grant funding from Genzyme, Merck and Takeda. MK has received funding for travel and speaker honoraria from Bayer, Novartis, Merck, Biogen Idec and Teva Pharmaceutical Industries Ltd. and serves on scientific advisory boards for Biogen Idec, Merck Serono, Roche, Novartis and Gilead. CT has a collaboration contract with ADx Neurosciences, Quanterix and Eli Lilly, performed contract research or received grants from AC-Immune, Axon Neurosciences, Bioconnect, Biogen, Biorchestra, Brainstorm Therapeutics, Celgene, EIP Pharma, Eisai, Novo Nordisk, PeopleBio, Roche, Toyama, and Vivoryon. She serves on editorial boards of Medidact Neurologie/Springer, Alzheimer Research and Therapy, Neurology: Neuroimmunology & Neuroinflammation, and is editor of a Neuromethods book Springer. Research of CET is supported by the European Commission (Marie Curie International Training Network, grant agreement no. 860197 (MIRIADE), Innovative Medicines Initiatives 3TR (Horizon 2020, grant no. 831434) and JPND (bPRIDE), National MS Society (Progressive MS alliance) and Health Holland, the Dutch Research Council (ZonMW), Alzheimer Drug Discovery Foundation, The Selfridges Group Foundation, Alzheimer Netherlands, Alzheimer Association. CT is recipient of ABOARD, which is a public–private partnership receiving funding from ZonMW (#73305095007) and Health~Holland, Topsector Life Sciences & Health (PPP-allowance; #LSHM20106). ABOARD also receives funding from Edwin Bouw Fonds and Gieskes-Strijbisfonds. HT has participated in meetings sponsored by or received honoraria for acting as an advisor/speaker for Alexion, Bayer, Biogen, Celgene, Fresenius, Genzyme-Sanofi, Janssen, Merck, Novartis, Roche, Siemens and Teva. LMV has served at scientific advisory boards, participated in meetings sponsored by, received speaking honoraria or travel funding or research grants from Roche, Sanofi, Merck, Biogen, Bristol Myers, and Novartis. MAVW has received research grants from The Binding Site, Siemens Healthineers and Sebia Inc, has participated in an advisory board for Myeloma360. HZ has served in scientific advisory boards and/or as a consultant for Abbvie, Alector, Annexon, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Pinteon Therapeutics, Red Abbey Labs, Passage Bio, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave; has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). FD has participated in meetings sponsored by or received honoraria for acting as an advisor/speaker for Alexion, Almirall, Biogen, Celgene, Genzyme-Sanofi, Merck, Novartis Pharma, Roche, and Teva. His institution has received research grants from Biogen and Genzyme Sanofi. He is section editor of the MSARD Journal (Multiple Sclerosis and Related Disorders). JW, BK, and RS have nothing to disclose.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Janette Walde  https://orcid.org/0000-0002-1241-5292
https://orcid.org/0000-0002-1241-5292
Klaus Berek  https://orcid.org/0000-0003-2755-2043
https://orcid.org/0000-0003-2755-2043
Georgina Arrambide  https://orcid.org/0000-0002-2657-5510
https://orcid.org/0000-0002-2657-5510
Sharmilee Gnanapavan  https://orcid.org/0000-0003-2817-9922
https://orcid.org/0000-0003-2817-9922
Michael Khalil  https://orcid.org/0000-0002-5350-3328
https://orcid.org/0000-0002-5350-3328
Charlotte Teunissen  https://orcid.org/0000-0002-4061-0837
https://orcid.org/0000-0002-4061-0837
Florian Deisenhammer  https://orcid.org/0000-0003-4541-8841
https://orcid.org/0000-0003-4541-8841
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Harald Hegen, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Janette Walde, Department of Statistics, Faculty of Economics and Statistics, University of Innsbruck, Innsbruck, Austria.
Klaus Berek, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Georgina Arrambide, Centre d’Esclerosi Múltiple de Catalunya, Department of Neurology/Neuroimmunology, Hospital Universitari Vall d’Hebron, Universitat Autónoma de Barcelona, Barcelona, Spain.
Sharmilee Gnanapavan, Blizard Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London, UK.
Batia Kaplan, Laboratory of Hematology, Sheba Medical Center, Ramat Gan, Israel.
Michael Khalil, Department of Neurology, Medical University of Graz, Graz, Austria.
Ruba Saadeh, Department of Laboratory Medicine and Pathology and Department of Neurology, Mayo Clinic, Rochester, MN, USA.
Charlotte Teunissen, Neurochemistry Laboratory, Department of Clinical Chemistry, Amsterdam Neuroscience, Vrije Universiteit, Amsterdam UMC, Amsterdam, The Netherlands.
Hayrettin Tumani, CSF Laboratory, Department of Neurology, University of Ulm, Ulm, Germany.
Luisa M Villar, Immunology Department, Hospital Universitario Ramón y Cajal, Madrid, Spain.
Maria Alice V Willrich, Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN, USA.
Henrik Zetterberg, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy, University of Gothenburg, Mölndal, Sweden/Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, Sweden/Department of Neurodegenerative Disease, Queen Square Institute of Neurology, University College London, London, UK/UK Dementia Research Institute, University College London, London, UK/Hong Kong Center for Neurodegenerative Diseases, Hong Kong, China.
Florian Deisenhammer, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
References
- 1. Stangel M, Fredrikson S, Meinl E, et al. The utility of cerebrospinal fluid analysis in patients with multiple sclerosis. Nat Rev Neurol 2013; 9(5): 267–276. [DOI] [PubMed] [Google Scholar]
- 2. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17(2): 162–173. [DOI] [PubMed] [Google Scholar]
- 3. Arrambide G, Tintore M, Espejo C, et al. The value of oligoclonal bands in the multiple sclerosis diagnostic criteria. Brain 2018; 141(4): 1075–1084. [DOI] [PubMed] [Google Scholar]
- 4. Freedman MS, Thompson EJ, Deisenhammer F, et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: A consensus statement. Arch Neurol 2005; 62(6): 865–870. [DOI] [PubMed] [Google Scholar]
- 5. Konen FF, Schwenkenbecher P, Jendretzky KF, et al. The increasing role of kappa free light chains in the diagnosis of multiple sclerosis. Cells 2021; 10(11): 3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bradwell AR, Carr-Smith HD, Mead GP, et al. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem 2001; 47(4): 673–680. [PubMed] [Google Scholar]
- 7. Velthuis HT, Knop I, Stam P, et al. N Latex FLC—New monoclonal high-performance assays for the determination of free light chain kappa and lambda. Clin Chem Lab Med 2011; 49(8): 1323–1332. [DOI] [PubMed] [Google Scholar]
- 8. Leurs CE, Twaalfhoven H, Lissenberg-Witte BI, et al. Kappa free light chains is a valid tool in the diagnostics of MS: A large multicenter study. Mult Scler 2020; 26(8): 912–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Presslauer S, Milosavljevic D, Huebl W, et al. Validation of kappa free light chains as a diagnostic biomarker in multiple sclerosis and clinically isolated syndrome: A multicenter study. Mult Scler 2016; 22(4): 502–510. [DOI] [PubMed] [Google Scholar]
- 10. Gurtner KM, Shosha E, Bryant SC, et al. CSF free light chain identification of demyelinating disease: Comparison with oligoclonal banding and other CSF indexes. Clin Chem Lab Med 2018; 56(7): 1071–1080. [DOI] [PubMed] [Google Scholar]
- 11. Senel M, Mojib-Yezdani F, Braisch U, et al. CSF free light chains as a marker of intrathecal immunoglobulin synthesis in multiple sclerosis: A blood-CSF barrier related evaluation in a large cohort. Front Immunol 2019; 10: 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bernardi G, Biagioli T, Malpassi P, et al. The contribute of cerebrospinal fluid free light-chain assay in the diagnosis of multiple sclerosis and other neurological diseases in an Italian multicenter study. Mult Scler 2021; 28: 1364–1372. [DOI] [PubMed] [Google Scholar]
- 13. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst Rev 2021; 10(1): 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001; 50(1): 121–127. [DOI] [PubMed] [Google Scholar]
- 15. Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.” Ann Neurol 2005; 58(6): 840–846. [DOI] [PubMed] [Google Scholar]
- 16. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69(2): 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Teunissen C, Menge T, Altintas A, et al. Consensus definitions and application guidelines for control groups in cerebrospinal fluid biomarker studies in multiple sclerosis. Mult Scler 2013; 19(13): 1802–1809. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21(11): 1539–1558. [DOI] [PubMed] [Google Scholar]
- 19. Reitsma JB, Glas AS, Rutjes AW, et al. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005; 58(10): 982–990. [DOI] [PubMed] [Google Scholar]
- 20. Viechtbauer W, Cheung MW-L. Outlier and influence diagnostics for meta-analysis. Res Synth Methods 2010; 1(2): 112–125. [DOI] [PubMed] [Google Scholar]
- 21. Hedges LV, Pigott TD. The power of statistical tests for moderators in meta-analysis. Psychol Methods 2004; 9(4): 426–445. [DOI] [PubMed] [Google Scholar]
- 22. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2021. https://www.r-project.org/ [Google Scholar]
- 23. mada: Meta-Analysis of Diagnostic Accuracy. Comprehensive R Archive Network. https://CRAN.R-project.org/package=mada
- 24. Desplat-jégo S, Feuillet L, Pelletier J, et al. Quantification of immunoglobulin free light chains in cerebrospinal fluid by nephelometry. J Clin Immunol 2005; 25(4): 338–345. [DOI] [PubMed] [Google Scholar]
- 25. Presslauer S, Milosavljevic D, Brücke T, et al. Elevated levels of kappa free light chains in CSF support the diagnosis of multiple sclerosis. J Neurol 2008; 255(10): 1508–1514. [DOI] [PubMed] [Google Scholar]
- 26. Duranti F, Pieri M, Centonze D, et al. Determination of κFLC and κ Index in cerebrospinal fluid: A valid alternative to assess intrathecal immunoglobulin synthesis. J Neuroimmunol 2013; 263(1–2): 116–120. [DOI] [PubMed] [Google Scholar]
- 27. Menéndez-Valladares P, García-Sánchez MI, Cuadri Benítez P, et al. Free kappa light chains in cerebrospinal fluid as a biomarker to assess risk conversion to multiple sclerosis. J Exp Transl Clin 2015; 1: 2055217315620935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pieri M, Storto M, Pignalosa S, et al. KFLC Index utility in multiple sclerosis diagnosis: Further confirmation. J Neuroimmunol 2017; 309: 31–33. [DOI] [PubMed] [Google Scholar]
- 29. Bayart JL, Muls N, van Pesch V. Free Kappa light chains in neuroinflammatory disorders: Complement rather than substitute. Acta Neurol Scand 2018; 138(4): 352–358. [DOI] [PubMed] [Google Scholar]
- 30. Christiansen M, Gjelstrup MC, Stilund M, et al. Cerebrospinal fluid free kappa light chains and kappa index perform equal to oligoclonal bands in the diagnosis of multiple sclerosis. Clin Chem Lab Med 2018; 57: 210–220. [DOI] [PubMed] [Google Scholar]
- 31. Schwenkenbecher P, Konen FF, Wurster U, et al. The persisting significance of oligoclonal bands in the dawning era of kappa free light chains for the diagnosis of multiple sclerosis. Int J Mol Sci 2018; 19(12): 3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Valencia-Vera E, Martinez-Escribano Garcia-Ripoll A, Enguix A, et al. Application of κ free light chains in cerebrospinal fluid as a biomarker in multiple sclerosis diagnosis: Development of a diagnosis algorithm. Clin Chem Lab Med 2018; 56(4): 609–613. [DOI] [PubMed] [Google Scholar]
- 33. Altinier S, Puthenparampil M, Zaninotto M, et al. Free light chains in cerebrospinal fluid of multiple sclerosis patients negative for IgG oligoclonal bands. Clin Chim Acta 2019; 496: 117–120. [DOI] [PubMed] [Google Scholar]
- 34. Crespi I, Vecchio D, Serino R, et al. K index is a reliable marker of intrathecal synthesis, and an alternative to IgG index in multiple sclerosis diagnostic work-up. J Clin Med 2019; 8(4): 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Emersic A, Anadolli V, Krsnik M, et al. Intrathecal immunoglobulin synthesis: The potential value of an adjunct test. Clin Chim Acta 2019; 489: 109–116. [DOI] [PubMed] [Google Scholar]
- 36. Schwenkenbecher P, Konen FF, Wurster U, et al. Reiber’s diagram for kappa free light chains: The new standard for assessing intrathecal synthesis? Diagnostics 2019; 9(4): 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Duell F, Evertsson B, Al Nimer F, et al. Diagnostic accuracy of intrathecal kappa free light chains compared with OCBs in MS. Neurol Neuroimmunol Neuroinflamm 2020; 7(4), e775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gudowska-Sawczuk M, Tarasiuk J, Kułakowska A, et al. Kappa free light chains and IgG combined in a novel algorithm for the detection of multiple sclerosis. Brain Sci 2020; 10(6): 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vecchio D, Bellomo G, Serino R, et al. Intrathecal kappa free light chains as markers for multiple sclerosis. Sci Rep 2020; 10(1): 20329–20326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sanz Diaz CT, de Las Heras Flórez S, Carretero Perez M, et al. Evaluation of kappa index as a tool in the diagnosis of multiple sclerosis: Implementation in routine screening procedure. Front Neurol 2021; 12: 676527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ferraro D, Trovati A, Bedin R, et al. Cerebrospinal fluid kappa and lambda free light chains in oligoclonal band-negative patients with suspected multiple sclerosis. Eur J Neurol 2020; 27(3): 461–467. [DOI] [PubMed] [Google Scholar]
- 42. Ferraro D, Bedin R, Natali P, et al. Kappa index versus CSF oligoclonal bands in predicting multiple sclerosis and infectious/inflammatory CNS disorders. Diagnostics 2020; 10(10): 856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Crespi I, Sulas MG, Mora R, et al. Combined use of kappa free light chain index and isoelectrofocusing of cerebro-spinal fluid in diagnosing multiple sclerosis: Performances and costs. Clin Lab 2017; 63(3): 551–559. [DOI] [PubMed] [Google Scholar]
- 44. Agnello L, Sasso Lo B, Salemi G, et al. Clinical use of κ free light chains index as a screening test for multiple sclerosis. Lab Med 2020; 51(4): 402–407. [DOI] [PubMed] [Google Scholar]
- 45. Vasilj M, Kes VB, Vrkic N, et al. Relevance of KFLC quantification to differentiate clinically isolated syndrome from multiple sclerosis at clinical onset. Clin Neurol Neurosurg 2018; 174: 220–229. [DOI] [PubMed] [Google Scholar]
- 46. Reiber H, Zeman D, Kušnierová P, et al. Diagnostic relevance of free light chains in cerebrospinal fluid—The hyperbolic reference range for reliable data interpretation in quotient diagrams. Clin Chim Acta 2019; 497: 153–162. [DOI] [PubMed] [Google Scholar]
- 47. Cavalla P, Caropreso P, Limoncelli S, et al. Kappa free light chains index in the differential diagnosis of Multiple Sclerosis from Neuromyelitis optica spectrum disorders and other immune-mediated central nervous system disorders. J Neuroimmunol 2020; 339: 577122. [DOI] [PubMed] [Google Scholar]
- 48. Gaetani L, Di Carlo M, Brachelente G, et al. Cerebrospinal fluid free light chains compared to oligoclonal bands as biomarkers in multiple sclerosis. J Neuroimmunol 2020; 339: 577108. [DOI] [PubMed] [Google Scholar]
- 49. Berek K, Bsteh G, Auer M, et al. Kappa-free light chains in CSF predict early multiple sclerosis disease activity. Neurol Neuroimmunol Neuroinflamm 2021; 8(4): e1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rosenstein I, Rasch S, Axelsson M, et al. Kappa free light chain index as a diagnostic biomarker in multiple sclerosis: A real-world investigation. J Neurochem 2021; 159(3): 618–628. [DOI] [PubMed] [Google Scholar]
- 51. Passerini G, Dalla Costa G, Sangalli F, et al. Free light chains and intrathecal B cells activity in multiple sclerosis: A prospective study and meta-analysis. Mult Scler Int 2016; 2016: 2303857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Süße M, Reiber H, Grothe M, et al. Free light chain kappa and the polyspecific immune response in MS and CIS—Application of the hyperbolic reference range for most reliable data interpretation. J Neuroimmunol 2020; 346: 577287. [DOI] [PubMed] [Google Scholar]
- 53. Puthenparampil M, Altinier S, Stropparo E, et al. Intrathecal K free light chain synthesis in multiple sclerosis at clinical onset associates with local IgG production and improves the diagnostic value of cerebrospinal fluid examination. Mult Scler Relat Disord 2018; 25: 241–245. [DOI] [PubMed] [Google Scholar]
- 54. Süße M, Feistner F, Grothe M, et al. Free light chains kappa can differentiate between myelitis and noninflammatory myelopathy. Neurol Neuroimmunol Neuroinflamm 2020; 7(6): e892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Saadeh RS, Bryant SC, McKeon A, et al. CSF kappa free light chains: Cutoff validation for diagnosing multiple sclerosis. Mayo Clin Proc 2022; 97(4): 738–751. [DOI] [PubMed] [Google Scholar]
- 56. Sáez MS, Rojas JI, Lorenzón MV, et al. Validation of CSF free light chain in diagnosis and prognosis of multiple sclerosis and clinically isolated syndrome: Prospective cohort study in Buenos Aires. J Neurol 2019; 266(1): 112–118. [DOI] [PubMed] [Google Scholar]
- 57. Hassan-Smith G, Durant L, Tsentemeidou A, et al. High sensitivity and specificity of elevated cerebrospinal fluid kappa free light chains in suspected multiple sclerosis. J Neuroimmunol 2014; 276(1–2): 175–179. [DOI] [PubMed] [Google Scholar]
- 58. Presslauer S, Milosavljevic D, Huebl W, et al. Kappa free light chains: Diagnostic and prognostic relevance in MS and CIS. PLoS ONE 2014; 9(2): e89945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reiber H. Dynamics of brain-derived proteins in cerebrospinal fluid. Clin Chim Acta 2001; 310(2): 173–186. [DOI] [PubMed] [Google Scholar]
- 60. Deisenhammer F, Bartos A, Egg R, et al. Guidelines on routine cerebrospinal fluid analysis. Report from an EFNS task force. Eur J Neurol 2006; 13(9): 913–922. [DOI] [PubMed] [Google Scholar]
- 61. Hegen H, Walde J, Milosavljevic D, et al. Free light chains in the cerebrospinal fluid. Comparison of different methods to determine intrathecal synthesis. Clin Chem Lab Med 2019; 57: 1574–1586. [DOI] [PubMed] [Google Scholar]
- 62. Levraut M, Ayrignac X, Bigaut K, et al. Kappa free light chains intrathecal synthesis biomarkers are efficient tools for the diagnosis of multiple sclerosis: A large multicenter cohort study (S19.003). Neurology 2022; 98(Suppl 18): 1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Süße M, Hannich M, Petersmann A, et al. Kappa free light chains in cerebrospinal fluid to identify patients with oligoclonal bands. Eur J Neurol 2018; 25(9): 1134–1139. [DOI] [PubMed] [Google Scholar]
- 64. Dobson R, Ramagopalan S, Davis A, et al. Cerebrospinal fluid oligoclonal bands in multiple sclerosis and clinically isolated syndromes: A meta-analysis of prevalence, prognosis and effect of latitude. J Neurol Neurosurg Psychiatry 2013; 84(8): 909–914. [DOI] [PubMed] [Google Scholar]
- 65. Hegen H, Milosavljevic D, Schnabl C, et al. Cerebrospinal fluid free light chains as diagnostic biomarker in neuroborreliosis. Clin Chem Lab Med 2018; 56(8): 1383–1391. [DOI] [PubMed] [Google Scholar]
- 66. Tjernberg I, Johansson M, Henningsson AJ. Diagnostic performance of cerebrospinal fluid free light chains in Lyme neuroborreliosis—A pilot study. Clin Chem Lab Med 2019; 57(12): 2008–2018. [DOI] [PubMed] [Google Scholar]
- 67. Gastaldi M, Zardini E, Scaranzin S, et al. Autoantibody diagnostics in neuroimmunology: Experience from the 2018 Italian neuroimmunology association external quality assessment program. Front Neurol 2019; 10: 1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hegen H, Walde J, Berek K, et al. Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A consensus statement. Mult Scler 2022; 29(2): 182–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental material, sj-pdf-10-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental material, sj-pdf-11-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental material, sj-pdf-12-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental material, sj-pdf-13-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental material, sj-pdf-14-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental material, sj-pdf-15-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental material, sj-pdf-16-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental material, sj-pdf-17-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental material, sj-pdf-18-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental material, sj-pdf-19-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental material, sj-pdf-2-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental material, sj-pdf-3-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental material, sj-pdf-4-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental material, sj-pdf-5-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental material, sj-pdf-6-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental material, sj-pdf-7-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental material, sj-pdf-8-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal
Supplemental material, sj-pdf-9-msj-10.1177_13524585221134213 for Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis by Harald Hegen, Janette Walde, Klaus Berek, Georgina Arrambide, Sharmilee Gnanapavan, Batia Kaplan, Michael Khalil, Ruba Saadeh, Charlotte Teunissen, Hayrettin Tumani, Luisa M Villar, Maria Alice V Willrich, Henrik Zetterberg and Florian Deisenhammer in Multiple Sclerosis Journal