Abstract
Objective
Colorectal cancer (CRC) is the third most common cancer and the second largest cause of cancer-related death worldwide. Current CRC screening in various countries involves stool-based faecal immunochemical testing (FIT) and/or colonoscopy, yet public uptake remains sub-optimal. This review assessed the literature regarding acceptability of alternative CRC screening modalities compared to standard care in average-risk adults.
Method
Systematic searches of MEDLINE, EMBASE, CINAHL, Cochrane and Web of Science were conducted up to February 3rd, 2022. The alternative interventions examined were computed tomography colonography, flexible sigmoidoscopy, colon capsule endoscopy and blood-based biomarkers. Outcomes for acceptability were uptake, discomfort associated with bowel preparation, discomfort associated with screening procedure, screening preferences and willingness to repeat screening method. A narrative data synthesis was conducted.
Results
Twenty-one studies met the inclusion criteria. Differences between intervention and comparison modalities in uptake did not reach statistical significance in most of the included studies. The findings do suggest FIT as being more acceptable as a screening modality than flexible sigmoidoscopy. There were no consistent significant differences in bowel preparation discomfort, screening procedure discomfort, screening preference and willingness to repeat screening between the standard care and alternative modalities.
Conclusion
Current evidence comparing standard colonoscopy and stool-based CRC screening with novel modalities does not demonstrate any clear difference in acceptability. Due to the small number of studies available and included in each screening comparison and lack of observed differences, further research is needed to explore factors influencing acceptability of alternative CRC modalities that might result in improvement in population uptake within different contexts.
Keywords: colorectal cancer screening, faecal occult blood test, faecal immunochemical test, flexible sigmoidoscopy, colon capsule endoscopy, computed tomography colonography, blood-based biomarker
Introduction
Colorectal cancer (CRC) is the third most common cancer and the second largest cause of cancer-related death worldwide, with over 1.9 million new cases causing 935,000 deaths in 2020 globally. 1 Screening for CRC can be effective at reducing mortality, but uptake remains suboptimal. 2 Several tests can be used to screen for CRC, including stool-based tests and colonoscopy.
The faecal immunochemical test (FIT) is currently most commonly used to screen for CRC and uses antibodies to detect human blood in the stool. Colonoscopy is considered the gold standard of CRC screening due to its ability to examine the whole colon while simultaneously detecting and removing polyps. 2 Population-based colonoscopy screening has not been considered to be practicable in several countries due to the cost, capacity and expertise required 3 whilst it has been implemented in others with relatively limited coverage of the population at risk. For example, colonoscopy-based but opportunistic screening is used in the United States and Poland, rather than a population-based screening programme. 4 Stool-based screening may have significant false negatives depending on the threshold used for detection in a particular screening programme. 3 Hence, there is a need for an effective as well as patient-centred and less invasive screening test that is acceptable to participants. 5
There are several alternative technologies that have been investigated for colorectal screening, including flexible sigmoidoscopy (FS), computed tomography (CT) colonography, colon capsule endoscopy and blood-based biomarkers, 5 which may have adequate sensitivity and specificity and fulfil criteria 6 to be used as a screening tool. Most of these are less invasive and/or often perceived as more patient-friendly than colonoscopy. 5 CRC screening uptake is consistently low among the underserved sections of the population. 7 Socioeconomic, ethnic and sociocultural factors also play a role in non-adherence with CRC screening. Individuals from areas with higher levels of social deprivation were less likely to participate in screening. 8 Zhu (2021) 9 reported that psychosocial barriers such as unpleasantness, embarrassment, pain and fear about a positive result were the most commonly reported barriers to colonoscopy screening among the Hispanic population.
Alternative technologies for CRC screening require systematic investigation of patient acceptability for their efficacy to be translated to effectiveness at a population level. Common parameters used in previous CRC screening studies to determine acceptability have included screening uptake, bowel preparation discomfort, screening procedure discomfort and screening preference. 10 There are limited studies assessing the acceptance of alternative technologies among average-risk populations. 11 Lin and colleagues 12 suggested that participants preferred CT colonography to colonoscopy in 16 of the 19 studies included in the review, but a pooled difference was not calculated. Khalid-de Bakker 13 reviewed comparative uptake of a range of CRC modalities in average-risk populations. Their study suggested higher uptake for stool-based modalities. Zhu et al. 14 conducted a meta-analysis comparing uptake between CT colonography and colonoscopy and found no significant difference. However, their review was limited by comparing one alternative modality to colonoscopy. The purpose of the current systematic review was to examine the acceptability of four alternative CRC screening methods currently available, with published data on their use, compared to standard care (colonoscopy and FIT).
Methods
The systematic review was registered on PROSPERO (reg. no. CRD42020203971) and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. 15 Throughout all stages of the search, data extraction and quality appraisal, 15% of studies were double-checked for consistency by another member of the team (SG). All discrepancies were resolved through discussion. Data duplication was managed by removing duplications using a reference management software package (EndNote X9) and Rayyan, 16 followed by manual checking.
Search strategy
The literature from 1985 to February 3rd, 2022, was searched on electronic databases Medline, Embase, Cochrane, CINAHL and Web of Science using the terms listed in Table 1. A list of the search terms for the different databases are listed in Appendix 1 (see online Supplemental material). Studies published before 1985 were not included because the alternative interventions were not used in clinical practice prior to this date.
Table 1.
Population, intervention, comparator, outcomes (PICO) and search terms.
| PICO | Description | Search terms |
|---|---|---|
| Population | Participants aged 45 to 86 years and who were at average risk of CRC | exp Colorectal Neoplasms/ or ((bowel or colorectral or colon) or adj3 (carcinoma* or neoplasm* or cancer)).mp or ("early detection of cancer" or early screening).mp or mass screening/ or (screen* or detection or test*) |
| Intervention | Colon capsule endoscopy, CT colonography, FS, blood biomarkers | (capsule endoscopy* or colon capsule or virtual camera or video endoscopy).mp or virtual endoscopy.mp or exp Colonography, Computed Tomographic/ or (virtual colonoscopy or CT colonoscopy).mp or Sigmoidoscopy/ or (flexible sigmoid* or flexible sigmoidoscopy).mp or blood testmp. or Hematologic tests/ or (epi procolon or septins or msept9).mp or septin9.mp or (blood bio* or blood-based or blood-based biomarker or liquid bio*).mp |
| Comparison | FOBT, FIT, colonoscopy | exp Colonoscopy/ or Occult Blood/ or fecal immunochemical testmp or faecal immunochemical testmp |
| Outcome | Acceptability - uptake, discomfort associated with bowel preparation, discomfort associated with screening procedure, screening preference, willingness to repeat screening modality | (acceptability* or acceptance).mp or (adherence* or attend* or attendance*) or (engage or engagement or interest or willing).mp or (uptake* or screening uptake).mp or (compliance* or complete*).mp or (intend or visit or choice or choose or chose).mp or patient preference/ or patient participation or participate*.mp or (knowledge or understanding or comprehension).mp or (decision making or decide or attitude or belief).mp or (perception or perceive or interest or value or decisional conflict).mp or (anxiety/ or discomfort).mp or (embarrassment or pain or experience).mp or satisfaction.mp or Personal Satisfaction/confidence* or fear or worry.mp |
Eligibility criteria
Inclusion criteria were: (1) participants aged 45–86 years; (2) participants with average risk of CRC; (3) studies that compared colon capsule endoscopy, CT colonography, flexible sigmoidoscopy and/or blood biomarkers (e.g. Septin 9 or Epi proColon) with FOBT, FIT and/or colonoscopy. The exclusion criteria were: (1) studies that used a decision aid to increase CRC uptake; (2) participants who were previously non-adherent to screening and received a tailored intervention to encourage screening; (3) studies that did not report primary study data or used simulation models. This review was not limited to randomised controlled trials (RCTs); it also included studies that reported participants’ perceptions and preferences to better understand acceptance of screening tests.
Selection process
Databases were searched with fixed search terms (Table 1) and all results were exported and saved onto EndNote. Articles were cross-referenced to look for relevant articles. Full texts of potentially eligible studies were reviewed. Discrepancies that arose were resolved by agreement between the two reviewers. If no agreement could be reached a third reviewer (SD) was consulted.
Data extraction and synthesis
A standardised form was used to extract the following study details: authors, year of publication, country, study design, screening intervention, screening comparator and study outcomes. Due to the heterogeneity of included studies, a narrative approach was used to synthesise key findings. 17 A p-value of <0.05 was used as a cut-off to determine significance of results reported.
Quality assessment
One author assessed the quality of all included studies using the Cochrane risk-of-bias (RoB 2) tool 18 to assess randomised studies and the ROBINS_I tool 19 for non-randomised studies. The Cochrane tool 18 assesses the likelihood of bias in studies across five domains: (1) randomisation bias, (2) intended intervention bias, (3) missing data bias, (4) outcome bias and (5) reporting bias. The risk of bias was judged as low, high or some concerns. Seven domains assessed using the ROBINS_I tool 19 were: (1) confounding bias, (2) participant selection bias, (3) intervention classification bias, (4) deviations from intended intervention bias, (5) missing data bias, (6) outcome bias and (7) reported bias. The risk of bias was judged as low, moderate or high. An RCT was graded higher than an observational study when evaluating the same outcome measure. Evidence from observational studies was used where no RCT data were available.
Results
Study selection
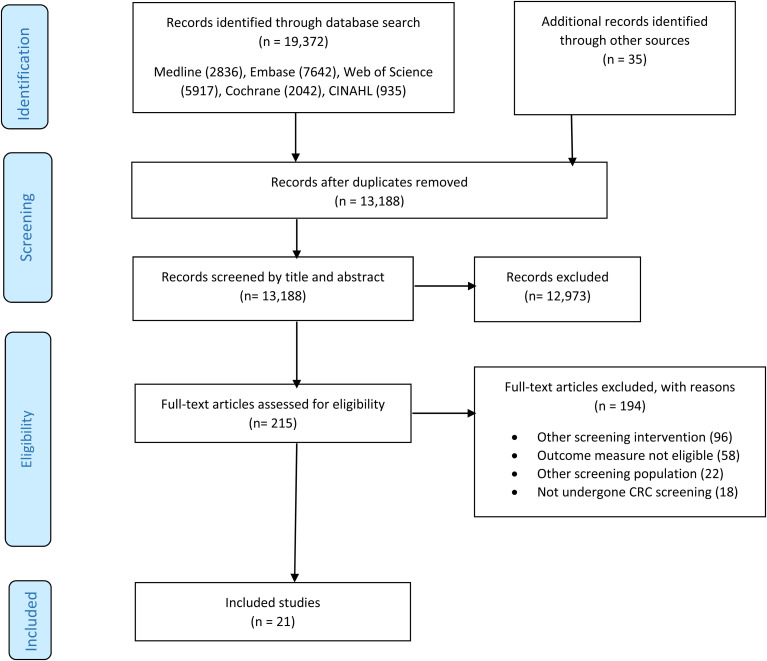
The initial search yielded 19,372 articles (Figure 1). After removing duplicates, 13,188 underwent title and abstract screening. Two-hundred and fifteen articles were assessed for full-text eligibility, of which 21 studies were included in the final analysis. Three of these used the same population cohort to assess uptake and measure acceptability,31,34,36 but the findings were reported in separate studies.
Figure 1.
PRISMA flow diagram depicting study selection process.
Study characteristics
Key characteristics of the included studies are outlined in Table 2. Twelve studies were RCTs20–31 and eight studies were observational.32–40 To assess acceptability, studies compared participants who had completed a screening intervention or a comparator. Eight studies compared CT colonography and colonoscopy,20–24,34–37,38 five studies compared FS and FIT,25–28,39 four studies compared FS and colonoscopy,21,26,36–37 four studies compared blood-based biomarker tests and FIT,29–32 two studies compared colon capsule endoscopy and colonoscopy,33,40 two studies compared CT colonography and FOBT21,23 and one study compared CT colonography and FIT. 24
Table 2.
Key characteristics of included studies.
| Study, (Country) | Study design | CRC screening intervention and comparator | Sample | Outcome measure | Summary of key findings | Quality appraisal |
|---|---|---|---|---|---|---|
| Scott et al. (2004) (Australia)
20
Forbes et al (2006) (Australia) 21 Stoop et al (2011) (Netherlands) 22 You et al (2015) (Canada) 23 Sali et al (2016) (Italy) 24 |
RCT RCT RCT RCT RCT |
CT colonography vs
colonoscopy CT colonography vs colonoscopy CT colonography vs colonoscopy CT colonography vs colonoscopy CT colonography vs colonoscopy |
Sample size invitees: CT colonography (n = 359), colonoscopy
(n = 350). Age of participants: 50–55 years (53.0%), 65–69 years (47.0%). Gender: male (50.0%), female (50.0%). Sample size invitees: CT colonography (n = 215), colonoscopy (n = 214). Age of participants: 50–54 years (49.5%), 65–69 years (50.5%). Gender: male (50.1%), female (40.9%). Sample size invitees: CT colonography (n = 2920), colonoscopy (n = 5924). Age of participants: 50–59 years (45.9%), 60–75 years (54.2%). Gender: male (67.1%), female (32.9%). Sample size invitees: CT colonography (n = 65), colonoscopy (n = 66). Age of participants: 50–70 years. Mean age: 58.7 years. Gender: male (52.5%), female (47.5%). Sample size invitees: r-CT colonography (n = 2395), f-CT colonography (n = 2430), colonoscopy (n = 1036). Age of participants: 54–60 years (61.5%), 61–65 years (38.5%). Gender: male (46.4%), female (53.6%). |
Uptake Uptake Uptake Uptake Uptake |
CT colonography (18.1% 65/359), colonoscopy (16.3%, 57/350),
p = 0.82, no significant
difference. CT colonography (16.3%, 35/214), colonoscopy (17.8%, 38/214), no significant difference. CT colonography (34%, 982/2920) had highest uptake compared to colonoscopy (22%, 1276/5924), (relative risk [RR] 1·56, 95% CI 1·46–1·68; p < 0·001). CT colonography (76.9%), colonoscopy (80.3%), no p-value as trial was stopped early. r-CT colonography (28.1% 674/2395), f-CT colonography (25.2%, 612/2430), colonoscopy (14.8%, 153/1036). All differences between groups were statistically significant (P < .001). |
High
risk High risk High risk High risk High risk |
| Kirkoen et al (2017) (Norway)
25
Segnan et al (2007) (Italy) 26 Hol et al (2009) (Netherlands) 27 Randel et al (2021) (Norway) 28 |
RCT RCT RCT RCT |
FS vs FIT FS vs FIT FS vs FIT FS vs FIT |
Sample size invitees: FS (1700), FIT (1439). Age of participants: 50–74 Sample size invitees: FIT (6075), FS (6018). Age of participants: 55–59 years (59.5%), 60–64 years (40.5%). Gender: male (47.7%), female (52.3%) Sample size invitees: FS (5000), FIT (5007). Age of participants: 50–74 Sample size invitees: FS (69,165) FIT (70,096). Age of participants: 50–74 Gender: male (49.3%), female (50.7%). |
Uptake Uptake Uptake Uptake |
FS (52%), FIT (54%), no significant
difference. FS: 32.3% (1944/6018), FIT: 32.3% (1965/6075), no significant difference. 61.5% (CI, 60.1 to 62.9%) for FIT and 32.4% (CI, 31.1 to 33.7%) for FS screening. FIT 1st round had highest uptake (58.4%) compared to FS (52.1%), p < 0.05. |
High risk High risk High risk High risk |
| Symonds et al (2019) (Australia)
29
Young et al (2021) (Australia) 30 Liles et al (2016) (USA) 31 Ioannou et al (2021) (USA) 32 |
RCT RCT RCT Observational study |
Blood-based vs FIT Blood-based vs FIT Blood-based vs FIT Blood-based vs FIT |
Sample size invitees: Blood-based (585), FIT (588). Gender:
female (50.7%), male (49.3%). Age of participants:
50–74 Sample size invitees: blood test (293), FIT (292). Age of participants: 50–74. Gender: male (53.3%), female (47.7%) Sample size invitees: Epi-proColon (203), FIT (210). Age of participants: 50–75. Gender: male (39.9%), female (60.1%) Ethnicity: Caucasian (85.7%), Others (14.3%). Sample size invitees: 460. Age of participants: >50 years. Gender: male (39%), female (61%) |
Uptake Uptake Uptake Uptake |
Blood-based test (5.3%, 31/585), FIT (3.6%, 21/588),
p > 0.05. Blood-based test (13.3%, 39/293), FIT (12.0%, 35/292), 13.3%, p = 0.88. Blood-based test had highest uptake: (99.5%, 202/203), FIT (88.1%, 185.210), p < 0.001. Of 460 participants, none chose colonoscopy, 30 (6.5%) chose FIT and 430 (93.5%) chose blood-based test No p-value stated. |
High risk High risk High risk Moderate risk |
| Forbes et al (2006) (Australia)
21
You et al (2015) (Canada) 23 |
RCT RCT |
CT colonography vs FOBT CT colonography vs FOBT |
Sample size invitees: CT colonography (215), FOBT
(234). Sample size invitees: CT colonography (65), FOBT (67). |
Uptake Uptake |
FOBT had highest uptake: (27.4%, 64/234), CT colonography
(16.3%, 35/215), p = 0.005. CT colonography: (76.9%, 50/65), FOBT (64.2%, 43/67). |
High risk High risk |
| Segnan et al (2007) (Italy) 26 | RCT | FS vs colonoscopy | Sample size invitees: FIT (6075), FS (6018), colonoscopy (6021). | Uptake | FS: 32.3% (1944/6018), colonoscopy: 26.5% (1597/6021), (OR, 0.74; 95% CI: 0.68–0.80), | High risk |
| Groth et al (2012) (Germany) 33 | Observational study |
Capsule endoscopy vs colonoscopy | Sample size invitees: 2150. Age of participants: >55
years. Gender: male, (49.3%), female (50.7%) |
Uptake | Capsule endoscopy: (4.2%, 90/2150), colonoscopy: (1.6%, 34/2150). | Low risk |
| Sali et al (2016) (Italy) 24 | RCT | CT colonography vs FIT | Sample size invitees: r-CT colonography (2617), f-CT colonography (2625), FIT (9288). Age of participants: 54–65. | Uptake |
FIT had highest uptake (50.4%), r-CT colonography: (28.1%), f-CT colonography: (25.2%). All differences between groups were statistically significant (P < .001). | High risk |
| Wijkerslooth et al (2011) (Netherlands)
34
Gareen et al (2015) (USA) 35 |
Observational
study Observational study |
CT colonography vs colonoscopy CT colonography vs colonoscopy |
Post-study questionnaire: CT colonography (n = 801/982),
colonoscopy (n = 1009/1276). Age of participants: 50–74
years. Sample size invitees: 2310, participants. Age of participants: 55–86 years. Mean age: 58.4 years. |
Bowel preparation discomfort Bowel preparation discomfort |
More burdensome in colonoscopy than CT colonography: (61% vs
16%, p < 0.001). CT colonography participants reported more discomfort (81.3% vs 27.8%, p < 0.001) and more embarrassment (42.5% vs 26.0%, p < 0.001). |
High risk High risk |
| Nicholson and Korman (2005) (Australia)
36
Senore et al (2011) (Italy) 37 |
Observational study Observational study |
FS vs colonoscopy FS vs colonoscopy |
Sample size invitees: FS (191), colonoscopy (256). Gender:
male (45%), female (55%). Sample size invitees: FS (1696), colonoscopy (1382). |
Bowel preparation discomfort Bowel preparation discomfort |
BP ranked the worst part of procedure FS: 31%, colonoscopy
78% (p < 0.02). BP symptom moderate/severe: FS (3.8%), colonoscopy (15.1%), not significant. |
Low risk Moderate risk |
| Scott et al (2004) (Australia)
20
Forbes et al (2006) (Australia) 21 Pickhardt et al (2003) (USA) 38 Wijkerslooth et al (2011) (Netherlands) 34 |
RCT RCT Observational study Observational study |
CT colonography vs
colonoscopy CT colonography vs colonoscopy CT colonography vs colonoscopy CT colonography vs colonoscopy |
Participants returned post-study questionnaire: CT
colonography (n = 56), colonoscopy
(n = 95). Post-study questionnaire: CT colonography (n = 37/38), colonoscopy (n = 62/63). Age of participants: 50–54 years and 65–69 years. Sample size invitees: 1233 (81.5% returned post-study questionnaire). Age of participants: 50–79 years Participants returned post-study questionnaire: CT colonography (n = 801/982), colonoscopy (n = 1009/1276). Age of participants: 50–74 years. |
Screening
discomfort Screening discomfort Screening discomfort Screening discomfort |
Acceptability measured using median 100-point analogue
scores (0 = most favourable, 100 = least favourable). Pain:
CT computed tomography (23), colonoscopy (7.1).
Satisfaction: CT computed tomography (6.5), colonoscopy
(4.6). Embarrassment: CT computed tomography (10.2),
colonoscopy (7.8). Acceptability measured using median 100-point analogue scores (0 = most favourable, 100 = least favourable). Pain score: CT colonography (20), colonoscopy (4.5). Satisfaction score: CT colonography (10), colonoscopy (4). Embarrassment score: CT colonography (6), colonoscopy (4). CT colonography participants reported more discomfort (54.3% vs 38.1%, p < 0.001) and more acceptable in terms of convenience (68.3% vs 24.1%, p < 0.001). CT colonography participants reported more pain (72% vs 47%, p < 0.001), more embarrassment (8% vs 5%, p < 0.001). |
High
risk High risk Low risk High risk |
| Forbes et al (2006) (Australia)
21
Nicholson and Korman (2005) (Australia) 36 Senore et al (2011) (Italy) 37 |
RCT Observational study Observational study |
FS vs
colonoscopy FS vs colonoscopy FS vs colonoscopy |
Sample size invitees: FS (39), colonoscopy
(63). Sample size invitees: FS (191), colonoscopy (256). Gender: male (45%), female (55%). Sample size invitees: FS (1696), colonoscopy (1382). |
Screening
discomfort Screening discomfort Screening discomfort |
Acceptability measured using median 100-point analogue
scores (0 = most favourable, 100 = least favourable). Pain:
FS (18), colonoscopy (4.5). Satisfaction: FS (6),
colonoscopy (4). Embarrassment: FS (10), colonoscopy
(4). Colonoscopy more comfortable (75% vs 18%; P < 0.001), embarrassment score not significantly different. No pain associated with colonoscopy and most individuals had a pain score of less than 3 (11-point scale) for FS. No significant difference with pain and embarrassment levels. |
High risk Low risk Moderate risk |
| Hol et al (2010) (Netherlands) 39 | Observational study | FS vs FIT | Post-study questionnaire: FS (852/1124), FIT (530/659). Age of participants: 50–74 | Screening discomfort | FS participants reported greater discomfort and
embarrassment mean scores (p <
0.001). |
Moderate risk |
| Scott et al (2004) (Australia)
20
Pickhardt et al (2003) (USA) 38 Gareen et al (2015) (USA) 35 |
RCT Observational study Observational study |
CT colonography vs colonoscopy CT colonography vs colonoscopy CT colonography vs colonoscopy |
62 participants returned post-study questionnaire. Age of
participants: 50–54 years and 65–69
years. Sample size invitees: 1233 (81.5% returned post-study questionnaire). Sample size invitees: 2310 participants. |
Screening preference Screening preference Screening preference |
CT colonography (39%), colonoscopy (61%), p = 0.075, not
significant. CT colonography participants reported greater preference (49.8% vs 41.1%, p = 0.004). CT colonography: 46.6%, (95% confidence interval [CI]: 44.5% −48.7%), colonoscopy: 25.0%, (95% CI: 23.3%–26.9%). |
High risk Low risk High risk |
| Groth et al (2012) (Germany)
33
Voska et al (2019) (Czech Republic) 40 |
Observational study Observational study |
Capsule endoscopy vs colonoscopy Capsule endoscopy vs colonoscopy |
Sample size invitees: 147 Sample size invitees: 225 |
Screening preference Screening preference |
Capsule endoscopy (70.6%), colonoscopy
(29.4%) Capsule endoscopy (47.0%), colonoscopy (53.0%). |
Low risk Moderate risk |
| Scott et al (2004) (Australia)
20
Forbes et al (2006) (Australia) 21 Wijkerslooth et al (2011) (Netherlands) 34 Gareen et al (2015) (USA) 35 |
RCT RCT Observational study Observational study |
CT colonography vs colonoscopy CT colonography vs colonoscopy CT colonography vs colonoscopy CT colonography vs colonoscopy |
Post-study questionnaire: CT colonography (n = 56),
colonoscopy (n = 95). Post-study questionnaire: CT colonography (n = 37/38), colonoscopy (n = 62/63). Participants returned post-study questionnaire: CT colonography (n = 801/982), colonoscopy (n = 1009/1276). Sample size invitees: 2310 participants. |
Willingness to repeat Willingness to repeat Willingness to repeat Willingness to repeat |
Acceptability measured using median 100-point analogue
scores (0 = most favourable, 100 = least favourable).
Colonoscopy (4.5), CT colonography
(11.0). Acceptability measured using median 100-point analogue scores (0 = most favourable, 100 = least favourable). CT colonography (10), Colonoscopy (4). CT colonography (93%), colonoscopy (96%), p = 0.99, not significant. Colonoscopy participants reported greater willingness to screen (96.6% vs 79%%, p < 0.001). |
High risk High risk High risk High risk |
| Kirkoen et al (2017) (Norway)
25
Hol et al (2010) (Netherlands) 39 |
RCT Observational study |
FS vs FIT FS vs FIT |
Post-study questionnaire: FS (528), FIT (356) Age of participants: 50–74 Post-study questionnaire: FS (852/1124), FIT (530/659). Age of participants: 50–74 |
Willingness to repeat Willingness to repeat |
FS (90%), FIT (95%), not statistically
significant. FIT (94.0%), FS (83.8%). |
High risk Moderate risk |
| Forbes et al (2006) (Australia)
21
Nicholson and Korman (2005) (Australia) 36 |
RCT Observational study |
FS vs colonoscopy FS vs colonoscopy |
Sample size invitees: FS (39), colonoscopy
(63). Sample size invitees: FS (191), colonoscopy (256). |
Willingness to repeat Willingness to repeat |
Acceptability measured using median 100-point analogue
scores (0 = most favourable, 100 = least favourable). FS
(5), Colonoscopy (4). FS (97.5%), colonoscopy (99.5%). |
High risk Low risk |
| Groth et al (2012) (Germany)
33
|
Observational study | Capsule endoscopy vs colonoscopy | Sample size invitees: 147 |
Willingness to repeat | Capsule endoscopy (87%), colonoscopy (94%). | Low risk |
CT: computed tomography, BP: bowel preparation, r-CT: reduced computed tomography, f-CT: full computed tomography, FS: flexible sigmoidoscopy, FIT: faecal immunochemical test.
Study quality
All of the randomised studies obtained a high risk of bias from the Cochrane RoB 2 tool, 18 as shown in Table 3, since participants were aware of their assigned intervention during the study. According to the ROBINS_I tool 19 shown in Table 4, two studies were assessed to be at high risk,34–35 four studies moderate risk32,37,39–40 and three studies low risk.33,36,38 In two studies, participants were informed of their screening result prior to completing the questionnaire, which might have influenced the responses.39–40
Table 3.
Risk of bias in randomised studies assessed by Cochrane Risk of bias 2.
| Randomisation bias | Intended intervention bias | Missing data bias | Outcome bias | Reporting bias | Overall bias | |
|---|---|---|---|---|---|---|
| Scott 2004 20 | + | − | + | + | + | − |
| Forbes 2006 21 | + | − | + | + | + | − |
| Stoop 2011 22 | + | − | + | + | + | − |
| You 2015 23 | + | − | − | − | − | − |
| Sali 2016 24 | + | − | + | + | + | − |
| Kirkoen 2017 25 | + | − | + | + | + | − |
| Segnan 2007 26 | + | − | + | + | + | − |
| Hol 2009 27 | + | − | + | + | + | − |
| Randel 2021 28 | + | − | + | + | + | − |
| Symonds 2019 29 | + | − | + | + | + | − |
| Young 2021 30 | + | − | + | + | + | − |
| Liles 2016 31 | + | − | + | + | + | − |
+ = Low risk, – = High risk.
Table 4.
Risk of bias in non-randomised studies assessed by ROBINS_I tool.
| Confounding bias | Participant selection bias | Intervention classification bias | Deviations from intervention bias | Missing data bias | Outcome bias | Reported bias | Overall bias | |
|---|---|---|---|---|---|---|---|---|
| Ioannou 32 2021 | Low | Low | Low | Low | Low | Low | Moderate | Moderate |
| Groth 33 2012 | Low | Low | Low | Low | Low | Low | Low | Low |
| Wijkerslooth 34 2011 | Low | Low | Low | Low | Low | Low | High | High |
| Gareen 35 2015 | Low | Low | Low | Low | Low | Low | High | High |
| Nicholson 36 2005 | Low | Low | Low | Low | Low | Low | Low | Low |
| Senore 37 2011 | Low | Low | Low | Low | Low | Low | Moderate | Moderate |
| Pickhardt 38 2003 | Low | Low | Low | Low | Low | Low | Low | Low |
| Hol 39 2010 | Low | Low | Low | Low | Low | Low | Moderate | Moderate |
| Voska 40 2019 | High | Low | Low | Low | Low | Low | Moderate | Moderate |
Uptake
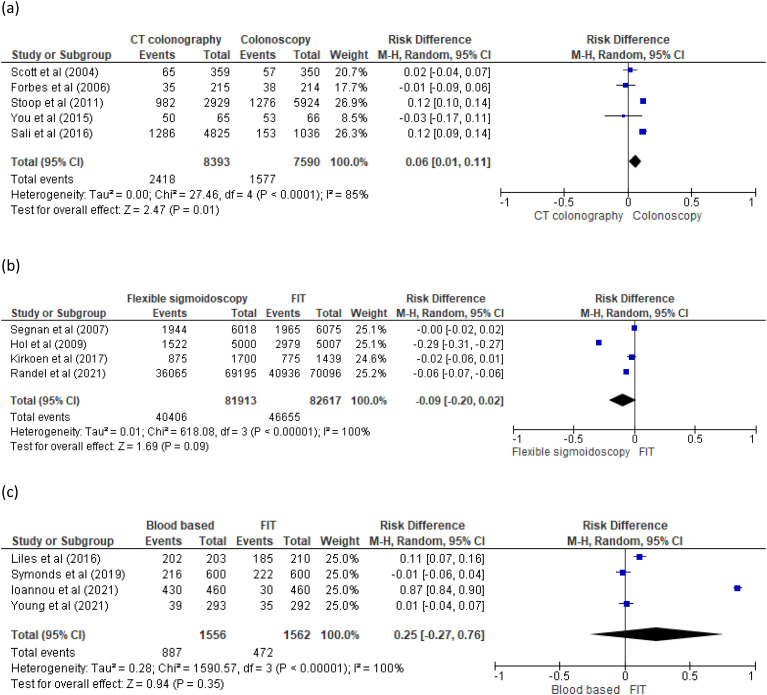
Five RCT studies compared uptake between CT colonography and colonoscopy20–24 (Figure 2(a)). In two studies,20–21 differences in observed uptake were not statistically significant. In a third study by You, 23 the trial was stopped early; there was insufficient statistical power to detect relevant differences in uptake. In Stoop’s study, 22 differences in observed uptake were statistically higher in CT colonography than colonoscopy (34% vs. 22%, p < 0.001). Similarly, differences in observed uptake in Sali’s study 24 were significantly higher in both reduced (28.1%, p < .001) and full-preparation (25.2%, p < .001) CT colonography over colonoscopy (14.8%).
Figure 2.
(a) Pooled results of uptake for computed tomography (CT) colonography and colonoscopy. (b) Pooled results of uptake for flexible sigmoidoscopy (FS) and FIT. (c) Pooled results of uptake for blood based and faecal immunochemical test (FIT).
Four RCT studies compared uptake of FS and FIT25–28 (Figure 2(b)). Uptake was higher with FIT compared to FS in two studies,27–28 which totalled 150,000 participants. The other two studies25–26 found no significant differences, but their combined sample size only accounted for a tenth of the total. In this review, Hol’s study 27 was the only comparator of FS and FIT which included socio-economic status as a baseline characteristic. The results found participants from a higher socio-economic group were more likely to take part in both FS and FIT screening (p < 0.05). Three RCTs29–31 and one observational 32 study compared uptake of blood-based test and FIT (Figure 2(c)). In two of these studies,29–30 differences in observed uptake were not significant. In the third study, by Liles, 31 differences in observed uptake were higher in blood-based test than FIT (99.5% vs. 88.1%, p < 0.001). In the fourth study, by Ioannou, 32 there was no p-value stated to determine statistical difference.
Two RCTs compared uptake of CT colonography and FOBT.21,23 Differences in observed uptake were not significant in Forbes 21 study and in You’s study 23 the trial was stopped early. Segnan’s RCT 26 was the only study that compared FS and colonoscopy uptake. After adjustment for demographic variables, the uptake was significantly higher in FS than colonoscopy (OR, 0.74; 95 percent CI: 0.68–0.80). Groth’s observational study 33 was the only study that compared colon capsule endoscopy and colonoscopy uptake. The differences in observed uptake were not significant. Sali’s RCT 24 was the only study that compared CT colonography and FIT. The differences in observed uptake were higher with FIT (50.4%, p < 0.001) than both reduced (28.1%) and full-preparation (25.2%) CT colonography.
Bowel preparation associated discomfort
Two observational studies34–35 compared bowel preparation discomfort associated with CT colonography and colonoscopy. In Wijkerslooth’s study, 34 bowel preparation being found burdensome was significantly higher for colonoscopy than CT colonography (73% vs. 32%, p < 0.001). They suggest this may be due to the increased fluid intake before colonoscopy, as opposed to the limited bowel preparation in CT colonography. In Gareen’s study, 35 differences in bowel preparation discomfort were not statistically significant. Two observational studies36–37 compared bowel preparation discomfort of FS and colonoscopy. In Nicholson’s study, 36 bowel preparation discomfort was ranked the worst aspect in both colonoscopy (78%) and FS (31%) procedures (p < 0.02). In Senore’s study, 37 differences in bowel preparation discomfort scores were not statistically significant.
Screening procedure associated discomfort
Four studies compared screening procedure discomfort between CT colonography and colonoscopy.20–21,34,38 In two studies, discomfort was significantly higher for CT colonography than colonoscopy (p value<0.001).34,38 In the other two studies, differences in median pain scores were not statistically significant.20–21 Three studies compared screening procedure discomfort between FS and colonoscopy.21,36–37 In two studies, differences in pain scores were not significant.21,37 In Nicholson’s study, 36 differences in discomfort were significantly higher for FS than colonoscopy (p < 0.001). Hol’s study 39 was the only study that compared pain scores between FS and FIT. The mean pain score was unsurprisingly significantly higher with FS than FIT (p < 0.001).
Screening preference
Three studies compared screening preference between CT colonography and colonoscopy.20,35,38 In two studies,20,35 differences in screening preference were not significant. A questionnaire was used to capture participants’ reasons for choosing a certain modality.20,33 The reasons participants chose colonoscopy in Scott’s study 20 included there was no obligation for a second procedure, they expected a more detailed examination and a preference for sedation. Conversely, the reasons participants chose CT colonography in Scott’s study 20 included they expected it to take less time, be less painful, be less risky and not require sedation. In Pickhardt’s study, 38 differences in screening preference were significantly higher for CT colonography than colonoscopy (p = 0.004).
Two studies compared screening preference between capsule endoscopy and colonoscopy.33,40 In both studies, differences in screening preference were not statistically significant. In Groth’s study, 33 the reasons participants preferred capsule colonoscopy were captured from questionnaires, and included that it sounded more pleasant, were afraid of colonoscopy pain, were afraid of sedation, and were afraid of colonoscopy problems. The reasons participants’ favoured colonoscopy were because it allowed for biopsy and polypectomy in a single procedure and is the standard method.
Willingness to repeat screening modality at recommended screening interval
Four studies compared willingness to repeat screening between CT colonography and colonoscopy.20–23,34–35 In three of the studies, there was no significant difference in willingness to repeat.20–21,34 In Gareen's study, 35 the willingness to repeat colonoscopy was significantly higher than CT colonography (96.6% vs. 79%%, p < 0.001). Two studies compared willingness to repeat screening between FS and FIT25,29 and neither study found a significant difference. Two studies compared willingness to repeat screening between FS and colonoscopy21,36 and found no significant difference. The only study that evaluated willingness to repeat screening between colon capsule endoscopy and colonoscopy was Groth's 33 and showed no significant difference.
Discussion
This systematic review suggests that though there was no significant overall difference in acceptability between alternative modalities of screening, FIT seemed more acceptable than FS as evidenced by higher uptake and less discomfort experienced by participants. This review’s findings are in agreement with Stracci, 41 who indicated FIT as being a more widely accepted screening test than FS.
The findings of Hol’s study 27 are comparable to those of the English Bowel Screening Programme study, 42 which found those participants in the least deprived areas were more likely to participate (53.2%) in FS screening than those in the most deprived areas (32.7%). Previous synthesis of evidence has included these modalities in non-screening cohorts, e.g. for early detection of CRC in symptomatic patient groups where other and willingness to accept discomfort.11–12,43 Mutneja's meta-analysis 44 did not include Kirkoen’s study 25 and compared an additional two studies that were not eligible in this review. The bowel preparation requirement was found to be a common barrier to completing a colonoscopy. The percentage of participants in Wijkerslooth’s study 34 who declined screening due to inconvenience of bowel preparation was significantly higher for colonoscopy than for CT colonography. This review's findings are similar to those of Cash's randomised trial, 45 which found no significant differences in screening preference between colonoscopy, colon capsule endoscopy and CT colonography.
This systematic review has a number of strengths. Firstly, a range of alternative screening modalities were compared to current standard of care screening by colonoscopy and FIT. Secondly, this review compared several parameters of acceptability measures, which included uptake, bowel preparation, screening discomfort, screening preference and willingness to repeat modality. Thirdly, this review focused on average-risk CRC individuals to understand their views of screening as opposed to those at high risk, who may be more motivated and consequently more likely to participate in screening anyway. Lastly, this review focused on studies of actual screening participants rather than studies of hypothetical screening scenarios or discrete choice experiments.
There are some limitations to this systematic review. There can be no definitive conclusion drawn on acceptability of alternative modality, because only a limited number of studies that fitted the inclusion criteria were possible to analyse. Secondly, the 21 studies included in the review were heterogeneous in study design, screening comparison and sample size, which were all limiting factors for why a meta-analysis was not conducted. Thirdly, studies differed as to where participants completed the post-screening questionnaire: at their homes or in hospital. Completing the questionnaire in a hospital setting may potentially influence a participant’s response. Studies varied as to whether or not they informed participants of their screening result before completing the questionnaire, which could potentially influence participants’ responses. Finally, there was no uniform reporting on the acceptability measures, which included use of a Likert scale, percentages and median 100-point analogue score. This made it difficult to interpret the significance of the results.
Due to the limited number of studies in each screening comparison, no definitive conclusion can be drawn on most acceptable alternative modality. The lack of significance in the study outcomes could be due to the specific nature of study population, small study sample sizes, mixture of study designs, different healthcare systems and limited context of demographic differences in the populations studied (different countries, ages, and socio-economic status). Other factors such as insurance status in some jurisdictions may also have an influence on screening modality preference.46–47 However, this research adds to the limited evidence regarding bowel preparation acceptance, screening preference and willingness to repeat modality for non-invasive modalities. In the future, larger well-designed studies are needed comparing alternative CRC modalities with FIT and/or colonoscopy in order to facilitate meaningful comparison and complete a meta-analysis. Further qualitative studies are needed to explore compliance with bowel preparation, participants’ screening preferences, and reasons for non-uptake in standard screening and alternative modality. In addition, future studies should include qualitative research analysing the acceptability of alternative screening modalities among individuals who are less likely to engage in routine CRC screening.
Several factors need to be considered before the consideration of colon capsule endoscopy and blood-based screening as population-based screening. These include a cost-effectiveness analysis, resource availability, views of healthcare organisations, practical implementation, need for subsequent second procedures and patient preferences. We believe this review is relevant to inform the context when there is increasing focus on blood-based cancer screening (including multiple cancer screening and early detection) tests as well as colon capsule endoscopy as potential screening modalities for CRC. It highlights the complex interplay between the effectiveness and acceptability of various tests and in different populations.
Supplemental Material
Supplemental material, sj-docx-1-msc-10.1177_09691413221109999 for Acceptability of alternative technologies compared with faecal immunochemical test and/or colonoscopy in colorectal cancer screening: A systematic review by Omar Ali, Sunnia Gupta, Kate Brain, Kate J Lifford, Shantini Paranjothy and Sunil Dolwani in Journal of Medical Screening
Supplemental Material
Supplemental material, sj-docx-2-msc-10.1177_09691413221109999 for Acceptability of alternative technologies compared with faecal immunochemical test and/or colonoscopy in colorectal cancer screening: A systematic review by Omar Ali, Sunnia Gupta, Kate Brain, Kate J Lifford, Shantini Paranjothy and Sunil Dolwani in Journal of Medical Screening
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Tenovus Cancer Care.
ORCID iD: Omar Ali https://orcid.org/0000-0002-2778-3418
Supplemental material: Supplemental material for this article is available online.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2.Issa IA, Noureddine M. Colorectal cancer screening: an updated review of the available options. J Gastro 2017; 23: 5086–5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katsoula A, Paschos P, Haidich AB, et al. Diagnostic accuracy of fecal immunochemical test in patients at increased risk for colorectal cancer. J Am Med Ass 2017; 177: 1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut 2015; 64: 1637–1649. [DOI] [PubMed] [Google Scholar]
- 5.Tepus M, Yau T. Non-invasive colorectal cancer screening: an overview. Gast Tum 2020; 7: 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson JMG, Jungner G. Principles and practice of screening for disease. World Health Organization 1968. [Google Scholar]
- 7.Huang JL, Fang Y, Liang M, et al. Approaching the hard-to-reach in organized colorectal cancer screening: an overview of individual, provider and system level coping strategies. AIMS Pub Health 2017; 4: 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayhand KN, Handorg EA, Gonzalez ET, et al. Effect of neighborhood and individual-level socioeconomic factors on colorectal cancer screening adherence. Int J Environ 2021; 18: 4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu X, Parks PD, Weiser E, et al. Barriers to utilization of three colorectal cancer screening options – data from a national survey. Prev Med Rep 2021; 24: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrari A, Neefs I, Hoeck S, et al. Towards novel non-invasive colorectal cancer screening methods: a comprehensive review. Cancers (Basel) 2021; 13: 1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghanouni A, Smith SG, Halligan S, et al. Public perceptions and preferences for CT colonography or colonoscopy in colorectal cancer screening. Patient Educ Couns 2012; 89: 116–121. [DOI] [PubMed] [Google Scholar]
- 12.Lin OS, Kozarek RA, Gluck M, et al. Preference for colonoscopy versus computerized tomographic colonography: a systematic review and meta-analysis of observational studies. J Gen Intern Med 2012; 27: 1349–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khalid-de Bakker C, Jonkers D, Smits K, et al. Participation in colorectal cancer screening trials after first-time invitation: a systematic review. Endoscopy 2011; 43: 1059–1086. [DOI] [PubMed] [Google Scholar]
- 14.Zhu H, Li F, Tao K, et al. Comparison of the participation rate between CT colonography and colonoscopy in screening population: a systematic review and meta-analysis of randomized controlled trials. Br J Radiol 2020; 93(1105): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J 2021; 372(71): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan — a web and mobile app for systematic reviews. Syst Rev 2016; 5(210): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Popay J, Roberts H, Sowden A, et al. Guidance on the conduct of narrative synthesis in systematic reviews: a product from the ESRC methods programme. Lancaster, UK: University of Lancaster, 2006. [Google Scholar]
- 18.Sterne JAC, Savović J, Page MJ, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J 2019; 366(4898): 1–8. [DOI] [PubMed] [Google Scholar]
- 19.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J 2016; 355(4919): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott RG, Edwards JT, Fritschi L, et al. Community-based screening by colonoscopy or computed tomographic colonography in asymptomatic average-risk subjects. Am J Gastroenterol 2004; 99: 1145–1151. [DOI] [PubMed] [Google Scholar]
- 21.Forbes GM, Mendelson RM, Edwards JT, et al. A comparison of colorectal neoplasia screening tests: a multicentre community–based study of the impact of consumer choice. Med J Aust 2006; 184: 546–550. [DOI] [PubMed] [Google Scholar]
- 22.Stoop EM, de Haan MC, de Wijkerslooth TR, et al. Participation and yield of colonoscopy versus non-cathartic CT colonography in population-based screening for colorectal cancer: a randomised controlled trial. Lancet Oncol 2012; 13: 55–64. [DOI] [PubMed] [Google Scholar]
- 23.You JJ, Liu Y, Kirby J, et al. Virtual colonoscopy, optical colonoscopy, or fecal occult blood testing for colorectal cancer screening: results of a pilot randomized controlled trial. Trials 2015; 16(1): 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sali L, Mascalchi M, Falchini M, et al. Reduced and full-preparation CT colonography, fecal immunochemical test, and colonoscopy for population screening of colorectal cancer: a randomized trial. J NCI 2015; 108(2): 1–8. [DOI] [PubMed] [Google Scholar]
- 25.Kirkøen B, Berstad P, Botteri E, et al. Acceptability of two colorectal cancer screening tests: pain as a key determinant in sigmoidoscopy. Endoscopy 2017; 49: 1075–1086. [DOI] [PubMed] [Google Scholar]
- 26.Segnan N, Senore C, Andreoni B, et al. Comparing attendance and detection rate of colonoscopy with sigmoidoscopy and FIT for colorectal cancer screening. Gastroenterology 2007; 132: 2304–2312. [DOI] [PubMed] [Google Scholar]
- 27.Hol L, van Leerdam ME, van Ballegooijen M, et al. Screening for colorectal cancer: randomised trial comparing guaiac-based and immunochemical faecal occult blood testing and flexible sigmoidoscopy. Gut 2009; 59: 62–68. [DOI] [PubMed] [Google Scholar]
- 28.Randel KR, Schult AL, Botteri E, et al. Colorectal cancer screening with repeated fecal immunochemical test versus sigmoidoscopy: baseline results from a randomized trial. Gastroenterology 2021; 160: 1085–1096. [DOI] [PubMed] [Google Scholar]
- 29.Symonds EL, Hughes D, Flight I, et al. A randomized controlled trial testing provision of fecal and blood test options on participation for colorectal cancer screening. Cancer Prev Res 2019; 12: 631–640. [DOI] [PubMed] [Google Scholar]
- 30.Young GP, Chen G, Wilson CJ, et al. “Rescue” of nonparticipants in colorectal cancer screening: a randomized controlled trial of three noninvasive test options. Cancer Prev Res 2021; 14: 803–810. [DOI] [PubMed] [Google Scholar]
- 31.Liles EG, Coronado GD, Perrin N, et al. Uptake of a colorectal cancer screening blood test is higher than of a fecal test offered in clinic: a randomized trial. Cancer Treat Res Commun 2017; 10: 27–31. [Google Scholar]
- 32.Ioannou S, Sutherland K, Sussman DA, et al. Increasing uptake of colon cancer screening in a medically underserved population with the addition of blood-based testing. BMC Cancer 2021; 21(1): 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Groth S, Krause H, Behrendt R, et al. Capsule colonoscopy increases uptake of colorectal cancer screening. BMC Gastroenterol 2012; 12(1): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Wijkerslooth TR, de Haan MC, Stoop EM, et al. Burden of colonoscopy compared to non-cathartic CT-colonography in a colorectal cancer screening programme: randomised controlled trial. Gut 2011; 61: 1552–1559. [DOI] [PubMed] [Google Scholar]
- 35.Gareen I, Siewert B, Vanness D, et al. Patient willingness for repeat screening and preference for CT colonography and optical colonoscopy in ACRIN 6664: the national CT colonography trial. Patient Prefer Adherence 2015; 9: 1043–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicholson FB, Korman MG. Acceptance of flexible sigmoidoscopy and colonoscopy for screening and surveillance in colorectal cancer prevention. J Med Screen 2005; 12: 89–95. [DOI] [PubMed] [Google Scholar]
- 37.Senore C, Correale L, Regge D, et al. Flexible sigmoidoscopy and CT colonography screening: patients’ experience with and factors for undergoing screening - insight from the Proteus colon trial. Radiology 2018; 286: 873–883. [DOI] [PubMed] [Google Scholar]
- 38.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. NEJM 2003; 349: 2191–2200. [DOI] [PubMed] [Google Scholar]
- 39.Hol L, de Jonge V, van Leerdam ME, et al. Screening for colorectal cancer: comparison of perceived test burden of guaiac-based faecal occult blood test, faecal immunochemical test and flexible sigmoidoscopy. EJC 2010; 46: 2059–2066. [DOI] [PubMed] [Google Scholar]
- 40.Voska M, Zavoral M, Grega T, et al. Accuracy of colon capsule endoscopy for colorectal neoplasia detection in individuals referred for a screening colonoscopy. Gastroenterol Res Pract 2019: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stracci F, Zorzi M, Grazzini G. Colorectal cancer screening: tests, strategies, and perspectives. Public Health Front 2014; 2: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koo S, Neilson LJ, Von Wagner C, et al. The NHS bowel cancer screening program: current perspectives on strategies for improvement. Risk Manag Healthc Policy 2017; 10: 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deding U, Valdivia PC, Koulaouzidis A, et al. Patient-reported outcomes and preferences for colon capsule endoscopy and colonoscopy: a systematic review with meta-analysis. Diagnostics 2021; 11(9): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mutneja H, Agrawal R, Bhurwal A, et al. Comparative effectiveness of fecal immunochemical tests versus flexible sigmoidoscopy for colorectal cancer screening: a systematic review and meta-analysis of randomized clinical trials. JGLD 2021; 30(2): 267–273. [DOI] [PubMed] [Google Scholar]
- 45.Cash BD, Fleisher MR, Fern S, et al. Multicentre, prospective, randomised study comparing the diagnostic yield of colon capsule endoscopy versus CT colonography in a screening population (the TOPAZ study). Gut 2020; 70: 2115–2122. [DOI] [PubMed] [Google Scholar]
- 46.Zhu X, Parks PD, Weiser E, et al. National survey of patient factors associated with colorectal cancer screening preferences. Cancer Prev Res 2021; 14: 603–614. [DOI] [PubMed] [Google Scholar]
- 47.Wolf RL, Basch CE, Zybert P, et al. Patient test preference for colorectal cancer screening and screening uptake in an insured urban minority population. J Community Health 2015; 41: 502–508. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-msc-10.1177_09691413221109999 for Acceptability of alternative technologies compared with faecal immunochemical test and/or colonoscopy in colorectal cancer screening: A systematic review by Omar Ali, Sunnia Gupta, Kate Brain, Kate J Lifford, Shantini Paranjothy and Sunil Dolwani in Journal of Medical Screening
Supplemental material, sj-docx-2-msc-10.1177_09691413221109999 for Acceptability of alternative technologies compared with faecal immunochemical test and/or colonoscopy in colorectal cancer screening: A systematic review by Omar Ali, Sunnia Gupta, Kate Brain, Kate J Lifford, Shantini Paranjothy and Sunil Dolwani in Journal of Medical Screening