Abstract
The application of black soldier fly (BSF), Hermetia illucens based technology to process organic wastes presents a practical option for organic waste management by producing feed materials (protein, fat), biodiesel, chitin and biofertilizer. Therefore, BSF organic wastes recycling is a sustainable and cost-effective process that promotes resource recovery, and generates valuable products, thereby creating new economic opportunities for the industrial sector and entrepreneurs. Specifically, we discussed the significance of BSF larvae (BSFL) in the recycling of biowaste. Despite the fact that BSFL may consume a variety of wastes materials, whereas, certain lignocellulosic wastes, such as dairy manure, are deficient in nutrients, which might slow BSFL development. The nutritional value of larval feeding substrates may be improved by mixing in nutrient-rich substrates like chicken manure or soybean curd residue, for instance. Similarly, microbial fermentation may be used to digest lignocellulosic waste, releasing nutrients that are needed for the BSFL. In this mini-review, a thorough discussion has been conducted on the various waste biodegraded by the BSFL, their co-digestion and microbial fermentation of BSFL substrate, as well as the prospective applications and safety of the possible by-products that may be generated at the completion of the treatment process. Furthermore, this study examines the present gaps and challenges on the direction to the efficient application of BSF for waste management and the commercialization of its by-products.
Keywords: Food waste, manure, waste management, fermentation, black soldier fly
Introduction
Food security risks
Food security is critical for the survival of all humans and animals. It is considered to be reached when all people have access to enough nutritious food that is available at all times and meets their dietary needs and food preferences in order to enjoy an active and healthy life (Clapp et al., 2022; Mc Carthy et al., 2018). It is becoming more difficult for the gradually increasing world’s population to meet its expanding needs for meat, milk and dairy products, as well as water and land resources (Barrett, 2021; Kwasek, 2013). The biggest threats to food security are climate change, biodiversity loss in agriculture and the spread of plant and animal diseases. These factors will have an unfavourable influence on food security across the world (Clark et al., 2020; Drew et al., 2020).
Changes in climate poses a major challenge to the food and nutritional security across the globe. Human activities have had a considerable impact on the global atmospheric composition during the last several decades, resulting in dramatic climatic shifts throughout the world (Malhi et al., 2021). Greenhouse gases (GHGs), such as methane (CH4), carbon dioxide (CO2) and nitrous oxide (N2O) concentrations, have risen by 150, 40 and 20% since the year 1750, respectively (Zheng et al., 2019). Emissions of CO2, a major GHG, risen from 22.2 billion metric tonnes in 1990 to 36.2 billion metric tonnes in 2014 (Clark et al., 2020; Malhi et al., 2021). The worldwide average temperatures are forecast to rise by 2°C by 2100, due to the increase in the GHG emission from land clearing and deforestation, fossil fuels, agrochemical and fertilizer, enteric fermentation of ruminants and livestock manure (Clark et al., 2020; McGlade and Ekins, 2015; Welsby et al., 2021), whereas, agricultural productivity is very sensitive to even 2°C increase, which has huge consequences for poverty and food security (Clark et al., 2020; Malhi et al., 2021). The rise in CO2 concentration leads to greater plant growth and production because of an increase in photosynthesis, but an increase in temperature negates this impact by increasing crop respiration and evapotranspiration, increasing insect infestation, shifting the vegetation flora and shortening crop duration (Berhane, 2018; Malhi et al., 2021). Moreover, the change in the climate conditions has affected soil microbial populations and their enzymatic activity (Malhi et al., 2021; Willett et al., 2019).
One of the most critical determinants of a human’s destiny is access to clean water. The world’s water resources are estimated to be roughly 1385 million km3 (Alterta Water Portal, 2012; Kwasek, 2013; Souissi et al., 2019). It was estimated that 97% of the world’s water resources are salt water, whereas just 3% are fresh water, and 69% of fresh water is found in glaciers and icecaps, whereas 30% is found in the ground water (Chang, 2019; Kwasek, 2013; United Nations, 2020). Therefore, quantity of accessible drinking water accounts for 1% of the world’s total available water resources (Nebraska–Lincoln, 2014). The climate change results in the decrease of water resources globally. Due to climate change, it is predicted that the demand for water would rise in conjunction with the growth of the human population. On a global scale, the quantity of water necessary to feed the globe in 2050 would represent a 4500 km3/year rise from the current 7000 km3/year requirement (Berhane, 2018; Souissi et al., 2019). The demand of water is increasing at alarming rate due to the increase in the population, especially in developing countries. It was noted that in order to produce 1 kg of beef, 15,415 L water required; for 1 kg of chicken, 4325 L of water is needed; for one egg, 196 L water required and for 1 kg of wheat, 1500 L of water are required (Ebikeme, 2013). The water scarcity is a major problem restricting global food production growth, according to the Food and Agriculture Organization (FAO). The agriculture sector consumes 70% of the world’s fresh water supplies, and climate change is exacerbating the situation (Kwasek, 2013). Agricultural production, environmental protection and global food security are all threatened as a consequence of global climate change. Crop and animal production, water balances, agricultural inputs, natural resources and other agricultural system components are all influenced by it. It has serious repercussions in undeveloped countries because to a lack of capacity to adjust to changing environmental conditions (Berhane, 2018). It has an impact on crop growth and livestock and poultry production, water availability and the water balance in the soil, either directly or indirectly.
Multiple sources of GHG emissions are produced by the global food system. Among the most significant are the burning of fossil fuels, which release CO2 (McGlade and Ekins, 2015); manufacture and use of fertilizers and other agrichemicals, which release CO2, N2O and CH4; land clearing and urbanization/deforestation emit CO2 and N2O; fermentation in the ruminant digestive system (cattle, goat and sheep), which releases CH4 and livestock manure, which emit N2O and CH4 (Clark et al., 2020). It has determined that emissions from fossil fuels are the major driver of global warming, 89% of worldwide CO2 emissions originated from fossil fuels and industry (Olivier and Peters, 2020; Soeder, 2021; Welsby et al., 2021). Moreover, the production of 110 million tonnes of chemical fertilizer globally contributes to 14–17% of the total GHG (Gerber et al, 2013; Ravindran, 2013), causing significant environmental pollution and climate change. The world’s food sector produced 17 billion tonnes of CO2 annually (Clark et al., 2020), the animal-based food production accounted for 57% of total emissions, whereas plant-based food production accounted for 29% (Charles, 2021; Clark et al., 2020; Xu et al., 2021). Crops other than food, such cotton, were responsible for the remaining of the emissions (Charles, 2021).
Moreover, it is predicted that the world human population will rise from 7.6 to 8.6 billion by 2030, 9.8 billion by 2050 and 11.2 billion by 2100 (Skaf et al., 2021; United Nations, 2017). The level of urbanization will increase up to 70% in 2050 (Estrada et al., 2011), projected as a third key determinant of future demands for expansion is income increase, which implies an average growth of 2.9% during the period of 2005–2050 (Mensbrugghe et al., 2009). This increase in the population, urbanization and income will change the nutritional consumption habit, thus resulting in the profound change of the global food system. The demand for animal protein, such as meat and milk, is expected to be 58 and 70% higher, respectively, in 2050 compared to the demand in 2010. This increased consumption will be supposedly higher in the developing countries, as the population pressure is expected to be higher in that part of the world (Estrada et al., 2011; Makkar et al., 2014).
Currently, 40% of the world’s ice-free land is utilized for agriculture, whereas 70% of total agricultural land is utilized to feed our livestock. Additionally, around 70% of total freshwater is used for agriculture, and 30% of all grains are fed to domestic animals (soyabean: 80%; corn: 50%) (FAO, 2009; Foley et al., 2011). Furthermore, between 2012 and 2013, around 795 million tonnes of cereals were produced worldwide, of which approximately 1/3 was used to feed livestock. However, in 2050, it is expected that these numbers will increase, meaning that around 50% of 1315 million tonnes of grown cereals will be used as a protein source for livestock (Rapatsa and Moyo, 2017; Rehman and Hollah, 2020). Therefore, an alternative source of protein used to feed livestock that generate less GHGs and alternative biofertilizer to replace chemical fertilizers is needed to safeguard the earth environment and better use resources to feed the human population.
Progress in bioconversion of livestock manure (dairy and chicken)
With the increase of intensive and centralized livestock production, people are more aware of and pay attention to the rational management of livestock manure (dairy and chicken). The tonnes of manure generated by intensive dairy and chicken farming will spill into the waterways from leaking into manure pits, fields, seepage lagoons and pits, or applied on the field as fertilizer (Petersen et al., 2007; Rico et al., 2015). Intensive livestock and poultry farming generates a massive quantity of manure with high organic matter and mineral loading and has an environmental impact (Ledda et al., 2016). The contribution of livestock manure to environmental pollution is mainly related to the ammonia and nitrogen oxides released into the atmosphere, leaching nitrate into ground and surface water (Ledda et al., 2016). In addition, according to the data from the Intergovernmental Panel on Climate Change, in the storage process of manure, the production of methane and other GHGs is 21 times higher than the global warming potential of carbon dioxide (Rico et al., 2015). Moreover, the production of chicken results in the production of manure, of which nutrients need to be appropriately recycled. Otherwise, they can represent potential environmental and human health hazards as a source of undesired elements (nutrients and heavy metals), components (including veterinary drugs), insects and insect pests, and pathogens.
The present manure management strategies result in serious eutrophication of rivers and lakes, which is characterized by the high concentration of undesired nutrients caused by the ecological imbalance of the water system (Salvador et al., 2016), causing unusually high levels of algae and aquatic plant growth, such as water hyacinth (Girotto and Cossu, 2017). This reduces the oxygen content in the water and seriously impacts the survival of other organisms in the system. In addition, surface and groundwater can be contaminated by the leaching and runoff of manure nutrients, which increases the need for water purification treatment to provide safe drinking water (Ledda et al., 2016; Riaño and García-González, 2015). The direct discharge of groundwater seepage channel and faeces, usually in the bypass flow through the slit, puts human and animal health at great risk since livestock and poultry manure contain the large number of pathogens (bacteria, viruses and parasites). Some natural zoonotic pathogens, for example, Escherichia coli, Salmonella, Tuberculosis bovi, Leptospira, Campylobacter, Shigella, Listeria, Hepatitis A, Cryptosporidium, Nipah virus, Rotavirus and Avian influenza, may be transmitted to humans, causing systemic or local infection (Stanley et al., 1998; Venglovsky et al., 2009).
Current dairy manure (DM) management practices include decomposition, anaerobic digestion and composting, aiming to meet environmental regulations. They are expensive and can also lead to diseases, unpleasant odours, GHG emissions, global air and groundwater pollution, particularly in developing and underdeveloped countries (Aguirre-Villegas and Larson, 2017; Lim et al., 2016). In recent eras, the poultry industry has been greatly adjusted to meet the growing demand for a cheap and safe source of eggs and meat. In the past 3 years, the poultry industry recorded more than a 5% annual growth rate, compared to the 3% growth rate of pig meat and 1.5% of the bovine industry. Total global meat production increased from 15 to 30% in the last three decades (Wiedemann et al., 2017). According to statistics, in 2010, the world’s chicken stocks were valued at nearly 20 billion (Nie et al., 2015). With the chicken sales business opportunities to expand, the number of chickens is increasing, followed by many chicken manure (CHM) (Ghafoor et al., 2016). Thus, it can be stated that the growth of the livestock and poultry industry and the trends in intensification and concentration have caused some environmental problems related to manure management.
Conventional methods are mostly used for the disposal of livestock manure, which include processing to biofertilizer (compost, direct application and microbial fermentation), feed and transformation into energy sources (methane, electricity and direct burn). Recent research reported that manure could be used to produce ethanol, but this technology needs to be further investigated and demonstrated (Aguirre-Villegas and Larson, 2017; Lim et al., 2016). Bio-organic fertilizers show good application prospects, but the composting process is complex, and there is a lack of research on the mechanism of biological processes. Consequently, efficient treatment solutions are needed to lessen organic waste’s environmental and economic impacts
Developments in food waste bioconversion
Food waste is a significant component of the organic waste created at an increasing pace across the world (Kim et al., 2021). The rise in living standards and the human population has resulted in an ever-increasing need for food; as a result, food waste is rising at an alarming rate and poses several socio-economic issues (Kim et al., 2021). Meeting the growing demand for food, feed and fuel and, in parallel, handling wastes, particularly organic wastes, has become a major global challenge (Gold et al., 2018; Kumar et al., 2018; Lucifero, 2016), with the rapid increase in the worldwide population, namely 9.7 billion people living on earth in 2050, this situation is expected to aggravate (Duarte et al., 2021; Surendra et al., 2016).
As a result, food waste is gradually growing, contributing to many socio-economic problems. According to the FAO, food waste is defined as wasted food, often during the retail and consumption phases (Liu et al., 2016, 2020). The globe generates more than 2.1 billion tonnes of municipal solid waste every year; 45% of municipal solid waste is made up of food waste; however, only approximately 16% of that waste gets recycled, and more than 46% is wasted (Brás et al., 2020; Deus et al., 2019; Verisk Maplecroft, 2019). Global food systems are becoming increasingly unbalanced due to the growth in food waste, and in the case of hazardous waste, it has the potential to negatively impact human health, biodiversity and ecosystems (Djekic et al., 2019; Verisk Maplecroft, 2019). Food waste is being paid more and more consideration due to its adverse social, environmental and economic impacts (Kim et al., 2021; Loke and Leung, 2015). At present, about 1/3 of food production is equivalent to waste or loss of 1.3 tonnes, with noteworthy environmental, that is, GHG emissions and economic footprints. Without regard to land use variation, the yearly GHG emissions equivalent to food consumption are estimated to be 33 tonnes of carbon dioxide (Cardoen et al., 2015; Kumar et al., 2018). Every year, around 1.3 billion tonnes of food waste is generated, valued at up to 7.5 billion US$ (Li and Shimizu, 2021; Tomberlin et al., 2015). In addition, organic waste handling, especially in developing and underdeveloped countries, has become a severe problem. In the next 25 years, food waste is predicted to rise, mostly due to the expanding economies and populations of Asian countries, for example, between 2005 and 2025, there is a potential that the amount of food waste created in Asian countries would increase from 278 to 416 million tonnes (Kim et al., 2021; Melikoglu et al., 2013).
The management of food waste is more challenging due to its natural and rapid biodegradability (Singh and Kumari, 2019; Yin et al., 2014). The environmental problems associated with the massive generation of food wastes attract considerable attention (Cuadros et al., 2011; Somroo et al., 2019). Meanwhile, food waste is high in water-insoluble components such as protein, fat and carbohydrate, making it a potential environmental hazard (Kim et al., 2021; Li et al., 2016). Moreover, putrefaction may occur easily because of these water-insoluble materials (Chen et al., 2020; Li et al., 2013). This may make it possible to be a suitable medium for microbial growth, with the formation of foul-smelling. Moreover, food waste is currently used as fertilizer, dumped in landfills and burnt to generate carbon dioxide (Li et al., 2013; Prateep Na Talang and Sirivithayapakorn, 2021). Food waste is now disposed of mostly by landfill disposal, incineration and anaerobic digestion; however, new environmentally friendly methods of managing it are required.
Challenges of current organic waste management
Meeting the growing demand for food, feed and fuel and, in parallel, handling a significant amount of waste, particularly organic waste, has become a key global challenge (Surendra et al., 2016). Due to the perishable characteristics, low calorific value and high organic matter content, organic waste is not always easy to dispose of in a sanitary landfill or by incineration. Moreover, landfill gases or GHGs are produced by breaking down organic waste in landfills. In terms of composition, landfill gases are approximately 50% CH4, 50% CO2 and a small quantity of non-methane organic compounds (Coskuner et al., 2020; US EPA, 2021). Methane is a powerful GHG that traps heat in the atmosphere 28–36 times more efficiently than CO2 (US EPA, 2021; Valta et al., 2019). Landfill gases (CH4 and CO2) also lead to air pollution, smog and aggravating asthma (Ali et al., 2021; Gies, 2016).
Managing organic wastes is more challenging due to their large natural and rapid biodegradability (Nanda and Berruti, 2021; Yin et al., 2014). Existing organic waste management practices, specifically landfilling and waste disposal/stabilization through anaerobic digestion and composting, comply with environmental regulations, but are high in costs and additionally induce adverse effects on the environment, such as groundwater and surface water pollution, GHG emissions and animal/human health issues (de Titto and Savino, 2019; Singh et al., 2020; Yin et al., 2014). On the other hand, due to the rapid growth of the global population, it is believed that relative affluence has led to a sharp increase in the demand for food, feed and fuel, as well as the production of wastes. As a response to the growing pressure of organic wastes, major environmental issues are believed to arise in the near future. Therefore, the development of resource utilization strategies for organic waste in the recycling economy is crucial and needs to meet zero waste concepts in the future.
Potential innovative approach for organic waste management
In a growing global food, feed and fuel demand and increasing concern in organic waste management, the use of insects to effectively transform organic waste into these resources seem to be innovative and promising (Ites et al., 2020; Purkayastha and Sarkar, 2021; Wang et al., 2017). Moreover, the organic waste management with insect produce less GHGs emission (Parodi et al., 2020; Van Huis and Oonincx, 2017), reduce ground and surface water pollution by degrading antibiotics (Liu et al., 2021; Mei et al., 2022) and reduce pathogen content (Awasthi et al., 2020). In the context of organic waste valorization, a promising new strategy is a use of organic waste for the production of insects usable for feed and food, recognized as a rich source of protein and fat, which can also be utilized for the production of biodiesel (Rehman et al., 2018; Shorstkii et al., 2020; Singh and Kumari, 2019). Thus, insects represent a single potentially valuable solution for two problems, simultaneously: solving the problem of the increasing organic wastes, which may cause serious environmental issues if not appropriately managed, and the rising demand for feed, food and fuel (Salomone et al., 2017; Smetana et al., 2016).
The application of black soldier fly larvae (BSFL) for organic waste treatment is getting significant attention among insect researchers nowadays (Kim et al., 2021). The resource insect black soldier fly (BSF), Hermetia illucens, can efficiently convert livestock manure (poultry or dairy), along with other organic waste, and produce larval biomass rich in protein and fat, which can be used directly or after drying as animal feed (Liu et al., 2017; Shorstkii et al., 2020). In addition, the larval biomass can be used to separate protein and fat, where fat can be used for biodiesel production and larval protein for animal feed use (Rehman et al., 2019; Wang et al., 2017; Zhu et al., 2019). The remaining residue of manure after BSFL growth can be used as multi-purpose fertilizer (Ma et al., 2018; Mazza et al., 2020; Newton et al., 2005b). Thus, BSFL can be used in solving organic waste pollution. Simultaneously, BSFL can ease the shortage of protein and fat used in animal feed, supporting liquid energy production. It has good prospects with simple operation, low cost, high efficiency and high added value, and it can realize the utilization of animal manures and other organic wastes. The schematic presentation of animal feed and liquid energy production from the proposed BSFL production unit on livestock farm facilities is shown in Figure 1.
Figure 1.
Schematic presentation of animal feed and liquid energy production from proposed BSFL production unit.
Purpose of mini-review
Non-vector insects like the BSF larvae are being employed widely to reduce the weight of organic wastes (Ji-bin et al., 2020; Lalander et al., 2019; Rehman et al., 2019). Raw or processed, the BSF prepupae may be utilized for a variety of commercial reasons, including the production of protein-rich animal feed, the extraction of lipids and the extraction of chitin (Liu et al., 2017; Somroo et al., 2019; Wang et al., 2017, 2020). The former reviews on the BSF and solid waste management were covered BSF artificial rearing methodologies, BSFL waste stabilization performances under diverse environmental circumstances, and the impacts of micronutrients and associated microorganism populations (Purkayastha and Sarkar, 2021), the biowaste composition on BSF process performance and effects of BSFL gut microbe on fly larval development (Gold et al., 2018) and organic waste treatment by BSFL and their bioconversion efficiencies (Kim et al., 2021; Kumar et al., 2018). Moreover, significance of BSF larvae in organic waste treatment, as well as their life cycle patterns, eating preferences and environmental factors that impact their survival was reviewed by Singh and Kumari, (2019).
However, the present review summarizes the effects of co-digestion of various organic waste, their mixing ration and microbial fermentation on development of BSFL. The authors have also attempted to analyze the current limits, research gaps and future directions connected with this technology through this literature evaluation. BSF larvae composting is a complete strategy that convert the organic wastes with the aforementioned value larval biomass; moreover, the technological innovations might enhance the system’s efficiency and industrialization of the BSF technology. As a result, the current study is an amalgamation of BSF larval applications for societal benefit that are considered as alternative source of protein for humans, livestock, aquaculture, poultry and pets (Rehman et al., 2021).
Hermetia illucens, BSF
The H. illucens generally known as BSF is one of five genera in the Hermetiinae subfamily of the Diptera order. The Chaetohermetia, Patagiomyia, Notohermetia and Chaetosargus are the other four genera; H. illucens is the widely disseminated species. This species may be found across the world’s warm and tropical temperate zones, from the Neotropics through the Australasian, Nearctic, Palaearctic and Afro-tropical climates (Devon Brits et al., 2016; Woodley, 2001). The BSF are huge, slender and black, with brownish-coloured wings and an antenna (with three segments) that protrudes outwards from the top of their heads. Like other dipteran species, the adult body is separated into three parts: the head, the thorax and the abdomen (Oonincx et al., 2016; Üstüner et al., 2003). The eyes are uncovered and widely distant from one another (Oonincx et al., 2016). The abdomen is divided into five parts, each with white abdominal patches. Generally speaking, females are smaller than males, but they have longer terminalia than male genitalia, and their wings are also larger than those of males. The size of both the female body and wings is generally between 12 and 20 mm and 8 and 14.8 mm, respectively (Oonincx et al., 2016; Singh and Kumari, 2019; Üstüner et al., 2003).
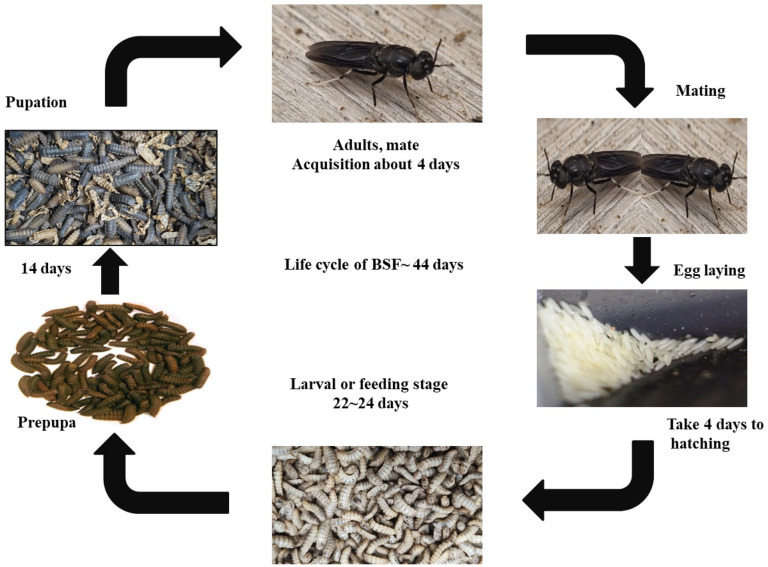
Life cycle of BSF
The five stages of the BSF’s life cycle may be summarized as follows: egg, larva, prepupa, pupa and adult (Figure 2). Between eclosion and oviposition is 3–4 days, flies never laid eggs on the moist rotting material directly (Diclaro and Kaufman, 2009). Sheppard et al. (2002) determined that the larval stage lasts between 22 and 24 days at 27°C, and then converted into prepupae that travel to a dry and well-protected pupation location (Diener et al., 2011). At the end of the larval stage, the prepupae moves to a dry and appropriate pupation place and transforms into a pupa (the next metamorphosis stage) (Diener et al., 2011). The pupa molts and results in the emergence of an adult fly. Upon reaching sexual maturity, the adult females may mate and deposit their eggs in dry cracks and cervices near the feed stream (Diener, 2010). Neither pests nor disease carriers, mature flies are harmless. The adult flies live on the fat that was accumulated during their larval stage throughout development; hence, adult BSF need nothing other than water to survive (Banks, 2014; Furman et al., 1959). Female BSF oviposits just around the edges of the larval feeding substrate, rather than directly on the feed itself (Booth and Sheppard, 1984). Therefore, they do not spread pathogens from the wastes (Banks et al., 2014).
Figure 2.
Life cycle of black soldier fly, Hermetia illucens.
Biological physiognomies of BSF
While prominently inhabiting the equatorial tropics, like United States, Europe and Asia. (Martínez-Sánchez et al., 2011; Sheppard et al., 2002; Tomberlin et al., 2002), H. illucens can be currently found worldwide (Brammer and von Dohlen, 2007). The BSFL are voracious eaters of decayed fruits and vegetables, animal manure and municipal organic waste (MOW) (Diener et al., 2015; Paz et al., 2015; Salomone et al., 2017). Its larvae are saprophagous, have complex feeding habits, large appetite and strong resistance to the harsh environment. In addition, the nutritional value of its prepupa is high, and it has the habit of migrant property. All these traits make the BSFL an excellent candidate for converting organic waste.
Mating and oviposition of BSF have a close relationship with the environment, illumination intensity, illumination period, temperature and humidity, which can all impact the mating and oviposition. The mating of BSF needs enough light to be stimulated so that this activity may be influenced when days are cloudy (Sheppard et al., 2002; Tomberlin et al., 2002). As low light levels prevent BSF from mating throughout the winter, it is necessary to use artificial lighting (Zhang et al., 2010). Light-emitting diode lamps, halogens lamps and other light-emitting diodes can be employed as artificial light sources; however, rare earth lights did not produce mating (Zhang et al., 2010).
The temperature and relative humidity have been identified as crucial for the growth and development of BSF. BSFs are eurythermal insect that can withstand a wide temperature range (15°C–47°C) (Singh and Kumari, 2019). More than 96% of oviposition occurred in temperatures between 27.5°C and 37.5°C and 60% relative humidity (Holmes, 2010; Sheppard et al., 2002). According to another team of researchers, the ideal relative humidity for BSF growth under laboratory circumstances is between 50 and 90% (Bosch et al., 2020; Diener et al., 2009). The optimal culturing temperature is 27°C; either higher or lower temperatures will reduce larvae survival and adult eclosion rate (Tomberlin et al., 2009). At 27°C and 30°C, the average survival rate of 4- to 6-day-old larvae to adults was 74–97%, whereas it was just 0.1% at 36°C (Tomberlin et al., 2009). It was also discovered that if temperature and humidity are not properly controlled, it might harm egg eclosion and colony development (Ji-bin et al., 2020; Purkayastha and Sarkar, 2021). It was shown that species treated to 70% relative humidity have 2–3 days longer life spans than those subjected to 25% relative humidity (Holmes et al., 2012; Miranda et al., 2020).
Role of BSF as an organic waste manager
In the family Stratiomyida and order Diptera, H. illucens is perhaps the best member. The BSFL, called as ‘phoenix worms’, is utilized for organic waste bioconversion, pest control and animal, poultry and fish feed supplementation (Rehman et al., 2019, 2021; Sheppard, 1992). The BSFL are grown on a variety of substrates, including DM (Myers et al., 2008; Rehman et al., 2017b), poultry manure (Rehman et al., 2019; Sheppard et al., 1994), pig manure (Newton et al., 2005a) and food wastes (Barry, 2004; Ites et al., 2020; Rehman et al., 2017b). The insects are now distributed from the tropical areas into warm and moderate temperate regions of Europe, Asia, North and South America and Australia due to worldwide trade and business (Čičková et al., 2015). Because of its wide distribution and the advantages for easy management of breeding colony (Sheppard et al., 2002), there is considerable global interest in mass rearing of BSF production for novel protein used in the poultry, aquaculture and livestock feed, and using fat for replacing less sustainable edible fats (e.g. in margarine) (Smetana et al., 2020) and biodiesel production (Rehman et al., 2018).
Black soldier fly as a promising bioconversion agent
The BSF is a promising bioconversion agent for organic wastes (Figure 3) and a potential nutrient-rich source of protein for livestock, poultry, pets and aquaculture (Rehman and Hollah, 2020; Veldkamp et al., 2021). BSFL could comprise as much as 43% of their body weight in protein, and they are rich in minerals and amino acids (Liu et al., 2017; Zhu et al., 2019). The BSFL’s ability to develop on a wide range of substrates has made it one of the most appealing insects for biotransformation on a large scale since the larvae may develop on everything from organic waste to manure (Lievens et al., 2021). In addition, these environmentally friendly methods provide intriguing options for waste-based nutrient recovery (Grossule et al., 2020). Waste composting, nutrient recovery and revenue creation are key components of the BSF larvae treatment system (Purkayastha and Sarkar, 2022; Villa et al., 2021). Because of its low cost, low maintenance, simple operation, minimum land requirements, low ecological impact and more scalable economic potential, it is a significantly better option than other composting systems (Singh and Kumari, 2019). The BSF farming/rearing is sustainable with efficient reproduction capacity (one clutch can produce up to 500 eggs), high feed conversion ratio (FCR) (1 kg of beef production requires 10 kg of feed, whereas 1 kg of BSFL requires just 1.5 kg feed), clean food production is faster and cheaper, excellent nutritional value, and regulatory approval to be used as food and feed (Skrobonja, 2020).
Figure 3.
Schematic showing black soldier fly as a promising bioconversion agent.
According to Van Huis et al. (2013), the BSF is not a pest as it is not attracted to the feed due to the lack of functioning mouth parts and thus cannot contact unsanitary organic wastes. Therefore, H. illucens does not act as a disease vector (Makkar et al., 2014). In addition, BSFL can grow on organic waste, specifically manure, and can potentially reduce harmful bacteria, such as E. coli or salmonella, which would tremendously reduce the harm on the environment caused by livestock manure (Erickson et al., 2004; Liu et al., 2008). One study advised that the larvae comprise natural antibiotics used in maggot debridement treatment for cleaning human wounds, a technique progressively practiced because of drug-resistant bacterial infections (Sherman and Wyle, 1996). When common housefly larvae (Musca domestica) are cultivated simultaneously with the BSFL population, the housefly population was reduced from 94 to 100% (Sheppard et al., 1994). The BSF uses dry mass more efficiently than house fly larvae and makes manure more liquid, which is not suitable for the growth and development of house fly larvae (Sheppard, 1983). Therefore, BSFL could potentially assist to manage the housefly populations in livestock farmhouses and homes with deprived sanitation and hygiene, thus improving the health conditions of the farm animals and in the human population, as housefly is considered as a significant vector of diseases (Makkar et al., 2014; Newton et al., 2005b). Figure 3 shows BSF as promising bioconversion agent.
Black soldier fly in organic waste bioconversion
More research has shown the potential of BSFL to grow effectively on animal manure and thus reduce organic waste. Bondari and Sheppard (1981) grew BSFL on CHM at a commercial cage layer house and recorded that BSFL waste mass reduction was 30% of dry manure mass, and larval biomass had 17% dry weight and 83% water content. Lardé (1990) fed coffee pulp to H. illucens L. with 29.8% dry mass reduction. Newton et al. (2005a) fed 169 kg fresh pig manure on BSFL. The total population of 45,000 BSFL was added into 169 kg of fresh pig manure and resulted in 68 kg of dry larvae mass and 41.6 kg of the dry mass of the manure residues. In this study, the dry mass reduction of pig manure was 39%, with a FCR of 9.6 and bioconversion of 4%. Dierenfeld and King (2008) developed H. illucens L. on a commercial diet resulting in a dry mass reduction rate of 27%.
Myers et al. (2008) studied the growth of BSFL on cow manure under normal conditions and reported low conversion efficiency, BSFL survival rate of 71–85%, the development time of 26–30 days, as well as the mean individual fresh larval mass of 140–170 mg and dry mass reduction of 33%. Sealey et al. (2011) discovered that the BSFL grown on cow manure and fish organs could substitute 50% fishmeal without influencing the growth characteristics and taste. Diener et al. (2009) used commercial chicken feed to investigate the life history traits fluctuation by offering different feeding rates to the larvae of BSF. It was recorded that feeding rates influence the survival rate, development time (16–42 days), individual larvae weight (80–150 mg) and dry mass reduction (23–44%). Li et al. (2011) explored the potential of BSF growth on DM and observed a 53% reduction rate, and in this study, about 1248.6 g of fresh cow manure was used in 21 days by 1200 BSFL.
Another study was designed to evaluate the feasibility of using the BSFL as a treatment for MOW in nations with a low and moderate income (Diener et al., 2011). The study reported that BSFL could reduce around 68% of the dry mass of MOW with 5.8 FCR and 12% bioconversion. Gobbi et al. (2013) used hen feed and meat meal for the BSFL development and found out that a more acidic medium of meat meal was unfavourable for BSF growth. These investigations resulted in a 93 and 40% survival rate, the development time of 15 and 33 days and dry mass reduction of 70 and 31%, feeding hen feed and meat meal to BSFL, respectively. Oonincx et al. (2015a) used dry cow, chicken and pig manure to develop BSFL and found out that the dry manure used after storage was not favourable for the development of the larvae. The authors reported survival rates was 82–97% at a development time of 214–144 days with the fresh individual larval weight of 50–70 mg. In another study, Oonincx et al. (2015b) used different food waste formulations varying in the ratio of protein and fat to grow BSFL. Here, the authors recorded 75–85% survival rate, a development period of 21–37 days and FCR of 1.4–2.6. Moreover, Manurung et al. (2016) developed larvae of BSF on dry rice straw and recorded the life history traits, amongst others, the survival rate (51.2–98.3%), the development time (38–54 days) and dry mass reduction (9–31%). Sheppard et al., (1994) calculated different life-history traits of BSF and found that BSFL converts CHM to BSFL biomass, which contains 42% protein and 35% fat. Here, the BSF can consume 50% of the manure while digesting 62% nitrogen element with a mean larvae biomass of 220 mg. A review of the progress of BSF bioconversion research is presented in Table 1. Banks et al. (2014) and Rehman et al. (2017b) reported 190–300 mg of individual prepupa fresh weight with 25.2–54.6% waste reduction, 2.1–22.3% bioconversion and FCR of 2–15.6 (Table 2).
Table 1.
Comparison of selected parameters of black soldier fly bioconversion experiments feed with various single and co-digestion mixture of various waste feeding substrates (NA: not available).
| References | Feed source | Survival rate (%) | Development time (day) | Mean fresh larval mass (mg) | Dry mass (%) of live weight BSFL | Dry mass reduction (%) | Temperature (°C) | Humidity (%) | Feed conversion ratio | Bioconversion (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Somroo et al. (2019) | Soybean curd residues (SCRs) | 95.4–98.0 | 16.1–17.7 | 126.4–146.4 | 19.8–23.7 | 23.7 | 27 | 70 | 8-9.8 | 5.0–6.9 |
| Lim et al. (2019) | Waste coconut endosperm and SCRs | NA | 19–23 | 32.5–67.5 | NA | 68 | 25–27 | 65–70 | NA | 14 |
| Cai et al. (2018) | Sewage sludge and Chicken manure (CHM) | 90–99.7 | 12–30 | 78–111 | NA | 58–64 | 25–30 | 60 | NA | NA |
| Rehman et al. (2017b) | Dairy manure and SCR | 91–99 | 19–23 | 63–123 | 21.4–26.5 | 26–72 | 27 | 60–70 | 4.2–6.2 | 6.3–15.2 |
| Rehman et al. (2017a) | Dairy manure and CHM | 89.5–98.4 | 18–22 | 60–100 | 19.5–23.2 | 43–55 | 27 | 60–70 | 5.6–10.3 | 4.1–9.8 |
| Manurung et al. (2016) | Rice straw | 51.2–98.3 | 38–54 | NA | NA | ~9–31 | 28 | 70 | NA | NA |
| Oonincx et al. (2015b) | Artificial feed | 75–85 | 21–37 | NA | 32.9–35.6 | NA | 28 | 70 | 1.4–2.6 | NA |
| Oonincx et al. (2015a) | Cow manure | 87.8 | 214.5 | 70 | 20.3 | NA | 28 | 70 | NA | NA |
| Chicken manure | 82.2 | 144 | 50 | 20.6 | NA | 28 | 70 | NA | NA | |
| Pig manure | 97 | 144 | 60 | 20.2 | NA | 28 | 70 | NA | NA | |
| Gobbi et al. (2013) | Hen feed | 93 | 15 | NA | NA | ~70 | 27 | NA | NA | NA |
| Gobbi et al. (2013) | Meat meal | 40 | 33 | NA | NA | ~31 | 25 | NA | NA | NA |
| Diener et al. (2011) | MOW | NA | NA | NA | NA | 68 | NA | NA | 5.8 | 12 |
| Li et al. (2011) | Dairy manure | NA | 31 | NA | NA | 53 | 27 | NA | NA | NA |
| Sealey et al. (2011) | Dairy manure | NA | ~120 | NA | NA | NA | 25 | NA | NA | NA |
| Diener et al. (2009) | Chicken feed | NA | 16–42 | 80–150 | 33–40 | 23–44 | 27 | NA | NA | NA |
| Myers et al. (2008) | Dairy manure | 71–85 | 26–30 | 140–170 | NA | 33–58 | NA | NA | NA | NA |
| Dierenfeld and King (2008) | Commercial diet | NA | NA | NA | 27 | NA | NA | NA | NA | NA |
| Newton et al. (2005a) | Swine manure | NA | NA | NA | NA | ~39 | NA | NA | 9.6 | ~4 |
| Lardé (1990) | Coffee pulp | NA | NA | NA | NA | 30 | NA | NA | NA | NA |
| Bondari and Sheppard (1981) | Chicken manure | NA | NA | NA | 17 | 30 | NA | NA | NA | NA |
Table 2.
Comparison of waste mass reduction, bioconversion and feed conversion ratio of black soldier fly conversion experiments on wet mass base.
| References | Feed source | Wet waste mass reduction (%) | Bioconversion (%) | FCR |
|---|---|---|---|---|
| Rehman et al. (2017a) | Dairy manure and soybean curd residue | 63.2–76.6 | 6.3–12.4 | 6.3–10.1 |
| Banks et al. (2014) | Fresh human faeces | 25.2–54.6 | 2.1–22.3 | 2–15.6 |
| Sheppard et al. (1994) | Chicken manure | ~50 | ~4 | ~13 |
It has been shown that BSFL can digest the waste, but when mixed with another kind of waste that has a higher nutritional value, the survival rate, development time, larval weight, bioconversion, FCR and waste reduction are significantly improved (Table 1). When up to 50% of faecal sludge was replaced with agri-market waste, BSFL developed at the same rate as when cultivated solely on faecal sludge; however, when 25% of agri-market waste was replaced with faecal sludge, BSFL grew at the same rate as when grown on 100% agri-market waste (Diener, et al., 2011). Multiple studies have established that co-digestion of various organic wastes is key to improving nutritional profiles and body weight of BSFL. Rehman et al. (2017a, 2017b, 2019) found a new co-digestion process of DM, CHM and soyabean curd residue (SCR) to develop BSFL. In the experimental group treated with substrate comprising of 40% DM and 60% CHM, average utilization of cellulose, hemicellulose and lignin was significantly increased by 53.22, 61.19 and 42.23% respectively. In comparison, cellulose, hemicellulose and lignin consumption were lower in the experimental group fed with DM, 49.89%, 49.77% and 31.95%, respectively. In terms of commercialization, the development of BSFL on combined-formulations of different types of wastes has proved significantly more encouraging and promising than on a single-type waste stream. Figure 4 shows the schematic summary of BSFL role in organic waste bioconversion.
Figure 4.
Schematic summary of black soldier fly larvae role in organic waste bioconversion.
Impact of microbe on organic waste bioconversion with H. illucens larvae
The microbe was used during organic wastes bioconversion with BSFL. A significant positive fluctuation of various parameters, that is, survival rate, development time, larval weight gain, waste reduction and FCR, were found (Table 3). Previously reported investigations, in which microbes were utilized for assistant of BSFL in dairy and CHM by Rehman et al. (2019), in CHM by Yu et al. (2011) and Zheng et al. (2012) for the conversion of rice straw. The ability of the BSFL to use different organic materials as food sources may be due to their symbiotic microbiome. The gut microbiota is not only important for digestion but also for the host’s physiology, development and immune system, which prevents the colonization of pathogens (Balzola et al., 2010; Lanan et al., 2016; Seong et al., 2008). The larvae of BSF usually obtain nutrients from decomposing plants, animal matter and faeces. The BSF is known to feed on used MOWs (Diener et al., 2011), vegetable wastes (Paz et al., 2015), CHM, DM and pig manure (Li et al., 2011; Oonincx et al., 2015; Rehman et al., 2017b; Zhou et al., 2013), rice straw (Manurung et al., 2016), restaurant wastes (Zheng et al., 2012), the coffee pulp (Lardé, 1990), food wastes and restaurant waste (Salomone et al., 2017). Since insects play an important role in reprocessing of biological wastes, the bacterial assortment of the insect gut was investigated to elucidate the microbial community assembly of different hosts and to find the bacterial genes encoding enzymes, for example, proteases, lipases, cellulases, pectinases and xylanases, naturally engaged in degradation of organic compounds (Kudo, 2009; Yang et al., 2015; Zhu et al., 2019). In particular, the amount of residual protein and other nutrients from the larvae of various insects can subsequently be used as a good animal feed (Makkar et al., 2014). The gut and waste microflora is also a source of isolation and identification of new bacteria, yeast, protists and archaea (Hongoh, 2010; Ohkuma, 2008; Shinzato et al., 2007). In one study, the intestinal bacterial community of the BSFL was shown to be dependent on their diet, with food waste fed BSFL having a more complex gut microbiota than the BSFL when fed on cooked feed and calf forage (Jeon et al., 2011). Thus, bacterial community composition depends on different nutrient sources. Identification of bacterial strains in the BSF larval gut represented a unique bacteria species, unlike other insects’ intestinal microflora (Jeon et al., 2011). These indicate that bacterial community structures in BSF larvae are unique, which allows them to degrade a wide range of food sources.
Table 3.
Comparison of selected parameters of black soldier fly waste bioconversion experiments and microbial fermented feeding substrate (NA: not available).
| References | Feed source | Survival rate (%) | Development time (day) | Mean larval mass (mg/larvae) | Dry mass reduction (%) | BSFL protein (%) | BSFL fat (%) | Feed conversion ratio | Bioconversion (%) |
|---|---|---|---|---|---|---|---|---|---|
| Wong et al. (2020) | Waste coconut endosperm and Saccharomyces cerevisiae | NA | 15.5 | 42.0 | NA | NA | 42.50 | NA | 11 |
| Waste coconut endosperm | NA | 13.5 | 3.3 | NA | NA | 45 | NA | 10 | |
| Rehman et al. (2019) | Dairy manure and chicken manure (CHM) mixture and Bacillus subtilis | 99.1 | 19.0 | 25.9 | 48.7 | NA | NA | 4.5 | 22.3 |
| Dairy manure and CHM mixture | 94.6 | 20.6 | 16.4 | 41.9 | NA | NA | 6.2 | 16.3 | |
| Somroo et al. (2019) | Soybean curd residues and Lactobacillus buchneri | 98.0 | 16.1 | 34.7 | 55.7 | 55.3 | 30.0 | 8.0 | 9.0 |
| Soybean curd residues | 95.4 | 17.3 | 25.0 | 49.0 | 52.9 | 26,1 | 9.8 | 5.0 | |
| Abduh et al. (2017) | Rubber seeds and microbe | NA | 12.0 | NA | NA | 28.6–55.2 | 18.9–28.3 | NA | NA |
| Zheng et al. (2012) | Rice straw and dairy manure (DM) mixtures and microbes | NA | NA | 86.2–115.5 | NA | NA | 35.7–39.6 | NA | NA |
| Yu et al. (2011) | CHM and B. subtilis | 99.3 | 7.7 | 94.6 | NA | NA | NA | NA | NA |
| CHM | 98 | 12.3 | 60.6 | NA | NA | NA | NA | NA |
Wong et al. (2020) investigated S. cerevisiae, for fermentation of waste coconut endosperm prior to providing for BSFL feeding. The BSFL development rate and waste-to-biomass transformation were observed to be at their highest levels at 2.5 wt% yeast in feeding substrate fermentation, with 42.5 mg/larvae and 11.5%, respectively. The larval development rate on the control waste coconut endosperm was only 3.3 mg/larva when compared to the absence of yeast. It was assumed that, because of yeast’s ability to decompose monosaccharides found in waste coconut endosperm, it would be easier for the body to digest and absorb nutrients from it (Raksasat et al., 2020).
Rehman et al. (2017a, 2017b, 2019) developed a cellulose-degrading mechanism to assists BSFL in the transformation of cellulose in DM. In the best mixing ratio between DM and CHM studied (2:3) (DM40), cellulose-degrading bacteria were added. When using BSF larvae with microbial assistance, the best results were achieved in terms of manure reduction (48.7%), bioconversion (10.1%), food conversion ratio (4.5%) and the usage of nutrients (protein 71.2% and fat 67.8%). BSFL and corresponding bacteria from the eggs and gut co-transformed CHM were used by researchers. The co-conversion of CHM with BSFL assisted by egg and gut-associated bacteria increases the waste reduction rate and larval weight gain significantly compared to the control (Mazza et al., 2020).
The large-scale co-digestion of CHM with BSFL gut microbes strain was isolated from the guts of surface-disinfected larvae (BSF-CL) for producing larvae as feedstuff and organic fertilizer, resulted in the reduction of CHM by 40.5%. In comparison, the control group CHM was reduced by 35.8%. By comparison, the BSFL’s weight improved by 15.9%, the BSFL’s conversion rate improved by 12.7% and the CHM reduction rate improved by 13.4%, as compared to control group with no B. subtilis, BSF-CL (Xiao et al., 2018). The effect of L. buchneri on SCR bioconversion with BSFL was evaluated to produce protein and fats for human, livestock and poultry consumption. The L. buchneri (108 cfu/ml) was used to pretreat the SCR, and then BSFL was used to transform it. The BSFL development on SCR and L. buchneri had a significantly higher waste reduction (55.7%), bioconversion rate (6.9%), FCR (8.0), protein content (55.3%) and fat content (30.0%), than SCR (49.0, 5.0, 9.8, 52.8 and 26.1%, respectively) and artificial feed (43.9, 3.9, 11.1, 50.3 and 24.3%, respectively) (Somroo et al., 2019).
The B. subtilis species (S15, S16 and S19) from the BSFL gut were isolated and used for the fermentation of CHM prior to feeding to the BSFL, according to Yu et al. (2011). It was found that BSFL development on B. subtilis-fermented CHM had a higher prepupal weight and a shorter development period than the BSFL fed with non-inoculated CHM. The S15-treated CHM from the isolated B. subtilis species generated the highest prepupal weight of 94.6 mg/prepupa and the shortest development time of 7.67 days (Raksasat et al., 2020). The BSFL mass incremental rate and substrate reduction rate both increased by 15.9 and 40.5%, respectively, when BSFL was provided B. subtilis-inoculated CHM instead of control CHM, according to Xiao et al. (2018). Symbiotic bacteria of B. subtilis may help BSFL digest non-digestible food while delivering the needed nutrients for BSFL development through fermentation, which might assist in the process of digestion (Mazza et al., 2020).
Safety of BSFL used as food and feed
The H. illucens are regarded to be the most potential fly species for use as a feed and food source because of their high nutritional value (van der Fels-Klerx et al., 2020). As a result, in order to be economically successful and to contribute to a green circular economy, BSF larvae should be reared on substrates that have not much or no alternate value as feed or in the production of foods (van der Fels-Klerx et al., 2020). It is possible to employ them to transform organic waste into useful biomass, and because of their chemical composition, they are an important element in many industrial applications (Smets et al., 2020; Surendra et al., 2020). To utilize BSFL as a feed ingredient, it is necessary to ensure that they are chemically safe. Because bioaccumulation of different (in)organic chemicals (e.g. heavy metals, mycotoxins, pesticides, etc.) in BSFL might pose a safety issue, the composition of their rearing substrate is one of the most important considerations for safety (Zhang et al., 2021). The European Union currently prohibits using several organic waste streams as BSFL-rearing substrates (except for pre-consumed vegetable and fruit wastes) because of safety knowledge gaps (Lievens et al., 2021).
The organic wastes may include pharmaceuticals, agriculture pesticide, heavy metals and toxins including dioxins, polychlorinated biphenyls (PCBs) and polyaromatic hydrocarbons (PAHs). As a consequence, this new industry is concerned about the transfer and accumulation of these contaminants to BSFL in the feed and food chain (Lievens et al., 2021; Wang et al., 2021). More research has been carried out to establish the safety of BSFL when it is used as food and feed (Bessa et al., 2021; Diener et al., 2015; Gao et al., 2017; Makkar et al., 2014). BSFL treatment has been shown to lower the number of microorganisms in the substrate, according to prior investigations (Erickson et al., 2004; Lalander et al., 2013; Liu et al., 2008). More importantly, according to the findings of the recent research that have been completed so far, dioxins, PCBs and PAHs, as well as certain pesticides, antibiotics and mycotoxins, did not accumulate in the BSFL (Bessa et al., 2021; Bosch et al., 2017; Lalander et al., 2016; Lievens et al., 2021; Peguero et al., 2021; Purschke et al., 2017; van der Fels-Klerx et al., 2020). Cadmium, lead and zinc had no influence on the physiology of BSFL, although cadmium was shown to accumulate in the body (Diener et al., 2015). Further research found that heavy metals, particularly cadmium and lead, had a negative impact on the development, and these metals accumulated in high concentrations in the BSFL that exceed maximum permissible limits of animal feed regulations (Lievens et al., 2021; Purschke et al., 2017; Wu et al., 2020). In another research, cadmium and chromium were shown to accumulate in the larval and prepupae stages of the BSF development (Gao et al., 2017). A reduction in biowaste volume during BSF treatment causes heavy metals to accumulate in the residue, which may restrict its use as a soil conditioner or biofertilizer. It is possible that bacteria present in and on the larvae may provide a risk to the safety of animal feed, and that germs present in the residue could offer a harm to human and environmental health (Lalander et al., 2013; Yukesh Kannah et al., 2020). Various studies show that inoculating biowaste with bacteria can improve the overall performance of the BSFL process (Ji-bin et al., 2020; Mazza et al., 2020; Rehman et al., 2019; Xiao et al., 2018). Therefore, in order to use BSFL as food and feed, it is required to monitor heavy elements (such as cadmium and lead) and pathogenic microorganisms in both the BSFL and substrate residues.
Conclusion
The BSFL waste digestion technology can provide commercially viable high-value product (protein, fat, biodiesel, chitin and melanin) that can contribute to ecological and economical sustainable resource recuperation from waste management model. Various environmental issues may be reduced by using BSFL to process organic waste, such as GHG emissions and the generation of hazardous compounds associated with landfills and incineration. The composition of organic wastes used as feeding substrate for BSFL can affect the biochemical accumulation (protein and fat) in the BSFL biomass. Organic wastes with a high proportion of fibres (cellulose, hemicellulose and lignin) inhibit the growth of BSFL, resulting in limited larval size because of a shortage of key nutrients. Therefore, BSFL feeding substrates’ nutrient content has recently been improved by investigating the co-digestion of various organic wastes and the impacts of microorganisms on BSF organic wastes valorization technology. Furthermore, due to its less complicated and easy application, low-technical needs, low-area requirements/minimal ecological footprints and better-suited economic potential, BSF larval composting was shown more viable and cost-effective than other composting techniques. This mini-review presents a complete overview of current information on the artificial rearing of BSF, the influence of different kinds of wastes as well as their co-digestion or waste stabilization performance, the impact of various organic waste fermentation with microorganisms in conjunction with BSFL and the uses of this technology for commercialization. The content in this article may be used to gain a better knowledge of the BSF’s function and to design a scalable system for turning waste into high-value products.
The following are some of the recommendations for future research into the development and use of BSFL-based waste digestion technology:
Investigating how BSF waste treatment technology might be applied in novel ways to address organic waste issues.
To further enhance BSFL organic waste digestion technology, it may be necessary to investigate low-cost and nutrient-rich substrates that can be blended with organic wastes that are deficient in adequate nutrients.
The role of microbe in BSF treatment/organic waste fermentation may be explored for the sustainable development of technology.
Research on how well BSFL can biorefine organic wastes into valuable resources may be done using the nutrients in larval-feeding substrates and the biochemicals (protein, fat, fatty acids, amino acids, chitin and melanin) in recovered BSFL biomasses.
Effects of environmental conditions on BSF breeding operation must be investigated to improve BSF artificial breeding.
The bioaccumulation cross-tropic contaminations and infectious/harmful microorganism are reasons that some countries have banned the use of insects grown on organic wastes as livestock and poultry feed; therefore, future investigations in this area may be beneficial for the use of BSFL development on organic wastes in livestock and poultry-compound feed formulation.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Kashif ur Rehman  https://orcid.org/0000-0003-4954-3172
https://orcid.org/0000-0003-4954-3172
Kemal Aganovic  https://orcid.org/0000-0002-5850-8777
https://orcid.org/0000-0002-5850-8777
References
- Abduh MY, Jamilah M, Istiandari P, et al. (2017) Bioconversion of rubber seeds to produce protein and oil-rich biomass using black soldier fly larva assisted by microbes. Journal of Entomology and Zoology Studies 5: 591–597. [Google Scholar]
- Aguirre-Villegas HA, Larson RA. (2017) Evaluating greenhouse gas emissions from dairy manure management practices using survey data and lifecycle tools. Journal of Cleaner Production 143: 169–179. [Google Scholar]
- Ali Y, Jokhio DH, Dojki AA, et al. (2021) Adoption of circular economy for food waste management in the context of a developing country. Waste Management and Research 40: 676–684. [DOI] [PubMed] [Google Scholar]
- Alterta Water Portal (2012) World water facts. Available at: https://albertawater.com/interesting-facts/world (accessed 29 January 2022).
- Balzola F, Bernstein C, Ho GT, et al. (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks IJ. (2014) To Assess the Impact of Black Soldier Fly (Hermetia illucens) Larvae on faecal Reduction in Pit Latrines. London School of Hygiene and Tropical Medicine Thesis, University of London. [Google Scholar]
- Banks IJ, Gibson WT, Cameron MM. (2014) Growth rates of black soldier fly larvae fed on fresh human faeces and their implication for improving sanitation. Tropical Medicine & International Health 19: 14–22. [DOI] [PubMed] [Google Scholar]
- Barrett CB. (2021) Overcoming global food security challenges through science and solidarity. American Journal of Agricultural Economics 103: 422–447. [Google Scholar]
- Barry T. (2004) Evaluation of the economic, social, and biological feasibility of bioconverting food wastes with the black soldier fly (Hermetia illucens). UNT Digital Library. University of Texas. Available at: http://digital.library.unt.edu/ark:/67531/metadc4639/m1/107/ (accessed 12 December 2016). [Google Scholar]
- Berhane A. (2018) Climate change and variability impacts on agricultural productivity and food security. Journal of Climatology & Weather Forecasting 6: 240. [Google Scholar]
- Bessa LW, Pieterse E, Marais J, et al. (2021) Food safety of consuming black soldier fly (Hermetia illucens) larvae: Microbial, heavy metal and cross-reactive allergen risks. Foods 10: 1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondari K, Sheppard DC. (1981) Soldier fly larvae as feed in commercial fish production. Aquaculture 24: 103–109. [Google Scholar]
- Booth DC, Sheppard C. (1984) Oviposition of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae): eggs, masses, timing, and site characteristics. Environmental Entomology 13: 421–423. [Google Scholar]
- Bosch G, Oonincx DGAB, Jordan HR, et al. (2020) Standardisation of quantitative resource conversion studies with black soldier fly larvae. Journal of Insects as Food and Feed 6: 95–109. [Google Scholar]
- Bosch G, van der Fels-Klerx HJ, De Rijk TC, et al. (2017) Aflatoxin B1 tolerance and accumulation in black soldier fly larvae (hermetia illucens) and yellow mealworms (tenebrio molitor). Toxins 9: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brammer CA, von Dohlen CD. (2007) Evolutionary history of Stratiomyidae (Insecta: Diptera): The molecular phylogeny of a diverse family of flies. Molecular Phylogenetics and Evolution 43: 660–673. [DOI] [PubMed] [Google Scholar]
- Brás I, Silva E, de Lemos LT. (2020) Feasibility of using municipal solid wastes rejected fractions as fuel in a biomass power plant. Environment Protection Engineering 46: 53–62. [Google Scholar]
- Cai M, Hu R, Zhang K, et al. (2018) Resistance of black soldier fly (Diptera: Stratiomyidae) larvae to combined heavy metals and potential application in municipal sewage sludge treatment. Environmental Science and Pollution Research 25: 1559–1567. [DOI] [PubMed] [Google Scholar]
- Cardoen D, Joshi P, Diels L, et al. (2015) Agriculture biomass in India: Part 2. Post-harvest losses, cost and environmental impacts. Resources, Conservation and Recycling 101: 143–153. [Google Scholar]
- Chang H. (2019) Water and Climate Change. International Encyclopedia of Geography. John Wiley & Sons, Ltd: 1–6. DOI: 10.1002/9781118786352. [DOI] [Google Scholar]
- Charles K. (2021) Food production emissions make up more than a third of global total | New Scientist. Available at: https://www.newscientist.com/article/2290068-food-production-emissions-make-up-more-than-a-third-of-global-total/ (accessed 3 April 2022).
- Chen C, Chaudhary A, Mathys A. (2020) Nutritional and environmental losses embedded in global food waste. Resources, Conservation and Recycling 160: 104912. [Google Scholar]
- Čičková H, Newton GL, Lacy RC, et al. (2015) The use of fly larvae for organic waste treatment. Waste Management 35: 68–80. [DOI] [PubMed] [Google Scholar]
- Clapp J, Moseley WG, Burlingame B, et al. (2022) Viewpoint: The case for a six-dimensional food security framework. Food Policy 106: 102164. [Google Scholar]
- Clark MA, Domingo NGG, Colgan K, et al. (2020) Global food system emissions could preclude achieving the 1.5° and 2°C climate change targets. Science 370: 705–708. [DOI] [PubMed] [Google Scholar]
- Coskuner G, Jassim MS, Nazeer N, et al. (2020) Quantification of landfill gas generation and renewable energy potential in arid countries: Case study of Bahrain. Waste Management and Research 38: 1110–1118. [DOI] [PubMed] [Google Scholar]
- Cuadros F, López-Rodríguez F, Ruiz-Celma A, et al. (2011) Recycling, reuse and energetic valuation of meat industry wastes in Extremadura (Spain). Resources, conservation and recycling 55: 393–399. [Google Scholar]
- de Titto E, Savino A. (2019) Environmental and health risks related to waste incineration. Waste Management and Research 37: 976-986. [DOI] [PubMed] [Google Scholar]
- Deus RM, Bezerra BS, Battistelle RAG. (2019) Solid waste indicators and their implications for management practice. International Journal of Environmental Science and Technology 16: 1129–1144. [Google Scholar]
- Devon Brits by, Pryke JS, Villet MH. (2016) Improving feeding efficiencies of black soldier fly larvae, Hermetia illucens (L., 1758) (Diptera: Stratiomyidae: Hermetiinae) through feeding environment manipulation for industrial mass rearing. Journal of Entomology and Zoology Studies 3: 147–152. [Google Scholar]
- Diclaro JW, Kaufman PE. (2009) Black soldier fly Hermetia illucens Linnaeus (Insecta: Diptera: Stratiomyidae). University of Florida IFAS Extension. Available at: https://edis.ifas.ufl.edu/publication/IN830 (accessed 7 June 2022).
- Diener S. (2010) Valorisation of organic solid waste using the black soldier fly, Hermetia illucens, in low and middle-income countries. Doctoral Thesis. ETH Zurich. DOI: 10.3929/ETHZ-A-6559779 [DOI] [Google Scholar]
- Diener S, Solano NMS, Gutiérrez FR, et al. (2011) Biological treatment of municipal organic waste using black soldier fly larvae. Waste and Biomass Valorization 2: 357–363. [Google Scholar]
- Diener S, Zurbrugg C, Gutierrez FR, et al. (2011) Black soldier fly lavae for organic waste treatment: Prospects and constraints. In: Proceedings of the WasteSafe 2011, 2nd international conference on solid waste management in the developing countries, Khulna, Bangladesh, 13–15 February 2011, pp. 1–8. [Google Scholar]
- Diener S, Zurbrügg C, Tockner K. (2009) Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Management & Research 27: 603–610. [DOI] [PubMed] [Google Scholar]
- Diener S, Zurbrügg C, Tockner K. (2015) Bioaccumulation of heavy metals in the black soldier fly, Hermetia illucens and effects on its life cycle. Journal of Insects as Food and Feed 1: 261–270. [Google Scholar]
- Dierenfeld ES, King JD. (2008) Digestibility and mineral availability of Phoenix worms, Hermetia illucens, ingested by mountain chicken frogs, Leptodactylus fallax. J Herpetol Med Surg 18: 100–105. [Google Scholar]
- Djekic I, Operta S, Djulancic N, et al. (2019) Quantities, environmental footprints and beliefs associated with household food waste in Bosnia and Herzegovina. Waste Management and Research 37: 1250–1260. [DOI] [PubMed] [Google Scholar]
- Drew J, Cleghorn C, Macmillan A, et al. (2020) Healthy and climate-friendly eating patterns in the New Zealand context. Environmental Health Perspectives 128: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte CM, Bruhn A, Krause-Jensen D. (2021) A seaweed aquaculture imperative to meet global sustainability targets. Nature Sustainability 5: 185–193. [Google Scholar]
- Ebikeme C. (2013) Water world | Eyes on environment | Learn science at scitable. Available at: https://www.nature.com/scitable/blog/eyes-on-environment/water_world/ (accessed 29 January 2022).
- Erickson MC, Islam M, Sheppard C, et al. (2004) Reduction of Escherichia coli O157:H7 and Salmonella enterica serovar enteritidis in chicken manure by larvae of the black soldier fly. Journal of Food Protection 67: 685–690. [DOI] [PubMed] [Google Scholar]
- Estrada A, Coates Estrada R, Meritt D, Jr, et al. (2011) World livestock 2011: Livestock in food security. Roma (Italia): FAO. [Google Scholar]
- FAO (2009) The state of food and agriculture 2009: Livestock in the balance. Roma (Italia): FAO. [Google Scholar]
- Foley JA, Ramankutty N, Brauman KA, et al. (2011) Solutions for a cultivated planet. Nature 478: 337–342. [DOI] [PubMed] [Google Scholar]
- Furman DP, Young RD, Catts PE. (1959) Hermetia illucens (Linnaeus) as a factor in the natural control of Musca domestica Linnaeus. Journal of Economic Entomology 52: 917–921. [Google Scholar]
- Gao Q, Wang X, Wang W, et al. (2017) Influences of chromium and cadmium on the development of black soldier fly larvae. Environmental Science and Pollution Research 24: 8637–8644. [DOI] [PubMed] [Google Scholar]
- Gerber PJ, Steinfeld H, Henderson B, et al. (2013) Tackling Climate Change Through Livestock: A Global Assessment of Emissions and Mitigation Opportunities. Rome: Food and Agriculture Organization of the United Nations. [Google Scholar]
- Ghafoor A, ur Rehman T, Munir A, et al. (2016) Current status and overview of renewable energy potential in Pakistan for continuous energy sustainability. Renewable and Sustainable Energy Reviews 60: 1332–1342. [Google Scholar]
- Gies E. (2016) Landfills have a huge greenhouse gas problem. Here’s what we can do about it. Available at: https://ensia.com/features/methane-landfills/ (accessed 4 December 2021).
- Girotto F, Cossu R. (2017) Animal waste: Opportunities and challenges. In: Lichtfouse E. (ed.) Sustainable Agriculture Reviews, vol. 22. Cham: Springer. [Google Scholar]
- Gobbi P, Martínez-Sánchez A, Rojo S. (2013) The effects of larval diet on adult life-history traits of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). European Journal of Entomology 110: 461. [Google Scholar]
- Gold M, Tomberlin JK, Diener S, et al. (2018) Decomposition of biowaste macronutrients, microbes, and chemicals in black soldier fly larval treatment: A review. Waste Management 82: 302–318. [DOI] [PubMed] [Google Scholar]
- Grossule V, Vanin S, Lavagnolo MC. (2020) Potential treatment of leachate by Hermetia illucens (Diptera, Stratyomyidae) larvae: Performance under different feeding conditions. Waste Management and Research 38: 537–545. [DOI] [PubMed] [Google Scholar]
- Holmes L. (2010)Role of abiotic factors on the development and life history of the black soldier fly, Hermetia illucens (L.) (Diptera: Stratiomyidae). Electronic Theses and Dissertations. 285. University of Windsor, Ontario, Canada. Available at: https://scholar.uwindsor.ca/cgi/viewcontent.cgi?article=1284&context=etd (accessed 7 June 2022). [Google Scholar]
- Holmes LA, Vanlaerhoven SL, Tomberlin JK. (2012) Relative humidity effects on the life history of Hermetia illucens (Diptera: Stratiomyidae). Environmental Entomology 41: 971–978. [DOI] [PubMed] [Google Scholar]
- Hongoh Y. (2010) Diversity and genomes of uncultured microbial symbionts in the termite gut. Bioscience, Biotechnology, and Biochemistry 74: 1145–1151. [DOI] [PubMed] [Google Scholar]
- Ites S, Smetana S, Toepfl S, et al. (2020) Modularity of insect production and processing as a path to efficient and sustainable food waste treatment. Journal of Cleaner Production 248: 119248. [Google Scholar]
- Jeon H, Park S, Choi J, et al. (2011) The intestinal bacterial community in the food waste-reducing larvae of Hermetia illucens. Current Microbiology 62: 1390–1399. [DOI] [PubMed] [Google Scholar]
- Ji-bin Z, Jia Z, Jia-hui LI, et al. (2020) Black soldier fly: A new vista for livestock and poultry manure management. Journal of Integrative Agriculture 19: 2–14. [Google Scholar]
- Kim CH, Ryu J, Lee J, et al. (2021) Use of black soldier fly larvae for food waste treatment and energy production in asian countries: A review. Processes 9: 161. [Google Scholar]
- Kudo T. (2009) Termite-microbe symbiotic system and its efficient degradation of lignocellulose. Bioscience, Biotechnology, and Biochemistry 73: 2561–2567. [DOI] [PubMed] [Google Scholar]
- Kumar S, Negi S, Mandpe A, et al. (2018) Rapid composting techniques in Indian context and utilization of black soldier fly for enhanced decomposition of biodegradable wastes: A comprehensive review. Journal of Environmental Management 227: 189–199. [DOI] [PubMed] [Google Scholar]
- Kwasek M. (2013) Threats to food security and common agricultural policy. Ekonomika Poljoprivrede 59: 701–713. [Google Scholar]
- Lalander C, Diener S, Magri ME, et al. (2013) Faecal sludge management with the larvae of the black soldier fly (Hermetia illucens): From a hygiene aspect. Science of the Total Environment 458–460: 312–318. [DOI] [PubMed] [Google Scholar]
- Lalander C, Diener S, Zurbrügg C, et al. (2019) Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). Journal of Cleaner Production 208: 211–219. [Google Scholar]
- Lanan MC, Rodrigues PAP, Agellon A, et al. (2016) A bacterial filter protects and structures the gut microbiome of an insect. The ISME Journal 10: 1866–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalander C, Senecal J, Gros Calvo M, et al. (2016) Fate of pharmaceuticals and pesticides in fly larvae composting. Science of the Total Environment 565: 279–286. [DOI] [PubMed] [Google Scholar]
- Lardé G. (1990) Recycling of coffee pulp by Hermetia illucens (Diptera: Stratiomyidae) larvae. Biological Wastes 33: 307–310. [Google Scholar]
- Ledda C, Schievano A, Scaglia B, et al. (2016) Integration of microalgae production with anaerobic digestion of dairy cattle manure: An overall mass and energy balance of the process. Journal of Cleaner Production 112: 103–112. [Google Scholar]
- Li Q, Zheng L, Qiu N, et al. (2011) Bioconversion of dairy manure by black soldier fly (Diptera: Stratiomyidae) for biodiesel and sugar production. Waste Management 31: 1316–1320. [DOI] [PubMed] [Google Scholar]
- Li S, Chen Y, Li K, et al. (2016) Characterization of physicochemical properties of fermented soybean curd residue by Morchella esculenta. International Biodeterioration and Biodegradation 109: 113–118. [Google Scholar]
- Li S, Zhu D, Li K, et al. (2013) Soybean curd residue: composition, utilization, and related limiting factors. ISRN Industrial Engineering 2013: 423590. [Google Scholar]
- Li X, Shimizu N. (2021) Effects of lipase addition, hydrothermal processing, their combination, and co-digestion with crude glycerol on food waste anaerobic digestion. Fermentation 7: 284. [Google Scholar]
- Lievens S, Poma G, De Smet J, et al. (2021) Chemical safety of black soldier fly larvae (Hermetia Illucens), knowledge gaps and recommendations for future research: A critical review. Journal of Insects as Food and Feed 7: 383–396. [Google Scholar]
- Lim JW, Mohd-Noor SN, Wong CY, et al. (2019) Palatability of black soldier fly larvae in valorizing mixed waste coconut endosperm and soybean curd residue into larval lipid and protein sources. Journal of Environmental Management 231: 129–136. [DOI] [PubMed] [Google Scholar]
- Lim SL, Lee LH, Wu TY. (2016) Sustainability of using composting and vermicomposting technologies for organic solid waste biotransformation: Recent overview, greenhouse gases emissions and economic analysis. Journal of Cleaner Production 111: 262–278. [Google Scholar]
- Liu C, Hotta Y, Santo A, et al. (2016) Food waste in Japan: Trends, current practices and key challenges. Journal of Cleaner Production 133: 557–564. [Google Scholar]
- Liu C, Mao C, Bunditsakulchai P, et al. (2020) Food waste in Bangkok: Current situation, trends and key challenges. Resources, Conservation and Recycling 157: 104779. [Google Scholar]
- Liu Q, Tomberlin JK, Brady JA, et al. (2008) Black soldier fly (Diptera: Stratiomyidae) larvae reduce Escherichia coli in dairy manure. Environmental Entomology 37: 1525–1530. [DOI] [PubMed] [Google Scholar]
- Liu X, Chen X, Wang H, et al. (2017) Dynamic changes of nutrient composition throughout the entire life cycle of black soldier fly. PLoS One 12: e0182601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke MK, Leung P. (2015) Quantifying food waste in Hawaii’s food supply chain. Waste Management and Research 33:1076–1083. [DOI] [PubMed] [Google Scholar]
- Lucifero N. (2016) Food loss and waste in the EU law between sustainability of well-being and the implications on food system and on environment. Agriculture and Agricultural Science Procedia 8: 282–289. [Google Scholar]
- Ma J, Lei Y, Rehman KU, et al. (2018) Dynamic effects of initial pH of substrate on biological growth and metamorphosis of black soldier fly (Diptera: Stratiomyidae). Environmental Entomology 47:159–165. [DOI] [PubMed] [Google Scholar]
- Makkar HPS, Tran G, Heuzé V, et al. (2014) State-of-the-art on use of insects as animal feed. Animal Feed Science and Technology 197: 1–33. [Google Scholar]
- Malhi GS, Kaur M, Kaushik P. (2021) Impact of climate change on agriculture and its mitigation strategies: A review. Sustainability (Switzerland) 13: 1318. [Google Scholar]
- Manurung R, Supriatna A, Esyanthi RR. (2016) Bioconversion of Rice straw waste by black soldier fly larvae (Hermetia illucens L.): Optimal feed rate for biomass production. Journal of Entomology and Zoology Studies 4: 1036–1041. [Google Scholar]
- Martínez-Sánchez A, Magaña C, Saloña M, et al. (2011) First record of Hermetia illucens (Diptera: Stratiomyidae) on human corpses in Iberian Peninsula. Forensic Science International 206: e76–e78. [DOI] [PubMed] [Google Scholar]
- Mazza L, Xiao X, Rehman KU, et al. (2020) Management of chicken manure using black soldier fly (Diptera: Stratiomyidae) larvae assisted by companion bacteria. Waste Management 102: 312–318. [DOI] [PubMed] [Google Scholar]
- Mc Carthy U, Uysal I, Badia-Melis R, et al. (2018) Global food security – issues, challenges and technological solutions. Trends in Food Science and Technology 77: 11–20. [Google Scholar]
- McGlade C, Ekins P. (2015) The geographical distribution of fossil fuels unused when limiting global warming to 2°C. Nature 517: 187–190. [DOI] [PubMed] [Google Scholar]
- Melikoglu M, Lin CSK, Webb C. (2013) Analysing global food waste problem: Pinpointing the facts and estimating the energy content. Central European Journal of Engineering 3: 157–164. [Google Scholar]
- Mensbrugghe D, Osorio-Rodarte I, Burns A, et al. (2009) How to feed the world in 2050: Macroeconomic environment, commodity markets – a longer term outlook. Food and Agriculture Organization of the United Nations. Available at: http://www.fao.org/3/a-ak967e.pdf (accessed 7 September 2016). [Google Scholar]
- Miranda CD, Cammack JA, Tomberlin JK. (2020) Mass production of the black soldier fly, Hermetia illucens (L.), (Diptera: Stratiomyidae) reared on three manure types. Animals 10: 1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers HM, Tomberlin JK, Lambert BD, et al. (2008) Development of black soldier fly (Diptera: Stratiomyidae) larvae fed dairy manure. Environmental Entomology 37: 11–15. [DOI] [PubMed] [Google Scholar]
- Nanda S, Berruti F. (2021) Municipal solid waste management and landfilling technologies: A review. Environmental Chemistry Letters 19: 1433–1456. [Google Scholar]
- Network NR-TGM (2014) World water distribution. Available at: https://snr.unl.edu/data/water/groundwater/realtime/waterdistribution.aspx (accessed 29 January 2022).
- Newton GL, Sheppard DC, Watson DW, et al. (2005. a) The black soldier fly, Hermetia illucens, as a manure management/resource recovery tool. In: Proceedings of the 2005 Symposium on the State of the Science of Animal Manure and Waste Management, San Antonio, TX, US, 5 January 2005, pp. 2–17. [Google Scholar]
- Newton L, Sheppard C, Watson DW, et al. (2005. b) Using the Black Soldier Fly, Hermetia illucens, as a Value-Added Tool for the Management of Swine Manure. Raleigh, NC: Animal and Poultry Waste Management Center, North Carolina State University. [Google Scholar]
- Nie H, Jacobi HF, Strach K, et al. (2015) Mono-fermentation of chicken manure: Ammonia inhibition and recirculation of the digestate. Bioresource Technology 178: 238–246. [DOI] [PubMed] [Google Scholar]
- Ohkuma M. (2008) Symbioses of flagellates and prokaryotes in the gut of lower termites. Trends in Microbiology 16: 345–352. [DOI] [PubMed] [Google Scholar]
- Olivier JGJ, Peters JAHW. (2020) Trends in Global CO2 and Total Greenhouse Gas Emissions. PBL Netherlands Environmental Assessment Agency. Available at: https://www.pbl.nl/sites/default/files/downloads/pbl-2020-trends-in-global- (accessed 2 April 2022).
- Oonincx D, Van Huis A, van Loon JJA. (2015) Nutrient utilisation by black soldier flies fed with chicken, pig, or cow manure. Journal of Insects as Food and Feed 1: 131–139. [Google Scholar]
- Oonincx DGAB, van Broekhoven S, Van Huis A, et al. (2015) Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS One 10: e0144601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oonincx DGAB, Volk N, Diehl JJE, et al. (2016) Photoreceptor spectral sensitivity of the compound eyes of black soldier fly (Hermetia illucens) informing the design of LED-based illumination to enhance indoor reproduction. Journal of Insect Physiology 95: 133–139. [DOI] [PubMed] [Google Scholar]
- Paz ASP, Carrejo NS, Rodríguez CHG. (2015) Effects of larval density and feeding rates on the bioconversion of vegetable waste using black soldier fly larvae Hermetia illucens (L.), (Diptera: Stratiomyidae). Waste and Biomass Valorization 6: 1059–1065. [Google Scholar]
- Peguero DA, Mutsakatira ET, Buckley CA, et al. (2021) Evaluating the microbial safety of heat-treated fecal sludge for black soldier fly larvae production in South Africa. Environmental Engineering Science 38: 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SO, Sommer SG, Béline F, et al. (2007) Recycling of livestock manure in a whole-farm perspective. Livestock Science 112: 180–191. [Google Scholar]
- Prateep Na Talang R, Sirivithayapakorn S. (2021) Environmental and financial assessments of open burning, open dumping and integrated municipal solid waste disposal schemes among different income groups. Journal of Cleaner Production 312: 127761. [Google Scholar]
- Purkayastha D, Sarkar S. (2021) Sustainable waste management using black soldier fly larva: a review. International Journal of Environmental Science and Technology. 1–26. DOI: 10.1007/s13762-021-03524-7 [DOI] [Google Scholar]
- Purkayastha D, Sarkar S. (2022) Black soldier fly larvae for treatment and segregation of commingled municipal solid waste at different environmental conditions. Journal of Environmental Management 302: 114060. [DOI] [PubMed] [Google Scholar]
- Purschke B, Scheibelberger R, Axmann S, et al. (2017) Impact of substrate contamination with mycotoxins, heavy metals and pesticides on the growth performance and composition of black soldier fly larvae (Hermetia illucens) for use in the feed and food value chain. Food Additives and Contaminants – Part A Chemistry, Analysis, Control, Exposure and Risk Assessment 34: 1410–1420. [DOI] [PubMed] [Google Scholar]
- Raksasat R, Lim JW, Kiatkittipong W, et al. (2020) A review of organic waste enrichment for inducing palatability of black soldier fly larvae: Wastes to valuable resources. Environmental Pollution 267: 115488. [DOI] [PubMed] [Google Scholar]
- Rapatsa MM, Moyo NAG. (2017) Evaluation of Imbrasia belina meal as a fishmeal substitute in Oreochromis mossambicus diets: Growth performance, histological analysis and enzyme activity. Aquaculture Reports 5: 18–26. [Google Scholar]
- Ravindran V. (2013) Main Ingredients Used in Poultry Feed Formulations. Poultry development Review. Rome: FAO. [Google Scholar]
- Rehman KU, Cai M, Xiao X, et al. (2017. a) Cellulose decomposition and larval biomass production from the co-digestion of dairy manure and chicken manure by mini-livestock (Hermetia illucens L.). Journal of Environmental Management 196: 458–465. [DOI] [PubMed] [Google Scholar]
- Rehman KU, Hollah C. (2020) Black soldier fly larval meal: Current status and future prospects used in poultry feed industry. Available at: https://www.feedplanetmagazine.com/english/black-soldier-fly-larval-meal-current-status-and-future-prospects-used-in-poultry-feed-industry/.html (accessed 13 November 2021).
- Rehman KU, Hollah C, Heinz V. (2021) Insects: Alternative source of protein and fat in livestock, pets and aquaculture feed. Feed Planet. Available at: https://feedplanetmagazine.com/blog/insects-alternative-source-of-protein-and-fat-in-livestock-pets-and-aquaculture-feed-1415 (accessed 3 April 2022). [Google Scholar]
- Rehman KU, Liu X, Wang H, et al. (2018) Effects of black soldier fly biodiesel blended with diesel fuel on combustion, performance and emission characteristics of diesel engine. Energy Conversion and Management 173: 489–498. [Google Scholar]
- Rehman KU, Rehman A, Cai M, et al. (2017. b) Conversion of mixtures of dairy manure and soybean curd residue by black soldier fly larvae (Hermetia illucens L.). Journal of Cleaner Production 154: 366–373. [Google Scholar]
- Rehman KU, Rehman RU, Somroo AA, et al. (2019) Enhanced bioconversion of dairy and chicken manure by the interaction of exogenous bacteria and black soldier fly larvae. Journal of Environmental Management 237: 75–83. [DOI] [PubMed] [Google Scholar]
- Riaño B, García-González MC. (2015) Greenhouse gas emissions of an on-farm swine manure treatment plant – comparison with conventional storage in anaerobic tanks. Journal of Cleaner Production 103: 542–548. [Google Scholar]
- Rico C, Montes JA, Muñoz N, et al. (2015) Thermophilic anaerobic digestion of the screened solid fraction of dairy manure in a solid-phase percolating reactor system. Journal of Cleaner Production 102: 512–520. [Google Scholar]
- Salomone R, Saija G, Mondello G, et al. (2017) Environmental impact of food waste bioconversion by insects: Application of life cycle assessment to process using Hermetia illucens. Journal of Cleaner Production 140: 890–905. [Google Scholar]
- Salvador S, Corazzin M, Piasentier E, et al. (2016) Environmental assessment of small-scale dairy farms with multifunctionality in mountain areas. Journal of Cleaner Production 124: 94–102. [Google Scholar]
- Sealey WM, Gaylord TG, Barrows FT, et al. (2011) Sensory analysis of rainbow trout, Oncorhynchus mykiss, fed enriched black soldier fly prepupae, Hermetia illucens. Journal of the World Aquaculture Society 42: 34–45. [Google Scholar]
- Seong WR, Nam YD, Chang HW, et al. (2008) Phylogenetic characterization of two novel commensal bacteria involved with innate immune homeostasis in Drosophila melanogaster. Applied and Environmental Microbiology 74: 6171–6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard C. (1983) House fly and lesser fly control utilizing the black soldier fly in manure management systems for caged laying hens. Environmental Entomology 12: 1439–1442. [Google Scholar]
- Sheppard DC. (1992) Large scale food production from animal manures with a non-pest native fly. Food Insects Newsletter 5: 3. [Google Scholar]
- Sheppard DC, Newton GL, Thompson SA, et al. (1994) A value added manure management system using the black soldier fly. Bioresource Technology 50: 275–279. [Google Scholar]
- Sheppard DC, Tomberlin JK, Joyce JA, et al. (2002) Rearing methods for the black soldier fly (Diptera: Stratiomyidae). Journal of Medical Entomology 39: 695–698. [DOI] [PubMed] [Google Scholar]
- Sherman RA, Wyle FA. (1996) Low-cost, low-maintenance rearing of maggots in hospitals, clinics, and schools. The American Journal of Tropical Medicine and Hygiene 54: 38–41. [DOI] [PubMed] [Google Scholar]
- Shinzato N, Muramatsu M, Matsui T, et al. (2007) Phylogenetic analysis of the gut bacterial microflora of the fungus-growing termite Odontotermes formosanus. Bioscience, Biotechnology and Biochemistry 71: 906–915. [DOI] [PubMed] [Google Scholar]
- Shorstkii I, Comiotto Alles M, Parniakov O, et al. (2020) Optimization of pulsed electric field assisted drying process of black soldier fly (Hermetia illucens) larvae. Drying Technology 40: 595–603. [Google Scholar]
- Singh A, Kumari K. (2019) An inclusive approach for organic waste treatment and valorisation using black soldier fly larvae: A review. Journal of Environmental Management 251: 109569. [DOI] [PubMed] [Google Scholar]
- Singh A, Tiwari R, Joshi P, et al. (2020) Insights into organic waste management practices followed by dairy farmers of Ludhiana District, Punjab: Policy challenges and solutions. Waste Management and Research 38: 291–299. [DOI] [PubMed] [Google Scholar]
- Skaf L, Buonocore E, Dumontet S, et al. (2021) Integrating environmental and socio-economic indicators to explore the sustainability of food patterns and food security in Lebanon. Current Research in Environmental Sustainability 3: 100047. [Google Scholar]
- Skrobonja E. (2020) 13 reasons why the black soldier fly is the future of food and feed. Available at: https://www.eatcrickster.com/blog/black-soldier-fly (accessed 1 February 2022).
- Smetana S, Leonhardt L, Kauppi SM, et al. (2020) Insect margarine: Processing, sustainability and design. Journal of Cleaner Production 264: 121670. [Google Scholar]
- Smetana S, Palanisamy M, Mathys A, et al. (2016) Sustainability of insect use for feed and food: Life cycle assessment perspective. Journal of Cleaner Production 137: 741–751. [Google Scholar]
- Smets R, Verbinnen B, Van De Voorde I, et al. (2020) Sequential extraction and characterisation of lipids, proteins, and chitin from black soldier fly (Hermetia illucens) larvae, prepupae, and pupae. Waste and Biomass Valorization 11: 6455–6466. [Google Scholar]
- Soeder DJ. (2021) Fossil Fuels and Climate Change. In: Soeder DJ. (ed.) Fracking and the Environment. South Dakota School of Mines & Technology, Rapid City, SD, USA. Cham: Springer. DOI: 10.1007/978-3-030-59121-2_9 [DOI] [Google Scholar]
- Somroo AA, Rehman KU, Zheng L, et al. (2019) Influence of Lactobacillus buchneri on soybean curd residue co-conversion by black soldier fly larvae (Hermetia illucens) for food and feedstock production. Waste Management 86: 114–122. [DOI] [PubMed] [Google Scholar]
- Souissi A, Mtimet N, Thabet C, et al. (2019) Impact of food consumption on water footprint and food security in Tunisia. Food Security 11: 989–1008. [Google Scholar]
- Stanley KN, Wallace JS, Currie JE, et al. (1998) The seasonal variation of thermophilic campylobacters in beef cattle, dairy cattle and calves. Journal of Applied Microbiology 85: 472–480. [DOI] [PubMed] [Google Scholar]
- Surendra KC, Olivier R, Tomberlin JK, et al. (2016) Bioconversion of organic wastes into biodiesel and animal feed via insect farming. Renewable Energy 98: 197–202. [Google Scholar]
- Surendra KC, Tomberlin JK, Van Huis A, et al. (2020) Rethinking organic wastes bioconversion: Evaluating the potential of the black soldier fly (Hermetia illucens (L.)) (Diptera: Stratiomyidae) (BSF). Waste Management 117: 58–80. [DOI] [PubMed] [Google Scholar]
- Tomberlin JK, Adler PH, Myers HM. (2009) Development of the black soldier fly (Diptera: Stratiomyidae) in relation to temperature. Environmental Entomology 38: 930–934. [DOI] [PubMed] [Google Scholar]
- Tomberlin JK, Sheppard DC, Joyce JA. (2002) Selected life-history traits of black soldier flies (Diptera: Stratiomyidae) reared on three artificial diets. Annals of the Entomological Society of America 95: 379–386. [Google Scholar]
- Tomberlin JK, Van Huis A, Benbow ME, et al. (2015) Protecting the environment through insect farming as a means to produce protein for use as livestock, poultry, and aquaculture feed. Journal of Insects as Food and Feed 1: 307–309. [Google Scholar]
- United Nations (2017) World population projected to reach 9.8 billion in 2050, and 11.2 billion in 2100 | UN DESA | United Nations Department of Economic and Social Affairs. Available at: https://www.un.org/development/desa/en/news/population/world-population-prospects-2017.html (accessed 24 July 2021).
- United Nations (2020) UN world water development report. Available at: https://www.unwater.org/publications/world-water-development-report-2020/ (accessed 29 January 2022).
- US EPA (2021) Basic information about landfill gas | US EPA. Available at: https://www.epa.gov/lmop/basic-information-about-landfill-gas (accessed 4 December 2021).
- Üstüner T, Hasbenli A, Rozkosný R. (2003) The first record of Hermetia illucens (Linnaeus, 1758) (Diptera: Stratiomyidae) from the near east. Studia Dipterologica 10: 181–185. [Google Scholar]
- Valta K, Sotiropoulos A, Malamis D, et al. (2019) Assessment of the effect of drying temperature and composition on the biochemical methane potential of in-house dried household food waste. Waste Management and Research 37: 461–468. [DOI] [PubMed] [Google Scholar]
- van der Fels-Klerx HJ, Meijer N, Nijkamp MM, et al. (2020) Chemical food safety of using former foodstuffs for rearing black soldier fly larvae (Hermetia illucens) for feed and food use. Journal of Insects as Food and Feed 6: 475–488. [Google Scholar]
- van Huis A, van Itterbeeck J, Klunder H, et al. (2013) Edible insects: future prospects for food and feed security. Rome: Food and agriculture organization of the United nations; (FAO). Available at: http://library.wur.nl/WebQuery/wurpubs/fulltext/258042 (accessed 8 June 2022). [Google Scholar]
- Veldkamp T, van Rozen K, Elissen H, et al. (2021) Bioconversion of digestate, pig manure and vegetal residue-based waste operated by black soldier fly larvae, Hermetia illucens L. (Diptera: Stratiomyidae). Animals 11: 3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venglovsky J, Sasakova N, Placha I. (2009) Pathogens and antibiotic residues in animal manures and hygienic and ecological risks related to subsequent land application. Bioresource Technology 100: 5386–5391. [DOI] [PubMed] [Google Scholar]
- Verisk Maplecroft (2019) Waste Generation and Recycling Indices 2019. Overview and findings. Available at: https://www.circularonline.co.uk/wp-content/uploads/2019/07/Verisk_Maplecroft_Waste_Generation_Index_Overview_2019.pdf (accessed 3 December 2021).
- Villa R, Muñoz HMB, Jawiarczyk N, et al. (2021) Black Soldier Fly Biorefinery: A Novel Upcycling Route for Municipal Biosolids. Elsevier. DOI: 10.1016/b978-0-323-85223-4.00019-1 [DOI] [Google Scholar]
- Wang H, Rehman KU, Liu X, et al. (2017) Insect biorefinery: a green approach for conversion of crop residues into biodiesel and protein. Biotechnology for Biofuels 10: 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Rehman KU, Feng W, et al. (2020) Physicochemical structure of chitin in the developing stages of black soldier fly. International Journal of Biological Macromolecules 149: 901–907. [DOI] [PubMed] [Google Scholar]
- Wang XB, Wu N, CAI RJ, et al. (2021) Changes in speciation, mobility and bioavailability of Cd, Cr and As during the transformation process of pig manure by black soldier fly larvae (Hermetia illucens). Journal of Integrative Agriculture 20: 1157–1166. [Google Scholar]
- Welsby D, Price J, Pye S, et al. (2021) Unextractable fossil fuels in a 1.5°C world. Nature 597: 230–234. [DOI] [PubMed] [Google Scholar]
- Wiedemann SG, McGahan EJ, Murphy CM. (2017) Resource use and environmental impacts from Australian chicken meat production. Journal of Cleaner Production 140: 675–684. [Google Scholar]
- Willett W, Rockström J, Loken B, et al. (2019) Food in the anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. The Lancet 393: 447–492. [DOI] [PubMed] [Google Scholar]
- Wong CY, Aris MNM, Daud H, et al. (2020) In-situ yeast fermentation to enhance bioconversion of coconut endosperm waste into larval biomass of Hermetia illucens: Statistical augmentation of larval lipid content. Sustainability (Switzerland) 12: 1558. [Google Scholar]
- Woodley NE. (2001) A world catalog of the Stratiomyidae (Insecta: Diptera). Myia 11: 1–484. [Google Scholar]
- Wu N, Wang X, Xu X, et al. (2020) Effects of heavy metals on the bioaccumulation, excretion and gut microbiome of black soldier fly larvae (Hermetia illucens). Ecotoxicology and Environmental Safety 192: 110323. [DOI] [PubMed] [Google Scholar]
- Xiao X, Mazza L, Yu Y, et al. (2018) Efficient co-conversion process of chicken manure into protein feed and organic fertilizer by Hermetia illucens L. (Diptera: Stratiomyidae) larvae and functional bacteria. Journal of Environmental Management 217: 668–676. [DOI] [PubMed] [Google Scholar]
- Xu X, Sharma P, Shu S, et al. (2021) Global greenhouse gas emissions from animal-based foods are twice those of plant-based foods. Nature Food 2: 724–732. [DOI] [PubMed] [Google Scholar]
- Yang Y, Yang J, Wu WM, et al. (2015) Biodegradation and mineralization of polystyrene by plastic-eating mealworms: Part 2. Role of gut microorganisms. Environmental Science and Technology 49: 12087–12093. [DOI] [PubMed] [Google Scholar]
- Yin J, Wang K, Yang Y, et al. (2014) Improving production of volatile fatty acids from food waste fermentation by hydrothermal pretreatment. Bioresource Technology 171: 323–329. [DOI] [PubMed] [Google Scholar]
- Yu G, Cheng P, Chen Y, et al. (2011) Inoculating poultry manure with companion bacteria influences growth and development of black soldier fly (Diptera: Stratiomyidae) larvae. Environmental Entomology 40: 30–35. [DOI] [PubMed] [Google Scholar]
- Yukesh Kannah R, Merrylin J, Poornima Devi T, et al. (2020) Food waste valorization: Biofuels and value added product recovery. Bioresource Technology Reports 11: 100524. [Google Scholar]
- Zhang J, Huang L, He J, et al. (2010) An artificial light source influences mating and oviposition of black soldier flies, Hermetia illucens. Journal of Insect Science 10: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Shi Z, Gao Z, et al. (2021) Identification of three metallothioneins in the black soldier fly and their functions in Cd accumulation and detoxification. Environmental Pollution 286: 117146. [DOI] [PubMed] [Google Scholar]
- Zheng L, Hou Y, Li W, et al. (2012) Biodiesel production from rice straw and restaurant waste employing black soldier fly assisted by microbes. Energy 47: 225–229. [Google Scholar]
- Zheng X, Streimikiene D, Balezentis T, et al. (2019) A review of greenhouse gas emission profiles, dynamics, and climate change mitigation efforts across the key climate change players. Journal of Cleaner Production 234: 1113-1133. [Google Scholar]
- Zhou F, Tomberlin JK, Zheng L, et al. (2013) Developmental and waste reduction plasticity of three black soldier fly strains (Diptera: Stratiomyidae) raised on different livestock manures. Journal of Medical Entomology 50: 1224–1230. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Rehman K ur, Yu Y, et al. (2019) De novo transcriptome sequencing and analysis revealed the molecular basis of rapid fat accumulation by black soldier fly (Hermetia illucens, L.) for development of insectival biodiesel. Biotechnology for Biofuels 12: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]