Abstract
Recent efforts have re-invigorated the Streptococcus pyogenes (Group A Streptococcus) vaccine development field, though scientific, regulatory and commercial barriers persist, and the vaccine pipeline remains sparse. There is an ongoing need to accelerate all aspects of development to address the large global burden of disease caused by the pathogen. Building on over 100 years of S. pyogenes vaccine development, there are currently eight candidates on a product development track, including four M protein-based candidates and four candidates designed around non-M protein antigens. These candidates have demonstrated proof of concept for protection against S. pyogenes in preclinical models, one has demonstrated safety and immunogenicity in a Phase 1 trial and at least four others are poised to soon enter clinical trials. To maintain momentum, the Strep A Vaccine Global Consortium (SAVAC) was established to bring together experts to accelerate global S. pyogenes vaccine development. This article highlights the past, present and future of S. pyogenes vaccine development and emphasizes key priorities, and the role of SAVAC, in advancing the field.
Subject terms: Bacterial infection, Bacterial infection, Pharmaceutics
Introduction
There is a long history of vaccine development against Streptococcus pyogenes, commensurate to the large global burden of disease caused by the pathogen1–3. However, the path to a successful candidate has been impeded by scientific, regulatory, and commercial barriers. Over the past 5 years, efforts at vaccine development have been re-invigorated. At the 2018 71st World Health Assembly, the need to prioritize a Strep A vaccine was recommended as an intervention that would effectively reduce the burden of rheumatic heart disease (RHD)4. The World Health Organization (WHO) and partners subsequently developed a research and development technology roadmap that has served as a valuable strategic guide for vaccine development5, and, in turn, the Strep A Vaccine Global Consortium (SAVAC) has attempted to action the roadmap6. The field has seen new investment, progress in the development of some existing vaccine candidates, and entry of new candidates to the pipeline. Nonetheless, the vaccine pipeline remains sparse, and there is an ongoing need to accelerate all aspects of vaccine development so that the compelling case for an effective vaccine can be met.
Vaccine history
Vaccine development and clinical studies date back well over 100 years. Vaccine antigen approaches have included inactivated whole cell, scarlet fever toxin, M protein and other non-M protein antigens7. A major focus of development since 1940 has been the M protein, a key virulence factor of S. pyogenes. This approach began with whole M protein, then was refined to N-terminal polypeptides and C-repeat peptides. Tens of thousands of study participants received M protein vaccines prior to the 1960s, including children. S. pyogenes pharyngitis human challenge studies of purified M protein in the 1970s demonstrated efficacy of up to 89%, with no serious adverse events detected in vaccinees8–10.
Despite these promising results, vaccine development faced a major obstacle in 1979 when the US Food and Drug Administration instituted a federal regulation (21 CFR 610.19) that prohibited “Group A streptococcus organisms or their derivatives” from vaccines because “Group A streptococcal antigens in bacterial vaccines and antigens may induce dangerous tissue reactions in humans”11. The panel cited a study by Massell et al.12 that was uncontrolled and involved the administration of partially purified type 3 M protein vaccine to 21 healthy siblings of patients with rheumatic fever. Two of the vaccinees developed rheumatic fever and one developed possible rheumatic fever. While concerns about multiple aspects of this study have been raised, the 1979 FDA regulation had the unintentional consequence of impeding future development of S. pyogenes vaccines. In 2006 the FDA revoked Subpart 610.1913, and currently the FDA does not provide specific requirements for a S. pyogenes vaccine.
From 2006–2016, S. pyogenes vaccine development has progressed slowly, still impacted by the lingering effects of the 1979 ruling. The major area of clinical development was in M protein-based vaccine development, although important pre-clinical advances also occurred in non M protein vaccines, including group A streptococcal C5a peptidase and others14. Early-phase clinical trials in healthy adult volunteers of 4 vaccine candidates were conducted: a 6-valent N-terminal M protein vaccine, a 26-valent N-terminal M protein vaccine, a 30-valent N-terminal M protein vaccine and a conserved C-repeat region M protein vaccine15–18. No serious safety signals were detected in these trials, and encouraging immunogenicity data were observed for all vaccine candidates.
Current pipeline
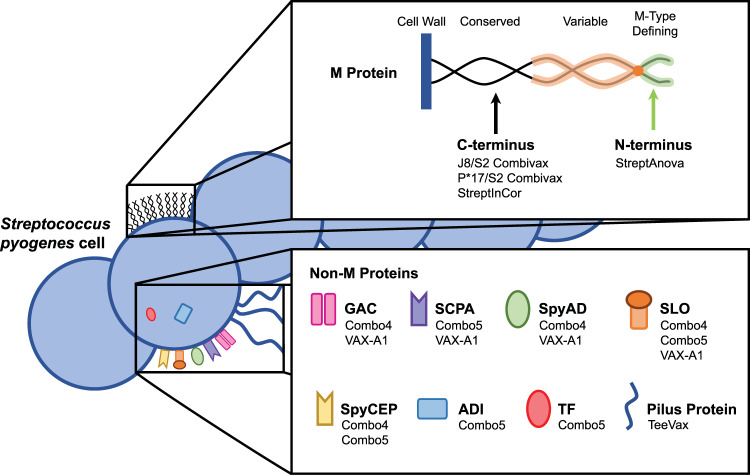
The current pipeline of S. pyogenes vaccine candidates on a product development track includes M protein-based concepts and candidates designed around non-M protein antigens. The following section provides an overview of both types of vaccine candidates, including their composition, theoretical coverage across S. pyogenes strains, demonstration of safety, immunogenicity and efficacy in various models and, where known, an indication of future development plans. Figure 1 presents a schematic of M protein and non-M protein antigens incorporated in the vaccine candidates of focus in this article.
Fig. 1.
S. pyogenes antigens: Schematic of M protein and non-M protein antigens and corresponding vaccine candidates.
M protein-based candidates
Building on the long history of M protein-based vaccine research, several current S. pyogenes vaccine candidates have been designed around various M protein antigens (see summary of M protein-based candidates, Table 1). Given the high number of emm types and the hypervariability of M protein N-terminal regions, the only current vaccine candidate targeting N-terminal epitopes employs a multivalent approach (i.e. the 30-valent StreptAnova). Other M protein-based vaccines incorporate peptides from the more conserved C-terminus, and two of these (J8/S2 combivax and P*17/S2 combivax) combine a non-M protein (i.e. peptide from SpyCEP) in their formulation.
Table 1.
S. pyogenes vaccine development pipeline: Overview of the most advanced M protein-based product development programs.
| CANDIDATE | DEVELOPER | CURRENT DEVELOPMENT PHASE |
ANTIGENS | ADJUVANT | THEORETICAL GLOBAL COVERAGEa | KEY REFERENCES |
|---|---|---|---|---|---|---|
| StreptAnova (30-valent) |
University of Tennessee/ Vaxent |
Phase 1a Completed 2020 | Four protein subunits comprising the N-terminal regions of M proteins from 30 S. pyogenes serotypes | Aluminium hydroxide |
48%19 Actual coverage may be higher (e.g. 80% in Africa) due to cross-opsonization21 |
18,20 |
| J8/S2 combivax | Griffith University/ University of Alberta |
Phase 1a Ongoing |
J8 peptide from the M protein C-terminus combined with a 20-mer B-cell epitope (K4S2) from SpyCEP | Aluminium hydroxide |
37%19 Actual coverage may be ~98% due to cross-reactivity of J8 alleles25 |
17,23,26,27 |
| P*17/S2 combivax | Griffith University/ University of Alberta |
Phase 1a Ongoing |
P*17 peptide from the M protein C-terminus combined with a 20-mer B-cell epitope (K4S2) from SpyCEP | Aluminium hydroxide |
37%19 Actual coverage may be ~98% due to cross-reactivity of J8 alleles25 |
24,27 |
| StreptInCor | University of São Paulo | Preclinical | 55-amino acid sequence peptide from the M5 protein conserved regions (C2 and C3) | Aluminium hydroxide |
23%19 An earlier study showed 71% identity between StreptInCor and several emm types30 |
29,31–33 |
SpyCEP streptococcal interleukin-8 proteas.
aBased on antigen presence across sample of 2,083 S. pyogenes genomes19.
StreptAnova, developed by Dale et al. at the University of Tennessee (USA) with commercialization partner Vaxent, is an emm-type specific, adjuvanted (alum) vaccine with four protein subunits comprising the N-terminal regions of M proteins from 30 S. pyogenes serotypes. This candidate is the farthest along the development pathway, having completed a Phase 1a trial (in 2020) that demonstrated significant immunogenicity towards most of the targeted antigens18. Moreover, the trial showed that StreptAnova was well-tolerated and did not elicit autoimmune or cross-reactive antibodies18. Although theoretical coverage of StreptAnova across S. pyogenes strains based on a large-scale genomic analysis is 48% on a global level (and ranging from 28% in East Africa to 75% in North America)19, the StreptAnova developers have suggested actual coverage may be significantly higher. When accounting for the ability of StreptAnova to elicit cross-opsonic antibodies against non-vaccine serotypes20 and employing an emm-cluster-based S. pyogenes typing system, it has been proposed that StreptAnova could provide hypothetical coverage of 80.3% of isolates in Africa21. Additional clinical trials for StreptAnova are planned, including a Phase 2 efficacy study, pending funding22.
J8/S2 combivax and P*17/S2 combivax are related vaccines in development by Good et al. at Griffith University (Australia) and University of Alberta (Canada). Both vaccine candidates contain K4S2, a peptide with a modified B-cell epitope from S. pyogenes cell envelope proteinase (SpyCEP), combined with one of two versions of the p145 peptide from the M protein C-terminus: J8 for J8/S2 combivax23 and P*17 for its namesake candidate24. Both peptides in each candidate are conjugated to the CRM197 carrier protein. Although the J8 allele of the M protein is only found in 37% of S. pyogenes genomes19, both candidates are still expected to provide ~98% coverage across S. pyogenes strains because the J8 alleles cross-react immunologically25. In mouse studies, J8/S2 combivax and P*17/S2 combivax protected against skin and systemic infection from hypervirulent CovR/S strains of S. pyogenes23. The P*17/S2 combivax vaccine or prototype versions have also been shown to protect against upper respiratory tract infection in mice when formulated with alum, liposomes26 or with the liposomal adjuvant, CAF0127. An earlier version of the vaccine, J8-DT/Alum (which lacked the S2 peptide and in which diphtheria toxoid was used as the carrier protein), was shown to be immunogenic and safe following a single injection in a ‘pilot’ Phase 1 trial17. Approval has been granted by Health Canada (the Canadian Regulator) to undertake a Phase Ia trial of J8/S2 combivax and P*17/S2 combivax and the trial has begun. Upon success of the Phase 1a trial, the developers plan to advance the development of the lead candidate to a Phase 1/2 trial in Australia. The development pathway of the vaccine will likely involve a human challenge study in Australia in 202328.
StreptInCor, from Guilherme et al. at the University of Sao Paulo (Brazil), is comprised of a 55-amino acid peptide from the M5 protein conserved regions (C2, C3) with B- and T-cell epitopes, adjuvanted with alum29. A large-scale genomic study showed that StreptInCor epitopes were found in 23% of S. pyogenes strains19, though an earlier study from the developers indicated that the StreptInCor sequence was 71% conserved amongst M protein sequences and that sequence differences do not affect opsonization, suggesting broad coverage30. In preclinical studies, StreptInCor has shown high levels of antigen-specific antibodies and survival against S. pyogenes infection challenge in mice as well as a lack of autoimmune reactions29,31. In minipigs, the candidate was well tolerated and displayed no harmful effects on heart tissue32. Studies in Wistar rats showed no evidence of toxicity after repeated intramuscular injections33. StreptInCor will be submitted to ANVISA (the Brazilian regulator) in early 2023 and the developer hopes to begin a clinical trial by the end of 202334.
Non-M protein-based candidates
Given the potential, but unproven, safety concerns of M protein-based vaccines, several S. pyogenes vaccine candidates are being designed around other antigens that provide broad coverage across S. pyogenes strains, and which have lower potential for cross-reactivity to host tissues (see summary of non-M protein-based candidates, Table 2). One of these antigens is Group A Carbohydrate (GAC), a surface polysaccharide comprising a poly-rhamnose backbone with an N-acetylglucosamine (GlcNAc) side chain. GAC is highly conserved and expressed in all S. pyogenes isolates35. Two groups have a vaccine candidate featuring GAC but each is using a different version of GAC and have conjugated their respective GAC antigens to different carrier proteins. The significance of these differences is yet to be fully understood, though preclinical results thus far indicate that both approaches may have their advantages (see below for details).
Table 2.
S. pyogenes vaccine development pipeline: Overview of the most advanced non-M protein-based product development programs.
| CANDIDATE | DEVELOPER | CURRENT DEVELOPMENT PHASE | ANTIGENS | ADJUVANT | THEORETICAL GLOBAL COVERAGEa | KEY REFERENCES |
|---|---|---|---|---|---|---|
| Combo4 |
GlaxoSmithKline/ GVGH |
Preclinical | SpyCEP, SLO and SpyAD recombinant proteins and native GAC conjugated to CRM197 carrier protein | Aluminium hydroxide | >99%19 | 41,43,44 |
| VAX-A1 | Vaxcyte | Preclinical |
SLO and SCPA recombinant proteins and modified GAC (Polyrhamnose) conjugated to SpyAD disease-specific carrier protein |
Aluminium hydroxide | >99%19 | 46 |
| Combo5 | University of Queensland | Preclinical | Trigger factor (TF), inactivated versions of arginine deiminase (ADI), SLO, SpyCEP and SCPA | Squalene-in-water emulsion containing cholesterol (SMQ) | >99%19 | 49,50,56 |
| TeeVax |
University of Auckland |
Preclinical | Multiple T-antigen domains from the pilus of the majority of S. pyogenes strains | Aluminium hydroxide | >95%36 | 36,51 |
SpyCEP streptococcal interleukin-8 protease, SLO streptolysin O, SpyAD putative surface exclusion protein, Spy0269, GAC Group A Carbohydrate, SCPA streptococcal C5a peptidase.
aBased on antigen presence across sample of 2,083 S. pyogenes genomes19.
Multiple S. pyogenes non-M protein antigens are also targeted by vaccine candidates. These proteins are highly conserved, being found in 95–99% of all characterized S. pyogenes isolates across the world19,35,36. Several of these proteins are noted below, with a brief description of their function. Streptolysin O is a secreted pore-forming toxin that is upregulated in virulent S. pyogenes isolates35. SpyCEP is a protease that drives immune evasion through cleavage of interleukin 837. SpyAD is a surface-exposed adhesin that mediates S. pyogenes interaction with host cells38. Group A streptococcal C5a peptidase (SCPA) is an enzyme expressed on the cell envelope that mediates resistance to phagocytosis by cleaving the chemotaxin C5a on leukocytes35. Trigger factor (TF) is a peptidyl-prolyl cis-trans isomerase that is essential for the secretion and maturation of the S. pyogenes cysteine protease39. Arginine deiminase (ADI) is an enzyme that contributes to colonization and modulation of host immune response through conversion of arginine to citrulline and ammonia40. Finally, although variable, T-antigens collectively form a structurally conserved backbone of the S. pyogenes pilus, which is involved in adhesion, colonization and immune evasion36. Vaccine candidates combining subsets of these proteins, with or without combination with GAC, are described below.
Combo4, from GSK Vaccines Institute for Global Health (GVGH), GSK Vaccines (Italy), contains the native S. pyogenes GAC conjugated to the CRM197 carrier protein, SLO, SpyCEP and SpyAD41. GVGH has presented data indicating that the native GAC can induce a higher anti-GAC IgG response than poly-rhamnose and greater binding of anti-GAC antibodies compared to anti-poly-rhamnose antibodies to a panel of Strep A strains42. Preclinical studies of Combo4 adjuvanted with alum demonstrated immuno-protection in mouse models and efficacy in opsonophagocytic killing assays using sera from immunized rabbits43,44. GVGH is currently conducting GMP manufacturing and toxicity studies with Combo4 and is planning a Phase 1 dose-escalation study in Australia45.
VAX-A1, from Vaxcyte (USA), is based on work from the Nizet group at University of California, San Diego. VAX-A1 contains GACPR, a modified version of GAC in which the GlcNAc side chain is removed, leaving the poly-rhamnose backbone46. In theory, GACPR may lower the risk of cross-immunogenicity compared to native GAC since the GlcNAc side chain on GAC has been implicated in provoking autoimmune cross-reactivity in RHD46. Moreover, the GACPR in Vax-A1 is conjugated to the S. pyogenes virulence factor SpyAD and this SpyAD-GACPR conjugate is combined with recombinant SLO and SPCA proteins and adjuvanted with alum46. Immunization of mice with VAX-A1 protected against S. pyogenes challenge in both a systemic infection model and localized skin infection model, with no observed signs of cross-reactivity to human heart or brain tissue epitopes46. Having initiated IND-enabling activities in late 2021, Vaxcyte is planning to provide guidance on expected timing for an IND application submission in the second half of 202247.
Combo5, from Walker et al. at the University of Queensland (Australia), contains five recombinant proteins: SLO, SpyCEP, SCPA, TF and ADI, adjuvanted with SMQ (a squalene-in-water emulsion containing a toll-like receptor 4 agonist and QS21)48. In addition to offering broad coverage across S. pyogenes strains48, the vaccine candidate was designed to exclude S. pyogenes antigens potentially linked to autoimmune complications49. In earlier studies using alum as adjuvant, Combo5 reduced the severity of pharyngitis and tonsillitis but did not protect against colonization in NHP49; in mice, the candidate protected against superficial skin infections but not invasive disease48,50. In contrast, adjuvanting Combo5 with SMQ conferred protection against invasive challenge in mice, potentially owing to a more balanced Th1/Th2 immune response compared to Combo5 adjuvanted with alum, which produced a Th2-biased response48. Interestingly, Combo5/SMQ protected mice against invasive challenge in the absence of opsonizing antibodies, suggesting that an opsonizing antibody response may not be a correlate of protection for non-M protein vaccines48.
TeeVax, from Thomas Proft and Jacelyn Loh’s group at University of Auckland (New Zealand), is a multivalent vaccine targeting T-antigens, the major protein component of the surface-exposed S. pyogenes pili that are involved in adhesion and colonisation of the host during infection36,51. This vaccine candidate is comprised of three recombinant proteins (TeeVax1, TeeVax2 and TeeVax3), each consisting of a fusion of 6 unique T-antigen domains36. Combination of all three proteins (TeeVax1-3) elicited a robust antibody response in rabbits that was reactive to all 18 T-antigens included in the three proteins and was cross-reactive to the three remaining sub-types not included in any of the proteins36. This cross-reactivity to all 21 T-antigens would be expected to provide >95% S. pyogenes strain coverage36. Immunization with TeeVax1 adjuvanted with alum produced opsonophagocytic antibodies in rabbits and conferred protective efficacy against invasive disease in humanized plasminogen transgenic mice36. The developers are currently testing TeeVax with different adjuvants and plan to conduct analyses of humoral and cellular immune responses to TeeVax to gain further knowledge about correlates of protection52.
Together, these eight M protein and non-M protein vaccine candidates serve as a diverse, albeit small, set of concepts and approaches for continued and future clinical testing. Though it may be tempting to compare the candidates against one another in terms of theoretical likelihood of technical or regulatory success, our view is that such an exercise would be premature based on current knowledge. Correlates of protection for a vaccine against S. pyogenes remain unknown, animal models are not validated and standardized, and there has not yet been head-to-head comparison of any of the candidates. Thus, identifying optimal antigens and formulations for efficacy and safety requires further clinical trial testing, which the field is poised to embark on over the coming years.
Future efforts
Despite a range of promising candidates, the pipeline of S. pyogenes vaccines remains relatively empty, especially when compared with pipelines for other infectious diseases that cause high global disease burden53. In an effort to attract more vaccine developers and investment to the field, SAVAC, established in 2019, has brought together S. pyogenes experts across multiple domains of expertise to fast track global S. pyogenes vaccine development6. SAVAC has highlighted the favourable cost effectiveness and return on investment of a S. pyogenes vaccine, as well as a series of key knowledge gaps. These gaps include: the scarcity of epidemiologic and economic data from low- and middle-income countries; incomplete understanding of measures of protection against S. pyogenes infection; the identification of immune correlates of protection against S. pyogenes infection, and the development of relevant new functional assays, the current absence of which represents a major impediment to vaccine development the identification of immune correlates of protection against S. pyogenes infection, and the development of relevant new functional assays still lacking and representing a major impediment to vaccine development54; the requirement for standardisation of safety surveillance; and the need for concerted advocacy efforts to raise the profile of the burden of S. pyogenes disease and how a vaccine could address this burden. The next phase of SAVAC’s work will be focused on filling these gaps by coordinating research to generate better burden of disease estimates, drawing together relevant stakeholders to establish guardrails for safety surveillance, boosting efforts around advocacy, and a range of other engagement activities. An innovative systematic framework outlining the properties of accurate and robust burden of disease data has been developed to guide vaccine development and evaluation and prioritize research and surveillance activities3. There is an opportunity to learn from the experience of vaccine development for SARS-CoV-253, including the use of vaccine technologies such as mRNA55 and accelerated or adaptive clinical trial designs. As lead S. pyogenes vaccine candidates approach clinical trials, SAVAC is anticipating the steps necessary to support the field over the next five years. These steps include: 1) preparing for vaccine efficacy trials in low- and middle-income countries by gathering the epidemiological, economic and societal data that is currently lacking, while simultaneously strengthening surveillance and laboratory activities as well as clinical trial capacity through a network of sentinel sites in low- and middle-income countries; 2) preparing industry by engaging with vaccine developers and manufacturers to highlight the need and commercial case for a Strep A vaccine and understanding the barriers to vaccine development with a view to accelerating the Strep A vaccine pipeline; and 3) preparing key non-industry stakeholders by engaging with relevant non-industry stakeholders (e.g., WHO, global funders, national policy makers, NITAGs, experts in laboratory and safety surveillance) to address barriers and enhance implementation efforts for a future Strep A vaccine.
Acknowledgements
The authors would like to acknowledge the members of the Strep A Vaccine Global Consortium (SAVAC; of which two of the authors [ACS, JHK] are co-Chairs of the SAVAC Executive Committee) for their commitment and support. We would also like to thank Nicole Revie (Shift Health) for her scientific illustration assistance. This work was funded through a grant from the Wellcome Trust awarded to the International Vaccine Institute (IVI) (Ref #215490/Z/19/Z).
Author contributions
D.R.W. and A.C.S. wrote the paper and led research efforts to gather source information. M.E.E.W. and J.L.E. contributed to research efforts. M.E.E.W., A.E.M., J.L.E. and J.H.K. reviewed and provided guidance on the draft.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miller, K. M. et al. The global burden of sore throat and group A Streptococcus pharyngitis: A systematic review and meta-analysis. EClinicalMedicine48,101458 10.1016/j.eclinm.2022.101458 (2022). [DOI] [PMC free article] [PubMed]
- 2.Watkins DA, et al. Global, Regional, and National Burden of Rheumatic Heart Disease, 1990–2015. N. Engl. J. Med. 2017;377:713–722. doi: 10.1056/NEJMoa1603693. [DOI] [PubMed] [Google Scholar]
- 3.Moore HC, et al. A Systematic Framework for Prioritizing Burden of Disease Data Required for Vaccine Development and Implementation: The Case for Group A Streptococcal Diseases. Clin. Infect. Dis. 2022;75:1245–1254. doi: 10.1093/cid/ciac291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.71st World Health Assembly Agenda item 12.8: Rheumatic Fever and Rheumatic Heart Disease https://www.who.int/about/governance/world-health-assembly/seventy-first (2018).
- 5.Vekemans J, et al. The Path to Group A Streptococcus Vaccines: World Health Organization Research and Development Technology Roadmap and Preferred Product Characteristics. Clin. Infect. Dis. 2019;69:877–883. doi: 10.1093/cid/ciy1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strep A Vaccine Global Consortium (SAVAC) https://savac.ivi.int. (International Vaccine Institute SAVAC Team, Accessed 14 January 2023).
- 7.Steer AC, Batzloff MR, Mulholland K, Carapetis JR. Group A streptococcal vaccines: facts versus fantasy. Curr. Opin. Infect. Dis. 2009;22:544–552. doi: 10.1097/QCO.0b013e328332bbfe. [DOI] [PubMed] [Google Scholar]
- 8.Fox EN, Waldman RH, Wittner MK, Mauceri AA, Dorfman A. Protective study with a group A streptococcal M protein vaccine. Infectivity challenge of human volunteers. J. Clin. Invest. 1973;52:1885–1892. doi: 10.1172/JCI107372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polly SM, Waldman RH, High P, Wittner MK, Dorfman A. Protective studies with a group A streptococcal M protein vaccine. II. Challange of volenteers after local immunization in the upper respiratory tract. J. Infect. Dis. 1975;131:217–224. doi: 10.1093/infdis/131.3.217. [DOI] [PubMed] [Google Scholar]
- 10.Waldman RH, Lee JD, Polly SM, Dorfman A, Fox EN. Group A streptococcal M protein vaccine: protection following immunization via the respiratory tract. Dev. Biol. Stand. 1975;28:429–434. [PubMed] [Google Scholar]
- 11.Office of the Federal Register National Archives and Records Administration. Bacterial vaccines and bacterial antigens with no U.S. standard of potency. Implementation of efficacy review - 42 Federal Register 58316. https://www.federalregister.gov/documents/2004/12/29/04-28322/biological-products-bacterial-vaccines-andtoxoids-implementation-of-efficacy-review(1977).
- 12.Massell BF, Honikman LH, Amezcua J. Rheumatic fever following streptococcal vaccination. Rep. three cases. JAMA. 1969;207:1115–1119. [PubMed] [Google Scholar]
- 13.Office of the Federal Register National Archives and Records Administration. 70 FR 72197 - Revocation of Status of Specific Products; Group A Streptococcus. (2005). [PubMed]
- 14.Cleary PP, Matsuka YV, Huynh T, Lam H, Olmsted SB. Immunization with C5a peptidase from either group A or B streptococci enhances clearance of group A streptococci from intranasally infected mice. Vaccine. 2004;22:4332–4341. doi: 10.1016/j.vaccine.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 15.Kotloff KL, et al. Safety and immunogenicity of a recombinant multivalent group a streptococcal vaccine in healthy adults: phase 1 trial. JAMA. 2004;292:709–715. doi: 10.1001/jama.292.6.709. [DOI] [PubMed] [Google Scholar]
- 16.McNeil SA, et al. Safety and immunogenicity of 26-valent group a streptococcus vaccine in healthy adult volunteers. Clin. Infect. Dis. 2005;41:1114–1122. doi: 10.1086/444458. [DOI] [PubMed] [Google Scholar]
- 17.Sekuloski S, et al. Evaluation of safety and immunogenicity of a group A streptococcus vaccine candidate (MJ8VAX) in a randomized clinical trial. PLoS One. 2018;13:e0198658. doi: 10.1371/journal.pone.0198658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pastural É, et al. Safety and immunogenicity of a 30-valent M protein-based group a streptococcal vaccine in healthy adult volunteers: A randomized, controlled phase I study. Vaccine. 2020;38:1384–1392. doi: 10.1016/j.vaccine.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Davies MR, et al. Atlas of group A streptococcal vaccine candidates compiled using large-scale comparative genomics. Nat. Genet. 2019;51:1035–1043. doi: 10.1038/s41588-019-0417-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dale JB, Penfound TA, Chiang EY, Walton WJ. New 30-valent M protein-based vaccine evokes cross-opsonic antibodies against non-vaccine serotypes of group A streptococci. Vaccine. 2011;29:8175–8178. doi: 10.1016/j.vaccine.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salie, T. et al. Systematic Review and Meta-analysis of the Prevalence of Group A Streptococcal emm Clusters in Africa To Inform Vaccine Development. mSphere5, e00429–20. 10.1128/mSphere.00429-20 (2020). [DOI] [PMC free article] [PubMed]
- 22.Personal communication from Jim Dale. (2021).
- 23.Pandey M, et al. Physicochemical characterisation, immunogenicity and protective efficacy of a lead streptococcal vaccine: Progress towards Phase i trial. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-14157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nordström T, et al. Enhancing Vaccine Efficacy by Engineering a Complex Synthetic Peptide To Become a Super Immunogen. J. Immunol. 2017;199:2794. doi: 10.4049/jimmunol.1700836. [DOI] [PubMed] [Google Scholar]
- 25.Pandey M, et al. Antibodies to the conserved region of the M protein and a streptococcal superantigen cooperatively resolve toxic shock-like syndrome in HLA-humanized mice. Sci. Adv. 2019;5:eaax3013. doi: 10.1126/sciadv.aax3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaman M, et al. Novel platform technology for modular mucosal vaccine that protects against streptococcus. Sci. Rep. 2016;6:39274–39274. doi: 10.1038/srep39274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozberk V, et al. Prime-pull immunization with a bivalent m-protein and spy-cep peptide vaccine adjuvanted with caf®01 liposomes induces both mucosal and peripheral protection from covr/s mutant streptococcus pyogenes. mBio. 2021;12:1–15. doi: 10.1128/mBio.03537-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Personal communication from Michael Good and Chris Davis. (2022).
- 29.Postol E, et al. StreptInCor: A Candidate Vaccine Epitope against S. pyogenes Infections Induces Protection in Outbred Mice. PLOS ONE. 2013;8:e60969–e60969. doi: 10.1371/journal.pone.0060969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Amicis KM, et al. Analysis of the coverage capacity of the StreptInCor candidate vaccine against Streptococcus pyogenes. Vaccine. 2014;32:4104–4110. doi: 10.1016/j.vaccine.2013.08.043. [DOI] [PubMed] [Google Scholar]
- 31.Guerino MT, et al. HLA class II transgenic mice develop a safe and long lasting immune response against StreptInCor, an anti-group A streptococcus vaccine candidate. Vaccine. 2011;29:8250–8256. doi: 10.1016/j.vaccine.2011.08.113. [DOI] [PubMed] [Google Scholar]
- 32.Postol E, et al. Group A Streptococcus Adsorbed Vaccine: Repeated Intramuscular Dose Toxicity Test in Minipigs. Sci. Rep. 2019 9:1. 2019;9:1–12. doi: 10.1038/s41598-019-46244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sá-Rocha LCD, et al. StreptInCor, a Group A Streptococcal Adsorbed Vaccine: Evaluation of Repeated Intramuscular Dose Toxicity Testing in Rats. Front. Cardiovascular Med. 2021;8:643317–643317. doi: 10.3389/fcvm.2021.643317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Personal communication from Luiza Guilherme. (2022).
- 35.Castro, S. A. & Dorfmueller, H. C. A brief review on Group A Streptococcus pathogenesis and vaccine development. R. Soc. Open Sci. 8, 201991. 10.1098/rsos.201991 (2021). [DOI] [PMC free article] [PubMed]
- 36.Loh JMS, et al. A multivalent T-antigen-based vaccine for Group A Streptococcus. Sci. Rep. 2021 11:1. 2021;11:1–10. doi: 10.1038/s41598-021-83673-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edwards RJ, et al. Specific C-terminal cleavage and inactivation of interleukin-8 by invasive disease isolates of Streptococcus pyogenes. J. Infect. Dis. 2005;192:783–790. doi: 10.1086/432485. [DOI] [PubMed] [Google Scholar]
- 38.Gallotta M, et al. SpyAD, a Moonlighting Protein of Group A Streptococcus Contributing to Bacterial Division and Host Cell Adhesion. Infect. Immun. 2014;82:2890–2901. doi: 10.1128/IAI.00064-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyon WR, Caparon MG. Trigger factor-mediated prolyl isomerization influences maturation of the Streptococcus pyogenes cysteine protease. J. Bacteriol. 2003;185:3661–3667. doi: 10.1128/JB.185.12.3661-3667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cusumano ZT, Watson ME, Jr., Caparon MG. Streptococcus pyogenes arginine and citrulline catabolism promotes infection and modulates innate immunity. Infect. Immun. 2014;82:233–242. doi: 10.1128/IAI.00916-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Benedetto, R. et al. Rational Design of a Glycoconjugate Vaccine against Group A Streptococcus. Int. J. Mol. Sci.21, 8558. 10.3390/ijms21228558 (2020). [DOI] [PMC free article] [PubMed]
- 42.Di Benedetto, R. et al. Design of an effective glycoconjugate vaccine against Group A Streptococcus. in Lancefield International Symposium on Streptococci and Streptococcal Disease (Stockholm, Sweden, 7-10 June 2022).
- 43.Bensi G, et al. Multi high-throughput approach for highly selective identification of vaccine candidates: The group a streptococcus case. Mol. Cell. Proteom. 2012;11:1–12. doi: 10.1074/mcp.M111.015693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kabanova A, et al. Evaluation of a Group A Streptococcus synthetic oligosaccharide as vaccine candidate. Vaccine. 2010;29:104–114. doi: 10.1016/j.vaccine.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 45.Personal communication from Danilo Gomes Moriel. (2022).
- 46.Gao NJ, et al. Site-Specific Conjugation of Cell Wall Polyrhamnose to Protein SpyAD Envisioning a Safe Universal Group A Streptococcal Vaccine. Infect. Microbes Dis. 2021;3:87–100. doi: 10.1097/IM9.0000000000000044. [DOI] [Google Scholar]
- 47.Vaxcyte. Corporate Presentation. (2022).
- 48.Rivera-Hernandez T, et al. Vaccine-Induced Th1-Type Response Protects against Invasive Group A Streptococcus Infection in the Absence of Opsonizing Antibodies. mBio. 2020;11:e00122–00120. doi: 10.1128/mBio.00122-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rivera-Hernandez T, et al. An experimental group a streptococcus vaccine that reduces pharyngitis and tonsillitis in a nonhuman primate model. mBio. 2019;10:1–10. doi: 10.1128/mBio.00693-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rivera-Hernandez T, et al. Differing Efficacies of Lead Group A Streptococcal Vaccine Candidates and Full-Length M Protein in Cutaneous and Invasive Disease Models. mBio. 2016;7:e00618–00616. doi: 10.1128/mBio.00618-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loh JMS, Lorenz N, Tsai CJ, Khemlani AHJ, Proft T. Mucosal vaccination with pili from Group A Streptococcus expressed on Lactococcus lactis generates protective immune responses. Sci. Rep. 2017;7:7174. doi: 10.1038/s41598-017-07602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Personal communication from Thomas Proft and Jacelyn Loh. (2022).
- 53.Parker EPK, Shrotri M, Kampmann B. Keeping track of the SARS-CoV-2 vaccine pipeline. Nat. Rev. Immunol. 2020;20:650. doi: 10.1038/s41577-020-00455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frost, H., Excler, J. L., Sriskandan, S. & Fulurija, A. Correlates of immunity to Group A Streptococcus – a pathway to vaccine development. npj Vaccines8, 1. 10.1038/s41541-022-00593-8 (2023). [DOI] [PMC free article] [PubMed]
- 55.Maruggi G, et al. Immunogenicity and protective efficacy induced by self-amplifying mRNA vaccines encoding bacterial antigens. Vaccine. 2017;35:361–368. doi: 10.1016/j.vaccine.2016.11.040. [DOI] [PubMed] [Google Scholar]
- 56.Rivera-Hernandez, T. et al. Vaccine-induced th1-type response protects against invasive group a Streptococcus infection in the absence of opsonizing antibodies. mBio11 (2020). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.