Abstract
Cognitive impairment has been observed in patients with various psychiatric disorders, including schizophrenia, major depressive disorder (MDD), and bipolar disorder (BD). Although modern therapeutic drugs can improve certain symptoms (i.e., psychosis, depression) in these patients, these drugs have not been found to improve cognitive impairment. The N-methyl-D-aspartate receptor antagonist (R,S)-ketamine has attracted attention as a rapidly acting antidepressant. In addition to its robust antidepressant effects, (R,S)-ketamine has been suggested to improve cognitive impairment in patients with MDD and BD, despite causing cognitive impairment in healthy control subjects. (R,S)-ketamine is a racemic mixture of equal amounts of (R)-ketamine (or arketamine) and (S)-ketamine (or esketamine). Arketamine has been found to have more potent antidepressant-like actions than esketamine in rodents. Interestingly, arketamine, but not esketamine, has been suggested to improve phencyclidine-induced cognitive deficits in mice. Furthermore, arketamine has been suggested to ameliorate cognitive deficits in rodent offspring after maternal immune activation. In the current article, it is proposed that arketamine has therapeutic potential for treating cognitive impairment in patients with psychiatric disorders. Additionally, the potential role of the gut–microbiome–brain axis in cognitive impairment in psychiatric disorders is discussed.
Keywords: Arketamine, Cognition, Esketamine, Gut microbiota, Ketamine
Introduction
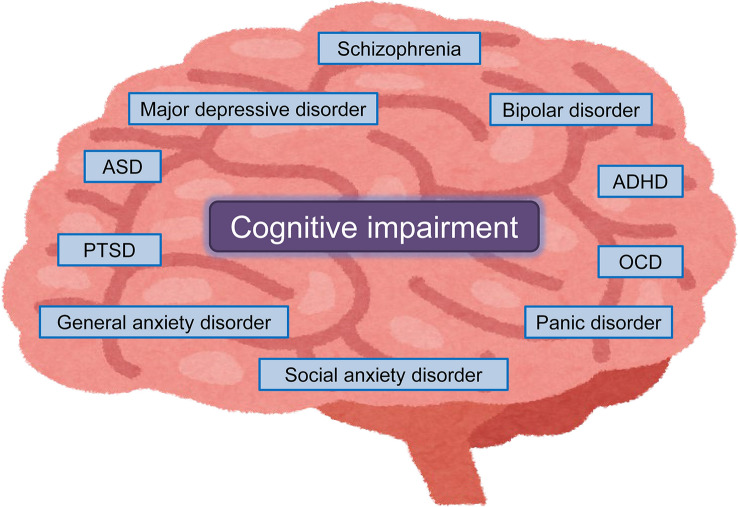
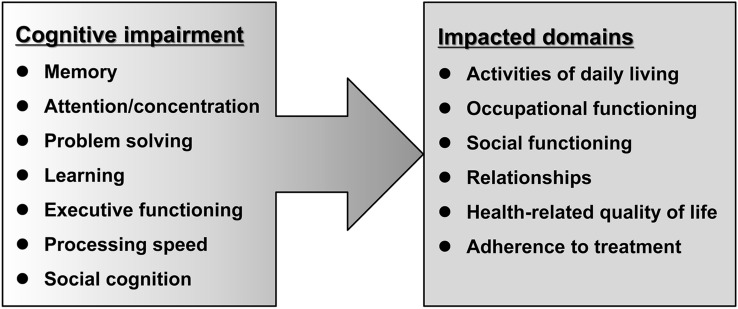
Cognitive impairment is common in patients with various psychiatric disorders, including schizophrenia, major depressive disorder (MDD), bipolar disorder (BD), autism spectrum disorder (ASD), post-traumatic stress disorder (PTSD), attention deficit/hyperactivity disorder (ADHD), obsessive–compulsive disorder (OCD), panic disorder, generalized anxiety disorder, and social anxiety disorder (Fig. 1) [1–3]. Several batteries, such as the Brief Assessment of Cognition in Schizophrenia (BACS), MATRICS Consensus Cognitive Battery (MCCB), Cambridge Neuropsychological Test Automated Battery (CANTAB), the Cogstate battery, and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) have been used to measure cognitive function in humans. Using the Cogstate battery, we previously reported that cognitive impairment in patients with schizophrenia was more severe than that in patients with MDD [4, 5]. The affected cognitive domains in patients with schizophrenia include memory, attention/concentration, problem solving, learning, executive function, processing speed, and social cognition. Furthermore, declines of these cognitive functions impact various domains, such as activities of daily living, occupational functioning, social functioning, relationships, health-related quality of life and adherence to treatment, resulting in increased direct and indirect costs associated with the treatment of schizophrenia (Fig. 2) [6]. A recent systematic review showed cognitive deficits in patients with MDD in the acute and remitted state [3]. Cognitive impairment of psychiatric disorders is not just a secondary consequence of perturbed affect, despite a close relationship between cognition and psychiatric symptoms. Although certain symptoms (i.e., psychosis, depression, anxiety) in patients with psychiatric disorders could be alleviated by the current therapeutic drugs, these drugs could not improve cognitive impairment [1]. Therefore, the development of novel therapeutic drugs for cognitive impairment is an unmet need [7–9].
Fig. 1.
Cognitive impairment in patients with psychiatric disorders. ADHD: attention deficits hyperactivity disorder. ASD: autism spectrum disorder. OCD: obsessive–compulsive disorder. PTSD: post-traumatic stress disorder. Some elements of the figure were created using resources from www.irasutoya.com
Fig. 2.
Impact of cognitive impairment in patients with schizophrenia. Cognitive functions affected in patients with schizophrenia include memory, attention/concentration, problem solving, learning, executive function, processing speed, and social cognition. Decline of cognitive functioning impacts the ability of individuals to carry out activities of daily living, occupational functioning, social functioning, relationships, health-related quality of life, and adherence to treatment. This figure was modified from reference [6]
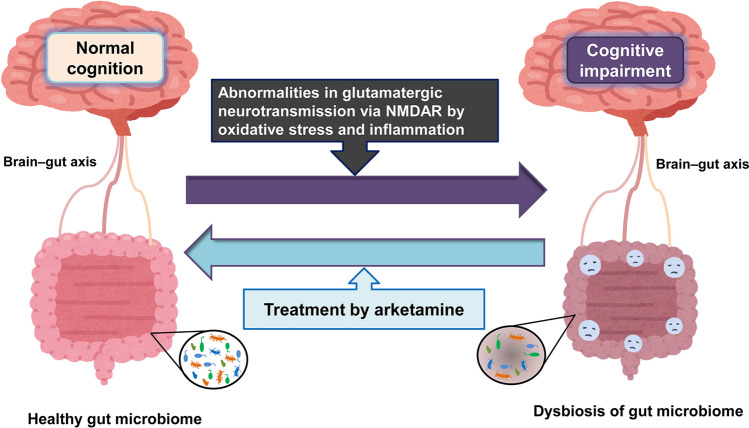
Glutamatergic neurotransmission via the N-methyl-D-aspartate receptor (NMDAR) regulates synaptic plasticity, memory, and cognition. Abnormalities in glutamatergic neurotransmission via the NMDAR play a role in cognitive impairment of psychiatric disorders (Fig. 3). In addition to psychomimetic and dissociative symptoms, NMDAR antagonists such as phencyclidine (PCP) and (R,S)-ketamine are known to cause cognitive impairment in rodents and humans [10–18]. There are several reports showing abnormalities in NMDAR-mediated amino acids (i.e., D-serine and L-serine) in patients with schizophrenia [19–22]. Recent mega-analysis of proton magnetic resonance spectroscopy (MRS) shows altered levels of glutamate in the brain from patients with schizophrenia [23]. Furthermore, abnormalities in NMDAR-mediated neurotransmission are implicated in mood disorders such as MDD and BD [24–29]. Meta-analyses of MRS studies showed altered levels of glutamate in the brain from patients with MDD or BD [30, 31]. Interestingly, there were significant correlations between cognitive functions and glutamate levels in the brain from first episode drug-naïve patients with MDD [32]. Collectively, it is likely that abnormalities in glutamatergic neurotransmission might play a role in the cognitive impairment in patients with psychiatric disorders (Fig. 3). Taken all together, previous research suggests that NMDAR could be a therapeutic target for cognitive impairment in patients with psychiatric disorders [14, 18, 33, 34].
Fig. 3.
Therapeutic potential of arketamine in cognitive impairment of psychiatric disorders. Abnormalities in glutamatergic neurotransmission via the NMDAR by oxidative stress and inflammation may play a role in the cognitive impairment and dysbiosis of gut microbiota in patients with psychiatric disorders. Accumulating evidence suggests that dysbiosis of gut microbiota may play a role in cognitive impairment in patients with psychiatric disorders. Therefore, it is likely that arketamine improves cognitive impairment in patients with psychiatric disorders through the gut–microbiota–brain axis. The figure was modified from references [36, 44]. Some elements of the figure were created using resources from www.irasutoya.com
It is widely recognized that (R,S)-ketamine causes cognitive impairment in healthy subjects [12, 13, 35]. However, increasing evidence suggests that (R,S)-ketamine may improve cognitive impairment in patients with mood disorders such as MDD and BD [36, 37]. In the current article, the author reviewed the therapeutic potential of (R,S)-ketamine and its enantiomer (R)-ketamine (or arketamine) for cognitive impairment in neuropsychiatric disorders. Furthermore, the author discussed the possible role of the gut–microbiota–brain axis in cognitive impairment of psychiatric disorders.
Brief history of (R,S)-ketamine and its enantiomers
In 1962, (R,S)-ketamine was synthesized as an alternative short-acting anesthetic of PCP [38]. In 1970, (R,S)-ketamine was approved for use as an anesthetic in the United States of America (USA). In 1985, (R,S)-ketamine was included on the World Health Organization’s List of Essential Medicines [39]. (R,S)-ketamine is a racemic mixture of equal amounts of (R)-ketamine (or arketamine) and (S)-ketamine (or esketamine). Esketamine has greater affinity for the NMDAR than arketamine. Because the anesthetic effect of esketamine in human volunteers was found to be more potent than that of arketamine [40, 41], esketamine has been used as an anesthetic in the European Union (EU) and China.
In the research field of mood disorders, (R,S)-ketamine has attracted attention as a rapidly acting antidepressant [36, 42–46]. In 2000, Berman et al. [47] reported that a single intravenous infusion of (R,S)-ketamine (0.5 mg/kg) produced rapidly acting and sustained antidepressant effects in drug-free patients with MDD. Subsequent studies confirmed robust antidepressant and anti-suicidal effects of (R,S)-ketamine (0.5 mg/kg) in treatment-resistant patients with MDD or BD [48–52]. (R,S)-ketamine has been widely used as an off-label treatment in the USA and EU, despite the current lack of safety data [53–55]. In 2019 and 2020, a nasal spray containing esketamine produced by Johnson and Johnson was approved in the USA and EU for treatment-resistant patients with MDD and people at high risk of suicide. However, there are several concerns about the efficacy and approval of esketamine nasal spray [56, 57].
An increasing number of preclinical studies has suggested that arketamine has greater potency and longer lasting antidepressant-like effects than esketamine in rodent models of depression, although the affinity of arketamine at NMDAR is less potent than that of esketamine [58–66]. Behavioral and biological abnormalities in rodents (i.e., hyperactivity, prepulse inhibition, dopamine release from the synaptic terminal, abuse liability, parvalbumin (PV)-immunoreactivity in the prefrontal cortex (PFC), and heat shock protein HSP-70 expression in the retrosplenial cortex) after injection of arketamine in animals were reduced compared with those after (R,S)-ketamine or esketamine [59, 67–71].
An open-label pilot study in Brazil reported that a single infusion of arketamine (0.5 mg/kg) caused rapid and sustained antidepressant effects in treatment-resistant patients with MDD [72]. Importantly, side effects (i.e., psychotomimetic and dissociative effects) of arketamine (0.5 mg/kg) in treatment-resistant patients with MDD [72] are substantially less severe than those of esketamine (0.2 and 0.4 mg/kg, i.v.) [73]. Taken together, previous findings suggest that arketamine might provide a novel antidepressant without the side effects of (R,S)-ketamine and esketamine. A phase 2 study of arketamine (or PCN-101) in treatment-resistant patients with MDD is currently being conducted by Perception Neuroscience, Inc. (New York, USA) [39, 46, 74].
It is well known that non-ketamine NMDAR antagonists/modulators do not produce ketamine-like robust antidepressant actions in patients with MDD, suggesting that NMDAR might not play a major role in the antidepressant effects of (R,S)-ketamine in patients with depression [36, 42, 75–77]. However, the precise molecular and cellular mechanisms underlying the antidepressant effects of arketamine remain elusive [36, 39, 46, 78–80].
Effects of (R,S)-ketamine and its enantiomers on cognition in healthy subjects
NMDAR antagonists such as PCP and ketamine are known to cause schizophrenia-like symptoms in healthy subjects, including cognitive impairment [12, 13]. In addition to positive and negative symptoms, intravenous administration of (R,S)-ketamine (0.5 mg/kg) produced cognitive impairments in healthy subjects [35]. Furthermore, intravenous administration of esketamine (0.1 mg/kg/min for 5 min and 0.006 mg/kg/min for 60 min) or (R,S)-ketamine (0.2 mg/kg/min for 5 min and 0.012 mg/kg/min for 60 min) to healthy subjects was found to produce significant psychopathological and neurocognitive impairment compared with placebo [81]. Interestingly, esketamine, but not (R,S)-ketamine, significantly increased the auditory alterations subscore of the five-dimensional questionnaire for the assessment of altered states of consciousness, suggesting that arketamine may have a potential protective effect against esketamine-induced psychotomimetic effects [81]. Furthermore, a single intranasal infusion of esketamine (84 mg) in healthy subjects caused a significant cognitive performance impairment at 40 min for all five Cogstate tests, although there were no changes between the esketamine group and the placebo group at 2, 4, or 6 h after infusion [82].
A recent meta-analysis showed acute impairment of cognition in healthy subjects after acute infusion of (R,S)-ketamine or esketamine [83]. Furthermore, verbal learning and memory are the functions most prominently affected in cognitive impairment caused by acute injection of (R,S)-ketamine or esketamine [83]. Thus, it is possible that (R,S)-ketamine and esketamine produce cognitive impairment in healthy subjects.
Effects of (R,S)-ketamine on cognitive impairment in patients with MDD or BD
Clinical studies suggest that (R,S)-ketamine may improve cognitive impairment in patients with mood disorders. Six repeated infusions of (R,S)-ketamine (0.5 mg/kg) were found to ameliorate cognitive impairment (i.e., processing speed) in treatment-resistant patients with MDD or BD [84–86]. A systematic review revealed that (R,S)-ketamine infusion led to significant improvements in cognitive impairment in treatment-resistant patients with MDD, and (R,S)-ketamine did not worsen cognitive function in depressed patients [87]. Furthermore, the improvement in working memory may be predictive of the anti-suicidal ideation response to (R,S)-ketamine in treatment-resistant patients with MDD [88]. Repeated infusion of (R,S)-ketamine (0.5 mg/kg) caused significant improvement of working memory in MDD patients with PTSD [89]. Interestingly, depression symptom severity and processing speed performance in patients with MDD or BD partially mediated the improvements in suicidal ideation after repeated infusion of (R,S)-ketamine [90]. A recent systematic review indicated potential procognitive effects of subanesthetic doses of (R,S)-ketamine among patients with depression, although there is evidence for immediate altered cognitive dysfunction in healthy subjects [91]. In addition, precognitive effects of (R,S)-ketamine were pronounced in cognitive domains of executive function. A short course of repeated infusion of (R,S)-ketamine (0.5 mg/kg) produced significant improvements in several cognitive domains, including attention, working memory, verbal memory, and visuospatial memory in treatment-resistant patients with MDD [92]. Taken together, these findings suggest that (R,S)-ketamine has beneficial effects on cognitive impairment in depressed patients, although further studies with larger sample sizes are needed.
Patients with MDD or BD typically exhibit a range of negative beliefs, such as worthlessness, hopelessness, and pessimism, and these conditions are considered to be a major public mental health concern [93, 94]. A recent study demonstrated that infusion of (R,S)-ketamine (0.5 mg/kg) improved depressive symptoms in treatment-resistant patients with MDD, and that the improvement was associated with changes in belief-updating processes [95]. These recent data provide new insights into the cognitive mechanisms of action of (R,S)-ketamine in mood disorders.
In contrast, Ochs-Ross et al. [96, 97] reported that intranasal injection of esketamine did not induce any changes in cognitive function of MDD patients from baseline, indicating a lack of beneficial effects of esketamine nasal spray on cognitive impairment.
Effects of ketamine enantiomers on PCP-induced cognitive deficits in rodents
It is well known that NMDAR antagonists such as PCP cause cognitive deficits in rodents. Using the novel object recognition test, we previously reported that repeated administration of PCP (10 mg/kg/day for 10 days) caused cognitive deficits in mice over a long period (more than 6 weeks), and that PCP-induced cognitive deficits could be improved by subsequent sub-chronic administration of clozapine, but not haloperidol [98]. Using the paradigm of PCP-induced cognitive deficits, we reported several candidates for cognitive impairment in psychiatric disorders [99–103].
We compared the effects of two ketamine enantiomers in a PCP-induced cognitive deficits model. Interestingly, PCP-induced cognitive deficits in mice were ameliorated after subsequent repeated intermittent administration of arketamine (10 mg/kg/day, twice weekly for 2 weeks), but not esketamine (10 mg/kg/day, twice weekly for 2 weeks) [104]. Western blot analysis showed decreased levels of brain-derived neurotrophic factor (BDNF) in the PFC and hippocampus of PCP-treated mice [104]. Furthermore, the beneficial effects of arketamine on cognitive deficits of PCP-treated mice were blocked by pretreatment with TrkB inhibitor ANA-12. These findings suggest that arketamine could ameliorate PCP-induced cognitive deficits via activation of BDNF-TrkB signaling in the brain [104]. Taken together, these findings suggest that arketamine could potentially provide a useful therapeutic drug for cognitive impairment in patients with schizophrenia (Fig. 3).
Cognitive impairment in the prodromal state of psychosis and the potential of arketamine
Cognitive impairment has been shown in the prodromal stage of psychosis [105–109]. Findings from meta-analyses support neurocognitive dysfunction as a potential detection and prognostic biomarker in individuals at clinical high risk (CHR) for psychosis [106, 107]. Therefore, it is important to treat cognitive impairment in individuals at CHR for psychosis to block the conversion to psychosis.
Epidemiological data suggest that maternal immune activation (MIA), such as maternal infection, might be associated with the risk of neuropsychiatric disorders, such as schizophrenia and ASD in offspring [110, 111]. During the coronavirus disease 2019 (COVID-19) pandemic, an increasing number of pregnant women have become infected with COVID-19 worldwide, and MIA induced by COVID-19 infection has been suggested as a risk factor for schizophrenia and ASD [112–114]. A cohort study shows that severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) exposure in utero may be associated with neurodevelopmental sequelae in some offspring [115].
Toll-like receptor-3 agonist polyriboinosinic–polyribocytidylic acid (poly[I:C]) has been used to establish a rodent model of MIA [116, 117]. Exposure of pregnant mice to poly(I:C) causes cognitive deficits in juvenile offspring [118–121], and these cognitive deficits in juvenile offspring appear to be similar to the prodromal stage of psychosis.
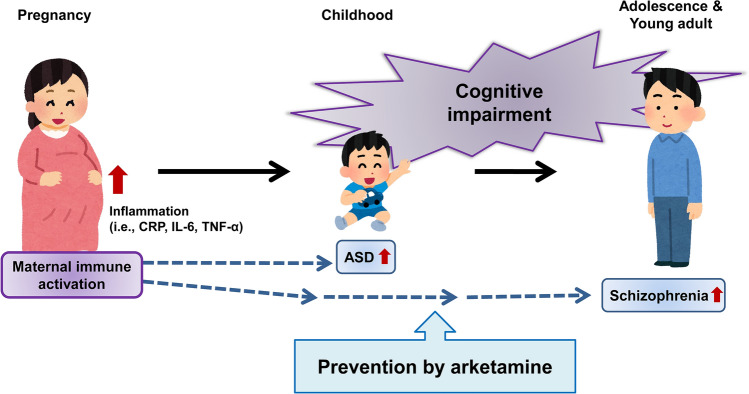
We investigated whether arketamine could prevent the development of psychosis-like phenotypes in adult offspring after MIA. We examined the effects of arketamine (10 mg/kg/day, twice weekly for 4 weeks) during juvenile and adolescent stages (P28–P56) on the development of cognitive deficits, loss of PV-immunoreactivity in the medial PFC (mPFC), and decreased dendritic spine density in the mPFC and hippocampus from adult offspring after prenatal poly(I:C) exposure [122]. Repeated intermittent administration of arketamine (10 mg/kg/day, twice weekly for 4 weeks) during juvenile and adolescent stages (P28–P56) significantly blocked the development of cognitive deficits, reduced PV-immunoreactivity in the prelimbic (PrL) of mPFC, and decreased dendritic spine density in the PrL of the mPFC, CA3, and dentate gyrus of the hippocampus from adult offspring after MIA. Furthermore, pretreatment with TrkB inhibitor ANA-12 significantly blocked the beneficial effects of arketamine on cognitive deficits of adult offspring after MIA [122]. These data suggest that repeated intermittent administration of arketamine during the juvenile and adolescent stages could prevent the development of psychosis in adult offspring after MIA through activation of BDNF-TrkB signaling. Therefore, it is possible that arketamine represents a useful prophylactic drug to prevent subsequent conversion from UHR to psychosis (Fig. 4).
Fig. 4.
Therapeutic potential of arketamine in subjects at ultra-high risk (UHR) for psychosis. Maternal immune activation (MIA), such as that caused by maternal infection, causes inflammatory events in pregnant women, resulting in higher levels of inflammatory biomarkers (i.e., C-reactive protein [CRP], IL-6, and TNF-α) in the blood and tissues. Epidemiological data suggest that MIA can increase the risk of ASD and schizophrenia in offspring. Because subjects at UHR for psychosis have cognitive impairment as a prodromal symptom, early intervention using arketamine may block the onset of neuropsychiatric disorders in subjects at UHR of psychosis. This figure was modified from Fig. 3 in reference [113]. Some elements of the figure were created using resources from www.irasutoya.com
Dysbiosis of gut microbiota and cognitive impairment of psychiatric disorders
Accumulating evidence suggests the role of dysbiosis of gut microbiota in a variety of psychiatric disorders [123–126]. A narrative review shows that intervention of gut microbiota can improve cognitive or brain function, suggesting a role of gut microbiota in cognitive performance [127]. A recent population-based study of middle-aged adults demonstrated that microbial community composition on the basis of beta-diversity was associated with all cognitive measures in multivariable-adjusted analysis [128], suggesting a role of gut microbiota in cognitive decline with aging. It has also been suggested that dysbiosis of gut microbiota may play a role in cognitive impairment in patients with psychiatric disorders such as schizophrenia, MDD, and BD [128–132].
In addition to rapid antidepressant-like effects, arketamine, (R,S)-ketamine, and (S)-norketamine have been suggested to improve abnormal composition of gut microbiota in mice with depression-like behaviors [133–138]. Furthermore, arketamine could ameliorate abnormal composition of gut microbiota in mouse models of multiple sclerosis [139] and postmenopausal osteoporosis [140]. Considering the beneficial effects of arketamine on dysbiosis of gut microbiota, it is likely that arketamine may improve cognitive impairment in patients of psychiatric disorders through the gut–microbiota–brain axis [36, 42, 44, 125, 126]. Therefore, it is of interest to investigate whether arketamine can improve cognitive impairment and abnormal composition of gut microbiota in patients with psychiatric disorders.
Conclusion and future directions
As stated in the introduction, cognitive impairment is shown in patients with a variety of psychiatric disorders. Neural mechanisms of cognitive impairment between schizophrenia and mood disorders such as MDD and BD may be different. However, a systematic review of MRS studies suggest that abnormal neurotransmission of glutamate and GABA (γ-aminobutyric acid) plays a role in cognitive impairment in patients with schizophrenia and mood disorders such as MDD and BD [141]. Given the role of glutamine–glutamate–GABA cycle in the brain [14, 16, 142], it is possible that abnormalities in the neurotransmission of glutamate and GABA may contribute to cognitive impairment in patients with psychiatric disorders such as schizophrenia and mood disorders. Nonetheless, further study is needed to ascertain the role of glutamate and GABA on cognitive impairment in patients with a variety of psychiatric disorders.
As discussed above, accumulating clinical data suggest that (R,S)-ketamine could improve cognitive impairment in patients with MDD or BD, although it causes cognitive impairment in healthy subjects. Preclinical data suggest that arketamine, but not esketamine, can improve PCP-induced cognitive deficits in rodents [104]. Furthermore, there is evidence that arketamine can ameliorate cognitive deficits in offspring after MIA through activation of BDNF-TrkB signaling [122]. Preclinical findings suggest that BDNF-TrkB signaling could play a role in the beneficial effects of arketamine in several animal models [36, 42–45, 66, 143–147]. However, the precise molecular and cellular mechanisms underlying the beneficial effects of arketamine remain elusive [36, 42–46, 148].
The COVID-19 pandemic began at the end of 2019, and continues to the present. The COVID-19 pandemic causes short-term and long-term mental health problems in survivors after SARS-CoV-2 infection [149–152]. A recent meta-analysis suggests that half of COVID-19 survivors have a high burden of either physical or mental sequelae (i.e., cognitive impairment) for up to at least 12 months [153]. Additionally, it may be useful to investigate whether arketamine can improve long-term mental sequelae in COVID-19 survivors.
Clinical trials of arketamine in healthy subjects and treatment-resistant patients with MDD are currently being conducted by several pharmaceutical companies, including Perception Neuroscience Inc. (USA), Otsuka Pharmaceutical Co., Ltd. (Japan), Jiangsu HengRui Medicine Co., Ltd. (China), and Jiangsu Enhua Pharmaceutical Co., Ltd. (China) [46]. Given the detrimental effects of cognitive impairment in patients with psychiatric disorders [1], it is of great interest to investigate whether arketamine could improve cognitive impairment in a number of psychiatric disorders, including schizophrenia, MDD, and BD. Finally, future clinical studies are needed to ascertain the efficacy of arketamine on cognitive impairment in patients with psychiatric disorders.
Acknowledgements
The author would like to thank my collaborators who are listed as the co-authors of our papers in the reference list. This study was supported by grants from Japan Society for the Promotion of Science (to K.H., 21H02846, 21H00184, 21H05612). The author thanks Benjamin Knight, MSc., from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Data availability
This article does not contain any original data.
Declarations
Conflict of interest
Dr. Hashimoto is the inventor of filed patent applications on “The use of R-ketamine in the treatment of psychiatric diseases”, “(S)-norketamine and salt thereof as pharmaceutical”, “R-ketamine and derivative thereof as prophylactic or therapeutic agent for neurodegeneration disease or recognition function disorder”, “Preventive or therapeutic agent and pharmaceutical composition for inflammatory diseases or bone diseases”, and “R-ketamine and its derivatives as a preventive or therapeutic agent for a neurodevelopmental disorder” by the Chiba University. Dr. Hashimoto has also received speakers’ honoraria, consultant fee, or research support from Abbott, Boehringer Ingelheim, Daiichi-Sankyo, Meiji Seika Pharma, Seikagaku Corporation, Dainippon-Sumitomo, Taisho, Otsuka, Murakami Farm and Perception Neuroscience.
References
- 1.Millan MJ, Agid Y, Brüne M, Bullmore ET, Carter CS, Clayton NS, Connor R, Davis S, Deakin B, DeRubeis RJ, Dubois B, Geyer MA, Goodwin GM, Gorwood P, Jay TM, Joëls M, Mansuy IM, Meyer-Lindenberg A, Murphy D, Rolls E, Saletu B, Spedding M, Sweeney J, Whittington M, Young LJ. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11(2):141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- 2.Gebreegziabhere Y, Habatmu K, Mihretu A, Cella M, Alem A. Cognitive impairment in people with schizophrenia: an umbrella review. Eur Arch Psychiatry Clin Neurosci. 2022;272(7):1139–1155. doi: 10.1007/s00406-022-01416-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kriesche D, Woll CFJ, Tschentscher N, Engel RR, Karch S. Neurocognitive deficits in depression: a systematic review of cognitive impairment in the acute and remitted state. Eur Arch Psychiatry Clin Neurosci. 2022 doi: 10.1007/s00406-022-01479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshida T, Suga M, Arima K, Muranaka Y, Tanaka T, Eguchi S, Lin C, Yoshida S, Ishikawa M, Higuchi Y, Seo T, Ueoka Y, Tomotake M, Kaneda Y, Darby D, Maruff P, Iyo M, Kasai K, Higuchi T, Sumiyoshi T, Ohmori T, Takahashi K, Hashimoto K. Criterion and construct validity of the CogState Schizophrenia Battery in Japanese patients with schizophrenia. PLoS One. 2011;6(5):e20469. doi: 10.1371/journal.pone.0020469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshida T, Ishikawa M, Niitsu T, Nakazato M, Watanabe H, Shiraishi T, Shiina A, Hashimoto T, Kanahara N, Hasegawa T, Enohara M, Kimura A, Iyo M, Hashimoto K. Decreased serum levels of mature brain-derived neurotrophic factor (BDNF), but not its precursor proBDNF, in patients with major depressive disorder. PLoS One. 2012;7(8):e42676. doi: 10.1371/journal.pone.0042676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitchen H, Rofail D, Herton L, Sacco P. Cognitive impairment associated with schizophrenia: a review of the humanistic burden. Adv Ther. 2012;29(2):148–162. doi: 10.1007/s12325-012-0001-4. [DOI] [PubMed] [Google Scholar]
- 7.Aquila R, Citrome L. Cognitive impairment in schizophrenia: the great unmet need. CNS Spectr. 2015;20(Suppl 1):35–39. doi: 10.1017/S109285291500070X. [DOI] [PubMed] [Google Scholar]
- 8.Keefe RS. Treating cognitive impairment in depression: an unmet need. Lancet Psychiatry. 2016;3(5):392–393. doi: 10.1016/S2215-0366(16)00095-X. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto K. Recent advances in the early intervention in schizophrenia: future direction from preclinical findings. Curr Psychiatry Rep. 2019;21(8):75. doi: 10.1007/s11920-019-1063-7. [DOI] [PubMed] [Google Scholar]
- 10.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148(10):1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 11.Neill JC, Barnes S, Cook S, Grayson B, Idris NF, McLean SL, Snigdha S, Rajagopal L, Harte MK. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharmacol Ther. 2010;128(3):419–432. doi: 10.1016/j.pharmthera.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Domino EF, Luby ED. Phencyclidine/schizophrenia: one view toward the past, the other to the future. Schizophr Bull. 2012;38(5):914–919. doi: 10.1093/schbul/sbs011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Javitt DC, Zukin SR, Heresco-Levy U, Umbricht D. Has an angel shown the way? Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Schizophr Bull. 2012;38(5):958–966. doi: 10.1093/schbul/sbs069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto K, Malchow B, Falkai P, Schmitt A. Glutamate modulators as potential therapeutic drugs in schizophrenia and affective disorders. Eur Arch Psychiatry Clin Neurosci. 2013;263(5):367–377. doi: 10.1007/s00406-013-0399-y. [DOI] [PubMed] [Google Scholar]
- 15.Wiescholleck V, Manahan-Vaughan D. Long-lasting changes in hippocampal synaptic plasticity and cognition in an animal model of NMDA receptor dysfunction in psychosis. Neuropharmacology. 2013;74:48–58. doi: 10.1016/j.neuropharm.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto K. Targeting of NMDA receptors in new treatments for schizophrenia. Expert Opin Ther Targets. 2014;18(9):1049–1063. doi: 10.1517/14728222.2014.934225. [DOI] [PubMed] [Google Scholar]
- 17.Cadinu D, Grayson B, Podda G, Harte MK, Doostdar N, Neill JC. NMDA receptor antagonist rodent models for cognition in schizophrenia and identification of novel drug treatments, an update. Neuropharmacology. 2018;142:41–62. doi: 10.1016/j.neuropharm.2017.11.045. [DOI] [PubMed] [Google Scholar]
- 18.Javitt DC. Cognitive impairment associated with schizophrenia: toward novel therapeutics. Annu Rev Pharmacol Toxicol. 2023;63:119–141. doi: 10.1146/annurev-pharmtox-051921-093250. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto K, Fukushima T, Shimizu E, Komatsu N, Watanabe H, Shinoda N, Nakazato M, Kumakiri C, Okada S, Hasegawa H, Imai K, Iyo M. Decreased serum levels of D-serine in patients with schizophrenia: evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. Arch Gen Psychiatry. 2003;60(6):572–576. doi: 10.1001/archpsyc.60.6.572. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto K, Engberg G, Shimizu E, Nordin C, Lindström LH, Iyo M. Reduced D-serine to total serine ratio in the cerebrospinal fluid of drug naive schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(5):767–769. doi: 10.1016/j.pnpbp.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Yamada K, Ohnishi T, Hashimoto K, Ohba H, Iwayama-Shigeno Y, Toyoshima M, Okuno A, Takao H, Toyota T, Minabe Y, Nakamura K, Shimizu E, Itokawa M, Mori N, Iyo M, Yoshikawa T. Identification of multiple serine racemase (SRR) mRNA isoforms and genetic analyses of SRR and DAO in schizophrenia and D-serine levels. Biol Psychiatry. 2005;57(12):1493–1503. doi: 10.1016/j.biopsych.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Maekawa M, Ohnishi T, Hashimoto K, Watanabe A, Iwayama Y, Ohba H, Hattori E, Yamada K, Yoshikawa T. Analysis of strain-dependent prepulse inhibition points to a role for Shmt1 (SHMT1) in mice and in schizophrenia. J Neurochem. 2010;115(6):1374–1385. doi: 10.1111/j.1471-4159.2010.07039.x. [DOI] [PubMed] [Google Scholar]
- 23.Merritt K, McGuire PK, Egerton A, 1H-MRS in Schizophrenia Investigators. Aleman A, Block W, Bloemen OJN, Borgan F, Bustillo JR, Capizzano AA, Coughlin JM, De la Fuente-Sandoval C, Demjaha A, Dempster K, Do KQ, Du F, Falkai P, Galinska-Skok B, Gallinat J, Gasparovic C, Ginestet CE, Goto N, Graff-Guerrero A, Ho BC, Howes OD, Jauhar S, Jeon P, Kato T, Kaufmann CA, Kegeles LS, Keshavan M, Kim SY, Kunugi H, Lauriello J, Liemburg EJ, Mcilwain ME, Modinos G, Mouchlianitis ED, Nakamura J, Nenadic I, Öngür D, Ota M, Palaniyappan L, Pantelis C, Plitman E, Posporelis S, Purdon SE, Reichenbach JR, Renshaw PF, Russell BR, Sawa A, Schaefer M, Shungu DC, Smesny S, Stanley JA, Stone JM, Szulc A, Taylor R, Thakkar K, Théberge J, Tibbo PG, van Amelsvoort T, Walecki J, Williamson PC, Wood SJ, Xin L, Yamasue H. Association of age, antipsychotic medication, and symptom severity in schizophrenia with proton magnetic resonance spectroscopy brain glutamate level: a mega-analysis of individual participant-level data. JAMA Psychiat. 2021;78(6):667–681. doi: 10.1001/jamapsychiatry.2021.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007;62(11):1310–1316. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev. 2009;61(2):105–123. doi: 10.1016/j.brainresrev.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Tokita K, Yamaji T, Hashimoto K. Roles of glutamate signaling in preclinical and/or mechanistic models of depression. Pharmacol Biochem Behav. 2012;100(4):688–704. doi: 10.1016/j.pbb.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Ohgi Y, Futamura T, Hashimoto K. Glutamate signaling in synaptogenesis and NMDA receptors as potential therapeutic targets for psychiatric disorders. Curr Mol Med. 2015;15(3):206–221. doi: 10.2174/1566524015666150330143008. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto K, Yoshida T, Ishikawa M, Fujita Y, Niitsu T, Nakazato M, Watanabe H, Sasaki T, Shiina A, Hashimoto T, Kanahara N, Hasegawa T, Enohara M, Kimura A, Iyo M. Increased serum levels of serine enantiomers in patients with depression. Acta Neuropsychiatr. 2016;28(3):173–178. doi: 10.1017/neu.2015.59. [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto K, Bruno D, Nierenberg J, Marmar CR, Zetterberg H, Blennow K, Pomara N. Abnormality in glutamine-glutamate cycle in the cerebrospinal fluid of cognitively intact elderly individuals with major depressive disorder: a 3-year follow-up study. Transl Psychiatry. 2016;6(3):e744. doi: 10.1038/tp.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moriguchi S, Takamiya A, Noda Y, Horita N, Wada M, Tsugawa S, Plitman E, Sano Y, Tarumi R, ElSalhy M, Katayama N, Ogyu K, Miyazaki T, Kishimoto T, Graff-Guerrero A, Meyer JH, Blumberger DM, Daskalakis ZJ, Mimura M, Nakajima S. Glutamatergic neurometabolite levels in major depressive disorder: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Mol Psychiatry. 2019;24(7):952–964. doi: 10.1038/s41380-018-0252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chabert J, Allauze E, Pereira B, Chassain C, De Chazeron I, Rotgé JY, Fossati P, Llorca PM, Samalin L. Glutamatergic and N-acetylaspartate metabolites in bipolar disorder: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Int J Mol Sci. 2022;23(16):8974. doi: 10.3390/ijms23168974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirayama Y, Takahashi M, Osone F, Hara A, Okubo T. Myo-inositol, glutamate, and glutamine in the prefrontal cortex, hippocampus, and amygdala in major depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2(2):196–204. doi: 10.1016/j.bpsc.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Chang CH, Lane HY, Tseng PT, Chen SJ, Liu CY, Lin CH. Effect of N-methyl-D-aspartate-receptor-enhancing agents on cognition in patients with schizophrenia: a systematic review and meta-analysis of double-blind randomised controlled trials. J Psychopharmacol. 2019;33(4):436–448. doi: 10.1177/0269881118822157. [DOI] [PubMed] [Google Scholar]
- 34.Kuo CY, Lin CH, Lane HY. Molecular basis of late-life depression. Int J Mol Sci. 2021;22(14):7421. doi: 10.3390/ijms22147421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51(3):199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 36.Wei Y, Chang L, Hashimoto K. Molecular mechanisms underlying the antidepressant actions of arketamine: beyond the NMDA receptor. Mol Psychiatry. 2022;27(1):559–573. doi: 10.1038/s41380-021-01121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan Y, Hashimoto K. Predictable biomarkers for rapid-acting antidepressant response to ketamine. In: Hashimoto K, Manto M, editors. New Rapid-acting Antidepressants. Switzerland: Springer Nature; 2021. pp. 31–48. [Google Scholar]
- 38.Domino EF. Taming the ketamine tiger. 1965. Anesthesiology. 2010;113(3):678–684. doi: 10.1097/ALN.0b013e3181ed09a2. [DOI] [PubMed] [Google Scholar]
- 39.Hashimoto K. Ketamine: anesthetic, psychotomimetic, antidepressant, or anthelmintic? Mol Psychiatry. 2022;27(8):3116–3118. doi: 10.1038/s41380-022-01587-7. [DOI] [PubMed] [Google Scholar]
- 40.White PF, Schüttler J, Shafer A, Stanski DR, Horai Y, Trevor AJ. Comparative pharmacology of the ketamine isomers. Studies in voluntters. Br J Anaesth. 1985;57(2):197–203. doi: 10.1093/bja/57.2.197. [DOI] [PubMed] [Google Scholar]
- 41.Hirota K, Lamber DG. Ketamine; history and role in anesthetic pharmacology. Neuropharmacology. 2022;216:109171. doi: 10.1016/j.neuropharm.2022.109171. [DOI] [PubMed] [Google Scholar]
- 42.Hashimoto K. Rapid-acting antidepressant ketamine, its metabolites and other candidates: a historical overview and future perspective. Psychiatry Clin Neurosci. 2019;73(10):613–627. doi: 10.1111/pcn.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang C, Yang J, Luo A, Hashimoto K. Molecular and cellular mechanisms underlying the antidepressant effects of ketamine enantiomers and its metabolites. Transl Psychiatry. 2019;9(1):280. doi: 10.1038/s41398-019-0624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hashimoto K. Molecular mechanisms of the rapid-acting and long-lasting antidepressant actions of (R)-ketamine. Biochem Pharmacol. 2020;177:113935. doi: 10.1016/j.bcp.2020.113935. [DOI] [PubMed] [Google Scholar]
- 45.Wei Y, Chang L, Hashimoto K. A historical review of antidepressant effects of ketamine and its enantiomers. Pharmacol Biochem Behav. 2020;190:172870. doi: 10.1016/j.pbb.2020.172870. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J, Yao W, Hashimoto K. Arketamine, a new rapid-acting antidepressant: a historical review and future directions. Neuropharmacology. 2022;21:109219. doi: 10.1016/j.neuropharm.2022.109219. [DOI] [PubMed] [Google Scholar]
- 47.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 48.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 49.Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA., Jr A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Su TP, Chen MH, Li CT, Lin WC, Hong CJ, Gueorguieva R, Tu PC, Bai YM, Cheng CM, Krystal JH. Dose-related effects of adjunctive ketamine in Taiwanese patients with treatment-resistant depression. Neuropsychopharmacology. 2017;42(13):2482–2492. doi: 10.1038/npp.2017.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fava M, Freeman MP, Flynn M, Judge H, Hoeppner BB, Cusin C, Ionescu DF, Mathew SJ, Chang LC, Iosifescu DV, Murrough J, Debattista C, Schatzberg AF, Trivedi MH, Jha MK, Sanacora G, Wilkinson ST, Papakostas GI. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD) Mol Psychiatry. 2020;25(7):1592–1603. doi: 10.1038/s41380-018-0256-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, Sos P, Wang G, Zarate CA, Jr, Sanacora G. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150–158. doi: 10.1176/appi.ajp.2017.17040472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.López-Díaz Á, Murillo-Izquierdo M, Moreno-Mellado E. Off-label use of ketamine for treatment-resistant depression in clinical practice: European perspective. Br J Psychiatry. 2019;215(2):447–448. doi: 10.1192/bjp.2019.102. [DOI] [PubMed] [Google Scholar]
- 54.Lengvenyte A, Strumila R, Olié E, Courtet P. Ketamine and esketamine for crisis management in patients with depression: Why, whom, and how? Eur Neuropsychopharmacol. 2022;57:88–104. doi: 10.1016/j.euroneuro.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 55.O'Brien B, Wilkinson ST, Mathew SJ. An update on community ketamine practices. Am J Psychiatry. 2022;179(5):393–394. doi: 10.1176/appi.ajp.21111086. [DOI] [PubMed] [Google Scholar]
- 56.Turner E. Esketamine for treatment-resistant depression: seven concerns about efficacy and FDA approval. Lancet Psychiatry. 2019;6(12):977–979. doi: 10.1016/S2215-0366(19)30394-3. [DOI] [PubMed] [Google Scholar]
- 57.Horowitz MA, Moncrieff J. Are we repeating mistakes of the past? A review of the evidence for esketamine. Br J Psychiatry. 2021;219(5):618. doi: 10.1192/bjp.2020.89. [DOI] [PubMed] [Google Scholar]
- 58.Zhang JC, Li SX, Hashimoto K. R (-)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav. 2014;116:137–141. doi: 10.1016/j.pbb.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 59.Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5(9):e632. doi: 10.1038/tp.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA, Jr, Gould TD. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fukumoto K, Toki H, Iijima M, Hashihayata T, Yamaguchi JI, Hashimoto K, Chaki S. Antidepressant potential of (R)-ketamine in rodent models: comparison with (S)-ketamine. J Pharmacol Exp Ther. 2017;361(1):9–16. doi: 10.1124/jpet.116.239228. [DOI] [PubMed] [Google Scholar]
- 62.Yang C, Qu Y, Abe M, Nozawa D, Chaki S, Hashimoto K. (R)-ketamine shows greater potency and longer lasting abtidepressant effects than its metabolites (2R,6R)-hydroxynorketamine. Biol Psychiatry. 2017;82(5):e43–e44. doi: 10.1016/j.biopsych.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 63.Yang C, Ren Q, Qu Y, Zhang JC, Ma M, Dong C, Hashimoto K. Mechanistic target of rapamycin-independent antidepressant effects of (R)-ketamine in a social defeat stress model. Biol Psychiatry. 2018;83(1):18–28. doi: 10.1016/j.biopsych.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 64.Chang L, Zhang K, Pu Y, Qu Y, Wang SM, Xiong Z, Ren Q, Dong C, Fujita Y, Hashimoto K. Comparison of antidepressant and side effects in mice after intranasal administration of (R, S)-ketamine, (R)-ketamine, and (S)-ketamine. Pharmacol Biochem Behav. 2019;181:53–59. doi: 10.1016/j.pbb.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 65.Zhu J, Hawkins E, Phillips K, Deshpande LS. Assessment of ketamine and its enantiomers in an organophosphate-based rat model for features of Gulf War illness. Int J Environ Res Public Health. 2020;17(13):4710. doi: 10.3390/ijerph17134710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yao W, Cao Q, Luo S, He L, Yang C, Chen J, Qi Q, Hashimoto K, Zhang JC. Microglial ERK-NRBP1-CREB-BDNF signaling in sustained antidepressant actions of (R)-ketamine. Mol Psychiatry. 2022;27(3):1618–1629. doi: 10.1038/s41380-021-01377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang C, Han M, Zhang JC, Ren Q, Hashimoto K. Loss of parvalbumin-immunoreactivity in mouse brain regions after repeated intermittent administration of esketamine, but not R-ketamine. Psychiatry Res. 2016;239:281–283. doi: 10.1016/j.psychres.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 68.Hashimoto K, Kakiuchi T, Ohba H, Nishiyama S, Tsukada H. Reduction of dopamine D2/3 receptor binding in the striatum after a single administration of esketamine, but not R-ketamine: a PET study in conscious monkeys. Eur Arch Psychiatry Clin Neurosci. 2017;267(2):173–176. doi: 10.1007/s00406-016-0692-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tian Z, Dong C, Fujita A, Fujita Y, Hashimoto K. Expression of heat shock protein HSP-70 in the retrosplenial cortex of rat brain after administration of (R, S)-ketamine and (S)-ketamine, but not (R)-ketamine. Pharmacol Biochem Behav. 2018;172:17–21. doi: 10.1016/j.pbb.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 70.Tan Y, Hashimoto K. Risk of psychosis after repeated intermittent administration of (S)-ketamine, but not (R)-ketamine, in mice. J Affect Disord. 2020;269:198–200. doi: 10.1016/j.jad.2020.03.040. [DOI] [PubMed] [Google Scholar]
- 71.Bonaventura J, Lam S, Carlton M, Boehm MA, Gomez JL, Solís O, Sánchez-Soto M, Morris PJ, Fredriksson I, Thomas CJ, Sibley DR, Shaham Y, Zarate CA, Jr, Michaelides M. Pharmacological and behavioral divergence of ketamine enantiomers: implications for abuse liability. Mol Psychiatry. 2021;26(11):6704–6722. doi: 10.1038/s41380-021-01093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leal GC, Bandeira ID, Correia-Melo FS, Telles M, Mello RP, Vieira F, Lima CS, Jesus-Nunes AP, Guerreiro-Costa LNF, Marback RF, Caliman-Fontes AT, Marques BLS, Bezerra MLO, Dias-Neto AL, Silva SS, Sampaio AS, Sanacora G, Turecki G, Loo C, Lacerda ALT, Quarantini LC. Intravenous arketamine for treatment-resistant depression: open-label pilot study. Eur Arch Psychiatry Clin Neurosci. 2021;271(3):577–582. doi: 10.1007/s00406-020-01110-5. [DOI] [PubMed] [Google Scholar]
- 73.Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, Tadic A, Sienaert P, Wiegand F, Manji H, Drevets WC, Van Nueten L. Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry. 2016;80(6):424–431. doi: 10.1016/j.biopsych.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 74.Chang L, Hashimoto K. Comments to behavioral tests for antidepressant-like actions of (2R,6R)-hydroxynorketamine by Bonaventura et al. Mol Psychiatry. 2022 doi: 10.1038/s41380-022-01766-6. [DOI] [PubMed] [Google Scholar]
- 75.Hashimoto K. Ketamine’s antidepressant action: beyond NMDA receptor inhibition. Expert Opin Ther Targets. 2016;20(11):1389–1392. doi: 10.1080/14728222.2016.1238899. [DOI] [PubMed] [Google Scholar]
- 76.Hashimoto K. Are NMDA and opioid receptors involved in the antidepressant actions of ketamine? Proc Natl Acad Sci USA. 2020;117(21):11200–11201. doi: 10.1073/pnas.2001264117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ma L, Hashimoto K. The role of hippocampal KCNQ2 channel in antidepressant actions of ketamine. Neuron. 2022;110(14):2201–2203. doi: 10.1016/j.neuron.2022.05.027. [DOI] [PubMed] [Google Scholar]
- 78.Jelen LA, Young AH, Stone JM. Ketamine: a tale of two enantiomers. J Psychopharmacol. 2021;35(2):109–123. doi: 10.1177/0269881120959644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scotton E, Antqueviezc B, Vasconcelos MF, Dalpiaz G, Paul Géa L, Ferraz Goularte J, Colombo R, Ribeiro Rosa A. Is (R)-ketamine a potential therapeutic agent for treatment-resistant depression with less detrimental side effects? A review of molecular mechanisms underlying ketamine and its enantiomers. Biochem Pharmacol. 2022;198:114963. doi: 10.1016/j.bcp.2022.114963. [DOI] [PubMed] [Google Scholar]
- 80.Wang X, Yang J, Hashimoto K. (R)-ketamine as prophylactic and therapeutic drug for neurological disorders: beyond depression. Neurosci Biobehav Rev. 2022;139:104762. doi: 10.1016/j.neubiorev.2022.104762. [DOI] [PubMed] [Google Scholar]
- 81.Passie T, Adams HA, Logemann F, Brandt SD, Wiese B, Karst M. Comparative effects of (S)-ketamine and racemic (R/S)-ketamine on psychopathology, state of consciousness and neurocognitive performance in healthy volunteers. Eur Neuropsychopharmacol. 2021;44:92–104. doi: 10.1016/j.euroneuro.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 82.Morrison RL, Fedgchin M, Singh J, Van Gerven J, Zuiker R, Lim KS, van der Ark P, Wajs E, Xi L, Zannikos P, Drevets WC. Effect of intranasal esketamine on cognitive functioning in healthy participants: a randomized, double-blind, placebo-controlled study. Psychopharmacology. 2018;235(4):1107–1119. doi: 10.1007/s00213-018-4828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhornitsky S, Tourjman V, Pelletier J, Assaf R, Li CR, Potvin S. Acute effects of ketamine and esketamine on cognition in healthy subjects: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2022;118:110575. doi: 10.1016/j.pnpbp.2022.110575. [DOI] [PubMed] [Google Scholar]
- 84.Zhou Y, Zheng W, Liu W, Wang C, Zhan Y, Li H, Chen L, Li M, Ning Y. Antidepressant effect of repeated ketamine administration on kynurenine pathway metabolites in patients with unipolar and bipolar depression. Brain Behav Immun. 2018;74:205–212. doi: 10.1016/j.bbi.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 85.Zhou Y, Zheng W, Liu W, Wang C, Zhan Y, Li H, Chen L, Li M, Ning Y. Neurocognitive effects of six ketamine infusions and the association with antidepressant response in patients with unipolar and bipolar depression. J Psychopharmacol. 2018;32(10):1118–1126. doi: 10.1177/0269881118798614. [DOI] [PubMed] [Google Scholar]
- 86.Shiroma PR, Thuras P, Wels J, Albott CS, Erbes C, Tye S, Kim KO. Neurocognitive performance of repeated versus single intravenous subanesthetic ketamine in treatment resistant depression. J Affect Disord. 2020;277:470–477. doi: 10.1016/j.jad.2020.08.058. [DOI] [PubMed] [Google Scholar]
- 87.Gill H, Gill B, Rodrigues NB, Lipsitz O, Rosenblat JD, El-Halabi S, Nasri F, Mansur RB, Lee Y, McIntyre RS. The effects of ketamine on cognition in treatment-resistant depression: a systematic review and priority avenues for future research. Neurosci Biobehav Rev. 2021;120:78–85. doi: 10.1016/j.neubiorev.2020.11.020. [DOI] [PubMed] [Google Scholar]
- 88.Chen X, Wang M, Hu Y, Zhan Y, Zhou Y, Zheng W, Liu W, Wang C, Zhong X, Li H, Lan X, Ning Y, Zhang B. Working memory associated with anti-suicidal ideation effect of repeated-dose intravenous ketamine in depressed patients. Eur Arch Psychiatry Clin Neurosci. 2021;271(3):431–438. doi: 10.1007/s00406-020-01221-z. [DOI] [PubMed] [Google Scholar]
- 89.Albott CS, Lim KO, Erbes C, Thuras P, Wels J, Tye SJ, Shiroma PR. Neurocognitive effects of repeated ketamine infusions in comorbid posttraumatic stress disorder and major depressive disorder. J Affect Disord. 2022;308:289–297. doi: 10.1016/j.jad.2022.04.066. [DOI] [PubMed] [Google Scholar]
- 90.Zhou Y, Wang C, Lan X, Li W, Chao Z, Wu K, McIntyre RS, Ning Y. Cognitive function mediates the anti-suicide effect of repeated intravenous ketamine in adult patients with suicidal ideation. Front Psychiatry. 2022;13:779326. doi: 10.3389/fpsyt.2022.779326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shiroma PR, Velit-Salazar MR, Vorobyov Y. A systematic review of neurocognitive effects of subanesthetic doses of intravenous ketamine in major depressive disorder, post-traumatic stress disorder, and healthy population. Clin Drug Investig. 2022;42(7):549–566. doi: 10.1007/s40261-022-01169-z. [DOI] [PubMed] [Google Scholar]
- 92.Phillips JL, Van Geel A, Burhunduli P, Vasudev D, Batten LA, Norris S, Talbot J, Ortiz A, Owoeye O, Blier P. Assessment of objective and subjective cognitive function in patients with treatment-resistant depression undergoing repeated ketamine infusions. Int J Neuropsychopharmacol. 2022;25(12):992–1002. doi: 10.1093/ijnp/pyac045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatry. 2008;165(8):969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- 94.Favaretto E, Bedani F, Offredi A, Schroffenegger M, Sassaroli S, Ruggiero G, Fagiolini A, Caselli G. Metacognitions and repetitive negative thinking in bipolar disorder and healthy controls: a comparative study. J Affect Disord. 2020;276:152–158. doi: 10.1016/j.jad.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 95.Bottemanne H, Morlaas O, Claret A, Sharot T, Fossati P, Schmidt L. Evaluation of early ketamine effects on belief-updating biases in patients with treatment-resistant depression. JAMA Psychiat. 2022;79(11):1124–1132. doi: 10.1001/jamapsychiatry.2022.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ochs-Ross R, Daly EJ, Zhang Y, Lane R, Lim P, Morrison RL, Hough D, Manji H, Drevets WC, Sanacora G, Steffens DC, Adler C, McShane R, Gaillard R, Wilkinson ST, Singh JB. Efficacy and safety of esketamine nasal spray plus an oral antidepressant in elderly patients with treatment-resistant depression-TRANSFORM-3. Am J Geriatr Psychiatry. 2020;228(2):121–141. doi: 10.1016/j.jagp.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 97.Ochs-Ross R, Wajs E, Daly EJ, Zhang Y, Lane R, Lim P, Drevets WC, Steffens DC, Sanacora G, Jamieson C, Hough D, Manji H, Singh JB. Comparison of long-term efficacy and safety of esketamine nasal spray plus oral antidepressant in younger versus older patients with treatment-resistant depression: post-hoc analysis of SUSTAIN-2, a long-term open-label phase 3 safety and efficacy study. Am J Geriatr Psychiatry. 2022;30(5):541–556. doi: 10.1016/j.jagp.2021.09.014. [DOI] [PubMed] [Google Scholar]
- 98.Hashimoto K, Fujita Y, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of clozapine, but not haloperidol. Eur J Pharmacol. 2005;519(1–2):114–117. doi: 10.1016/j.ejphar.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 99.Hashimoto K, Fujita Y, Ishima T, Hagiwara H, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of tropisetron: role of alpha-7 nicotinic receptors. Eur J Pharmacol. 2006;553(1–3):191–195. doi: 10.1016/j.ejphar.2006.09.055. [DOI] [PubMed] [Google Scholar]
- 100.Hashimoto K, Fujita Y, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of fluvoxamine: role of sigma-1 receptors. Neuropsychopharmacology. 2007;32(3):514–521. doi: 10.1038/sj.npp.1301047. [DOI] [PubMed] [Google Scholar]
- 101.Hashimoto K, Ishima T, Fujita Y, Matsuo M, Kobashi T, Takahagi M, Tsukada H, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of the novel selective alpha7 nicotinic receptor agonist SSR180711. Biol Psychiatry. 2008;63(1):92–97. doi: 10.1016/j.biopsych.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 102.Hashimoto K, Fujita Y, Ishima T, Chaki S, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of the glycine transporter-1 inhibitor NFPS and D-serine. Eur Neuropsychopharmacol. 2008;18(6):414–421. doi: 10.1016/j.euroneuro.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 103.Yoshimi N, Fujita Y, Ohgi Y, Futamura T, Kikuchi T, Hashimoto K. Effects of brexpiprazole, a novel serotonin-dopamine activity modulator, on phencyclidine-induced cognitive deficits in mice: a role for serotonin 5-HT1A receptors. Pharmacol Biochem Behav. 2014;124:245–249. doi: 10.1016/j.pbb.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 104.Tan Y, Fujita Y, Qu Y, Chang L, Pu Y, Wang S, Wang X, Hashimoto K. Phencyclidine-induced cognitive deficits in mice are ameliorated by subsequent repeated intermittent administration of (R)-ketamine, but not (S)-ketamine: role of BDNF-TrkB signaling. Pharmacol Biochem Behav. 2020;188:172839. doi: 10.1016/j.pbb.2019.172839. [DOI] [PubMed] [Google Scholar]
- 105.Fusar-Poli P, Deste G, Smieskova R, Barlati S, Yung AR, Howes O, Stieglitz RD, Vita A, McGuire P, Borgwardt S. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry. 2012;69(6):562–571. doi: 10.1001/archgenpsychiatry.2011.1592. [DOI] [PubMed] [Google Scholar]
- 106.Catalan A, Salazar de Pablo G, Aymerich C, Damiani S, Sordi V, Radua J, Oliver D, McGuire P, Giuliano AJ, Stone WS, Fusar-Poli P. Neurocognitive functioning in individuals at clinical high risk for psychosis: a systematic review and meta-analysis. JAMA Psychiat. 2021;78(8):859–867. doi: 10.1001/jamapsychiatry.2021.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Catalan A, Radua J, McCutcheon R, Aymerich C, Pedruzo B, González-Torres MÁ, Baldwin H, Stone WS, Giuliano AJ, McGuire P, Fusar-Poli P. Examining the variability of neurocognitive functioning in individuals at clinical high risk for psychosis: a meta-analysis. Transl Psychiatry. 2022;12(1):198. doi: 10.1038/s41398-022-01961-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haining K, Gajwani R, Gross J, Gumley AI, Ince RAA, Lawrie SM, Schultze-Lutter F, Schwannauer M, Uhlhaas PJ. Characterising cognitive heterogeneity in individuals at clinical high-risk for psychosis: a cluster analysis with clinical and functional outcome prediction. Eur Arch Psychiatry Clin Neurosci. 2022;272(3):437–448. doi: 10.1007/s00406-021-01315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Millman ZB, Roemer C, Vargas T, Schiffman J, Mittal VA, Gold JM. Neuropsychological performance among individuals at clinical high-risk for psychosis vs putatively low-risk peers with other psychopathology: a systematic review and meta-analysis. Schizophr Bull. 2022;48(5):999–1010. doi: 10.1093/schbul/sbac031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brown AS, Meyer U. Maternal immune activation and neuropsychiatric illness: a translational research perspective. Am J Psychiatry. 2018;175(11):1073–1083. doi: 10.1176/appi.ajp.2018.17121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guma E, Plitman E, Chakravarty MM. The role of maternal immune activation in altering the neurodevelopmental trajectories of offspring: a translational review of neuroimaging studies with implications for autism spectrum disorder and schizophrenia. Neurosci Biobehav Rev. 2019;104:141–157. doi: 10.1016/j.neubiorev.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 112.Hashimoto K. Risk of neuropsychiatric disorders in offspring of COVID-19-infected pregnant women and nutritional intervention. Eur Arch Psychiatry Clin Neurosci. 2021;271(2):387–389. doi: 10.1007/s00406-020-01148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hashimoto Y, Suzuki T, Hashimoto K. Mechanisms of action of fluvoxamine for COVID-19: a historical review. Mol Psychiatry. 2022;27(4):1898–1907. doi: 10.1038/s41380-021-01432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang R, Wu Z, Huang C, Hashimoto K, Yang L, Yang C. Deleterious effects of nervous system in the offspring following maternal SARS-CoV-2 infection during the COVID-19 pandemic. Transl Psychiatry. 2022;12(1):232. doi: 10.1038/s41398-022-01985-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Edlow AG, Castro VM, Shook LL, Kaimal AJ, Perlis RH. Neurodevelopmental outcomes at 1 year in infants of mothers who tested positive for SARS-CoV-2 during pregnancy. JAMA Netw Open. 2022;5(6):e2215787. doi: 10.1001/jamanetworkopen.2022.15787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Haddad FL, Patel SV, Schmid S. Maternal immune activation by poly I: C as a preclinical model for neurodevelopmental disorders: a focus on autism and schizophrenia. Neurosci Biobehav Rev. 2020;113:546–567. doi: 10.1016/j.neubiorev.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 117.Woods RM, Lorusso JM, Potter HG, Neill JC, Glazier JD, Hager R. Maternal immune activation in rodent models: a systematic review of neurodevelopmental changes in gene expression and epigenetic modulation in the offspring brain. Neurosci Biobehav Rev. 2021;129:389–421. doi: 10.1016/j.neubiorev.2021.07.015. [DOI] [PubMed] [Google Scholar]
- 118.Han M, Zhang JC, Yao W, Yang C, Ishima T, Ren Q, Ma M, Dong C, Huang XF, Hashimoto K. Intake of 7,8-dihydroxyflavone during juvenile and adolescent stages prevents onset of psychosis in adult offspring after maternal immune activation. Sci Rep. 2016;6:36087. doi: 10.1038/srep36087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fujita Y, Ishima T, Hashimoto K. Supplementation with D-serine prevents the onset of cognitive deficits in adult offspring after maternal immune activation. Sci Rep. 2016;6:37261. doi: 10.1038/srep37261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Matsuura A, Ishima T, Fujita Y, Iwayama Y, Hasegawa S, Kawahara-Miki R, Maekawa M, Toyoshima M, Ushida Y, Suganuma H, Kida S, Yoshikawa T, Iyo M, Hashimoto K. Dietary glucoraphanin prevents the onset of psychosis in the adult offspring after maternal immune activation. Sci Rep. 2018;8(1):2158. doi: 10.1038/s41598-018-20538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ma M, Ren Q, Yang J, Zhang K, Xiong Z, Ishima T, Pu Y, Hwang SH, Toyoshima M, Iwayama Y, Hisano Y, Yoshikawa T, Hammock BD, Hashimoto K. Key role of soluble epoxide hydrolase in the neurodevelopmental disorders of offspring after maternal immune activation. Proc Natl Acad Sci USA. 2019;116(14):7083–7088. doi: 10.1073/pnas.1819234116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tan Y, Fujita Y, Pu Y, Chang L, Qu Y, Wang X, Hashimoto K. Repeated intermittent administration of (R)-ketamine during juvenile and adolescent stages prevents schizophrenia-relevant phenotypes in adult offspring after maternal immune activation: a role of TrkB signaling. Eur Arch Psychiatry Clin Neurosci. 2022;272(4):693–701. doi: 10.1007/s00406-021-01365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McGuinness AJ, Davis JA, Dawson SL, Loughman A, Collier F, O'Hely M, Simpson CA, Green J, Marx W, Hair C, Guest G, Mohebbi M, Berk M, Stupart D, Watters D, Jacka FN. A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol Psychiatry. 2022;27(4):1920–1935. doi: 10.1038/s41380-022-01456-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shoubridge AP, Choo JM, Martin AM, Keating DJ, Wong ML, Licinio J, Rogers GB. The gut microbiome and mental health: advances in research and emerging priorities. Mol Psychiatry. 2022;27(4):1908–1919. doi: 10.1038/s41380-022-01479-w. [DOI] [PubMed] [Google Scholar]
- 125.Chang L, Wei Y, Hashimoto K. Brain-gut-microbiota axis in depression: a historical overview and future directions. Brain Res Bull. 2022;182:44–56. doi: 10.1016/j.brainresbull.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 126.Wei Y, Wang T, Liao L, Fan X, Chang L, Hashimoto K. Brain-spleen axis in health and diseases: a review and future perspective. Brain Res Bull. 2022;182:130–140. doi: 10.1016/j.brainresbull.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 127.Tooley KL. Effects of the human gut microbiota on cognitive performance, brain structure and function: a narrative review. Nutrients. 2020;12(10):3009. doi: 10.3390/nu12103009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Meyer K, Lulla A, Debroy K, Shikany JM, Yaffe K, Meirelles O, Launer LJ. Association of the gut microbiota with cognitive function in midlife. JAMA Netw Open. 2022;5(2):e2143941. doi: 10.1001/jamanetworkopen.2021.43941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zeng C, Yang P, Cao T, Gu Y, Li N, Zhang B, Xu P, Liu Y, Luo Z, Cai H. Gut microbiota: an intermediary between metabolic syndrome and cognitive deficits in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2021;106:110097. doi: 10.1016/j.pnpbp.2020.110097. [DOI] [PubMed] [Google Scholar]
- 130.Bioque M, González-Rodríguez A, Garcia-Rizo C, Cobo J, Monreal JA, Usall J, Soria V, PNECAT Group. Labad J. Targeting the microbiome-gut-brain axis for improving cognition in schizophrenia and major mood disorders: a narrative review. Prog Neuropsychopharmacol Biol Psychiatry. 2021;105:110130. doi: 10.1016/j.pnpbp.2020.110130. [DOI] [PubMed] [Google Scholar]
- 131.Dai W, Liu J, Qiu Y, Teng Z, Li S, Yuan H, Huang J, Xiang H, Tang H, Wang B, Chen J, Wu H. Gut microbial dysbiosis and cognitive impairment in bipolar disorder: current evidence. Front Pharmacol. 2022;13:893567. doi: 10.3389/fphar.2022.893567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dobielska M, Bartosik NK, Zyzik KA, Kowalczyk E, Karbownik MS. Mechanisms of cognitive impairment in depression. May probiotics help? Front Psychiatry. 2022;13:904426. doi: 10.3389/fpsyt.2022.904426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Qu Y, Yang C, Ren Q, Ma M, Dong C, Hashimoto K. Comparison of (R)-ketamine and lanicemine on depression-like phenotype and abnormal composition of gut microbiota in a social defeat stress model. Sci Rep. 2017;7(1):15725. doi: 10.1038/s41598-017-16060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang C, Qu Y, Fujita Y, Ren Q, Ma M, Dong C, Hashimoto K. Possible role of the gut microbiota-brain axis in the antidepressant effects of (R)-ketamine in a social defeat stress model. Transl Psychiatry. 2017;7(12):1294. doi: 10.1038/s41398-017-0031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ma L, Wang L, Chang L, Shan J, Qu Y, Wang X, Fujita Y, Hashimoto K. A role of microRNA-149 in the prefrontal cortex for prophylactic actions of (R)-ketamine in inflammation model. Neuropharmacology. 2022;219:109250. doi: 10.1016/j.neuropharm.2022.109250. [DOI] [PubMed] [Google Scholar]
- 136.Huang N, Hua D, Zhan G, Li S, Zhu B, Jiang R, Yang L, Bi J, Xu H, Hashimoto K, Luo A, Yang C. Role of Actinobacteria and Coriobacteriia in the antidepressant effects of ketamine in an inflammation model of depression. Pharmacol Biochem Behav. 2019;176:93–100. doi: 10.1016/j.pbb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 137.Wang Y, Jiang R, Wu Z, Zhou L, Xu J, Huang C, Yang L, Zhu B, Yan E, Liu C, Yang C. Gut microbiota is involved in the antidepressant-like effect of (S)-norketamine in an inflammation model of depression. Pharmacol Biochem Behav. 2021;207:173226. doi: 10.1016/j.pbb.2021.173226. [DOI] [PubMed] [Google Scholar]
- 138.Hua H, Huang C, Liu H, Xu X, Xu X, Wu Z, Liu C, Wang Y, Yang C. Depression and antidepressant effects of ketamine and its metabolites: The pivotal role of gut microbiota. Neuropharmacology. 2022;220:109272. doi: 10.1016/j.neuropharm.2022.109272. [DOI] [PubMed] [Google Scholar]
- 139.Wang X, Chang L, Wan X, Tan Y, Qu Y, Shan J, Yang Y, Ma L, Hashimoto K. (R)-ketamine ameliorates demyelination and facilitates remyelination in cuprizone-treated mice: a role of gut-microbiota-brain axis. Neurobiol Dis. 2022;165:105635. doi: 10.1016/j.nbd.2022.105635. [DOI] [PubMed] [Google Scholar]
- 140.Wan X, Eguchi A, Fujita Y, Ma L, Wang X, Yang Y, Qu Y, Chang L, Zhang J, Mori C, Hashimoto K. Effects of (R)-ketamine on reduced bone mineral density in ovariectomized mice: a role of gut microbiota. Neuropharmacology. 2022;213:109139. doi: 10.1016/j.neuropharm.2022.109139. [DOI] [PubMed] [Google Scholar]
- 141.Reddy-Thootkur M, Kraguljac NV, Lahti AC. The role of glutamate and GABA in cognitive dysfunction in schizophrenia and mood disorders - a systematic review of magnetic resonance spectroscopy studies. Schizophr Res. 2022;249:74–84. doi: 10.1016/j.schres.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hashimoto K. Abnormalities of the glutamine-glutamate-GABA cycle in the schizophrenia brain. Schizophr Res. 2014;156(2–3):281–282. doi: 10.1016/j.schres.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 143.Fujita A, Fujita Y, Pu Y, Chang L, Hashimoto K. MPTP-induced dopaminergic neurotoxicity in mouse brain is attenuated after subsequent intranasal administration of (R)-ketamine: a role of TrkB signaling. Psychopharmacology. 2020;2237(1):83–92. doi: 10.1007/s00213-019-05346-5. [DOI] [PubMed] [Google Scholar]
- 144.Fujita Y, Hashimoto Y, Hashimoto H, Chang L, Hashimoto K. Dextran sulfate sodium-induced inflammation and colitis in mice are ameliorated by (R)-ketamine, but not (S)-ketamine: a role of TrkB signaling. Eur J Pharmacol. 2021;897:173954. doi: 10.1016/j.ejphar.2021.173954. [DOI] [PubMed] [Google Scholar]
- 145.Qu Y, Shan J, Wang S, Chang L, Pu Y, Wang X, Tan Y, Yamamoto M, Hashimoto K. Rapid-acting and long-lasting antidepressant-like action of (R)-ketamine in Nrf2 knock-out mice: a role of TrkB signaling. Eur Arch Psychiatry Clin Neurosci. 2021;271(3):439–446. doi: 10.1007/s00406-020-01208-w. [DOI] [PubMed] [Google Scholar]
- 146.He T, Wu Z, Zhang X, Liu H, Wang Y, Jiang R, Liu C, Hashimoto K, Yang C. A bibliometric analysis of research on the role of BDNF in depression and treatment. Biomolecules. 2022;12(10):1464. doi: 10.3390/biom12101464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.He T, Wang D, Wu Z, Huang C, Xu X, Xu X, Liu C, Hashimoto K, Yang C. A bibliometric analysis of research on (R)-ketamine from 2002 to 2021. Neuropharmacology. 2022;218:109207. doi: 10.1016/j.neuropharm.2022.109207. [DOI] [PubMed] [Google Scholar]
- 148.Zhang K, Yao Y, Hashimoto K. Ketamine and its metabolites: potential as novel treatments for depression. Neuropharmacology. 2023;222:109305. doi: 10.1016/j.neuropharm.2022.109305. [DOI] [PubMed] [Google Scholar]
- 149.Groff D, Sun A, Ssentongo AE, Ba DM, Parsons N, Poudel GR, Lekoubou A, Oh JS, Ericson JE, Ssentongo P, Chinchilli VM. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. 2021;4:e2128568. doi: 10.1001/jamanetworkopen.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Damiano RF, Guedes BF, de Rocca CC, de Pádua SA, Castro LHM, Munhoz CD, Nitrini R, Filho GB, Miguel EC, Lucchetti G, Forlenza O. Cognitive decline following acute viral infections: literature review and projections for post-COVID-19. Eur Arch Psychiatry Clin Neurosci. 2022;272(1):139–154. doi: 10.1007/s00406-021-01286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hashimoto K. Mental health during the COVID-19 pandemic, impact of childhood trauma in psychiatric disorders, and predictable biomarkers for bipolar disorder. Eur Arch Psychiatry Clin Neurosci. 2022;272(5):753–755. doi: 10.1007/s00406-022-01445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hashimoto Y, Suzuki T, Hashimoto K. Comments to "Fluvoxamine and long COVID-19: a new role for sigma-1 receptor (S1R) agonists" by Khani and Entezari-Maleki. Mol Psychiatry. 2022;27(9):3563–3564. doi: 10.1038/s41380-022-01546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zeng N, Zhao YM, Yan W, Li C, Lu QD, Liu L, Ni SY, Mei H, Yuan K, Shi L, Li P, Fan TT, Yuan JL, Vitiello MV, Kosten T, Kondratiuk AL, Sun HQ, Tang XD, Liu MY, Lalvani A, Shi J, Bao YP, Lu L. A systematic review and meta-analysis of long term physical and mental sequelae of COVID-19 pandemic: call for research priority and action. Mol Psychiatry. 2023;28(1):423–433. doi: 10.1038/s41380-022-01614-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article does not contain any original data.