Abstract
Background
Up to date, the effect of cigarette smoke (CS) on the adhesive properties of the restorative materials bonded to tooth structure is still unclear. It is still questionable if it interferes with the durability and clinical success of these restorations. Few numbers of studies reported the assessment of microgaps of dental composite using cross-polarization optical coherence tomography (CP-OCT). The aim of this study was to evaluate the presence of interfacial microgaps between composite resin restoration and the tooth structure with or without exposure to CS under the CP-OCT.
Materials and methods
In this in-vitro study, a standardized round class-V cavities were prepared in twenty extracted, caries-free human molar teeth, and they were divided randomly into two groups (n = 10). Adhesive system (Gluma universal, Kulzer GmbH) was applied followed by filing the prepared cavities with flowable composite restoration (Charisma, Kulzer GmbH). Then, exposure to CS (40 cigarettes/day) was carried out for 14 days and the samples were stored in normal saline solution after each smoke exposure cycle. Next, all samples were immersed in ammoniac silver nitrate for 24 h, followed by immersion in a photo-developing solution for 8 h. Optical comparison was carried out by CP-OCT to assess microgaps percentage between smoke (SG) and non-smoke groups (NSG) at the axial walls (AW) and cavity floor (CF).
Results
Independent T-test showed that there was a significant difference between the two groups (SG, NSG) in AW microgaps percentage (p = 0.008), while there was no difference in CF (p = 0.15)
Conclusion
It can be inferred from the current finding that OCT can be used to predict the adaptability of the bonded restoration under the thermochemical influence. CS has a negative effect on the marginal integrity of the bonded polymeric restorations.
Keywords: Smoke, Dentin, OCT, Gap, Adhesive, Oral health, Restoration leakage
1. Introduction
Cigarette smoking is a major public health challenge with approximately 1 billion smokers all over the world [1]. It is well known that cigarette smoke (CS) has a direct effect on oral health as oral tissues are initially exposed to the heat generated by it [2]. In addition to heat, chemical toxins within the cigarette smoke vapor are harmful, including carbon monoxide, ammonia, nickel, arsenic, and heavy metals such as lead and cadmium [3]. Moreover, there are multiple negative effects on teeth and resin-based restorative materials, such as color instability and surface roughness. A previous study reported that CS increased water sorption and dental resin composite degradation [4]. It also modifies the physical properties (such as water sorption and solubility) of resin-based dental composites [4].
Several studies have evaluated the effect of smoking on the color stability and surface roughness of different restorative materials and dental tissues [[5], [6], [7], [8]]. Although the specific mechanism of discoloration after smoking has not been completely researched, brown pigments from the particulate phase of the CS are thought to be the main reason for staining teeth and resin composite materials [7]. When cigarettes lighted and puffed, tobacco is burned at temperatures ranging between 600 and 900 °C, resulting in a particulate phase containing nicotine [2]. Solid particles and liquid droplets suspended in the gas phase constitute the particulate phase. When the particulate phase is captured on a filter and the amount of water and nicotine present is subtracted, the result is nicotine-free dry particulate matter, often known as “tar” [9]. Tars contain pigmented compounds and metal ions, such as lead and cadmium [8]. Dentin is a heterogeneous, wet, and organic substrate compared to enamel, making bonding to this substrate more challenging [10,11]. Proper adhesion mandates intimate contact with the tooth surface when bonding restorative materials to dental substrates. The presence of any contamination on the surface may decrease bond strength. It was reported that samples that were exposed to CS demonstrated significantly lower bond strength values compared to those that were not exposed to CS, which may affect the long-term outcomes of the restorations [12].
Nowadays, composite restorations are commonly used because of their high esthetic properties. However, polymerization shrinkage is the major disadvantage like other resin materials. Dental composites are set by light polymerization, which mainly depends on a free radical polymerization reaction through polymer side chains crosslinking. This reaction leads to an observable volumetric shrinkage due to the formation of covalent bonds and reduction of the intra-molecular spaces [13]. Therefore, they are susceptible to marginal leakage during cavity restoration [[14], [15], [16]]. Additionally, some studies found some relation between polymerization shrinkage and dietary habits, such as consumption of soft drinks and smoking [17].
The introduction of a universal adhesive system has simplified the restorative techniques. Much attention should be given to dental adhesives as it is a vital link between dental composites and tooth substrate to obtain a gap-free and durable bonding with minimum microleakage and further marginal staining, tooth sensitivity, and secondary caries. Additionally, resin infiltration into demineralized dentin is required to obtain micromechanical adhesion [18].
Several invasive imaging techniques, including scanning electron microscopy, micro-computed tomography, and transmission electron microscope, have been utilized to assess the adaptation of the restoration to the cavity walls and floor [19,20]. One of the recent non-invasive methods that is also used for this purpose is optical coherence tomography (OCT). It is an emerging imaging technique that uses low-coherence light to capture two- and three-dimensional images at micrometer resolution [14]. Additionally, it has been used for medical imaging and industrial nondestructive testing [21,22]. OCT works by employing near-infrared light. The use of relatively long-wavelength light allows penetration through the scattering medium. OCT has wide applications in the fields of ophthalmology, dentistry, and dermatology [19,[23], [24], [25], [26]]. Various OCT systems have been used in the dental field such as polarized-sensitive OCT, swept-source OCT (SS-OCT), and cross-polarization OCT (CP-OCT) [16,26,27]. CP-OCT is considered the most recent OCT imaging modality, which is quite similar to SS-OCT, except that it eliminates specular reflections that form with mirror-like surfaces [28]. Thus, it is commonly used in studies investigating highly scattered surfaces, such as dental remineralization/demineralization [28]. In addition, it can be used to analyze the composition of non-metallic restorations [29]. Originally, OCT was used in 1991 in-vivo and in-vitro studies to obtain an image of hard and soft dental tissues [30]. Additionally, it has been utilized in interfacial microgap detection as well as internal restoration defects in 2D (B-scan) and 3D (C-scan) images [19,31]. It can also be used to detect dental caries and enamel cracks [29,32].
The association between tooth discoloration and tobacco smoking is well known. It has been proven that these stains develop because of the impregnation of tobacco contaminants into the tooth. This staining effect is positively influenced by the amount and duration of smoking [6,8]. To date, the effect of cigarette smoke on the adhesive properties of restorative materials bonded to enamel/dentin surfaces is still unclear. It is still questionable whether it interferes with the durability and clinical success of these restorations. To the best of our knowledge, few studies have reported quantitative gap measurements at the tooth–composite interface using CP-OCT. No study has been performed to measure the relationship between these gaps and CS using OCT.
This study aimed to detect the interfacial gaps formed under bonded polymeric restorations at the cavity floor (CF) or axial walls (AW), with and without CS, using CP-OCT. The null hypothesis was that CS does not affect composite adaptation or interfacial gap formation at the CF and AW of bonded restorations.
2. Material and methods
2.1. Materials used
In this study, GLUMA bond universal and Charisma flowable composite (Kulzer GmbH, Germany) were applied and cured according to the manufacturer's instructions using a light-emitting diode (LED) light-curing unit (1200 mW/cm2). The chemical compositions of the tested materials and the method of application are listed in Table-1.
Table-1.
List of the composition of the used materials in this study.
| Material (Manufacturer) | Composition | Direction of use |
|---|---|---|
| GLUMA bond universal (Heraeus Kulzer) |
4-META, 10-MDP, acetone, methacrylate monomers and Silane. | 1. Apply and rub the adhesive for 20 s. 2. Air dry with a gentle oil-free air flow until the adhesive film is immobilized and the surface become glossy. 3. Light cure for 10 s. |
| Charisma flow composite (Heraeus Kulzer) | 2,2-dimethoxy-1,2-diphenylethan-1-one, TEGDMA, CQ, Ba–Al–B–F–Si Glass and Pyrogenic SiO2 inorganic fillers (62% wt). The filler particle size is between 0.005 μm and 5 μm. | 1. Apply Charisma flow in thin layers (max. 2 mm thickness). 2. Photoactivate the composite for 20 s. |
Abbreviation: 4-META; 4-methacryloyloxyethy trimellitate anhydride, 10-MDP; 10-methacryloyloxydecyl dihydrogen phosphate, TEGDMA; triethylene glycol dimethacrylate, CQ; camphorquinone.
2.2. Specimen preparation
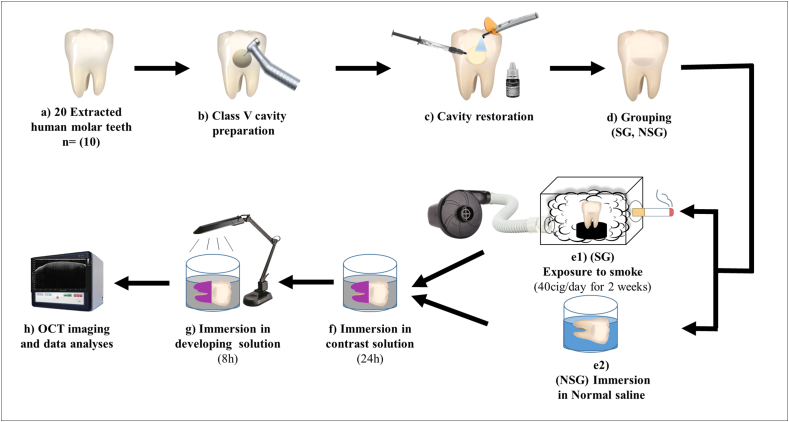
This study was approved by the Research Ethics Committee of King Abdulaziz University (code: 156-11-19). The sample size calculation was performed using a 0.05 alpha value and 90% power to detect a mean difference of 0.25 (Piface, https://homepage.stat.uiowa.edu/~rlenth/Power/oldversion.html The common standard deviation within a group was presumed to be 0.15. The expected sample size for each group should be at least 9 [33]. As a result, twenty freshly extracted non-carious human molars were collected from the oral surgery clinics of the university and were preserved for 2 weeks in distilled water at 4 °C before starting the experiment. The clinical crowns of the teeth were cleaned using tissue debris and calculus. Class V round cavities (4 mm diameter, 2 mm depth) were prepared on the cervical area of the facial surface of all teeth with cavity margins located in the enamel. Material application and photoactivation were performed as previously reported in prior investigations to maintain the repeatability of the restoration process [15,34]. All prepared specimens were bonded according to manufacturer instructions using a disposable microbrush in a self-etch mode followed by application of composite restoration (shade A2) and photoactivated using LED dental light cure in continuous irradiation mode. After cavity restoration, they were distributed equally into two groups (n = 10): the non-smoke group (NSG) and smoke group (SG). This was conducted using a simple random sampling technique, which involved the equal and random distribution of the specimens into two experimental groups. In the NSG group, the specimens were incubated in normal saline solution, which was changed daily, for 14 days at 37 °C. A schematic illustration of the research methodology is presented in Figure-1.
Fig. 1.
Schematic illustration of the implemented methodology in the current study.
2.3. Custom-made smoking device
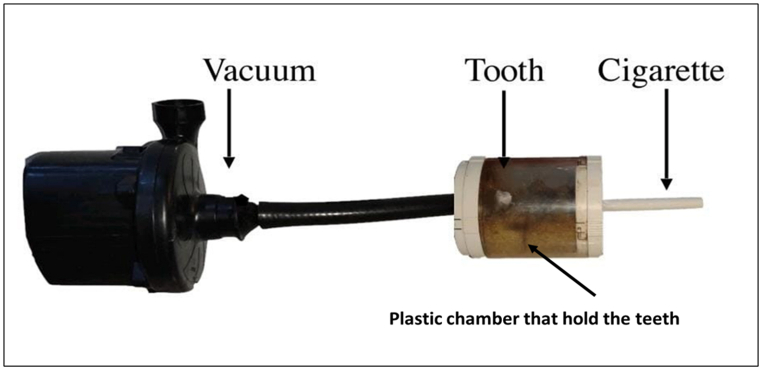
A simulating smoking device (Fig. 2) was developed to simulate tooth exposure to cigarette smoke in the oral cavity. The device consisted of two chambers with a hole created in the middle between them. A tube was inserted in the first chamber, which was connected to the second chamber that created a negative pressure, which aspirated the cigarette smoke into the chamber. This machine simulated suction action in the oral cavity. At the other end of the second chamber, a hole was prepared to accommodate the cigarette to be placed in the first chamber. The second chamber was designed to hold five specimens of the SG group at a time in a horizontal position using a wet sponge to simulate the humid environment of the oral cavity and to prevent dryness of the specimens. In the SG group, specimens were exposed to 40 cigarettes/day (Marlboro Red, Phillip Morris) for 14 days. Each cigarette was consumed for approximately 5 min. After each puff, the specimens were sprayed with normal saline solution to prevent dehydration. At the end of the smoke exposure, the specimens were stored in normal saline solution prior to the CP-OCT examination.
Fig. 2.
A simulating smoking device to simulate the smoking process in the oral cavity. It shows the positioning of the cigarette, which is connected to a chamber that hold the teeth inside. The chamber contains two holes; one is connected to the cigarette and the other is connected to the vacuum that induce a negative pressure during the experiment.
2.4. Cross-polarization OCT (CP-OCT)
CP-OCT (IVS-300; Santec, Japan) is a portable imaging system that was utilized to examine the restored cavities non-invasively using a scanning diode laser with 30 kHz scan rates and a continuous wavelength centered near 1310 nm, in the range of 100 nm wavelength range. It has a 12 μm axial resolution with an output power of ≤10 mW [28]. The technical specifications of the system are listed in Table-2.
Table-2.
Technical specifications of CP-OCT system.
| Cross-Polarization OCT (CP-OCT; IVS-300, Santec, Komaki, Aichi, Japan) | |
|---|---|
| Parameter | Specification |
| Wavelength | 1330 ± 30 nm |
| Scan rate | 30 ± 0.1 kHz |
| Axial resolution | ≤12 μm (in air) |
| Lateral resolution | 30 ± 7 μm (Spot size) |
| System sensitivity | >95 dB |
| Lateral scan area | ≥5 × 5 mm |
| Imaging depth range | 3 mm (in air) |
| Maximum Output Power | Less than 5 mW (Near-infrared Class 1 Laser) |
2.5. Contrast medium preparation and CP-OCT imaging
Following smoke exposure, all specimens were immersed in a contrast medium (ammoniacal silver nitrate solution) for 24 h. This solution was prepared in a dark room as follows: 25 mg of silver nitrate crystals (Sigma Chemical, USA) was dissolved in 25 mL of distilled water. Ammonium hydroxide (28%) (Sigma Chemical, USA) was then added to titrate the black, dark solution until it became clear and transparent. Next, the specimens were immersed in a photo-developing solution for 8 h under a fluorescent light source, which converted the silver ions into metallic deposits for CP-OCT assessment.
Subsequently, each specimen was lightly polished with SiC paper (2000 grit) and cleansed in an ultrasonic water bath (25 °C for 5 min) before being placed on a micrometer stage with the restoration surface positioned at 90° to the projected laser source from the scanning OCT probe with a standardized distance of 0.25–0.5 mm from probe. The cavity floor was parallel to the micrometer-stage base and subjected to tomographic scanning. Next, each specimen was scanned in the cervico-occlusal direction to obtain serial tomographic scans at 0.5 mm intervals [34,35].
2.6. Image analysis
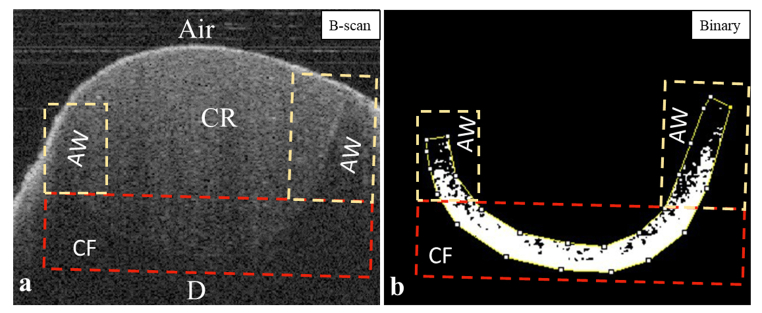
OCT data were analyzed according to the protocol described by Bakhsh et al. [[14], [15], [16]]. OCT scans were imported into ImageJ software (ImageJ 1.5 m9, National Institutes of Health, USA) and managed by a plug-in macro-file coded in the software to transform OCT data into a grayscale image based on each pixel's signal intensity value. The AW and CF were selected and cropped. Images were then converted to 8-bit, and a median filter was applied (1 px radius), followed by image binarization (black and white pixels) to determine the target pixels with significantly higher brightness compared to other dark pixels in the background. The target pixels transformed into black pixels on a white background along the cavity boundary were considered interfacial gaps. The gap percentage was calculated for both AW and CF (Fig. 3) separately, according to the following equation:
Fig. 3.
Representative OCT images that show image segmentation and analyses. a) OCT image shows composite restoration bonded to dentin. The tooth-restoration interface was being outlined in white (for illustrative purpose) and the axial walls (AW) with the cavity floor (CF) are segmented in 2 separate rectangles. b) Binarization function was applied to the original OCT data to obtain binary image (black and white image). Gap width was measured separately on the binary images in each rectangle (AW, CF) separately followed by calculating gap percentage. CR; composite resin, D; dentin.
2.7. Statistical analysis
The average percentage values obtained from the B-scans of each specimen were included in the statistical analysis. A parametric test was performed as the data distribution was normal. An independent t-test was used to compare the gap percentage between the groups. The software used for statistical analysis was the Statistical Package for Social Science (SPSS for Windows, Version 23, SPSS, USA) with a significance level of alpha = 0.05.
3. Results
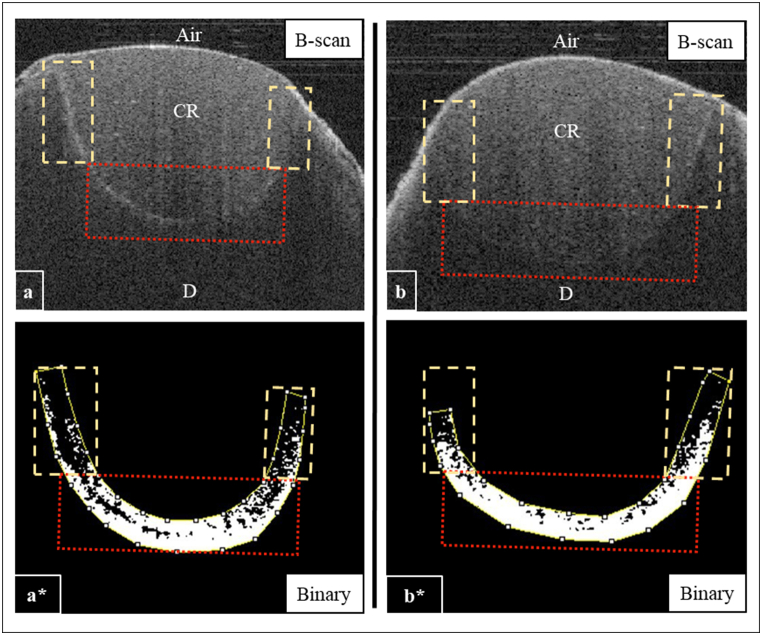
Quantitative analysis of B-scans demonstrated some areas with high signal intensity (intense backscattered reflection) with a remarkably bright pixel along with the AW of the cavities in the SG group. These areas mostly indicated the presence of interfacial gaps at the AW of the cavity; however, the appearance of CF in both groups showed minimal changes in the pixel intensity values, indicating a low interfacial gap percentage in these regions (Fig. 4). Representative CP-OCT images obtained from the NSG and SG are shown in Figure-4.
Fig. 4.
Representative CP-OCT images for SG (a) and NSG (b) groups. a) The CP-OCT image for the SG was showing a strong reflection at the composite-tooth interface. a*) A binary image for the selected axial walls (dashed rectangles) and cavity floor (dotted square). Image processing showed continuous dark cluster along the axial walls (dashed rectangles) and cavity floor (dotted square). b) B-scan image for NSG group. b*) A binary image for the selected areas; axial walls and the cavity floor. Image processing showed variable length of bright pixels at the composite-tooth interface, which transformed to black pixels after binarization. CR; composite resin, D; dentin.
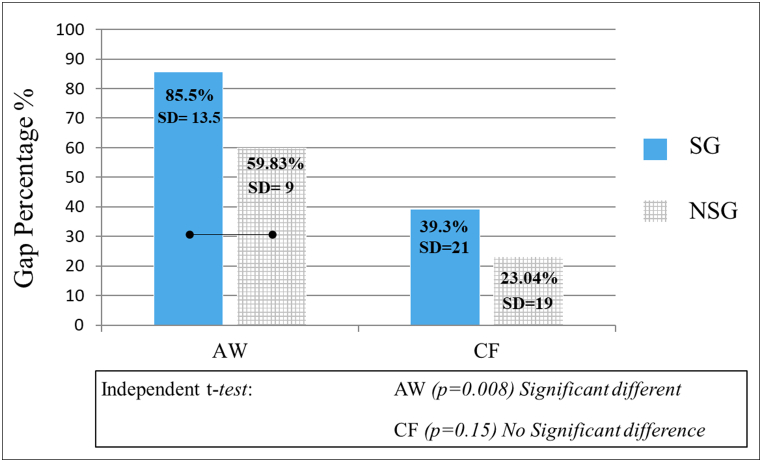
The independent t-test showed a significant difference between the two in microgap percentage in AW groups (NSG = 59.83 ± 9, SG = 85.5 ± 13.5%) (p = 0.008), while no difference was found at CF (NSG = 23.04 ± 19, SG = 39 ± 21) (p = 0.15), as shown in Figure-5.
Fig. 5.
Bar graph shows the significant difference in AW gap percentage between SG and NSG (p = 0.008), while there was no significant difference in CF gap percentage between the two groups (SG and NSG). Connected bars indicate there is a significant difference (p = 0.15). AW; axial wall, CF; cavity floor.
4. Discussion
CP-OCT is a non-invasive and nondestructive imaging tool that can assess composite adaptation, interfacial microgaps, and internal restoration defects using optical reflectivity indices. It works by projecting light from the OCT probe into the specimen and passes through different media with different compositions and refractive indices [14]. When the light traverses two media with a significant difference in the refractive index, some photons reflect, while others refract in the form of prominent peaks as backscattered signals. The location of the abrupt change in the signal intensity appears in the OCT image as bright pixels [16]. The refractive index of the tooth and resin composite is approximately 1.5–1.6 [11], while the refractive indices of air and water are in the range of 1–1.3. It is possible to detect specular reflection in the presence of a significant difference between the bonded media and SS-OCT [31]. However, silver nitrate was used as a contrasting agent with CP-OCT to intensify the appearance of the interfacial gap in the form of diffuse reflection [36].
Several in-vitro studies have assessed the effects of cigarette smoke on color stability, surface roughness, and bond strength of resin-bonded restorations [5]. Moreover, the loss of interfacial seal will result in low bond strength, according to some studies [14,37,38]. According to Theobaldo et al. and Silva et al. both studies demonstrated a reduction in bond strength values of their samples due to cigarette smoke exposure, which is in agreement with the results of our study [3,39]. This reduction in bond strength was attributed to heavy metal contamination (cadmium, lead, and arsenic), which may have interfered with the chemical interaction between the functional monomers 10-MDP in the adhesive and Ca hydroxyapatite in dentin, which is thought to be the major bonding mechanism [3]. Besides this cause, the reduced diameter of the particles from cigarette smoke makes it capable of being absorbed into the dentin hydroxyapatite based on the exposure. It has been reported that contamination by CS decreases the bond strength between dentin and composite resin, reducing the contact between the adhesive and dentin, thus reducing the bond strength values [39].
In contrast, previous studies have reported changes in the surface roughness of composite restorations following exposure to CS. It has been suggested that variations in temperature and the presence of solvents, oil, and water in CS may contribute to composite surface degradation [5,9]. Thus, temperature and humidity interactions in the environment can damage restorative materials [40]. It has been reported that an increase in temperature might decrease the viscosity of the resin, increasing shrinkage. It was also suggested that an increase in temperature may lead to a release of the resin monomer [41]. Another study concluded that CS did not affect the surface roughness of composite restorations; however, it influenced the surface gloss and increased the staining possibilities due to the interaction between CS and composite filler content [10].
The present study assessed the effect of CS on microgap formation during bonded polymeric restoration. In AW, the results demonstrated a significant percentage of microgap formation in the SG group compared to that in the NSG group. One of the possible factors that may cause such differences is the aspirated heat from the cigarettes. Loguercio et al. found that higher temperatures (above 50 °C) of acetone-based adhesives may result in lower bond strength values [13]. This finding agrees well with the results of the present study.
Moreover, the results of the current study showed that the NSG group followed a random pattern of microgap formation but was not as significant as the SG group, which is possibly due to polymerization shrinkage following curing. In general, polymerization shrinkage of conventional composite resins occurs when monomer molecules are converted to polymer structures by replacing van der Waals gaps with covalent connections, resulting in a reduction in free volume [42]. The type of resin matrix, concentration of monomers, and type of initiator all affect polymerization shrinkage because they determine the polymer structure of the composite resins. Additionally, the amount, type, and size of fillers affect the volumetric shrinkage of composite resins [14,16,31]. Owing to the reduced volume of monomers available for the curing reaction, increasing the number of fillers in the resin matrix reduces the overall shrinkage of conventional composite resins. However, it can increase the elastic modulus of the material and cause significant shrinkage [43,44]. In contrast, flowable composites include a larger amount of resin monomer than conventional packable composites. As a result, they may contract more during polymerization and cause more strain within the polymer than packable composites, resulting in more internal stresses and microleakage [45]. It appears that this fact applies to the utilized composite in this study, the Charisma flow composite, which contains 62% filler loading by weight according to the manufacturer, which would explain the obtained results and justify the presence of interfacial gaps in both groups, regardless of the smoking influence.
In the SG group, the microgaps were mostly detected along with the AW of the restorations, which were located close to the smoke vapor. In addition to heat, another factor that could contribute to microgap formation is the heavy metal deposition and chemical toxins that are included in the cigarette smoke vapor, which is composed of more than 5000 constituents, including carbon monoxide, ammonia, nickel, arsenic, and heavy metals such as lead and cadmium [2]. Silva et al. concluded that smoking could impair bonding to dentin because of the deposition of CS particles into dentinal tubules, which prevents complete impregnation of the adhesive and thus decreases the bond strength values. Moreover, they demonstrated that acid etching used in their study design could not remove cigarette smoke particles [39]. It is noteworthy that heat generated from cigarette smoke negatively impacts universal adhesives when they are utilized in the self-etch mode, and they become more prone to microleakage [46].
It is known that smoking cigarettes involves thermocyling that has a negative effect on class-V restorations. However, thermocyling is a part of the smoking process in real clinically relevant environment. The current study design is trying to simulate the oral environment by exposing the restored teeth to cigarette smoke while they are hydrated as if they were in smoker's patient mouth (SG group), while the second group (NSG group) were not subjected to heat (only kept hydrated) and considered as if they were in a mouth of a healthy patient. There are other factors that has to be considered, such as masticatory forces and chemical effect of the food and drinks, however these factors were neglected in this study in order to ensure no other factor would influence composite adaptation. Future study will consider these mechanical and chemical challenges.
Within the limitations of the study, which included a limited number of teeth and a narrow study design, and as there was a significant difference between the tested groups, the null hypothesis was rejected. Further studies that involve changing the rate of smoke flow, different bonding systems, composite materials and filing techniques as well as histological investigations are required to understand the influence of cigarette smoke on the adhesion of bonded restorations. It could also assist in identifying the contributing factors that may interfere with the longevity and clinical success of these resin-based restorations to aid in achieving better clinical performance.
5. Conclusions
It can be inferred from the current finding that OCT can be used to predict the adaptability of bonded restorations under the thermochemical influence. The results of this study indicate that smoke negatively affects the marginal integrity of the restoration. Patients should be informed that restored teeth are more prone to microleakage upon exposure to cigarette smoke.
Impact statement
Nowadays, the advancement in optics is remarkable in several fields including dentistry. Although the effect of cigarette smoke has been thoroughly investigated, viewing its negative impact on composite adaptation with aid of optical coherence tomography (OCT) has provided an additional insight by showing the gap location and its progression non-invasively. Based on the current finding, it would be beneficial to add OCT assessment to routine dental examination that would improve clinicians’ diagnosis and predication of restoration longevity.
Declarations
Author contribution statement
Roaa Abuljadayel: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Naif Hashem and Yousef Almaddah: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Turki Bakhsh: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research was funded by the authors.
Data availability statement
The data that has been used is confidential.
Declaration of interests statement
The authors declare no conflict of interest.
Acknowledgment
This study was partly supported partly by King Abdulaziz University, Jeddah, Saudi Arabia and partly by Tokyo Medical and Dental University, Tokyo, Japan.
References
- 1.Warren G.W., et al. Wiley Online Library; 2014. The 2014 Surgeon General's report:“The Health Consequences Of Smoking–50 Years Of Progress”: a Paradigm Shift in Cancer Care; pp. 1914–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eaton D., et al. Assessment of tobacco heating product THP1. 0. Part 2: product design, operation and thermophysical characterisation. Regul. Toxicol. Pharmacol. 2018;93:4–13. doi: 10.1016/j.yrtph.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Theobaldo J., et al. Effect of cigarette smoke on resin composite bond strength to enamel and dentin using different adhesive systems. Operat. Dent. 2016;41(3):E57–E63. doi: 10.2341/15-056-L. [DOI] [PubMed] [Google Scholar]
- 4.Mathias P., et al. Cigarette smoke: effects on water sorption and solubility of restorative dental composites. Gen. Dent. 2014;62(2):54–57. [PubMed] [Google Scholar]
- 5.Alandia-Roman C., et al. Effect of cigarette smoke on color stability and surface roughness of dental composites. J. Dent. 2013;41:e73–e79. doi: 10.1016/j.jdent.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Dalrymple A., et al. Assessment of enamel discoloration in vitro following exposure to cigarette smoke and emissions from novel vapor and tobacco heating products. Am. J. Dent. 2018;31(5):227–233. [PubMed] [Google Scholar]
- 7.Zanetti F., et al. Effects of cigarette smoke and tobacco heating aerosol on color stability of dental enamel, dentin, and composite resin restorations. Quintessence Int. 2019;50(2):156–166. doi: 10.3290/j.qi.a41601. [DOI] [PubMed] [Google Scholar]
- 8.Zhao X., et al. Effects of cigarette smoking on color stability of dental resin composites. Am. J. Dent. 2017;30(6):316–322. [PubMed] [Google Scholar]
- 9.Thielen A., Klus H., Müller L. Tobacco smoke: unraveling a controversial subject. Exp. Toxicol. Pathol. 2008;60(2–3):141–156. doi: 10.1016/j.etp.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Bakhsh T., et al. Advanced imaging of dentin microstructure. Biomed. Phy. Eng. Exp. 2021;7(5) doi: 10.1088/2057-1976/ac19cf. [DOI] [PubMed] [Google Scholar]
- 11.Bakhsh T.A. Ultrastructural features of dentinoenamel junction revealed by focused gallium ion beam milling. J. Microscopy. 2016;264(1):14–21. doi: 10.1111/jmi.12410. [DOI] [PubMed] [Google Scholar]
- 12.Abdalla A., Davidson C.L. Bonding efficiency and interfacial morphology of one-bottle adhesives to contaminated dentin surfaces. Am. J. Dent. 1998;11(6):281–285. [PubMed] [Google Scholar]
- 13.Subbiya A., et al. A review on polymerization shrinkage of resin composites. Eur. J. Mol. Clin. Med. 2020;7(5):1245–1250. [Google Scholar]
- 14.Bakhsh T.A., et al. Concurrent evaluation of composite internal adaptation and bond strength in a class-I cavity. J. Dent. 2013;41(1):60–70. doi: 10.1016/j.jdent.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Bakhsh T.A., et al. Non-invasive quantification of resin–dentin interfacial gaps using optical coherence tomography: validation against confocal microscopy. Dent. Mater. 2011;27(9):915–925. doi: 10.1016/j.dental.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Bakhsh T.A., et al. Does lining class-II cavities with flowable composite improve the interfacial adaptation? J. Adhes. Sci. Technol. 2020;34(4):400–416. [Google Scholar]
- 17.Saraswati W., Hadinata A.T.S., Sukaton S. The effect of self-etch and total-etch bonding systems application on microleakage of bulkfill flowable composite restoration in carbonated drink immersion. Conser. Dentis. J. 2019;9(2):87–92. [Google Scholar]
- 18.Perdigão J. Current perspectives on dental adhesion:(1) Dentin adhesion–not there yet. Jap. Dental Sci. Rev. 2020;56(1):190–207. doi: 10.1016/j.jdsr.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakhsh T.A. Optical comparison between micro‐CT and OCT in imaging of marginal composite adaptation: observational study. J. Microscopy. 2021;282(2):136–145. doi: 10.1111/jmi.12988. [DOI] [PubMed] [Google Scholar]
- 20.Nazari A., et al. 3D assessment of void and gap formation in flowable resin composites using optical coherence tomography. J. Adhesive Dent. 2013;15(3):237–243. doi: 10.3290/j.jad.a28623. [DOI] [PubMed] [Google Scholar]
- 21.Masthoff M., et al. RöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren. © Georg Thieme Verlag KG; 2019. Dental imaging–a basic guide for the radiologist. [Google Scholar]
- 22.Shirazi M.F., et al. Fast industrial inspection of optical thin film using optical coherence tomography. Sensors. 2016;16(10):1598. doi: 10.3390/s16101598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai T.-H., et al. Optical coherence tomography in gastroenterology: a review and future outlook. J. Biomed. Opt. 2017;22(12) doi: 10.1117/1.JBO.22.12.121716. [DOI] [PubMed] [Google Scholar]
- 24.O'leary S., et al. OCT image atlas of healthy skin on sun‐exposed areas. Skin Res. Technol. 2018;24(4):570–586. doi: 10.1111/srt.12468. [DOI] [PubMed] [Google Scholar]
- 25.Kim T.-H., Son T., Yao X. Functional OCT angiography reveals early physiological dysfunction of hyaloid vasculature in developing mouse eye. Exp. Biol. Med. 2019;244(10):819–823. doi: 10.1177/1535370219850787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marschall S., et al. Optical coherence tomography—current technology and applications in clinical and biomedical research. Anal. Bioanal. Chem. 2011;400(9):2699–2720. doi: 10.1007/s00216-011-5008-1. [DOI] [PubMed] [Google Scholar]
- 27.Bakhsh T.A. Optical comparison between micro-CT and OCT in imaging of marginal composite adaptation: observational study. J. Microsc. 2021;282(2):136–145. doi: 10.1111/jmi.12988. [DOI] [PubMed] [Google Scholar]
- 28.Bakhsh T., et al. Novel evaluation and treatment techniques for white spot lesions. An in vitro study. Orthod. Craniofac. Res. 2017;20(3):170–176. doi: 10.1111/ocr.12193. [DOI] [PubMed] [Google Scholar]
- 29.Turkistani A., Bakhsh T. Progression of enamel demineralisation around fissure sealants: optical coherence tomography study. Oral Health Prev. Dent. 2021;19(1):707–712. doi: 10.3290/j.ohpd.b2448625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang D., et al. Optical coherence tomography. Science. 1991;254(5035):1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakhsh T., Turkistani A. The effect of thermocycling on interfacial bonding stability of self-etch adhesives: OCT study. BioMed Res. Int. 2021:2021. doi: 10.1155/2021/5578539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimada Y., et al. Evaluation of dental caries, tooth crack, and age-related changes in tooth structure using optical coherence tomography. Jap. Dental Sci. Rev. 2020;56(1):109–118. doi: 10.1016/j.jdsr.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lenth R.V. Statistical power calculations. J. Anim. Sci. 2007;85(13 Suppl):E24–E29. doi: 10.2527/jas.2006-449. [DOI] [PubMed] [Google Scholar]
- 34.Bakhsh T.A., et al. Adaptation assessment of three bonded resin restorations at the cavity floor using cross-polarization optical coherence tomography. Photobiomodulation, Photomedicine, and Laser Surgery. 2019;37(5):318–324. doi: 10.1089/photob.2018.4553. [DOI] [PubMed] [Google Scholar]
- 35.Bakhsh T.A., et al. Effect of self-etch adhesives on the internal adaptation of composite restoration: a CP-OCT Study. Odontology. 2019;107(2):165–173. doi: 10.1007/s10266-018-0381-2. [DOI] [PubMed] [Google Scholar]
- 36.Bakhsh T., et al. Nondestructive evaluation of microleakage in restored primary teeth using CP-OCT. Niger. J. Clin. Pract. 2021;24(6):919. doi: 10.4103/njcp.njcp_442_20. 919. [DOI] [PubMed] [Google Scholar]
- 37.Omar H., Haggag S., Ghoneima A. The effect of cigarette smoke on the shear bond strength of metallic and ceramic orthodontic brackets: an in vitro study. Int. Orthod. 2020;18(1):121–126. doi: 10.1016/j.ortho.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Makishi P., et al. Assessment of self-adhesive resin composites: nondestructive imaging of resin–dentin interfacial adaptation and shear bond strength. Microsc. Microanal. 2015;21(6):1523–1529. doi: 10.1017/S1431927615015354. [DOI] [PubMed] [Google Scholar]
- 39.e Silva J.A., de Araujo E.M., Jr., Araujo E. Cigarette smoke affects bonding to dentin. Gen. Dent. 2010;58(4):326–330. [PubMed] [Google Scholar]
- 40.Ha J.-Y., et al. Simple heat treatment of zirconia ceramic pre-treated with silane primer to improve resin bonding. J. Nanosci. Nanotechnol. 2015;15(1):587–590. doi: 10.1166/jnn.2015.8359. [DOI] [PubMed] [Google Scholar]
- 41.Watts D., Alnazzawi A. Temperature-dependent polymerization shrinkage stress kinetics of resin-composites. Dent. Mater. 2014;30(6):654–660. doi: 10.1016/j.dental.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Cook W.D., Standish P.M. Polymerization kinetics of resin‐based restorative materials. J. Biomed. Mater. Res. 1983;17(2):275–282. doi: 10.1002/jbm.820170206. [DOI] [PubMed] [Google Scholar]
- 43.Labella R., et al. Polymerization shrinkage and elasticity of flowable composites and filled adhesives. Dent. Mater. 1999;15(2):128–137. doi: 10.1016/s0109-5641(99)00022-6. [DOI] [PubMed] [Google Scholar]
- 44.Miyazaki M., et al. Effect of filler content of light-cured composites on bond strength to bovine dentine. J. Dent. 1991;19(5):301–303. doi: 10.1016/0300-5712(91)90078-d. [DOI] [PubMed] [Google Scholar]
- 45.Braga R.R., Ballester R.Y., Ferracane J.L. Factors involved in the development of polymerization shrinkage stress in resin-composites: a systematic review. Dent. Mater. 2005;21(10):962–970. doi: 10.1016/j.dental.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 46.Vitória L.A., et al. ISRN Dentistry; 2013. Changes in Water Sorption and Solubility of Dental Adhesive Systems after Cigarette Smoke. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.