Abstract
Objectives
This study evaluated the effect of implementing a hierarchical pharmaceutical service pattern based on the knowledge–attitude–practice (KAP) intervention theory on patients with systemic lupus erythematosus.
Methods
Eligible patients were randomly divided into an intervention or control group. Pharmaceutical service classification criteria were formulated and used to provide patients with differing levels of pharmaceutical services. The classification scores and KAP levels of patients before and at various time points after the intervention were analyzed. The rates of acute attacks and adverse reactions, related clinical test indices, and disease activity were evaluated in both groups.
Results
After 9 months of intervention, the proportions of first- and second-level services in the intervention group declined by 14.43% and 3.94%, respectively, compared with the control group, and the rates of acute attacks and adverse reactions declined by 18.26% and 12.43%, respectively. The KAP level, clinical test indices, and disease activity were significantly different between the groups.
Conclusion
Providing patients with systemic lupus erythematosus with pertinent hierarchical pharmaceutical services based on the KAP theory was instrumental in changing patients’ behavior and contributed to facilitating disease self-management, thus improving the quality of pharmaceutical services.
Keywords: Knowledge–attitude–practice, hierarchical pharmaceutical service pattern, systemic lupus erythematosus, effect evaluation, disease activity, Systemic Lupus Erythematosus Disease Activity Index-2000
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease with a complicated pathogenesis that is clinically characterized by multisystem and organ involvement of the whole body, repeated relapses, and remissions. If left untreated, SLE can rapidly cause irreversible damage to the organs involved and even death.1,2 SLE treatment should follow the principles of early-stage and individualized treatment, the postponement of disease progression to the greatest extent, the mitigation of organ damage, and the improvement in the patient’s prognosis. In the short term, SLE treatment aims to control disease activity, improve clinical symptoms, and reduce disease activity as much as possible. In the long term, treatment goals are to prevent and reduce relapses, decrease adverse drug reactions, prevent and control organ damage triggered by the disease, achieve long-term persistent disease remission, lower the case fatality rate, and improve patients’ quality of life.3,4 Long-term medication use, disease control, and follow-up visits are ongoing processes; therefore, standardizing patients’ health management behaviors is especially important.5–7
Pharmaceutical care intervention involves providing patient education and counseling services and identifying and solving drug-related problems. Previous reports have confirmed the effectiveness of pharmaceutical care in driving adherence to drug therapy among patients with SLE, controlling the disease, and improving patients’ quality of life.8,9 However, data on the effectiveness of pharmaceutical care intervention in patients with SLE and the specific pattern of patient management of SLE remain limited. In addition, a systematic review of the impact of pharmaceutical interventions on the clinical and economic outcomes of patients with various diseases suggested that rheumatic diseases including SLE have not been the focus of pharmaceutical interventions.10
Knowledge–attitude–practice (KAP) intervention theory, a pattern that changes human health-related behaviors, highlights that individual behavior change can be divided into three processes: knowledge acquisition, belief generation, and behavior formation. The intervention has achieved significant effects in the prevention and management of various chronic diseases.11–14 The implementation of hierarchical pharmaceutical services can help pharmacists rapidly identify key interventions, and the service pattern has played a significant role in standardizing the content of and criteria for pharmaceutical services for chronic diseases.15,16 Therefore, providing patients with SLE with hierarchical pharmaceutical services based on KAP theory under the guidance of the SLE hierarchical therapeutic schedule is feasible and is predicted to be beneficial.
Methods
Case collection
Patients receiving treatment for SLE at the Rheumatology and Immunology Department from March 2019 to December 2019 who fulfilled the eligibility criteria were identified and recruited into the retrospective study. The inclusion criteria were patients 20 to 70 years of age who were diagnosed with SLE in accordance with the 1997 revised American College of Rheumatology criteria17 and who had been receiving medication for SLE for at least 1 month. The exclusion criteria were an inability to understand and express, cognitive impairment, or a significant psychiatric disorder. We de-identified all patient details. The reporting of this study conforms to STROBE guidelines.18
General patient information was collected and patients’ medical files were accessed. KAP scores were obtained from the KAP Questionnaire on Drug Use Behavior Risk of Chinese Residents designed by the Science and Technology Development Center of the Chinese Pharmaceutical Society (shown in the Supplement). The questionnaire included three dimensions: knowledge, attitude, and practice. The 5-point Likert scoring method was used for each item. The total score of the 28 items in the knowledge dimension ranged from 28 to 140 points, the score of the 11 items in the attitude dimension ranged from 11 to 55 points, and the score of the 24 items in the practice dimension ranged from 24 to 120 points. The SLE Disease Activity Index-2000 (SLEDAI-2K) was used as the measurement criterion for SLE activity;19 a high score on this scale represents poor disease control. Scores were accumulated according to recent 10-day conditions: a score ≥15 meant severe activity; a score of 10 to 14 represented moderate activity; a score of 5 to 9 denoted mild activity; and a score of 0 to 4 signified no activity.
This study was approved by the Committee on Medical Ethics of the First Affiliated Hospital of Soochow University (2019-090). Written informed consent was obtained from all participants before enrollment. Compensation claims or serious or lasting side effects from this type of pharmaceutical service have not been reported in China.
Randomized grouping
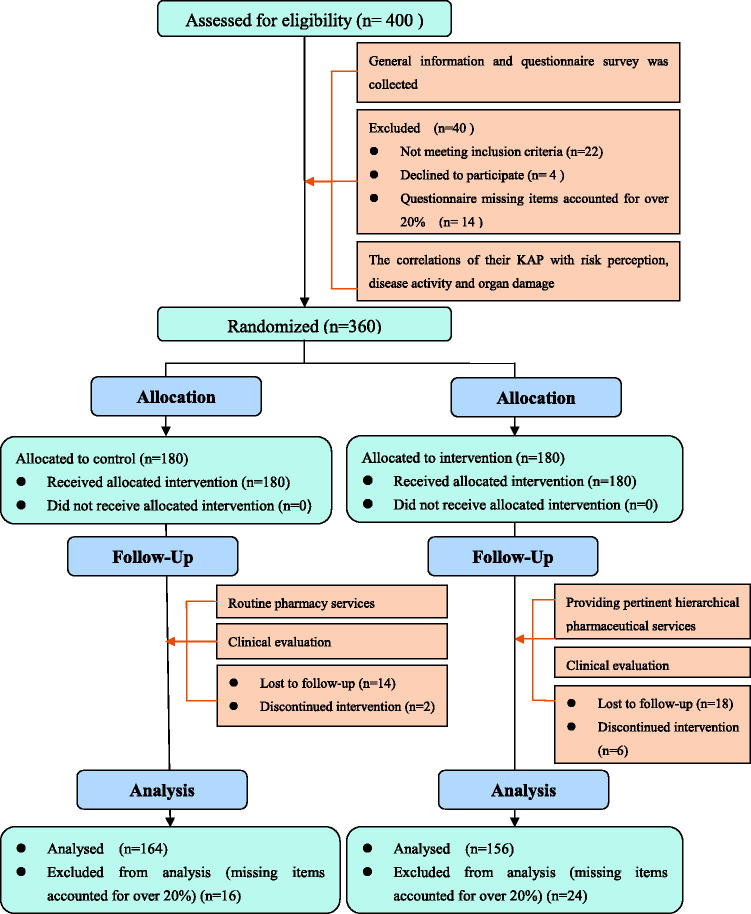
An investigator not involved in the clinical follow-up generated a random number table (1:1 ratio) using STATA 12.0 software (StataCorp LLC, College Station, Texas, USA). Eligible patients were then randomized to the intervention or control groups (Figure 1). Each patient was then followed up individually for a period of 9 months post-recruitment before completing the study.
Figure 1.
Patient inclusion and attrition.
Classification criteria and content
After forming the groupings, the pharmacist delivered pharmaceutical services at differing levels according to the drugs taken by the patient and the KAP level, and made dynamic adjustments based on changes in patients’ treatment conditions. Specific classification criteria are shown in Table 1.
Table 1.
Hierarchical pharmaceutical service criteria and content for patients with SLE.
| Main item | Level I | Level II | Level III |
|---|---|---|---|
| Criteria | |||
| Drug combination | Over 7 drugs are taken simultaneously or over 2 high-risk drugs are used | 3–6 drugs are taken simultaneously or one high-risk drug is used | Number of drugs taken simultaneously does not exceed 2 |
| Knowledge score | 113–140 | 57–112 | 28–56 |
| Attitude score | 45–55 | 23–44 | 11–22 |
| Practice scores | 97–120 | 49–96 | 24–48 |
| Content | |||
| Patient file | |||
| Frequency | On the grouping day | On the grouping day | On the grouping day |
| Focal point | Evaluate the patient’s health status, determine classification criteria, and develop the pharmaceutical service plan | Evaluate the patient’s health status, determine classification criteria, and develop the pharmaceutical service plan | Evaluate the patient’s health status, determine classification criteria, and develop the pharmaceutical service plan |
| Knowledge publicity and education | |||
| Frequency | Once per week | Once per month | Once per quarter |
| Focal point | Suitability of drug combination, basic knowledge of glucocorticoids, possible adverse reactions and preventive measures, consequences of continuous progression of disease, and significance of taking ancillary drugs | Suitability of drug combination, basic knowledge of glucocorticoids and consequences of continuous progression of disease | Basic knowledge of glucocorticoids |
| Belief cultivation | |||
| Frequency | Once per week | Once per month | Once per quarter |
| Focal point | Establishment of pharmacist–patient trust relationship, medication education among patient’s family members (family support) and sharing of positive cases (psychological support) | Establishment of pharmacist–patient trust relationship and sharing of positive cases (psychological support) | Establishment of pharmacist–patient trust relationship |
| Behavior guidance | |||
| Frequency | Once per week | Once per month | Once per quarter |
| Focal point | Medication adherence and handling of adverse reactions, evaluation of healthy lifestyle, and everyday index self-monitoring | Medication adherence and handling of adverse reactions and evaluation of healthy lifestyle | Medication adherence and handling of adverse reactions |
| Effect evaluation | |||
| Frequency | After pharmaceutical services last 9 months | After pharmaceutical services last 9 months | After pharmaceutical services last 9 months |
| Focal point | KAP and classification score, safety, and efficacy | KAP and classification score, safety, and efficacy | KAP and classification score, safety, and efficacy |
KAP: knowledge–attitude–practice, SLE: systemic lupus erythematosus.
During the treatment process, the pharmacist provided pertinent hierarchical pharmaceutical services to patients based on the KAP theory. Services mainly consisted of establishing a complete patient file including general information and medications taken (e.g., drug name, dosage, delivery method), evaluating the patient’s current KAP status, educating the patient about the disease and drugs used to treat the disease, cultivating belief, and guiding behavior. After the level of pharmaceutical services required by patients was determined, patients were provided with relevant services for 9 months. The focal point and frequency of services varied by level, as shown in Table 1.
Clinical evaluation indices
Evaluation was conducted before the intervention and 3, 6, and 9 months after the intervention, and score changes across KAP dimensions were recorded. Patients’ classification scores were statistically analyzed and dynamically adjusted according to their current status.
Patients were followed up 3, 6, and 9 months after the intervention. The occurrences of acute exacerbations and drug-related adverse reactions during follow-up visits were recorded and rates were calculated.
Patients’ clinical test indices and SLEDAI-2K scale scores were collected before and 9 months after the intervention. Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), complement C3, and complement C4 served as observational indices for changes in illness state and the therapeutic effect of treatment. The SLEDAI-2K can comprehensively reflect patients’ state of illness; disease activity was evaluated by including the comprehensive judgment of clinicians.
Statistical methods
Patients who returned questionnaires in which 20% or more items were missing were excluded from the data entry phase. Statistical analysis was conducted using SPSS 30.0 (IBM Corp., Armonk, NY, USA) and P < 0.05 indicated that a difference was statistically significant. The normality of measurement data was evaluated with the Shapiro–Wilk test. Two independent sample t-tests were adopted and data that followed a normal distribution were expressed as the mean ± standard deviation. Enumeration data were described using frequency or percentage and chi-square tests were performed. Pearson analysis was applied for correlations. Analysis of variance for repeated measurements was used to investigate the variation of clinical evaluation indices in the various scales.
Results
Patient identification and attrition
The patient flow is illustrated in Figure 1. A total of 320 patients—156 and 164 in the intervention and control groups, respectively—were ultimately analyzed in this study, with a sample loss rate of 20%. Baseline data for the two groups are shown in Table 2. Patients in the two groups were not significantly different in age, sex, and disease duration.
Table 2.
Analysis of basic information.
| Characteristics | Intervention group(n = 156) | Control group(n = 164) | t/χ2 | P |
|---|---|---|---|---|
| Age (years) | 44.39 ± 13.95 | 42.24 ± 13.65 | 1.395 | 0.164 |
| Sex | 0.257 | 0.612 | ||
| Male | 21 | 19 | ||
| Female | 135 | 145 | ||
| Mode of payment | 5.667 | 0.059 | ||
| Medicare | 43 | 29 | ||
| Self-pay | 112 | 135 | ||
| Educational level | 1.328 | 0.515 | ||
| Elementary education and below | 41 | 51 | ||
| Secondary education | 68 | 62 | ||
| Tertiary education and above | 47 | 51 | ||
| Disease duration (months) | 3.507 | 0.173 | ||
| ≤6 | 39 | 51 | ||
| 6–36 | 42 | 51 | ||
| ≥36 | 75 | 62 | ||
| Drug combination | 4.20 ± 1.32 | 4.06 ± 1.28 | 0.992 | 0.322 |
t: statistic of the t-test; χ2: statistic of the chi-square test.
KAP score
As shown in Table 3, the patients in the two groups were not significantly different in any KAP dimension before the intervention. After 3, 6, and 9 months of the intervention, significant differences were observed between the groups in each KAP dimension score at each time point (P < 0.01). The time effect was statistically significant (P < 0.01). Specifically, when intervention factors were not considered, each KAP dimension score changed over time. The difference in grouping effect between the two groups was statistically significant. Specifically, when the time factor was not considered, patients in various groups obtained different scores in each KAP dimension (P < 0.01). The time effect of each KAP dimension and the grouping effect interacted with one another in both groups (P < 0.01).
Table 3.
Analysis of variance results by KAP Dimension of the two groups before and after intervention.
| Main item | 0 months | 3 months | 6 months | 9 months | timeF | groupF | Time × groupF |
|---|---|---|---|---|---|---|---|
| Knowledge dimension | |||||||
| Intervention group | 89.21 ± 19.68 | 66.53 ± 16.31 | 52.66 ± 11.14 | 49.06 ± 14.43 | 676.17** | 144.41** | 146.20** |
| Control group | 89.62 ± 16.51 | 86.76 ± 16.06 | 79.40 ± 10.96 | 72.30 ± 14.28 | |||
| t | 0.202 | 11.170 | 21.641 | 14.477 | |||
| P | 0.840 | <0.001 | <0.001 | <0.001 | |||
| Attitude dimension | |||||||
| Intervention group | 38.74 ± 5.26 | 33.90 ± 4.77 | 30.90 ± 5.28 | 29.36 ± 7.31 | 369.70** | 35.64** | 43.78** |
| Control group | 38.77 ± 5.11 | 37.54 ± 5.23 | 35.85 ± 4.98 | 33.49 ± 4.98 | |||
| t | 0.053 | 6.501 | 8.638 | 5.919 | |||
| P | 0.958 | <0.001 | <0.001 | <0.001 | |||
| Practice dimension | |||||||
| Intervention group | 79.86 ± 27.66 | 64.26 ± 20.52 | 58.26 ± 19.12 | 55.83 ± 19.30 | 116.60** | 28.43** | 77.48** |
| Control group | 78.24 ± 26.91 | 77.32 ± 22.86 | 76.41 ± 20.39 | 73.49 ± 20.08 | |||
| t | 0.533 | 5.371 | 8.201 | 8.923 | |||
| P | 0.594 | <0.001 | <0.001 | <0.001 |
**P < 0.01; t: statistic of the t-test.
Classification scores
Before the intervention, the proportions of patients at different intervention levels in the two groups were not significantly different. The proportions of level I services in the intervention group after 6 and 9 months of the intervention declined by 13.43% and 14.43%, respectively, and those of level II services declined by 3.30% and 3.94%, respectively. The differences between the groups were statistically significant (P < 0.01). Results are shown in Table 4.
Table 4.
Patients’ classification scores before and after intervention.
| Months after intervention | Intervention group (n = 156) |
Control group (n = 164) |
χ2 | P | ||||
|---|---|---|---|---|---|---|---|---|
| Level I | Level II | Level III | Level I | Level II | Level III | |||
| 0 | 53 | 91 | 12 | 55 | 92 | 17 | 0.705 | 0.703 |
| 3 | 32 | 103 | 21 | 53 | 94 | 17 | 5.824 | 0.054 |
| 6 | 19 | 85 | 52 | 42 | 89 | 33 | 12.819 | 0.002 |
| 9 | 18 | 83 | 55 | 40 | 88 | 36 | 12.266 | 0.002 |
χ2: statistic of the chi-square test.
Rates of acute attacks and adverse drug reactions
In the initial 3-month intervention period, the rates of acute attacks and adverse drug reactions in the two groups were not significantly different. The rates of acute attacks in the intervention group were reduced by 11.15% and 18.26% after 6 and 9 months of the intervention, respectively, and adverse drug reaction rates were reduced by 11.99% and 12.43%, respectively. Differences with the control group were statistically significant (P < 0.05). The results are listed in Table 5.
Table 5.
Evaluation of clinical therapeutic effect before and after intervention.
| Main item | Intervention group(n = 156) | Control group(n = 164) | t/χ2 | P |
|---|---|---|---|---|
| Acute attack (%) | ||||
| 0–3 months | 53.84 | 55.49 | 0.087 | 0.768 |
| 3–6 months | 33.97 | 45.12 | 4.151 | 0.042 |
| 6–9 months | 25.64 | 43.90 | 11.719 | 0.001 |
| Adverse drug reactions (%) | ||||
| 0–3 months | 64.74 | 64.02 | 0.018 | 0.893 |
| 3–6 months | 41.67 | 53.66 | 4.608 | 0.032 |
| 6–9 months | 32.69 | 45.12 | 5.189 | 0.023 |
| ESR (abnormality rate, %) | ||||
| 0 months | 63.46 | 66.46 | 0.317 | 0.574 |
| 9 months | 21.79 | 31.70 | 3.997 | 0.046 |
| CRP (abnormality rate, %) | ||||
| 0 months | 59.61 | 56.70 | 0.279 | 0.598 |
| 9 months | 17.31 | 26.83 | 3.958 | 0.047 |
| C3 (abnormality rate, %) | ||||
| 0 months | 53.85 | 50.61 | 0.336 | 0.562 |
| 9 months | 14.74 | 23.78 | 4.647 | 0.031 |
| C4 (abnormality rate, %) | ||||
| 0 months | 48.72 | 46.95 | 0.100 | 0.752 |
| 9 months | 18.59 | 28.66 | 4.476 | 0.034 |
| SLEDAI-2K | ||||
| 0 months | 10.51 ± 5.10 | 10.59 ± 5.08 | 0.138 | 0.890 |
| 9 months | 4.69 ± 3.24 | 7.00 ± 3.94 | 5.720 | <0.001 |
ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; SLEDAI-2K: SLE Disease Activity Index-2000; C3: complement C3: C4: complement C4; t: statistic of the t-test; χ2: statistic of the chi-square test.
Clinical therapeutic effect
The two groups were not significantly different in the rates of abnormal clinical test indices and SLEDAI-2K scores before the intervention. After 9 months of the intervention, the levels of ESR, CRP, complement C3, complement C4, and disease activity of both groups improved, and the differences were statistically significant (P < 0.05). The results are displayed in Table 5.
Discussion
The focus of pharmaceutical care is to solve potential or actual medication problems during the process of medication consultation or education. Inspired by the graded diagnosis and treatment scheme, pharmacists in our hospital formulated graded pharmaceutical care standards and content for patients with SLE based on daily pharmaceutical care work, KAP intervention theory, and clinical practice. This allowed the implementation of systematic, real-time, and continuous pharmaceutical care for patients. The service consisted of three processes including disease and drug education, belief cultivation, and behavior guidance; specific content and the frequency of implementation of each level and project were defined based on these processes. The adjustment of the pharmaceutical intervention plan was primarily based on the patient’s grading score. Pharmacists identified and focused on patients with SLE who were at risk for acute attacks, upgraded or downgraded the adjustment according to the step treatment plan, and implemented an intervention to enhance disease control and reduce the risk of acute attacks in these patients.
Before the intervention, approximately three-quarters of patients had limited information (e.g., medication purpose, necessary precautions, and possible adverse reactions) about the drugs they were taking. Moreover, most patients were receiving several types of medications and often needed dose adjustments that resulted in poor compliance behaviors such as drug omission or self-withdrawal. After the intervention, patients had a clear understanding of their medication situation and also had confidence that they could overcome the disease; consequently, medication compliance was significantly improved among patients. In addition, the pharmacists participating in the study mastered the standards and content of graded pharmaceutical care and improved the quality and efficiency of the pharmaceutical care that they provided.
The standards and content in this study allowed a preliminary exploration of the graded pharmaceutical care model. This standardization facilitated implementation in the field of pharmaceutical care for patients with SLE, promoted the improvement and homogenization of pharmacists' professional technical competence and pharmaceutical care ability, and provided new ideas for the management of other chronic diseases.
Our study had some shortcomings. The study was single-blind because blinding pharmacists who performed the pharmaceutical intervention was impossible. However, we stipulated that pharmacists who participated in the intervention not contribute to data collection or analysis. Pharmacists who participated in data collection and analysis were unaware of the grouping of patients. In addition, the intervention time in this study was relatively short; follow-up time should be extended to further investigate the long-term impact of this pharmacologic intervention mode on disease control, prognosis, and the self-management ability of patients with SLE.
Conclusion
The correlation analysis of KAP level and risk perception, disease activity, and organ damage may provide a reference for formulating the content and standards of hierarchical pharmaceutical services and can deliver a convenient, effective, and specific tool for patient management of SLE. Investigating the effect of implementing hierarchical pharmaceutical services for patients with SLE may contribute new ideas to the implementation of SLE patient management patterns.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605231154749 for Implementation effect of a hierarchical pharmaceutical service pattern in patients with systemic lupus erythematosus by Yinhua Gong, Wei Wei, Wei Zhang, Qiang Han and Chunge Zhang in Journal of International Medical Research
Footnotes
Author contributions: Wei Wei, Wei Zhang, and Qiang Han collected the patient data on systemic lupus erythematosus. Yinhua Gong contributed to the analysis and manuscript writing. Chunge Zhang performed data analyses and was a major contributor to preparing and writing the manuscript. Chunge Zhang helped perform the analysis aided by constructive discussions. All authors read and approved the final manuscript.
The authors declare that they have no competing interests.
Funding: The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Scientific Research Project of Suzhou Science and Technology Bureau (No. SYSD2019177 & No. SYSD2018232) and the Jiangsu Pharmaceutical Association-Hospital Pharmacy Research Project (grant no. Q202201).
ORCID iD: Yinhua Gong https://orcid.org/0000-0002-5642-8714
References
- 1.Chinese Rheumatology Association; National Clinical Research Center for Dermatologic and Immunologic Diseases; Chinese Systemic Lupus Erythematosus Treatment and Research Group. [2020 Chinese guidelines for the diagnosis and treatment of systemic lupus erythematosus]. Zhonghua Nei Ke Za Zhi 2020; 59: 172–185. [DOI] [PubMed] [Google Scholar]
- 2.Keeling SO, Vandermeer B, Medina J, et al. Measuring disease activity and damage with validated metrics: a systematic review on mortality and damage in systemic lupus erythematosus. J Rheumatol 2018; 45: 1448–1461. [DOI] [PubMed] [Google Scholar]
- 3.Dörner T, Furie R. Novel paradigms in systemic lupus erythematosus. Lancet 2019; 393: 2344–2358. [DOI] [PubMed] [Google Scholar]
- 4.Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 Update of the EULAR Recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019; 78: 736–745. [DOI] [PubMed] [Google Scholar]
- 5.Harry O, Crosby LE, Smith AW, et al. Self-management and adherence in childhood-onset systemic lupus erythematosus: what are we missing? Lupus 2019; 28: 642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams EM, Egede L, Faith T, et al. Effective Self-Management Interventions for Patients With Lupus: Potential Impact of Peer Mentoring. Am J Med Sci 2017; 353: 580–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Twumasi AA, Shao A, Dunlop-Thomas C, et al. Exploring the Perceived Impact of the Chronic Disease Self-Management Program on Self-Management Behaviors among African American Women with Lupus: A Qualitative Study. ACR Open Rheumatol 2020; 2: 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliveira-Santos M, Verani JF, Camacho LA, et al. Effectiveness of pharmaceutical care for drug treatment adherence in patients with systemic lupus erythematosus in Rio de Janeiro, Brazil: study protocol for a randomized controlled trial. Trials 2016; 17: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliveira-Santos M, Verani JFS, Camacho LAB, et al. Effectiveness of pharmaceutical care for drug treatment adherence in women with lupus nephritis in Rio de Janeiro, Brazil: a randomized controlled trial. Lupus 2019; 28: 1368–1377. [DOI] [PubMed] [Google Scholar]
- 10.Melchiors AC, Correr CJ, Venson R, et al. An analysis of quality of systematic reviews on pharmacist health interventions. Int J Clin Pharm 2012; 34: 32–42. [DOI] [PubMed] [Google Scholar]
- 11.Lin C, Zhang CG, Shu LY, et al. Current status of knowledge-attitude-practice of patients with long-term oral administration of glucocorticoids in the rheumatic immunology department and the pharmaceutical monitoring. Chin J Hosp Pharm 2020; 40: 1020–1026. [Google Scholar]
- 12.Niroomand M, Ghasemi SN, Karimi-Sari H, et al. Diabetes knowledge, attitude and practice (KAP) study among Iranian in-patients with type-2 diabetes: A cross-sectional study. Diabetes Metab Syndr 2016; 10: S114–S119. [DOI] [PubMed] [Google Scholar]
- 13.Karbalaeifar R, Kazempour-Ardebili S, Amiri P, et al. Evaluating the effect of knowledge, attitude and practice on self-management in patients with type 2 diabetes. Acta Diabetol 2016; 53: 1015–1023. [DOI] [PubMed] [Google Scholar]
- 14.Mustafa RE, Mushtaq S, Akhtar N, et al. Assessment of knowledge, attitude and practice towards hepatitis among patients visiting the hepatitis clinic in tertiary care hospital, Rawalpindi, Pakistan. J Pak Med Assoc 2019; 69: 1136–1141. [PubMed] [Google Scholar]
- 15.Qin Q, Chen R, Zhang Y, et al. Clinical Evaluation and Implement of Grading Pharmaceutical Care on Patients with Asthma and COPD. Chin Pharm J 2017; 52: 1460–1464. [Google Scholar]
- 16.Li YY, Liu R, Xu Y. Establishment of a hierarchical pharmaceutical care system for perioperative blood glucose management in diabetic patients based on retrospective study. Pharm Care Res 2020; 20: 28–31. [Google Scholar]
- 17.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40: 1725. [DOI] [PubMed] [Google Scholar]
- 18.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 19.Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000[J]. J Rheumatol 2002; 29: 288–291. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605231154749 for Implementation effect of a hierarchical pharmaceutical service pattern in patients with systemic lupus erythematosus by Yinhua Gong, Wei Wei, Wei Zhang, Qiang Han and Chunge Zhang in Journal of International Medical Research