Abstract
Background: Remifentanil-induced postoperative hyperalgesia (RIH) refers to a state of hyperalgesia or aggravated pre-existing pain after remifentanil exposure. There has been considerable interest in understanding and preventing RIH. However, the mechanisms responsible for RIH are still not completely understood. Toll-like receptor 4 (TLR4), a classic innate immune receptor, has been detected in sensory neurons and participates in various nociceptive conditions, whereas its role in RIH remains unclear. Transient receptor potential ankyrin 1 (TRPA1) always serves as a nociceptive channel, whereas its role in RIH has not yet been investigated. This study aimed to determine whether the TLR4 signaling pathway in sensory neurons engaged in the development of RIH and the possible involvement of TRPA1 during this process. Methods: A rat model of remifentanil-induced postoperative hyperalgesia (RIH) was established, which presented decreased paw withdrawal mechanical threshold (PWMT) and paw withdrawal thermal latency (PWTL). The mRNA and protein expression levels of TLR4, phosphorylated NF-κB, and TRPA1 in the dorsal root ganglion (DRG) from RIH model were analyzed by real-time PCR, western blot, and immunofluorescence. The TLR4 antagonist TAK-242 and the TRPA1 antagonist HC-030031 were applied to determine the role of sensory neuron TLR4 signaling and TRPA1 in RIH. Results: Compared with control, PWMT and PWTL were significantly decreased in RIH model. Moreover, the mRNA and protein expression of TLR4 and TRPA1 in DRG were upregulated after remifentanil exposure together with increased NF-κB phosphorylation. TLR4 antagonist TAK-242 mitigated mechanical pain in RIH together with downregulated expression of TLR4, phosphorylated NF-κB, and TRPA1 in DRG neurons. In addition, TRPA1 antagonist HC-030031 also alleviated mechanical pain and decreased TRPA1 expression in RIH without affecting TLR4 signaling in DRG. Conclusions: Taken together, these results suggested that activation of TLR4 signaling pathway engaged in the development of RIH by regulating TRPA1 in DRG neurons. Blocking TLR4 and TRPA1 might serve as a promising therapeutic strategy for RIH.
Keywords: Remifentanil-induced postoperative hyperalgesia, opioids, toll-like receptor 4 signaling, transient receptor potential ankyrin 1, dorsal root ganglion
Introduction
Opioid-induced hyperalgesia (OIH) is defined as a paradoxical condition of nociceptive sensitization in patients who use opioids to treat acute and chronic pain.1,2 Clinical studies have confirmed that intraoperative use of high-dose opioid agonists, such as morphine and remifentanil, was associated with increased acute pain incidence after surgery, inducing perplexities for the clinical application of opioids.3–5 The mechanisms of OIH are complex, involving both central mechanisms and peripheral mechanisms.6–8 The latest research indicated that peripheral sensitization mainly leads to OIH during the early opioid post-infusion period, while central mechanisms might account for hyperalgesia during the late opioid post-infusion period, indicating that remifentanil might sensitize peripheral and central nociceptive signal to promote chronologically distinctive hyperalgesia.9 Among these clinical opioids, remifentanil was able to elevate postoperative pain scores featured by remifentanil-induced postoperative hyperalgesia (RIH) and increase opioid consumption. Although numerous contributory molecular and cellular mechanisms had been described in previous studies, the underlying molecular mechanism of OIH/RIH still needs further investigation.1,6
Recent studies have indicated that Toll-like receptor 4 (TLR4) might participate in the development of OIH.10,11 TLR4 is a pattern recognition receptor that initiates the innate immune response to protect the body from invasive pathogens.12 In addition, the process of TLR4 challenged by various endogenous or exogenous ligands, both in the sensory neuron and dorsal horn glias, would induce pain.13 Studies have reported that TLR4 and its downstream signals, such as NF-κB, play an important role in acute and chronic pain.14–16 Moreover, opioids could directly activate spinal microglial TLR4, promoting the release of neuroexcitatory immune mediators and following glial “priming”, exacerbating neuropathic pain after opioid treatment.17 Increasing researches revealed that initiating central sensitization in OIH depended on TLR4 signaling while inhibiting TLR4 or Tlr4 knockout prevented mechanical allodynia in OIH.10,11,17 However, the molecular underlying TLR4-mediated OIH/RIH remains unclear, especially focusing on peripheral sensitization mediated by sensory neurons.
The importance of transient receptor potential (TRP) ion channels in pain sensation has been emphasized by many studies.18,19 The transient receptor potential vanilloid 1 (TRPV1) channel and transient receptor potential ankyrin 1 (TRPA1) channel, which are mainly present in peripheral nociceptors, are well-known nociceptive TRP family members. TRPV1 and TRPA1 served as transducers and amplifiers for pain signals, mediating mechanical allodynia and thermal hyperpathia in neuropathic and inflammatory pain.20–22 Detailedly, TLR4 signaling might cause neuropathic and inflammatory pain by upregulating and sensitizing TRPV1 and TRPA1 in sensory neurons.22,23 The role of sensory neuron TRPV1 in RIH has been well established.24,25 However, whether TRPA1 participated in RIH remains unclear.
In this study, we proposed that TLR4 signaling pathway in sensory neurons modulated the development of RIH via upregulating the TRPA1 channel. To address this hypothesis, we first established a rat RIH model and assessed the expression changes of TLR4 signaling and TRPA1 in DRG neurons. Furtherly, pharmacological inhibition of TLR4 and TRPA1 was applied to test the role of sensory neuron TLR4-TRPA1 axis in RIH. This study might supply potential therapeutic strategies for the pain symptom in RIH and promote appropriate clinical applications of associated opioids.
Materials and methods
Animals
Male Sprague-Dawley (SD) rats aged 5–6 weeks, weighing 240–250 g, were purchased from Beijing Huafukang Bioscience Co. Ltd (Beijing, China). The rats were given water and food ad libitum and were housed in conditions of constant temperature (22 ± 2°C) and humidity (50 ± 15%) with a 12-h light/dark cycle. All animals were adapted to the laboratory environment for at least 3 days before the experiment. This study was approved by the Animal Welfare and Ethics Committee of China-Japan Friendship Hospital (No. zyrhyy21-20-01-4). All experimental procedures were performed in accordance with the guidelines of the Animal Welfare and Ethics Committee of China-Japan Friendship Hospital and the International Association for the Study of Pain.
Experiment protocol
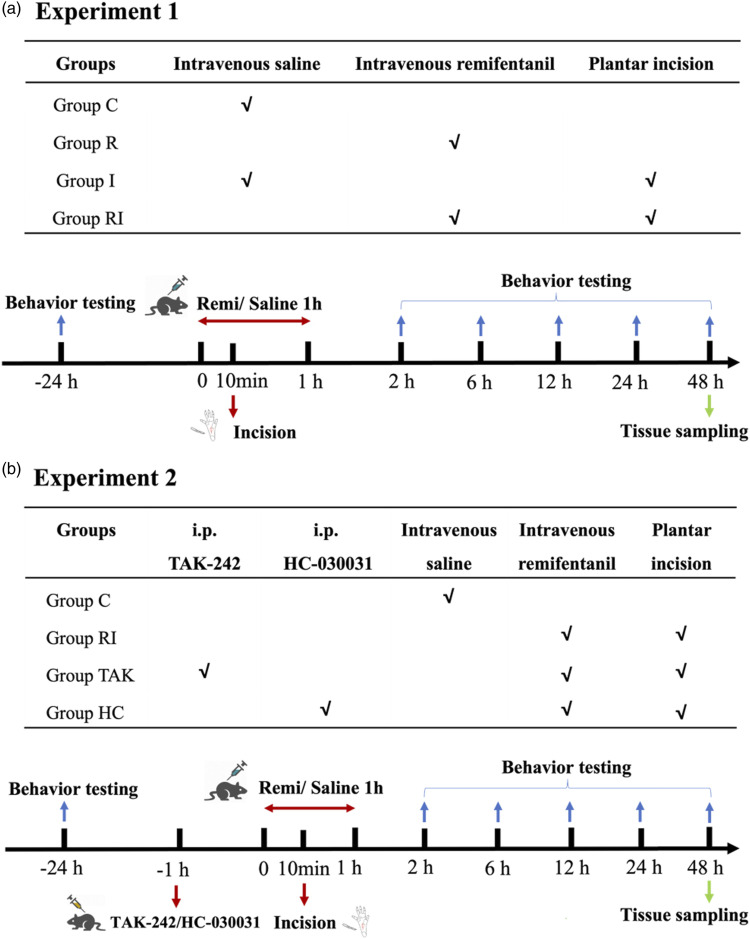
This study consisted of two experiments. A schematic diagram of the protocol is shown in Figure 1. In experiment 1 (Figure 1(a)), 24 rats were randomly divided into four groups (n = 6 for each group): group C (control), group R (rats with remifentanil infusion), group I (rats with plantar incision), and group RI (rats with remifentanil infusion and incision). According to previously reported procedures,26 rats received remifentanil infusion and a plantar incision to induce a model of RIH. Plantar incision was performed 10 min after remifentanil infusion. After the behavioral tests, the expressions of TLR4, NF-κB (phosphorylated p65 subunit), and TRPA1 were measured by using western blot analysis (n = 4 for each group) or immunofluorescence staining (n = 4 for each group). TRPA1 and TLR4 mRNA levels were detected by RT-PCR (n = 4 for each group).
Figure 1.
Schematic diagram of the experiment protocol. a. Experiment 1: To confirm the changes of pain behavior and protein expression after remifentanil exposure in RIH rats. b. Experiment 2: To explore the role of TLR4 signaling and TRPA1 after remifentanil exposure in RIH rats.
In experiment 2 (Figure 1(b)), 24 rats were randomly divided into four groups (n = 6 for each group): group C (control group), group RI (rats with RIH model), group TAK (i.p. 3 mg/kg TAK-242 in rats with RIH model), and group HC (i.p. 300 mg/kg HC-030031 in rats with RIH model). After the behavioral tests, the expressions of TLR4, NF-κB (phosphorylated p65 subunit), and TRPA1 were measured by western blot analysis (n = 4 for each group) or immunofluorescence staining (n = 4 for each group). Trpa1 and Tlr4 mRNA levels were detected by RT-PCR (n = 4 for each group).
Drugs preparation
TAK-242 (Selleck Chemicals, Shanghai, China) and HC-030031 (Selleck Chemicals, Shanghai, China) were dissolved in dimethyl sulfoxide (DMSO; Sigma Aldrich, USA) and diluted with saline. Rats in group TAK and group H were intraperitoneally (i.p.) injected with TAK-242 and HC-030031 1 h before remifentanil infusion, respectively.
Remifentanil (Yichang Renfu Pharmaceutical Co., Hubei, China) at a dose of 60 μg/kg was dissolved in 0.9% normal saline (NS) to a volume of 0.6 mL. After being anesthetized with 2% sevoflurane (Hengrui Pharmaceutical CO., Shanghai, China) using a nose mask, a 24-gauge cannula was inserted into the caudal vein and flushed with heparinized saline. The rats in groups R, RI, TAK, and HC were infused intravenously with remifentanil at a rate of 1.0 μg·kg−1·min−1 for 60 min26 using an infusion pump (Harvard Pump 11, Harvard Apparatus, USA). Intravenous saline was infused at a rate of 0.01 mL·min−1 for 60 min as control exposure.
Plantar incision
After being anesthetized with sevoflurane, rats in groups I, RI, TAK, and HC received plantar incisions. According to a previously described procedure,26 a 1-cm longitudinal incision through the skin and fascia of the right hind paw plantar, starting 0.5 cm from the edge of the heel and extending to the toes, was performed with a number 11 blade after disinfection with 75% alcohol. Then the plantar muscles and tendons were exposed and incised longitudinally. After hemostasis, the skin was sutured with 4–0 silk and covered with erythromycin ointment to prevent infection. Rats in groups C and R had a sham procedure without incision of the right paw.
Pain behavior tests
All measurements were performed in a quiet, temperature-controlled room at 24 h before plantar incision/remifentanil infusion (baseline) and at 2, 6, 12, 24, and 48 h. To evaluate mechanical allodynia, paw withdrawal mechanical threshold (PWMT) was measured using a digital electronic Von Frey (IITC-Life Science Instruments, CA, USA). Rats were placed individually in elevated, inverted transparent boxes with a steel mesh floor (1-cm grid) and allowed to habituate for approximately 15 min or until exploratory behavior ceased. The electronic Von Frey filament, consisting of a plastic tip fitted in a hand-held force transducer, was applied perpendicularly to the mid-plantar surface of the right hind paw with gradually increased force. A positive response was defined as lifting, shaking, or licking the paw following stimulation. The tests were performed 3 times with intervals of approximately 5 min, and the mean thresholds were calculated.
To evaluate thermal hyperalgesia, radiant heat was applied to the plantar surface of the right hind paw to measure paw withdrawal thermal latency (PWTL). Rats were placed into a moveable transparent plastic cage on an elevated clear glass plate and allowed to habituate for approximately 15 min. A radiant heat source (50-W projector lamp) mounted on a movable holder below the clear, smooth glass floor was placed directly under the plantar surface of the right hind paw. PWTL was defined as the time from the onset of the infrared heat stimulus to the paw withdrawal from the heat source. Before starting the experiment, the infrared heat intensity of the apparatus was adjusted to give an average PWTL of approximately 15 s, and the cutoff latency was set at 20 s to avoid tissue damage. The tests were performed 3 times with intervals of approximately 5 min, and the mean latencies were calculated.
Western blot analysis
After completing the last behavioral tests at 48 h, all rats were sacrificed under deep anesthesia (5% sevoflurane). According to the literature,27 the L3-L5 right DRGs were rapidly removed and stored in liquid nitrogen. Tissue samples were homogenized in lysis buffer, which was centrifuged at 12,000 r/min for 10 min at 4°C to collect the supernatant. The protein concentration was measured by BCA Protein Assay Kit (Solarbio, Beijing, China). Protein samples were separated on SDS-polyacrylamide gel electrophoresis, and the fractionated proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (Thermo Fisher Scientific, USA). After being blocked with 5% (w/v) skimmed milk for 1 h at room temperature, the membranes were incubated overnight at 4°C with the diluted primary antibodies. The primary antibodies used were: anti-TRPA1 (GTX54765, GeneTex, USA), anti-TLR4 (GTX57153, GeneTex, USA), anti- NF-κB (ab194726, Abcam, USA), and anti-GAPDH (ab8245, Abcam, USA). The membrane was washed with Tris-buffered saline with Tween buffer and incubated with the goat anti-mouse secondary antibody (ab150113, Abcam, USA) or goat anti-rabbit secondary antibody (ab150080 Abcam, USA) for 1 h at room temperature. The bands were scanned by ECL Western Blotting Substrate (Tanon, China) and analyzed by Image J software.
Immunofluorescence staining
Rats were terminally anesthetized with sevoflurane and perfused transcardially with PBS followed by 4% paraformaldehyde. After perfusion, the L3-5 DRGs were collected, post-fixed in 4% paraformaldehyde overnight at 4°C, and then dehydrated in sucrose. Later, tissues were embedded in OCT (Tissue-Tek, Japan) and serially sectioned in a cryostat into 15-μm-thick slices. The tissue sections were blocked with 10% goat serum in 0.3% Triton for 1 h at room temperature and incubated primary antibodies (anti-TRPA1, GTX54765, GeneTex, USA; anti-TLR4, GTX57153, GeneTex, USA) overnight in a wet box at 4°C. Subsequently, the sections were incubated with corresponding secondary antibodies for 1 h at room temperature. The stained sections were visualized under a fluorescence microscope (Leica Microsystems, Wetzlar, Germany). Three DRG tissues (L3-L5) in each tested rat were taken for IF staining and three sections were randomly selected in each tested DRG. Moreover, three rats were randomly selected in each group for IF evaluation. The images for each target were captured under same exposure time and fluorescence intensity in each image was quantized by Image J software for data analysis.
Real-time polymerase chain reaction (RT-PCR)
The expression of Trpa1 and Tlr4 mRNAs, with Gapdh mRNA as an internal control, were detected by RT-PCR. Briefly, total RNA was isolated with TRIzol (TakaRa Bio-technology, Dalian, China). Next, RNA was reverse-transcribed into cDNA with a cDNA Reverse Transcription kit. RT-PCR was performed using SYBR Green dye (Thermo Scientific Molecular Biology) according to the manufacturer’s instructions. The PCR thermocycling parameters were as follows: 50°C for 2 min, 94°C for 15 min, followed by 40 cycles at 94°C for 15 s, 58°C for 30 s, and 72°C for 30 s. The sequences of the oligonucleotide primers were listed as follows: Gapdh (forward: 5′-CAA GGC TGA GAA TGG GAA GC-3′; reverse: 5′-GAA GAC GCC AGT AGA CTC CA-3′), Tlr4 (forward: 5′-TAG CCA TTG CTG CCA ACA TC-3′; reverse: 5′-ACA CCA ACG GCT CTG GAT AA-3′), and Trpa1(forward: 5′-CCA CCC TGT GTG TAG GGA AT-3′; reverse: 5′-AAG GCC ATT CCA GGC TGT AT-3′). Relative quantification of the target gene was calculated with the 2–ΔΔCt method.
Statistical analysis
All data are presented as the mean ± standard deviation (SD). Data were analyzed for normal distribution using the D'Agostino and Pearson omnibus normality test and for homogeneity of variances using Bartlett’s test of sphericity. If data fulfilled the normal distribution and homogeneity of variance test, one-way or two-way analysis of variance (ANOVA) followed by the Tukey’s post hoc test was used to evaluate the presence of significant differences between groups, and Bonferroni-corrected repeated measures analysis of variance was used for intra-group comparison. If data did not follow a normal distribution and homogeneity of variance test, rank-sum test would be performed to measure data from multiple groups. GraphPad Prism 9.0 software (GraphPad Software, CA, USA) was used for all statistical analyses and graphing. p < .05 was considered to be statistically significant.
Results
Remifentanil facilitated pain behaviors and upregulated TLR4 signaling in DRGs
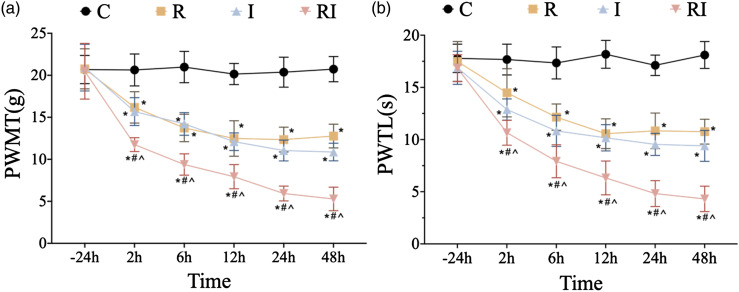
First, in experiment 1, pain behaviors were recorded to verify whether remifentanil exposure would aggravate mechanical allodynia and thermal hyperalgesia. There was no significant difference in the baselines of PWMT and PWTL among all groups before surgery (p > .05). Compared with the baseline, PWMT and PWTL did not present significant changes in the normal control group at the observed time points. Compared with the baseline and normal control group, both PWMT and PWTL were reduced from 2 h to 48 h in rats receiving either incision or remifentanil treatment (p < .05, n = 6; Figures 2(a)–(b)). Moreover, rats in group RIH displayed a significant decrease in PWMT and PWTL compared with the incision group, which started from 2 h and peaked at 48 h. These above results indicated that intraoperative remifentanil exposure facilitated hyperalgesia induced by plantar incision and therefore the rat model of remifentanil-induced postoperative hyperalgesia was successfully established.
Figure 2.
Time course of PWMT and PWTL after remifentanil and incision. PWMT, paw withdrawal mechanical threshold; PWTL, paw withdrawal thermal latency. a-b. Compared with control group, mechanical allodynia (a) and thermal hyperalgesia (b) were induced at 2 h, 6 h, 12 h, 24 h, and 48 h time points in group R (rats with remifentanil infusion), group I (rats with plantar incision), and group RI (rats with remifentanil infusion and incision). There was no statistically significant difference in PWMT and PWTL between group R and group I. Compared with group R and group I, mechanical allodynia (a) and thermal hyperalgesia (b) were aggravated in group RI, indicating a rat model of remifentanil-induced postoperative hyperalgesia was successfully established. All the data are means ± SD (n = 6) and analyzed by two-way ANOVA with Tukey’s post hoc comparisons. *p < .05 versus group C at the same time point; #p < .05 versus group R at the same time point; ^p < .05 versus group I at the same time point.
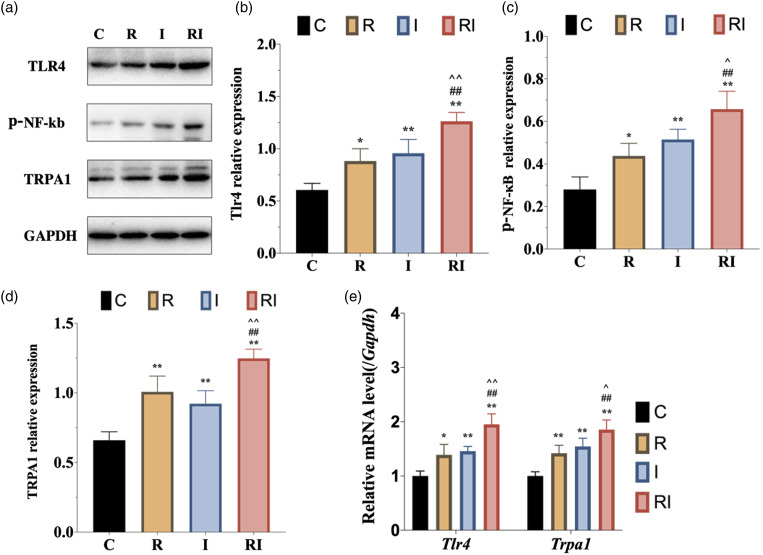
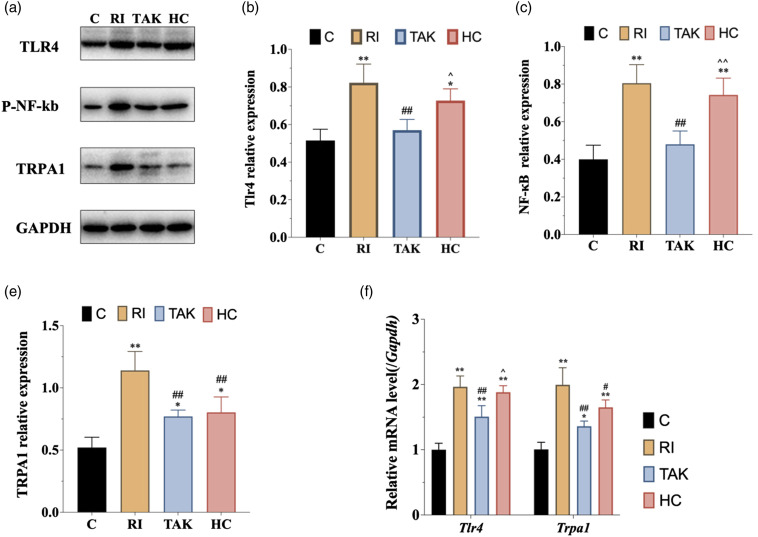
To determine the expression of TLR4 signaling in DRG neurons, we performed western blot to evaluate the protein levels of TLR4 and phosphorylated NF-κB, the activated form of NF-κB, in DRG. Western blot analysis exhibited a significant increase of TLR4 and phosphorylated NF-κB expression in DRG as remifentanil infusion and plantar incision compared with saline infusion. There was no significant difference in TLR4 or phosphorylated NF-κB expression between groups R and I (p > .05). Furthermore, TLR4 and phosphorylated NF-κB were upregulated in group RIH compared to group R or I (Figure 3(a)–(c)). Consistent with the results of Western blot analysis, the mRNA levels of Tlr4 exhibited a similar expression pattern (Figure 3(e)). We further performed immunofluorescence analysis to confirm the TLR4 signaling alteration in DRG neurons. As shown in Figure 4, TLR4 was mainly expressed in DRG neurons, and fluorescence intensity quantification further confirmed that RIH upregulated TLR4 in DRG (p < .01). Together, these results indicated that TLR4 signaling was activated in DRG neurons in the setting of remifentanil-induced hyperalgesia.
Figure 3.
Expression of TLR4 signaling and TRPA1 in DRG neurons in rats with RIH. a. Representative bands of Western blot for the expression of TLR4, p-NF-κB, and TPRA1 in DRG at 48 h after remifentanil exposure. b–d. Compared with the control group, the expression of TLR4, p-NF-κB, and TPRA1 were significantly upregulated in group R and group I. Moreover, the expression of TLR4, p-NF-κB, and TPRA1 were further increased in group RI. e. The mRNA expression of Tlr4 and Trpa1 were elevated significantly in rats with RIH model, when compared to the other three groups. All the data are expressed as means ± SD (n = 4) and analyzed by one-way ANOVA with Tukey’s post hoc comparisons. *p < .05, **p < .01 versus group C; #p < .05, ##p < .01 versus group R; ^p < .05, ^^p < .01 versus group I.
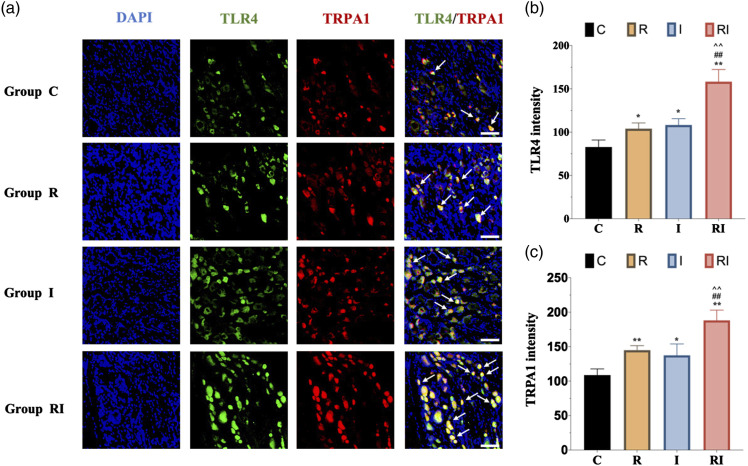
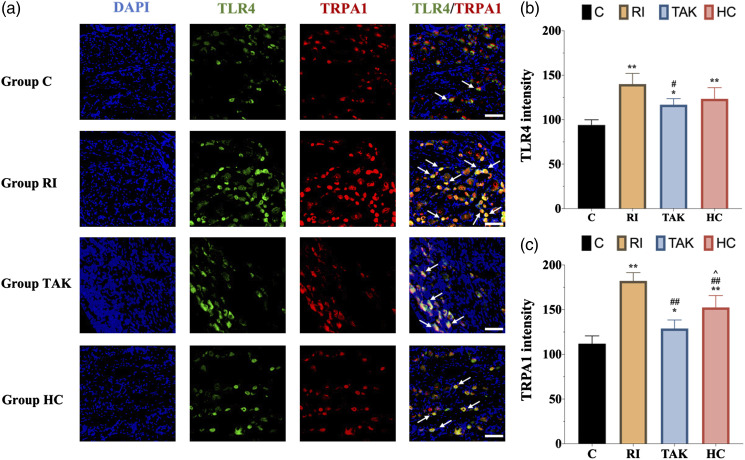
Figure 4.
Immunofluorescence of TLR4 and TRPA1 expression in DRG neurons. a. Representative immunofluorescence micrographs, with the scale bar equaling to 50 μm. b. The statistical results of TLR4 expression in DRG neurons. The expression of TLR4 in group RI was upregulated compared with any other groups. c. The expression of TRPA1 in group RI was upregulated compared with any other groups. All the data are expressed as means ± SD (n = 4) and analyzed by one-way ANOVA with Tukey’s post hoc comparisons. *p < 0.05, **p < 0.01 versus group C; ##p < 0.01 versus group R; ^^p < 0.01 versus group I.
The expression of TRPA1 was increased in remifentanil-induced hyperalgesia rats
To determine the changes in TRPA1 expression following remifentanil exposure, we performed western blot, RT-PCR, and immunofluorescence labeling in the DRG (Figure 3(a)) shows that the expression of TRPA1 was increased in both group R and group I as compared with the normal control group. However, there was no significant difference between group R and group I (Figure 3(d)). Furthermore, group RI showed a further upregulation of TRPA1 as compared with both group R (p < .01) and group I (p < .01). In addition, the mRNA level of Trpa1 in DRG was also detected. RT-PCR analysis revealed that compared with the normal control group, both group R and group I showed a dramatically increased mRNA level of Trpa1. However, no significant difference was detected between group R and group I. Furthermore, the mRNA level of Trpa1 was enhanced in the RIH group as compared with both I group and R group (Figure 3(e)). Furthermore, immunofluorescence labeling revealed that TRPA1 was abundantly and selectively expressed in DRG neurons and TRPA1-positive cells were almost co-stained with TLR4. Immunofluorescence intensity quantification further confirmed the upregulation of TRPA1 in group R and group I as compared with group C (p < .05), and in RIH group as compared with group R and group I (all p < .05). Together, the above results revealed significant upregulation of TRPA1 in DRG sensory neurons of RIH model.
Inhibition of TRPA1 alleviated mechanical hyperalgesia in RIH rats
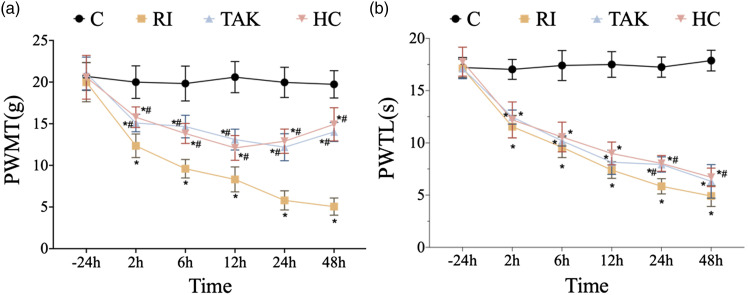
To clarify whether TRPA1 regulated RIH, TRPA1 antagonist HC-030031 was injected intraperitoneally, followed by pain behaviors recording. The results showed that compared with the rats in group RI, thresholds of PWMT in group HC were remarkably increased after TRPA1 antagonist injection. However, PWTL threshold elevation was only observed at 48 h between the group RIH and group HC (p < .05) (Figure 5).
Figure 5.
Effects of inhibiting TLR4 or TRPA1 on mechanical and thermal sensitivity. PWMT, paw withdrawal mechanical threshold; PWTL, paw withdrawal thermal latency. a-b. For rats in group TAK, TLR4 was inhibited by TAK-242. For rats in group HC, TRPA1 was inhibited by HC-030031. Compared with group RI, mechanical allodynia (a) was alleviated at 2 h, 6 h, 12 h, 24 h, and 48 h time points in group TAK and HC. However, thermal hyperalgesia (b) in group TAK and group HC showed no significant differences at most time points compared to that in group RI. All the data are means ± SD (n = 6) and analyzed by two-way ANOVA with Tukey’s post hoc comparisons. *p < .05 versus group C at the same time point; #p < .05 versus group RI at the same time point.
To further explore the expression of TLR4 and TRPA1 following HC-030031 administration in RIH model, western blot and immunofluorescence were implemented. The western blot analysis showed that the protein level of TRPA1 was significantly downregulated in group HC as compared with group RI (p < .01), while the expression levels of TLR4 and phosphorylated NF-κB in group HC were not altered by HC-030031 (p > .05) (Figure 6(a)–(d)). Similar to the results of the western blot, immunofluorescence and its scatter plot analysis also demonstrated a significant TRPA1 downregulation in DRG from group HC compared with group RI (p < 0.05) (Figure 7(a) and (c)). Meanwhile, RT-PCR analysis revealed that the mRNA level of Trpa1, but not Tlr4, was significantly decreased in DRG from group HC compared with group RI (Figure 6(e)).
Figure 6.
Expression of TLR4 signaling and TRPA1 in DRG neurons after inhibiting TLR4 or TRPA1 in RIH model. a. Representative bands of Western blot for the expression of TLR4, p-NF-κB, and TPRA1 in DRG at 48 h after remifentanil exposure. b-d. When compared with group RI, inhibition of TLR4 decreased the expression of TLR4 (b), p-NF-κB (c), and TRPA1 (d). However, inhibition of TRPA1 did not affect the expression of TLR4 (b) or p-NF-κB (c). e. Consistent with the protein expression trends of TLR4 and TPRA1 after inhibiting TLR4, the mRNA expression levels of Tlr4 and Trpa1 were significantly downregulated in group TAK compared with group RI. All the data are expressed as means ± SD (n = 4) and analyzed by one-way ANOVA with Tukey’s post hoc comparisons. *p < .05, **p < .01 versus group C; #p < .05, ##p < 0.01 versus group RI; ^p < .05, ^^p < .01 versus group TAK.
Figure 7.
Immunofluorescence of TLR4 and TRPA1 expression after inhibiting TLR4 or TRPA1 in DRG neurons. a. Representative immunofluorescence micrographs, with the scale bar equaling to 50 μm. b. The statistical results of TLR4 expression in DRG neurons. The upregulation of TLR4 in rats with RIH was inhibited after blocking TLR4. c. The statistical results of TRPA1 expression in DRG neurons. The expression of TRPA1 in group TAK and group HC were downregulated after blocking TLR4 or TRPA1 when compared with group RI. All the data are expressed as means ± SD (n = 4) and analyzed by one-way ANOVA with Tukey’s post hoc comparisons. *p < .05, **p < .01 versus group C; #p < .05, ##p < .01 versus group RI; ^p < 0.05 versus group TAK.
Inhibition of TLR4 alleviated mechanical hyperalgesia and downregulated the expression of TRPA1 in RIH rats
To further confirm the role of TLR4 signaling in RIH, we conducted intraperitoneal injection of TLR4 antagonist TAK-242 to block TLR4 signaling, then recorded pain-associated behavior. As shown in Figure 5, compared with the group RI, TAK-242 pretreatment presented significantly higher threshold of PWMT at different points (all p < .05) in group TAK. However, TAK-242 pretreatment in group TAK did not affect the threshold of PMWL when compared with group RI (p > .05). We further examined the protein expression of TLR4 and activated p-NF-κB. Both western blot and immunofluorescence showed that the protein expression of TLR4 was dramatically decreased in rats of group TAK at 48 h, together with decreased p-NF-κB content (Figure 6(a)–(c)). Similar results were obtained on the mRNA level of Tlr4 in group TAK compared with group RI (p < .05) (Figure 6(e)). These data indicated that TLR4 signaling mediated remifentanil-induced mechanical allodynia.
To figure out whether TRPA1 was the potential downstream regulatory target of TLR4 signaling, we further explored the expression of TRPA1 following TAK-242 administration. Both western blot (Figure 6(a) and (d)) and immunofluorescence (Figure 7) showed that the protein expression of TRPA1 was decreased in group TAK compared with group RI (p < .01). In addition, RT-PCR showed that the mRNA level of Trpa1 was downregulated after TAK-242 administration (p < 0.01) (Figure 6(e)).
Discussion
In the present study, we verified that sensory neurons’ TLR4 signaling pathway indeed participated in the progression of RIH via upregulating TRPA1 channel as follows: (1) Remifentanil-induced mechanical allodynia and thermal hyperalgesia, which were accompanied by upregulation of TLR4 signaling and TRPA1 in DRG neurons; (2) Inhibition of TLR4 attenuated mechanical allodynia in RIH and downregulated the expression of TLR4 signaling and TRPA1 in DRG neurons; (3) Inhibition of TRPA1 alleviated mechanical allodynia together with decreased TRPA1 expression in DRG,
Previous studies have proved that TLR4 signaling pathway in the spinal cord plays an important role in OIH.11,17 Moreover, Aguado D et al.10 found that the mechanical threshold after infusion of remifentanil was elevated in Tlr4-deficient mice as compared with wild type mice. Our study further identified that RIH upregulated the expression of TLR4 pathway in DRG neurons, and TLR4 antagonist mitigated mechanical allodynia in RIH model. All the above evidence suggested that opioids could induce the activation of TLR4 signaling in sensory neurons and subsequently lead to peripheral sensitization in RIH. Although opioids mainly exert analgesic effects by binding to μ-opioid receptors, multiple studies have verified that opioids can directly activate TLR4 signaling.28 It has been reported that opioids, including morphine and remifentanil, could non-stereo selectively bind to the TLR4/MD2 complex in glia and DRG neurons. In the current RIH model, opioids might activate the TLR4 downstream signals and finally phosphorylated and activated NF-κB in sensory neurons, which induced the release of proinflammatory mediators and regulated ion channels expression and/or functional status, then subsequently led to nociception and hyperalgesia.17,29 In addition, opioids could indirectly stimulate TLR4 by inducing endogenous TLR4 ligands, which could further challenge TLR4 in sensory neurons and glias.28 Moreover, previous studies confirmed that blocking TLR4 could significantly alleviate mechanical allodynia in OIH while the analgesic effects of opioids were still preserved.6,30 Given the above factors, the progression of OIH/RIH might be TLR4-dependent, and TLR4 could serve as a promising therapeutic target for OIH/RIH.
TRPA1, which is functionally expressed in peripheral pain-sensing neurons, has been recognized as a pivotal sensor of pain signals.19 It is known that the expression of TRPA1 in DRG was upregulated in various pain conditions, including inflammatory and neuropathic pain.31,32 In addition, either genetic deletion or pharmacological inhibition of TRPA1 mitigated pain responses.33,34 However, unlike that of TRPV1,24,25 the role of TRPA1 in RIH has not yet been thoroughly investigated. In Experiment 1, we confirmed that the TRPA1 expression in DRG neurons was upregulated in RIH model, revealing a link between increased sensory TRPA1 expression and RIH. In Experiment 2, we further discovered that the mechanical allodynia in RIH was significantly alleviated by TRPA1 antagonist HC-030031, indicating that TRPA1 underlies the ionic basis of mechanical allodynia in OIH. Collectively, our data suggested that TRPA1 upregulation in DRG neurons might mediate peripheral sensitization in RIH.
Interaction between TLR4 signals and TRPA1 has been investigated in prior studies, indicating that TLR4 could affect TRPA1 at both the gene expression and ion channel activity levels .35–37 Accumulating evidence suggested that activation of TLR4 signaling pathway upregulated TRPA1 expression and enhanced TRPA1 sensitivity.35,36 Further studies found that activation of TLR4 and its downstream signaling in sensory neurons triggered the production of neuroinflammatory mediators such as TNF-α and IL-6, which would further contribute to neuropathic and cancer pain via upregulating TRPA1.23,37 In this study, we observed that TLR4 co-expressed with TRPA1 in DRG sensory neurons. Moreover, we found that blocking TLR4 decreased TRPA1 expression in DRG neurons and mitigated mechanical sensitivity after remifentanil exposure. However, no significant changes in TLR4 signaling were observed as inhibiting TRPA1. All these data indicated that sensory neuron TLR4 signaling mediated RIH via upregulating the TRPA1 channel.
Notably, we found that both TLR4 and TRPA1 inhibition significantly alleviated mechanical allodynia rather than thermal hyperalgesia in RIH rats. Similar observations have been previously reported in inflammatory and neuropathic pain rodent models .38,39 Although TRPA1 serves as a key detector of mechanical and chemical stimuli, the thermal sensitivity of TRPA1 remains controversial.40 Recent evidence put forward the hypothesis that mammalian TRPA1 may be activated by noxious cold and noxious heat,41,42 which was not in accordance with this hypothesis. These data suggested that thermal hyperalgesia in RIH might result from other mechanisms independent of TLR4 and TRPA1, which should be further investigated in the following studies.
There are two limitations of our present study. First, TLR4-mediated upregulation of TRPA1 in the DRG neurons could not fully explain the thermal hyperalgesia in RIH, which could be further determined in the following studies. Second, TRPA1 activity and TRPA1 trafficking were not tested in the current study. Earlier studies have suggested that TRPA1 translocation to the plasma membrane might represent functional activation and sensitization.43,44 Therefore, further research could clarify the alteration of TRPA1 functional status in RIH and the role of TLR4 in this process.
Conclusions
The present study suggests that TLR4 signaling and TRPA1 in the DRG neurons probably play significant roles in developing mechanical allodynia in RIH. The results presented here also demonstrate that TLR4 signaling engages in RIH by amplifying the expression of TRPA1 in the DRG neurons.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Fundamental Research Funds for the Central Universities (No. 3332022079), and the Scientific Research Fund of China-Japan Friendship Hospital (No. 2017-RC-3 and No. 2018-2-QN-28).
ORCID iDs
Xiaowen Liu https://orcid.org/0000-0002-2013-7851
Ruisong Gong https://orcid.org/0000-0002-0595-3519
References
- 1.Colvin LA, Bull F, Hales TG. Perioperative opioid analgesia-when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet (London, England) 2019; 393(10180): 1558–1568. [DOI] [PubMed] [Google Scholar]
- 2.Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 2011; 14(2): 145–161. [PubMed] [Google Scholar]
- 3.Higgins C, Smith BH, Matthews K. Evidence of opioid-induced hyperalgesia in clinical populations after chronic opioid exposure: a systematic review and meta-analysis. Br J Anaesth 2019; 122(6): e114–e126. [DOI] [PubMed] [Google Scholar]
- 4.Neuman MD, Bateman BT, Wunsch H. Inappropriate opioid prescription after surgery. Lancet (London, England) 2019; 393(10180): 1547–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim Y, Bae H, Yoo S, Park SK, Lim YJ, Sakura S, Kim JT. Effect of remifentanil on postoperative analgesic consumption in patients undergoing shoulder arthroplasty after interscalene brachial plexus block: a randomized controlled trial. J Anesth 2022; 36(4): 506–513. [DOI] [PubMed] [Google Scholar]
- 6.Roeckel LA, Le Coz GM, Gaveriaux-Ruff C, Simonin F. Opioid-induced hyperalgesia: cellular and molecular mechanisms. Neuroscience 2016; 338: 160–182. [DOI] [PubMed] [Google Scholar]
- 7.Mercadante S, Arcuri E, Santoni A. Opioid-Induced Tolerance and Hyperalgesia. CNS Drugs 2019; 33(10): 943–955. [DOI] [PubMed] [Google Scholar]
- 8.Yi P, Pryzbylkowski P. Opioid induced hyperalgesia. Pain Med 2015; 16(Suppl 1): S32–S36. [DOI] [PubMed] [Google Scholar]
- 9.Horii Y, Matsuda M, Takemura H, Ishikawa D, Sawa T, Amaya F. Spinal and peripheral mechanisms individually lead to the development of remifentanil-induced hyperalgesia. Neuroscience 2020; 446: 28–42. [DOI] [PubMed] [Google Scholar]
- 10.Aguado D, Bustamante R, Gomez de Segura IA. Toll-like receptor 4 deficient mice do not develop remifentanil-induced mechanical hyperalgesia: An experimental randomised animal study. Eur J Anaesthesiol 2018; 35(7): 505–510. [DOI] [PubMed] [Google Scholar]
- 11.Araldi D, Bogen O, Green PG, Levine JD. Role of Nociceptor Toll-like Receptor 4 (TLR4) in Opioid-Induced Hyperalgesia and Hyperalgesic Priming. J Neurosci 2019; 39(33): 6414–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geng J, Shi Y, Zhang J, Yang B, Wang P, Yuan W, Zhao H, Li J, Qin F, Hong L, Xie C, Deng X, Sun Y, Wu C, Chen L, Zhou D. TLR4 signalling via Piezo1 engages and enhances the macrophage mediated host response during bacterial infection. Nat Commun 2021; 12(1): 3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Yang W, Zhu C, Sun S, Wu S, Wang L, Wang Y, Ge Z. Toll-like receptors and their role in neuropathic pain and migraine. Mol Brain 2022; 15(1): 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen S, Lim G, You Z, Ding W, Huang P, Ran C, Doheny J, Caravan P, Tate S, Hu K, Kim H, McCabe M, Huang B, Xie Z, Kwon D, Chen L, Mao J. Gut microbiota is critical for the induction of chemotherapy-induced pain. Nat Neurosci 2017; 20(9): 1213–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su W, Cui H, Wu D, Yu J, Ma L, Zhang X, Huang Y, Ma C. Suppression of TLR4-MyD88 signaling pathway attenuated chronic mechanical pain in a rat model of endometriosis. J Neuroinflammation 2021; 18(1): 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruno K, Woller SA, Miller YI, Yaksh TL, Wallace M, Beaton G, Chakravarthy K. Targeting toll-like receptor-4 (TLR4)-an emerging therapeutic target for persistent pain states. Pain 2018; 159(10): 1908–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, Fabisiak TJ, Anderson ND, Cheng K, Greene LI, Berkelhammer D, Zhang Y, Ellis AL, Yin HH, Campeau S, Rice KC, Roth BL, Maier SF, Watkins LR. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci U S A 2016; 113(24): E3441–E3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bamps D, Vriens J, de Hoon J, Voets T. TRP channel cooperation for nociception: therapeutic opportunities. Annu Rev Pharmacol Toxicol 2021; 61: 655–677. [DOI] [PubMed] [Google Scholar]
- 19.Moore C, Gupta R, Jordt SE, Chen Y, Liedtke WB. Regulation of pain and itch by TRP channels. Neurosci Bull 2018; 34(1): 120–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nassini R, Materazzi S, Benemei S, Geppetti P. The TRPA1 channel in inflammatory and neuropathic pain and migraine. Rev Physiol Biochem Pharmacol 2014; 167: 1–43. [DOI] [PubMed] [Google Scholar]
- 21.Min H, Cho WH, Lee H, Choi B, Kim YJ, Lee HK, Joo Y, Jung SJ, Choi SY, Lee S, Lee SJ. Association of TRPV1 and TLR4 through the TIR domain potentiates TRPV1 activity by blocking activation-induced desensitization. Mol Pain 2018; 14: 1744806918812636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y, Wang Y, Wang J, Fan Q, Zhu J, Yang L, Rong W. TLR4 mediates upregulation and sensitization of TRPV1 in primary afferent neurons in 2,4,6-trinitrobenzene sulfate-induced colitis. Mol Pain 2019; 15: 1744806919830018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Z, Wang S, Wu I, Mata M, Fink DJ. Activation of TLR-4 to produce tumour necrosis factor-alpha in neuropathic pain caused by paclitaxel. Eur J Pain 2015; 19(7): 889–898. [DOI] [PubMed] [Google Scholar]
- 24.Heles M, Mrozkova P, Sulcova D, Adamek P, Spicarova D, Palecek J. Chemokine CCL2 prevents opioid-induced inhibition of nociceptive synaptic transmission in spinal cord dorsal horn. J Neuroinflammation 2021; 18(1): 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song C, Liu P, Zhao Q, Guo S, Wang G. TRPV1 channel contributes to remifentanil-induced postoperative hyperalgesia via regulation of NMDA receptor trafficking in dorsal root ganglion. J Pain Res 2019; 12: 667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shu RC, Zhang LL, Wang CY, Li N, Wang HY, Xie KL, Yu YH, Wang GL. Spinal peroxynitrite contributes to remifentanil-induced postoperative hyperalgesia via enhancement of divalent metal transporter 1 without iron-responsive element-mediated iron accumulation in rats. Anesthesiology 2015; 122(4): 908–920. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Ni Y, Zhang W, Sun YE, Ma Z, Gu X. N-acetyl-cysteine attenuates remifentanil-induced postoperative hyperalgesia via inhibiting matrix metalloproteinase-9 in dorsal root ganglia. Oncotarget 2017; 8(10): 16988–17001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang P, Yang M, Chen C, Liu L, Wei X, Zeng S. Toll-like receptor 4 (TLR4)/opioid receptor pathway crosstalk and impact on opioid analgesia, immune function, and gastrointestinal motility. Front Immunol 2020; 11: 1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS, Thomas J, van Steeg K, Kopajtic TA, Loram LC, Sfregola C, GalEr E, Miles NE, Bland ST, Amat J, Rozeske RR, Maslanik T, Chapman TR, Strand KA, Fleshner M, Bachtell RK, Somogyi AA, Yin H, Katz JL, Rice KC, Maier SF, Watkins LR. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J Neurosci 2012; 32(33): 11187–11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah M, Anwar MA, Yesudhas D, Krishnan J, Choi S. A structural insight into the negative effects of opioids in analgesia by modulating the TLR4 signaling: An in silico approach. Sci Rep 2016; 6: 39271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu D, Sun M, Xu D, Ma X, Gao D, Yu H. Inhibition of TRPA1 and IL-6 signal alleviates neuropathic pain following chemotherapeutic bortezomib. Physiol Res 2019; 68(5): 845–855. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H, Chen H. TRPA1 involved in miR-141-5p-alleviated neuropathic pain induced by oxaliplatin. Neuroreport 2021; 32(3): 284–290. [DOI] [PubMed] [Google Scholar]
- 33.de Almeida AS, Pereira GC, Brum EDS, Silva CR, Antoniazzi CTdD, Ardisson-Araujo D, Oliveira SM, Trevisan G. Role of TRPA1 expressed in bone tissue and the antinociceptive effect of the TRPA1 antagonist repeated administration in a breast cancer pain model. Life Sci 2021; 276: 119469. [DOI] [PubMed] [Google Scholar]
- 34.Chung S, Kim H, Kim D, Lee JM, Lee CJ, Oh SB. Common bacterial metabolite indole directly activates nociceptive neuron through transient receptor potential ankyrin 1 channel. Pain 2022; 163(8): 1530–1541. [DOI] [PubMed] [Google Scholar]
- 35.Mukhopadhyay I, Kulkarni A, Aranake S, Karnik P, Shetty M, Thorat S, Ghosh I, Wale D, Bhosale V, Khairatkar-Joshi N. Transient receptor potential ankyrin 1 receptor activation in vitro and in vivo by pro-tussive agents: GRC 17536 as a promising anti-tussive therapeutic. PLoS One 2014; 9(5): e97005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Omar S, Clarke R, Abdullah H, Brady C, Corry J, Winter H, Touzelet O, Power UF, Lundy F, McGarvey LPA, Cosby SL. Respiratory virus infection upregulates TRPV1, TRPA1 and ASICS3 receptors on airway cells. PLoS One 2017; 12(2): e0171681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao D, Han DF, Wang SS, Lv B, Wang X, Ma C. Roles of tumor necrosis factor-alpha and interleukin-6 in regulating bone cancer pain via TRPA1 signal pathway and beneficial effects of inhibition of neuro-inflammation and TRPA1. Mol Pain 2019; 15: 1744806919857981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu TT, Wang RR, Tang YY, Wu YX, Yu J, Hou WW, Lou GD, Zhou YD, Zhang SH, Chen Z. TLR4 deficiency abrogated widespread tactile allodynia, but not widespread thermal hyperalgesia and trigeminal neuropathic pain after partial infraorbital nerve transection. Pain 2018; 159(2): 273–283. [DOI] [PubMed] [Google Scholar]
- 39.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006; 124(6): 1269–1282. [DOI] [PubMed] [Google Scholar]
- 40.Liu Q, Feng L, Han X, Zhang W, Zhang H, Xu L. The TRPA1 channel mediates mechanical allodynia and thermal hyperalgesia in a rat bone cancer pain model. Front Pain Res (Lausanne) 2021; 2: 638620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinica V, Zimova L, Barvikova K, Macikova L, Barvik I, Vlachova V. Human and Mouse TRPA1 Are Heat and Cold Sensors Differentially Tuned by Voltage. Cells 2019; 9(1): 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Talavera K, Startek JB, Alvarez-Collazo J, Boonen B, Alpizar YA, Sanchez A, Naert R, Nilius B. Mammalian Transient Receptor Potential TRPA1 Channels: From Structure to Disease. Physiol Rev 2020; 100(2): 725–803. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt M, Dubin AE, Petrus MJ, Earley TJ, Patapoutian A. Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron 2009; 64(4): 498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meng J, Wang J, Steinhoff M, Dolly JO. TNFalpha induces co-trafficking of TRPV1/TRPA1 in VAMP1-containing vesicles to the plasmalemma via Munc18-1/syntaxin1/SNAP-25 mediated fusion. Sci Rep 2016; 6: 21226. [DOI] [PMC free article] [PubMed] [Google Scholar]