Abstract
In the present study, we aimed to investigate the association between urate‐lowering drugs and cardiovascular events, primarily focusing on the risk of febuxostat and topiroxostat when compared with allopurinol in Japan. We conducted an observational study with a cohort design using the National Database of Health Insurance Claims and Specific Health Checkups of Japan, including new urate‐lowering drugs users between August 1, 2010, and March 31, 2018. Exposure and control groups were defined based on the first prescription of urate‐lowering drugs as follows: febuxostat or topiroxostat for exposure groups, allopurinol for the control group, and benzbromarone for the secondary control group. The primary outcome was cardiovascular events, defined as a composite of acute coronary syndrome, cerebral infarction, and cerebral hemorrhage. Hazard ratios were estimated using a Cox proportional hazards model. The number of patients in each exposure and control group was 1,357,671 in the febuxostat group, 83,683 in the topiroxostat group, 1,273,211 in the allopurinol group, and 258,786 in the benzbromarone group. The adjusted hazard ratios for the cardiovascular risk were 0.97 (95% confidence interval [CI]: 0.95–0.98) for febuxostat and 0.84 (95% CI: 0.78–0.90) for topiroxostat groups. The benzbromarone group exhibited similar results. No increased cardiovascular risk was observed with febuxostat or topiroxostat when compared with allopurinol in patients with hyperuricemia in Japan. These results provide real‐world evidence regarding the cardiovascular risk associated with urate‐lowering drugs, indicating that no additional safety‐related regulatory actions are warranted in Japan.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
The risk of cardiovascular death in patients with gout was higher in the febuxostat group than in the allopurinol group in the CARES trial; however, the extrapolation of these results to Japan remains unclear.
WHAT QUESTION DID THIS STUDY ADDRESS?
The specific aim of this study was to compare the risk of cardiovascular events associated with febuxostat and topiroxostat with that associated with allopurinol in Japan.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
The present study revealed that there was no observed risk of cardiovascular events with febuxostat and topiroxostat when compared with allopurinol in Japan.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
These results indicate that no additional safety measures are required to mitigate potential cardiovascular events associated with febuxostat and topiroxostat use in Japan.
INTRODUCTION
Febuxostat reduces serum uric acid through an inhibitory action of xanthine oxidase 1 and was first approved in the European Union in April 2008, 2 followed by the United States in February 2009 3 and Japan in January 2011. 4 In 2017, the US Food and Drug Administration (FDA) concluded that febuxostat could increase the risk of cardiovascular death and all‐cause mortality when compared with allopurinol, based on an analysis of post‐market clinical trial data (CARES trial). 5 , 6 This conclusion led to an update of the US febuxostat prescribing information in February 2019, with the addition of a boxed warning regarding cardiovascular death. 6 Furthermore, the use of febuxostat was limited to patients who were not effectively treated or experienced severe side effects with allopurinol. 6 Likewise, the European Medicine Agency recommended avoiding the use of febuxostat in patients with a history of major cardiovascular disease based on the findings of the CARES trial. 7
In July 2019, the febuxostat package insert was also revised in Japan to indicate the increased risk of cardiovascular deaths for providing an important precaution, 8 based on the results of the CARES trial and a postmarketing study in Japan, 9 as well as other related information, such as published literature 9 , 10 , 11 , 12 , 13 , 14 , 15 and Japanese clinical guidelines published by academic societies. 16 , 17 , 18 , 19 However, the extrapolability of the risk of cardiovascular events in the CARES trial to Japan remains unclear for the following three reasons: (1) lower cardiovascular risks have been reported in the Japanese population than in the European and American populations 20 ; (2) fewer Asian subjects were enrolled in the CARES trial (~70% White vs. ~3% Asians) 5 ; and (3) no increased cardiovascular death induced by febuxostat was detected in clinical trials conducted in Japan. 10 , 21 Therefore, to attain a deeper understanding of the cardiovascular risk of febuxostat in Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) designed and conducted an observational study using real‐world data in Japan to compare the risk of cardiovascular events associated with febuxostat, topiroxostat, and allopurinol.
METHODS
Study design and setting
We conducted an observational study with a cohort design using the National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB). 22 We deemed that the NDB would be the most appropriate database considering the objective of the present study to examine the low frequency of cardiovascular events, including late‐onset cases, based on the following reasons: (1) NDB is the largest database managed by the Ministry of Health, Labour, and Welfare (MHLW), collecting information on nation‐based medical claims from hospitals, clinics, pharmacies, and dental clinics in Japan; and (2) the long follow‐up period from hospitals where patients underwent treatment can be ensured. 23 , 24
Target population
As shown in Figure 1, among patients who were prescribed febuxostat, topiroxostat, allopurinol, benzbromarone, or probenecid between August 1, 2010, and March 31, 2018, we included those whose initial medical claims were recorded more than 8 months prior to the month of the first prescription to allow a look‐back period for assessing past prescriptions or a history of urate‐lowering drug usage. We also included topiroxostat in this study because its pharmacological action is similar to that of febuxostat (i.e., xanthine oxidase inhibitor) and its use in Japan in clinical settings. Furthermore, to select a more appropriate target population and minimize bias, patients who met the following criteria were excluded: (1) patients who were younger than 20 years of age on the date of the first prescription; (2) patients who had the date of admission for any cardiovascular events on the same date of the first prescription; (3) patients who were concomitantly prescribed two or more urate‐lowering drugs on the date of the first prescription; (4) patients who were prescribed probenecid on the date of the first prescription; (5) patients who were prescribed any anticancer drug prior to the date of the first prescription; and (6) patients with diseases that may cause renal dysfunction, patients who were prescribed renal‐related drugs, or patients on dialysis prior to the date of the first prescription (see Table S1 for more detailed reasons and Table S2 for the medicine codes of urate‐lowering drugs).
FIGURE 1.
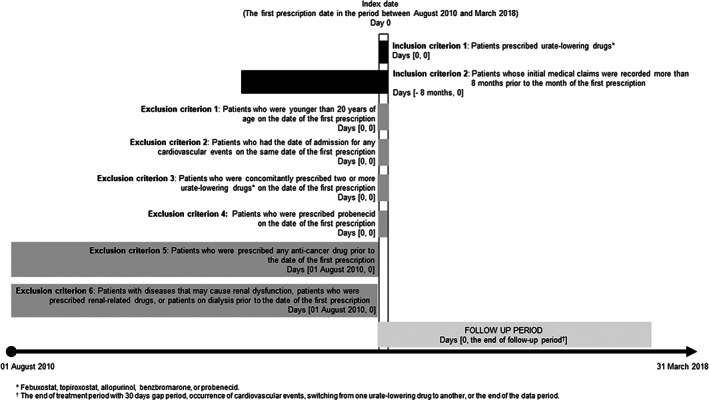
Study design
Exposure and control groups
Patients were categorized into exposure or control groups based on the type of urate‐lowering drug first prescribed: febuxostat or topiroxostat for exposure groups, allopurinol for the control group, and benzbromarone for the secondary control group. Allopurinol was selected as the primary control because it was also used as the control in the CARES trial. In addition to allopurinol, benzbromarone was established as the secondary control, given that allopurinol might reduce the risk of cardiovascular events and overall mortality. 25 , 26 However, such effects have not been widely reported with benzbromarone administration, and no cardiovascular‐related warnings are included on the package insert of benzbromarone in Japan, indicating that benzbromarone could be considered as a negative control.
The follow‐up period continued with the same urate‐lowering drug prescribed from the date of the first prescription. If the gap between the end date of the previous prescription and the start date of the next prescription was within 30 days, we considered it a continuous prescription period for the patient. The follow‐up period was also censored on observing the following events: occurrence of cardiovascular events (acute coronary syndrome, cerebral infarction, or cerebral hemorrhage; see below for more details), switching from one urate‐lowering drug to another (febuxostat, topiroxostat, allopurinol, benzbromarone, and probenecid), or the end of the data period (March 31, 2018).
Outcome definitions
The primary outcome of this study was the occurrence of cardiovascular events, including acute coronary syndrome, cerebral infarction, and cerebral hemorrhage, during the follow‐up period. Each event was defined by using algorithms (A, B, or C) using the medicine, diagnosis, and procedure codes (see Tables S2, S3, and S4, respectively). Algorithm A for acute coronary syndrome was defined based on observations from at least one of the following therapeutic interventions, that is, percutaneous coronary intervention, coronary artery bypass graft, intra‐aortic balloon pumping, percutaneous cardiopulmonary support, or thrombolysis within 30 days from and on the date of admission for acute coronary syndrome. Algorithm B for cerebral infarction was defined based on observations from at least one of the following examinations, that is, computed tomography, magnetic resonance imaging, or magnetic resonance angiography, as well as at least one of the following therapeutic interventions, namely, cerebro‐protective agents, injectable anti‐platelet agents, injectable anti‐coagulant agents, thrombolytic agents, anti‐edema agents, craniotomy, or mechanical thrombectomy within 30 days after and on the date of admission for cerebral infarction. Algorithm C for cerebral hemorrhage was defined based on observations from at least one of the following examinations, that is, computed tomography, magnetic resonance imaging, or magnetic resonance angiography, as well as at least one of the following therapeutic interventions, namely, anti‐edema drugs, injectable anti‐hypertensive drugs, or hematoma evacuation within 30 days of the date of admission for cerebral hemorrhage. These definitions were based on results of the outcome validation study for cardiovascular events by utilizing data from MID‐NET, another medical information database in Japan (data not shown), 27 , 28 with few minor modifications applied to the original definitions to ensure that data categories fitted to the NDB but not MID‐NET.
Furthermore, as a higher risk of cardiovascular death in the febuxostat group was observed in the CARES trial, cardiovascular death was set as the secondary outcomes in addition to an individual component of the primary outcome (acute coronary syndrome, cerebral infarction, and cerebral hemorrhage). “Cardiovascular death” was defined as a patient who met one of the algorithms (A, B, or C) described above and had the disease records for “death.” However, the investigation of cardiovascular death was for the exploratory purpose, as identifiability of “death” was expected to be low and has been less established in the analysis of NDB data.
Ethical considerations
As this study was conducted as an official activity of the PMDA under the Pharmaceuticals and Medical Devices Agency Law Article 15–5–(c) and (f), 29 it was not subject to review by institutional review boards. 30
Statistical analysis
Main analysis and secondary analysis
For the main analysis comparing the febuxostat group with the allopurinol group, or the topiroxostat group with the allopurinol group, the incidence rates (IRs) of outcomes (primary and secondary outcomes) in each group were calculated, followed by calculating the incidence rate ratio of the exposure groups to the control group (allopurinol). Crude and adjusted hazard ratios (aHRs) were also estimated using the Cox proportional hazards model with the following adjusted factors: age group, sex, the presence of diseases (acute coronary syndrome, stroke, heart failure, peripheral vascular disease, liver disease, dyslipidemia, diabetes mellitus, hypertension, arrhythmia, and gout <diagnosis with drug treatment for gout>), and drug prescription (anti‐platelet agents, anti‐coagulants, and colchicine; see Table S5 for more details of the covariates). For the secondary analysis comparing the febuxostat group with the benzbromarone group, or the topiroxostat group with the benzbromarone group, the analysis was the same as the main analysis for the primary outcome.
Sensitivity analysis
In the sensitivity analysis, we conducted two analyses of primary outcomes. The first focused on patients who were followed, regardless of the end of the prescription period or switching from one urate‐lowering drug to another, until the occurrence of cardiovascular events or censored at the end of the data period, whichever occurred first (sensitivity analysis 1). In the second analysis, we changed the exclusion criterion 6 in the main analysis to the criterion (patients with diseases that may cause severe renal dysfunction) for allowing to include patients with mild renal dysfunction for analysis (sensitivity analysis 2).
Subgroup analysis
We also conducted a subgroup analysis, in which the target population was limited to patients with a history of cardiovascular diseases, to consider the influence of the history of cardiovascular diseases on the primary outcome.
All analyses were conducted using the SAS statistical software (version 9.4; SAS Institute).
RESULTS
Study flow and patient characteristics
Figure 2 presents a flow diagram to establish the target population. The number of patients prescribed urate‐lowering drugs between August 1, 2010, and March 31, 2018, was 8,190,879. After applying inclusion and exclusion criteria as described in “Section 2,” the number of eligible patients decreased to 2,973,351, which was largely affected by the inclusion criteria 2, “patients whose initial medical claims were recorded more than 8 months prior to the month of the first prescription” and by the exclusion criteria 6, “patients with diseases that may cause renal dysfunction, patients who were prescribed renal‐related drugs, or patients on dialysis prior to the date of the first prescription.” The number of eligible patients in the exposure and control groups was 1,357,671 in the febuxostat group, 83,683 in the topiroxostat group, 1,273,211 in the allopurinol group, and 258,786 in the benzbromarone group. The median and quartile ranges (QRs) of a follow‐up period for each group were 245 days (QR: 85–714) for the febuxostat group, 167 days (QR: 64–404) for the topiroxostat group, 213 days (QR: 70–790) for the allopurinol group, and 145 days (QR: 61–545) for the benzbromarone group. No major differences in patient characteristics were observed between the exposure and control groups, except for the higher prevalence of heart failure in the febuxostat group and the shorter follow‐up periods in the topiroxostat and benzbromarone groups (Table 1).
FIGURE 2.
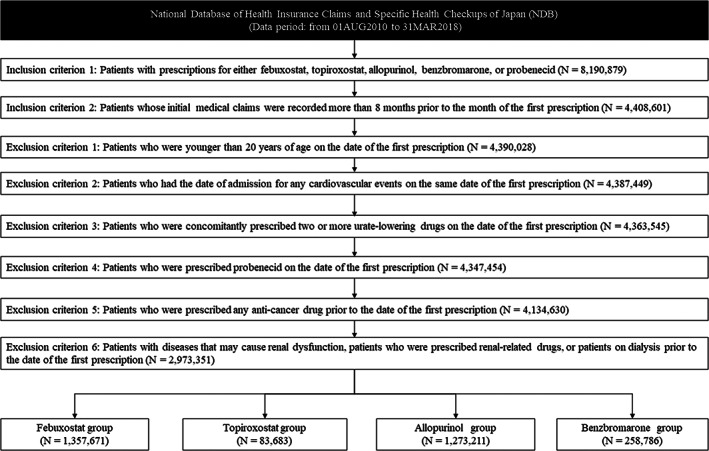
Study flow chart for the cohort identification
TABLE 1.
Patient characteristics
| Febuxostat | Standardized difference with allopurinol | Topiroxostat | Standardized difference with allopurinol | Allopurinol | Benzbromarone | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (N = 1,357,671) | (N = 83,683) | (N = 1,273,211) | (N = 258,786) | |||||||
| Age group, n (%) | ||||||||||
| 20–24 | 11,330 | 0.80% | 0.000 | 816 | 1.00% | 0.021 | 9788 | 0.80% | 2546 | 1.00% |
| 25–29 | 22,997 | 1.70% | 0.016 | 1782 | 2.10% | 0.045 | 19,189 | 1.50% | 5339 | 2.10% |
| 30–34 | 38,593 | 2.80% | 0.012 | 2926 | 3.50% | 0.052 | 32,798 | 2.60% | 8817 | 3.40% |
| 35–39 | 64,287 | 4.70% | 0.005 | 4507 | 5.40% | 0.037 | 59,019 | 4.60% | 15,426 | 6.00% |
| 40–44 | 96,209 | 7.10% | 0.020 | 6610 | 7.90% | 0.050 | 84,354 | 6.60% | 20,755 | 8.00% |
| 45–49 | 108,195 | 8.00% | 0.023 | 7583 | 9.10% | 0.062 | 93,930 | 7.40% | 22,599 | 8.70% |
| 50–54 | 110,968 | 8.20% | 0.011 | 7228 | 8.60% | 0.025 | 100,771 | 7.90% | 23,133 | 8.90% |
| 55–59 | 112,781 | 8.30% | 0.011 | 6936 | 8.30% | 0.011 | 108,861 | 8.60% | 24,370 | 9.40% |
| 60–64 | 126,679 | 9.30% | 0.040 | 7247 | 8.70% | 0.061 | 133,936 | 10.50% | 28,888 | 11.20% |
| 65–69 | 151,568 | 11.20% | 0.006 | 9011 | 10.80% | 0.019 | 144,823 | 11.40% | 28,467 | 11.00% |
| 70–74 | 133,929 | 9.90% | 0.020 | 7803 | 9.30% | 0.040 | 133,709 | 10.50% | 24,480 | 9.50% |
| 75–79 | 126,674 | 9.30% | 0.014 | 7421 | 8.90% | 0.028 | 123,554 | 9.70% | 21,041 | 8.10% |
| 80–84 | 111,998 | 8.20% | 0.004 | 6425 | 7.70% | 0.015 | 103,286 | 8.10% | 16,115 | 6.20% |
| 85–89 | 83,720 | 6.20% | 0.017 | 4531 | 5.40% | 0.017 | 73,864 | 5.80% | 10,163 | 3.90% |
| 90–94 | 43,162 | 3.20% | 0.012 | 2193 | 2.60% | 0.024 | 37,626 | 3.00% | 5006 | 1.90% |
| 95–99 | 12,708 | 0.90% | 0.000 | 584 | 0.70% | 0.022 | 11,724 | 0.90% | 1435 | 0.60% |
| 100– | 1873 | 0.10% | 0.026 | 80 | 0.10% | 0.026 | 1979 | 0.20% | 206 | 0.10% |
| Sex, n (%) | ||||||||||
| Male | 1,093,792 | 80.60% | 0.020 | 68,016 | 81.30% | 0.003 | 1,036,863 | 81.40% | 224,558 | 86.80% |
| Female | 263,879 | 19.40% | 0.020 | 15,667 | 18.70% | 0.003 | 236,348 | 18.60% | 34,228 | 13.20% |
| Presence of disease at baseline, n (%) | ||||||||||
| Acute coronary syndrome a | 89,762 | 6.60% | 0.092 | 4072 | 4.90% | 0.019 | 57,919 | 4.50% | 8001 | 3.10% |
| Stroke a | 230,674 | 17.00% | 0.049 | 13,616 | 16.30% | 0.030 | 193,017 | 15.20% | 30,520 | 11.80% |
| Heart failure a | 314,052 | 23.10% | 0.124 | 15,579 | 18.60% | 0.013 | 229,860 | 18.10% | 32,226 | 12.50% |
| Peripheral vascular disease a | 142,382 | 10.50% | 0.075 | 9192 | 11.00% | 0.092 | 106,256 | 8.30% | 17,678 | 6.80% |
| Liver disease | 262,308 | 19.30% | 0.025 | 16,474 | 19.70% | 0.015 | 258,129 | 20.30% | 42,810 | 16.50% |
| Dyslipidemia | 301,938 | 22.20% | 0.039 | 19,179 | 22.90% | 0.056 | 262,489 | 20.60% | 43,921 | 17.00% |
| Diabetes | 127,994 | 9.40% | 0.024 | 7490 | 9.00% | 0.011 | 110,695 | 8.70% | 16,564 | 6.40% |
| Hypertension | 672,252 | 49.50% | 0.036 | 38,936 | 46.50% | 0.024 | 607,512 | 47.70% | 102,900 | 39.80% |
| Arrhythmia | 92,640 | 6.80% | 0.086 | 3910 | 4.70% | 0.005 | 61,109 | 4.80% | 8587 | 3.30% |
| Gout | ||||||||||
| Disease code + NSAIDs | 16,942 | 1.20% | 0.040 | 1272 | 1.50% | 0.066 | 10,617 | 0.80% | 4320 | 1.70% |
| Disease code + Steroid | 14,697 | 1.10% | 0.042 | 1028 | 1.20% | 0.052 | 9110 | 0.70% | 3496 | 1.40% |
| Drug prescription at baseline, n (%) | ||||||||||
| Anti‐platelet agents | 195,871 | 14.40% | 0.011 | 9653 | 11.50% | 0.075 | 178,001 | 14.00% | 26,933 | 10.40% |
| Anti‐coagulants | 160,032 | 11.80% | 0.092 | 5315 | 6.40% | 0.098 | 114,821 | 9.00% | 14,672 | 5.70% |
| Colchicine | 32,089 | 2.40% | 0.027 | 2504 | 3.00% | 0.064 | 25,902 | 2.00% | 8345 | 3.20% |
| Follow‐up period, days | ||||||||||
| Mean (SD) | 479.4 | (±524.9) | 0.098 | 293.2 | (±310.4) | 0.479 | 537.3 | (±650.0) | 434.5 | (±591.8) |
| Median (Q1–Q3) | 245 | (85–714) | 167 | (64–404) | 213 | (70–790) | 145 | (61–545) | ||
Abbreviations: NSAIDs, nonsteroidal anti‐inflammatory drugs; SD, standard deviation.
Baseline indicates the period before the first prescription. As for other diseases or drug prescriptions without this mark, baseline indicates the 90 days prior to the first prescription.
Risk assessment
The results of the cardiovascular risk analysis are shown in Table 2. The IRs were similar among the three groups, and the aHRs were 0.97 (95% confidence interval [CI]: 0.95–0.98) in the febuxostat group and 0.84 (95% CI: 0.78–0.90) in the topiroxostat group.
TABLE 2.
Risk of cardiovascular events in the febuxostat group vs. the allopurinol group or the topiroxostat group vs. the allopurinol group
| N | Total follow‐up period in person‐years | Number of events | IR | IRR | cHR (95% CI) | aHR a (95% CI) | |
|---|---|---|---|---|---|---|---|
| Febuxostat | 1,357,671 | 1781,989.8 | 23,043 | 0.013 | 1.05 | 1.04 (1.02–1.06) | 0.97 (0.95–0.98) |
| Topiroxostat | 83,683 | 67,178.6 | 708 | 0.011 | 0.86 | 0.80 (0.74–0.86) | 0.84 (0.78–0.90) |
| Allopurinol | 1,273,211 | 1873,101.0 | 23,062 | 0.012 | Reference | Reference | Reference |
Abbreviations: aHR, adjusted hazard ratio; cHR, crude hazard ratio; CI, confidence interval; IR, incidence rate; IRR, incidence rate ratio.
Adjusted for age group, sex, presence of diseases (acute coronary syndrome, stroke, heart failure, peripheral vascular disease, liver disease, dyslipidemia, diabetes mellitus, hypertension, arrhythmia, and gout), and drug prescription (anti‐platelet agents, anti‐coagulants, and colchicine).
Considering the secondary outcome, the results of individual components of the primary outcome were consistent with the results of the primary outcome. Furthermore, the aHRs for cardiovascular death were 0.90 (95% CI: 0.84–0.96) in the febuxostat group and 0.61 (95% CI: 0.44–0.84) in the topiroxostat group (Table S6). Considering results for the secondary analysis (compared with the benzbromarone group), the aHR for the primary outcome was 0.96 (95% CI: 0.93–1.00) in the febuxostat group and 0.84 (95% CI: 0.77–0.91) in the topiroxostat group (Table S7). Furthermore, the results of sensitivity analyses 1 and 2 revealed similar tendencies to those of the main analysis (Tables 3 and 4). The subgroup analysis results limited to patients with a history of cardiovascular diseases were also consistent with the main analysis results (aHR in the febuxostat group: 0.91, 95% CI: 0.87–0.96, aHR in the topiroxostat group: 0.61, 95% CI: 0.50–0.75; Table S8).
TABLE 3.
Sensitivity analysis 1: Risk of cardiovascular events in the febuxostat group vs. the allopurinol group or the topiroxostat group vs. the allopurinol group on changing the censuring events
| N | Total follow‐up period in person‐years | Number of events | IR | IRR | cHR (95% CI) | aHR a (95% CI) | |
|---|---|---|---|---|---|---|---|
| Febuxostat | 1,357,671 | 3,768,059.6 | 40,156 | 0.011 | 1.06 | 1.02 (1.01–1.03) | 0.97 (0.96–0.98) |
| Topiroxostat | 83,683 | 139,498.3 | 1274 | 0.009 | 0.91 | 0.83 (0.78–0.87) | 0.87 (0.83–0.92) |
| Allopurinol | 1,273,211 | 5,646,585.6 | 56,757 | 0.010 | Reference | Reference | Reference |
Abbreviations: aHR, adjusted hazard ratio; cHR, crude hazard ratio; CI, confidence interval; IR, incidence rate; IRR, incidence rate ratio.
Adjusted for age group, sex, presence of diseases (acute coronary syndrome, stroke, heart failure, peripheral vascular disease, liver disease, dyslipidemia, diabetes mellitus, hypertension, arrhythmia, and gout), and drug prescription (anti‐platelet agents, anti‐coagulants, and colchicine).
TABLE 4.
Sensitivity analysis 2: Risk of cardiovascular events in the febuxostat vs. the allopurinol group or the topiroxostat group vs. the allopurinol group in the cohort, excluding patients with diseases that may cause severe renal dysfunction
| N | Total follow‐up period in person‐years | Number of events | IR | IRR | cHR (95% CI) | aHR a (95% CI) | |
|---|---|---|---|---|---|---|---|
| Febuxostat | 1,736,801 | 2,326,712.3 | 32,411 | 0.014 | 1.08 | 1.06 (1.05–1.08) | 0.97 (0.95–0.98) |
| Topiroxostat | 108,865 | 90,863.8 | 1099 | 0.012 | 0.94 | 0.87 (0.82–0.93) | 0.88 (0.82–0.93) |
| Allopurinol | 1,488,664 | 2,206,022.4 | 28,433 | 0.013 | Reference | Reference | Reference |
Abbreviations: aHR, adjusted hazard ratio; cHR, crude hazard ratio; CI, confidence interval; IR, incidence rate; IRR, incidence rate ratio.
Adjusted for age group, sex, presence of diseases (acute coronary syndrome, stroke, heart failure, peripheral vascular disease, liver disease, dyslipidemia, diabetes mellitus, hypertension, arrhythmia, and gout), drug prescription (anti‐platelet agents, anti‐coagulants, and colchicine), and presence of disease codes related to “mild to moderate renal dysfunction.”
DISCUSSION
In the present study, the aHR for cardiovascular events, composed of acute coronary syndrome, cerebral infarction, and cerebral hemorrhage, in the febuxostat group compared to the allopurinol group was ~1 (aHR: 0.97, 95% CI: 0.95–0.98), indicating that the cardiovascular risk of febuxostat is similar to that of allopurinol. This finding was supported by sensitivity and subgroup analyses (see Tables 3 and 4; Table S8). In addition, we detected no increase in the cardiovascular risk associated with febuxostat when compared with that of benzbromarone. To further examine the risk of febuxostat, we compared cardiovascular death between febuxostat and allopurinol and detected no increased risk of cardiovascular death associated with febuxostat (aHR: 0.90, 95% CI: 0.84–0.96). These results are consistent with the findings of the Febuxostat versus Allopurinol Streamlined Trial (FAST) and CARES trial in terms of cardiovascular events but not cardiovascular death, 5 , 31 and might be, in part, influenced by the different conditions of the target study populations (CARES trial: patients with gout and history of cardiovascular disease and FAST trial: patients with gout and at least one additional cardiovascular risk factor; vs. the present study: patients with hyperuricemia) and study design (CARES trial and FAST trial: randomized clinical trial; vs. the present study: observational study with secondary use of real‐world data). The study results may also be affected by pharmacogenetic factors because differences on the risks of gout and urate‐lowering drug responses among ethnic populations including Japanese have been reported. 32 Moreover, the results of death based on NDB used in the present study should be carefully interpreted, as such events in the secondary utilization of claim‐based databases in Japan may be less comprehensive and accurate than those in clinical trials (e.g., not‐covered for costs of postmortem procedures), including some errors as reported. 33
Considering topiroxostat, the aHR of cardiovascular events was 0.84 (95% CI: 0.78–0.90), suggesting that the risk of topiroxostat is lower than that of allopurinol. This observation was supported by secondary, sensitive, and subgroup analyses (even in evaluating cardiovascular death). In addition, the aHR for topiroxostat was lower than that for febuxostat; however, further investigation is warranted to conclude the clinical impact of these results, as the number of outcomes and total follow‐up period in person‐years for topiroxostat were smaller than those observed with febuxostat. The shorter follow‐up period of topiroxostat could be due to the later approval and launch of topiroxostat than allopurinol and febuxostat in Japan and may be not enough to capture events occurred in longer term.
Given that the NDB used in the present study is a nation‐based database in Japan, the results would reflect the general situation in Japan. However, it is difficult to exclude the possibility that other unexpected factors, which were not adjusted in the present study, could have impacted the study results.
The PMDA conducted a safety assessment of the risk of febuxostat and topiroxostat based on the present study results and other available data, including spontaneous adverse drug reaction reports, literature, and the results of the FAST trial, and concluded that no additional regulatory actions are currently warranted. The study results were valuable for the PMDA to consider appropriate regulatory actions, taking into account the safety profiles of these drugs in the real‐world clinical scenario in Japan.
In conclusion, no increased cardiovascular risk associated with febuxostat or topiroxostat when compared with allopurinol was confirmed in Japan. The potential differences of the cardiovascular risk among populations should be recognized in clinical practice for the proper use of urate‐lowering drugs.
AUTHOR CONTRIBUTIONS
S.S., H.S., C.I., R.K., Y.N., T.I., Y.O., and Y.U. wrote the manuscript. S.S., H.S., C.I., R.K., T.I., Y.O., and Y.U. designed the research. S.S. and H.S. performed the research. S.S. and K.K. analyzed the data.
FUNDING INFORMATION
No funding was received for this work.
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
Supporting information
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
Table S7
Table S8
ACKNOWLEDGMENT
None.
Sawada S, Kajiyama K, Shida H, et al. Cardiovascular risk of urate‐lowering drugs: A study using the National Database of Health Insurance Claims and Specific Health Checkups of Japan. Clin Transl Sci. 2023;16:206‐215. doi: 10.1111/cts.13439
Past presentation on this research: A part of this article was included in an official Pharmaceuticals and Medical Devices Agency (PMDA) report available on the PMDA website (https://www.pmda.go.jp/files/000239435.pdf).
REFERENCES
- 1. Okamoto K, Eger BT, Nishino T, Kondo S, Pai EF, Nishino T. An extremely potent inhibitor of xanthine oxidoreductase. Crystal structure of the enzyme‐inhibitor complex and mechanism of inhibition. J Biol Chem. 2003;278:1848‐1855. [DOI] [PubMed] [Google Scholar]
- 2. ADENURIC 80 mg film‐coated tablets. Product Information. Menarini International Operations Luxembourg S.A. https://www.ema.europa.eu/en/documents/product‐information/adenuric‐epar‐product‐information_en.pdf. Accessed June 3, 2022. [Google Scholar]
- 3. ULORIC (febuxostat) tablet for oral use. Label (Highrights of prescribing information). Takeda Pharmaceuticals America, Inc. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/021856s013lbl.pdf. Accessed June 3, 2022. [Google Scholar]
- 4. Feburic® Tablets 10mg, 20mg, 40mg. Package insert. Teijin Pharma Limited. [in Japanese]. https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/470310_3949003F1023_1_15. Accessed June 3, 2022. [Google Scholar]
- 5. White WB, Saag KG, Becker MA, et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med. 2018;378:1200‐1210. [DOI] [PubMed] [Google Scholar]
- 6. FDA adds Boxed Warning for increased risk of death with gout medicine Uloric (febuxostat). FDA Drug Safety Communication. Food and Drug Administration. https://www.fda.gov/drugs/drug‐safety‐and‐availability/fda‐adds‐boxed‐warning‐increased‐risk‐death‐gout‐medicine‐uloric‐febuxostat. Accessed April 27, 2022. [Google Scholar]
- 7. Adenuric . Procedural steps taken and scientific information after the authorisation. European Medicine Agency. https://www.ema.europa.eu/en/documents/procedural‐steps‐after/adenuric‐epar‐procedural‐steps‐taken‐scientific‐information‐after‐authorisation_en.pdf. Accessed April 27, 2022. [Google Scholar]
- 8. Febuxostat . Revision of precautions. Pharmaceuticals and Medical Devices Agency. https://www.pmda.go.jp/files/000230437.pdf. Accessed April 27, 2022. [Google Scholar]
- 9. Report on investigation results. Pharmaceuticals and Medical Devices Agency. https://www.pmda.go.jp/files/000230432.pdf. Accessed April 27, 2022. [Google Scholar]
- 10. Kojima S, Matsui K, Hiramitsu S, et al. Febuxostat for cerebral and cardiorenovascular events prevention study. Eur Heart J. 2019;40:1778‐1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang M, Solomon DH, Desai RJ, et al. Assessment of cardiovascular risk in older patients with gout initiating febuxostat versus allopurinol: population‐based cohort study. Circulation. 2018;138:1116‐1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Foody J, Turpin RS, Tidwell BA, Lawrence D, Schulman KL. Major cardiovascular events in patients with gout and associated cardiovascular disease or heart failure and chronic kidney disease initiating a xanthine oxidase inhibitor. Am Health Drug Benefits. 2017;10:393‐401. [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang T, Pope JE. Cardiovascular effects of urate‐lowering therapies in patients with chronic gout: a systematic review and meta‐analysis. Rheumatology (Oxford). 2017;56:1144‐1153. [DOI] [PubMed] [Google Scholar]
- 14. Bredemeier M, Lopes LM, Eisenreich MA, et al. Xanthine oxidase inhibitors for prevention of cardiovascular events: a systematic review and meta‐analysis of randomized controlled trials. BMC Cardiovasc Disord. 2018;18:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cuenca JA, Balda J, Palacio A, Young L, Pillinger MH, Tamariz L. Febuxostat and cardiovascular events: a systematic review and meta‐analysis. Int J Rheumatol. 2019;2019:1076189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clinical Guidelines for the Management of Hyperuricemia and Gout. 3rd ed. Japanese Society of Gout and Nucleic Acid Metabolism. [in Japanese]. https://minds.jcqhc.or.jp/docs/gl_pdf/G0001086/4/Clinical_Practice_Guidelines_of_Hyperuricemia_and_Gout.pdf. Accessed April 27, 2022. [Google Scholar]
- 17. Guidelines for Diagnosis and Treatment of Acute and Chronic Heart Failure (2017 Revision). Japanese Circulation Society. [in Japanese]. https://www.j‐circ.or.jp/cms/wp‐content/uploads/2017/06/JCS2017_tsutsui_h.pdf Accessed April 27, 2022. [Google Scholar]
- 18. JCS 2018 Guideline on Diagnosis of Chronic Coronary Heart Disease. Japanese Circulation Society. [in Japanese] https://www.j‐circ.or.jp/cms/wp‐content/uploads/2020/02/JCS2018_yamagishi_tamaki.pdf. Accessed April 27, 2022. [DOI] [PubMed] [Google Scholar]
- 19. Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. Japan Atherosclerotic Society. [in Japanese] https://www.jstage.jst.go.jp/article/naika/107/1/107_73/_pdf. Accessed April 27, 2022. [Google Scholar]
- 20. Hisamatsu T, Miura K. Trends in mortality and morbidity of cardiovascular disease in Japan (Wagakoku ni okeru shinshikkan no shibouritsu/rikanritsu no doukou) [in Japanese]. Jpn J Cardiovasc Dis Prevent. 2018;53:1‐8. [Google Scholar]
- 21. Kimura K, Hosoya T, Uchida S, et al. Febuxostat therapy for patients with stage 3 CKD and asymptomatic hyperuricemia: a randomized trial. Am J Kidney Dis. 2018;72:798‐810. [DOI] [PubMed] [Google Scholar]
- 22. Guideline of National Database of Health Insurance Claims and Specific Health Checkups of Japan. https://www.mhlw.go.jp/content/12400000/000923325.pdf. Accessed April 27, 2022. [Google Scholar]
- 23. Kubota K, Kamijima Y, Sato T, et al. Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the Japanese national claims database. BMJ Open. 2015;5:e006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Komamine M, Kajiyama K, Ishiguro C, Uyama Y. Cardiovascular risks associated with dipeptidyl peptidase‐4 inhibitors monotherapy compared with other antidiabetes drugs in the Japanese population: a nationwide cohort study. Pharmacoepidemiol Drug Saf. 2019;28:1166‐1174. [DOI] [PubMed] [Google Scholar]
- 25. MacIsaac RL, Salatzki J, Higgins P, et al. Allopurinol and cardiovascular outcomes in adults with hypertension. Hypertension. 2016;67:535‐540. [DOI] [PubMed] [Google Scholar]
- 26. Weisman A, Tomlinson GA, Lipscombe LL, Perkins BA, Hawker GA. Association between allopurinol and cardiovascular outcomes and all‐cause mortality in diabetes: a retrospective, population‐based cohort study. Diabetes Obes Metab. 2019;21:1322‐1329. [DOI] [PubMed] [Google Scholar]
- 27. Research group on characterization and methods of extraction and analysis of MID‐NET data, under the research on regulatory science of pharmaceuticals and medical devices supported by Japan Agency for Medical Research and Development, Grant No. 17mk0101088h0001: FY2017‐FY2019 [in Japanese]. https://amedfind.amed.go.jp/amed/index?now=Thu+Nov+03+16%3A14%3A25+JST+2022. Accessed April 27, 2022.
- 28. Tanigawa M, Kohama M, Nonaka T, et al. Validity of identification algorithms combining diagnostic codes with other measures for acute ischemic stroke in MID‐NET®. Pharmacoepidemiol Drug Saf. 2022;31:524‐533. [DOI] [PubMed] [Google Scholar]
- 29. Act on the Pharmaceuticals and Medical Devices Agency (Act No. 192 of 2002) [in Japanese]. https://elaws.e‐gov.go.jp/document?lawid=414AC0000000192. Accessed April 27, 2022.
- 30. Pharmaceuticals and Medical Devices Agency . MIHARI Project [in Japanese]. https://www.pmda.go.jp/safety/surveillance‐analysis/0045.html. Accessed April 27, 2022.
- 31. Mackenzie IS, Ford I, Nuki G, et al. Long‐term cardiovascular safety of febuxostat compared with allopurinol in patients with gout (FAST): a multicentre, prospective, randomised, open‐label, non‐inferiority trial. Lancet. 2020;396:1745‐1757. [DOI] [PubMed] [Google Scholar]
- 32. Alrajeh KY, Roman YM. Pharmacogenetic perspective for optimal gout management. Future Pharmacol. 2022;2:135‐152. [Google Scholar]
- 33. Ooba N, Setoguchi S, Ando T, et al. Claims‐based definition of death in Japanese claims database: validity and implications. PLoS One. 2013;8:e66116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
Table S7
Table S8


