Abstract
Background
Greater attainment of ideal cardiovascular health (ICH) and lower serum aldosterone are associated with lower diabetes risk. Higher levels of ICH are associated with lower aldosterone. The mediational role of aldosterone in the association of ICH with incident diabetes remains unexplored. Thus, we examined the mediational role of aldosterone in the association of 5 ICH components (smoking, diet, physical activity, body mass index [BMI], and cholesterol) with incident diabetes. Additionally, we investigated the mediational role of glucose and blood pressure (BP) in the association of aldosterone with incident diabetes in an African American (AA) cohort.
Methods
We conducted a prospective cohort analysis among AA adults, aged 21–94 years, in the Jackson Heart Study. Data on ICH, aldosterone, and cardiometabolic risk factors were collected at exam 1 (2000–2004). Diabetes (fasting glucose ≥ 126 mg/dL, physician diagnosis, use of diabetes drugs, or glycated hemoglobin ≥ 6.5%) was assessed at exams 1 through 3 (2009–2012). ICH metrics were defined by American Heart Association 2020 goals for smoking, dietary intake, physical activity, BMI, total cholesterol, BP and glucose. The number of ICH metrics attained at exam 1, excluding BP and fasting glucose, were summed (0–2, vs. 3+). R Package Mediation was used to examine: 1) The mediational role of aldosterone in the association of ICH with incident diabetes; and 2) the mediational role of BP and glucose in the association of aldosterone with incident diabetes.
Results
Among 2,791 participants (mean age: 53±12, 65% female) over a median of 7.5 years, there were 497 incident diabetes cases. Risk of incident diabetes was 37% (HR: 0.63, 95%CI: 0.47, 0.84) lower in 3+ ICH category compared to 0–2 ICH category. Aldosterone mediated 6.98% (95% CI: 1.8%, 18.0%) of the direct effect of ICH on incident diabetes. A 1-unit increase in log-aldosterone was associated with a 44% higher risk of diabetes (HR 1.44, 95%CI 1.25–1.64). BP and glucose mediated 16.3% (95% CI: 7.0%, 31.0%) and 19.7% (95% CI: 6.5%, 34.0%) of the association of aldosterone with incident diabetes, respectively.
Conclusion
Aldosterone is a mediator of the association of ICH with incident diabetes, whereas BP and glucose are mediators of the association of aldosterone with incident diabetes, emphasizing the importance of the renin-angiotensin-aldosterone system and ICH in lowering risk of diabetes in AA populations.
Keywords: Aldosterone, Ideal cardiovascular health, Diabetes, African Americans
1. Introduction
The incidence of type 2 diabetes (T2D) continues to rise among African Americans (AAs), while it has plateaued among non-Hispanic whites (NHWs) [1]. T2D is already more prevalent and associated with higher morbidity and mortality among AAs compared to NHWs [2]. Thus, examining novel risk factors to ameliorate the disparities in incidence, prevalence and mortality in T2D among AAs is critical. One such novel risk factor is aldosterone, which has been associated with higher incidence of diabetes, cardiovascular disease (CVD) and all-cause mortality among AAs [3], [4], [5]. In 2010, the American Heart Association introduced a set of 7 cardiovascular health metrics (Life's Simple 7 [LS7] also known as ideal cardiovascular health [ICH]), divided into 4 health behaviors (smoking, weight, physical activity, and diet) and 3 health factors (BP, total cholesterol, and glycemia) [6]. The individual components are stratified into poor, intermediate and ideal levels (Supplemental Table S1) and then stratified into scoring systems. Higher attainment of ICH metrics has been associated with lower risk of incident diabetes among AAs in the Jackson Heart Study (JHS), Multi-Ethnic Study of Atherosclerosis and the REasons for Geographic and Racial Differences in Stroke (REGARDS) study [3,[7], [8], [9]]. Additionally, higher attainment of ICH was associated with lower aldosterone among AAs in the JHS [10].
While associations between ICH, aldosterone and T2D are well established, to our knowledge, there has been no investigation of the potential mediating effect of aldosterone in the association of ICH with incident T2D. Given the underlying physiological importance of aldosterone in the development or aberration of insulin resistance and beta cell dysfunction [11], [12], [13], [14], it is plausible that a primary mechanism by which higher ICH lowers incident T2D is mediated via reductions in aldosterone. To evaluate this hypothesis, we examined aldosterone as a mediator in the association of ICH with incident T2D among AAs in the JHS (Fig. 1, Panel A). Additionally, we analyzed the association of aldosterone with incident T2D among AAs with blood pressure and glucose as mediators (Fig. 1, Panel B).
Fig. 1.
Conceptual Model.
Panel A shows the association of 5 components of American Heart Association's Life's Simple 7 with incident type 2 diabetes and mediation by aldosterone. Panel B shows the association of aldosterone with incident type 2 diabetes and mediation by blood pressure and glucose. The figure was created with BioRender.com.
2. Methods
2.1. Study participants
The JHS is a prospective study of the development and progression of CVD in a cohort of 5306 AA adults, aged 21–94 years from the tri-county area of the Jackson, Mississippi metropolitan area. Enrollment and baseline examinations were performed 2000-2004 with two in-person follow-up examinations in 2005–2008 and 2009–2013. Details about the study design, recruitment and methods have been described elsewhere [15]. Participants were excluded for known diabetes at baseline (n = 1152), missing data on baseline diabetes status (n = 66), ICH metrics (n = 721), aldosterone (n = 6), covariates (n = 18) or incident diabetes status (n = 552). After these exclusions, 2791 participants were included in the analytic cohort (Supplemental Figure S1). The study was approved by the institutional review boards of the University of Mississippi Medical Center, Jackson State University, and Tougaloo College. All participants provided informed consent.
2.2. Exposures
The primary exposure was ICH, assessed using five of the seven ICH metrics which included cigarette smoking status, diet, physical activity (PA), body mass index (BMI), and total cholesterol. Blood pressure (BP) and glucose metrics were excluded from the primary mediation analysis as they are downstream of aldosterone in the conceptual model, but are included as mediators of the aldosterone to incident diabetes analysis [Fig. 1] [16], [17], [18].
2.3. Ascertainment of ICH metrics
2.3.1. Total cholesterol
Total cholesterol was measured by the cholesterol oxidase method (Roche COBAS Fara analyzer; Roche Diagnostics), as previously described [19]. Ideal total cholesterol was defined as an untreated total cholesterol <200 mg/dL; intermediate total cholesterol defined by a level of 200 to 239 mg/dL or if treated with anti-hyperlipidemic medications to goal (<200 mg/dL); and poor was defined by a level of ≥240 mg/dL [6].
2.3.2. Body mass index
BMI was calculated by dividing weight (kilograms) by the square of height (meters). BMI <25 kg/m2 was considered ideal; 25 to 29.9 kg/m2 was intermediate; and ≥30 kg/m2 was poor [6].
2.3.3. Physical activity
PA was assessed using an interviewer-administered PA questionnaire at baseline, modified from the Baeke PA survey [20]. This instrument was identical to the one used during the Kaiser Physical Activity survey, which showed good validity and reliability in a multiethnic sample [21]. Exercise was reported by the average amount of time per week spent, and metabolic equivalent levels were defined for each activity. Moderate activity was defined as 3 to 6 metabolic equivalents and vigorous activity as >6 metabolic equivalents. Ideal PA was defined as ≥150 min of moderate-intensity activity or ≥75 min of vigorous activity or ≥150 min of combined moderate-intensity and vigorous activity per week; intermediate PA was defined as 1 to 149 min of moderate-intensity activity or 1 to 74 min of vigorous activity or 1 to 149 min of combined moderate-intensity and vigorous activity per week; poor PA was defined as no amount of activity (0 min per week) [6].
2.3.4. Smoking
Cigarette smoking was self-reported, and participants were asked about the quantity and duration of smoking. Participants who had never smoked or who quit smoking more than 12 months preceding the clinic visit were considered ideal; former smokers who quit smoking within 12 months of the clinic visit were considered intermediate; and current smokers were considered poor [6].
2.3.5. Diet
In the JHS, dietary intake was assessed using the Delta Nutrition Intervention Research Initiative food frequency questionnaire with 158 items administered in-person by trained AA interviewers [22,23]. The questionnaire had some slight differences compared with the AHA categorization regarding units of servings, requiring modification of the metrics, as has been done previously [6,8,24]. The dietary components used to compute the AHA score, based on a 2000-kcal diet, were: fruits and vegetables of 4.5 cups/d or more, two 3.5-oz servings or more per week of non-fried fish, fiber-rich whole grains of three 1-oz-equivalent servings/d or more, sodium less than 1500 mg/d, and sugar sweetened beverages of 450 kcal/week or less (36 oz). Participants were given one point per dietary component at goal for a total score ranging from 0 to 5. Ideal diet was defined by a diet including 4 to 5 components; intermediate diet, 2 to 3 components; and poor diet, 0 to 1 component [6].
2.3.6. Ideal cardiovascular health (ICH)
Each baseline metric was evaluated separately using poor, intermediate, and ideal categories [7]. Based on preclinical and clinical studies we hypothesized that BMI, dietary intake, smoking, physical activity and total cholesterol are upstream of aldosterone and impact levels of serum aldosterone, whereas BP and glucose are downstream of aldosterone [12,16,17,25]. Based on this we excluded BP and glucose from the score categorization, and thus included 5 components in the ICH score. Categorical scores were calculated by summing the number of ideal levels of ICH metrics each participant attained at baseline and categorizing participants into 0–2 ICH versus 3+ ICH metrics, similar to prior analyses [26,27]. Sensitivity analyses were performed with participants categorized 0–1, 2 and 3+ ICH metrics.
2.4. Mediator
2.4.1. Aldosterone
Fasting blood samples were drawn at baseline in the supine position and processed using a standardized protocol. Plasma and serum were prepared from samples by sedimentation in a refrigerated centrifuge within two hours of blood collection, stored at −70 °C and sent to central laboratories (University of Minnesota) [15,19]. Serum aldosterone was measured by radioimmunoassay (Siemens) and the intra-assay coefficients of variation were 8.7% and 6.2% for low and high concentrations, respectively.
2.4.2. Blood pressure
BP was measured twice at 5-minute intervals by using Hawksley random zero sphygmomanometer in sitting position and averaged for analyses at baseline. Ideal BP was defined as untreated systolic BP (SBP) <120 mmHg and diastolic BP (DBP) <80 mmHg; intermediate BP was defined as systolic BP of 120–139 mmHg or diastolic BP of 80–89 mmHg, or if treated with antihypertensive medications to goal (<120/<80 mmHg); and poor BP was defined as systolic BP of ≥140 mmHg or a diastolic BP ≥90 mmHg [6,7].
2.4.3. Glucose
At baseline, fasting plasma glucose was measured by the glucose oxidase colorimetric method using a Vitros 950 or 250 (Ortho Clinical Diagnostics analyzer; Ortho Clinical Diagnostics, Raritan, NJ). Ideal glucose was defined as an untreated fasting plasma glucose of <100 mg/dl; intermediate glucose was defined as a fasting plasma glucose of 100–125 mg/dL untreated or if treated to goal with anti-hyperglycemic medications (<100 mg/dL); and poor was defined as a fasting plasma glucose of ≥126 mg/dL [6].
2.5. Outcome
2.5.1. Incident diabetes
T2D was defined as HbA1c ≥ 6.5%, fasting blood glucose ≥ 126 mg/dl, taking diabetes medications or with a self-reported physician diagnosis [28].
2.6. Covariates
Baseline information was obtained during clinic visit of exam 1 or at home using standardized questionnaires including: demographics (age, sex), occupation (management/professional versus not), level of education (<high school versus ≥high school), alcohol use (in the past 12 months versus not), and current prescription medication usage. Estimated glomerular filtration rate (eGFR) was derived using the Chronic Kidney Disease Epidemiology Collaboration equation (ml/min/1.73 m2) [29]. EGFR was included as a covariate due to an inverse association with aldosterone [30].
2.7. Statistical analysis
Baseline characteristics of participants were summarized in ICH 0–2 vs. ≥ 3 categories using Student's t-test for normally distributed continuous variables, Mann-Whitney U test for non-normally distributed continuous variables, and Chi-square test for categorical variables.
2.8. Survival analyses
The time of incident diabetes was defined as the midpoint between the last examination without diabetes and the examination at which diabetes developed among participants without diabetes at exam 1. For participants who remained free of diabetes, the follow-up time was censored at their last available visit. Kaplan-Meier curves were generated to compare time (years) to diabetes between 0 and 2 ICH and ≥ 3 ICH categories. Cox proportional-hazards regression models were used to model the association of ICH metrics with incident diabetes adjusted for age, sex, education, occupation, alcohol use, and estimated glomerular filtration rate (eGFR).
2.9. Mediation analyses
To examine the role of aldosterone as a potential mediator in the pathway between ICH metrics and incident T2D, mediation analyses were performed using the “mediation” R package [31]. Aldosterone plays a central role in the homeostatic regulation of BP [16,17], modulating the reabsorption of sodium in the kidneys, thereby indirectly influencing water balance, BP, and blood volume. Aldosterone has been shown to exert its effects via mineralocorticoid receptors and G-protein estrogen receptors and results in vasoconstriction and sodium and water retention causing increased BP. Aldosterone is well established in the pathophysiology of BP, thus we excluded BP from ICH metrics while examining the mediation effect of aldosterone in incident T2D. Recent and accumulating evidence emphasizes the significance of aldosterone in glucose metabolism and metabolic syndrome [16]. Aldosterone excess may impair insulin secretion and insulin action [32]. Underlying mechanisms linking aldosterone and insulin resistance are related to the inhibitory effects of aldosterone on insulin signaling and insulin-stimulated glucose uptake via glut-4 translocation in adipocytes, skeletal muscle, and vascular smooth muscle cells, as well as a reduction in adiponectin and peroxisome proliferator-activated receptor-gamma [12,33]. Epidemiologic data supporting the association of aldosterone with T2D has been published in various racial/ethnic groups [7,8,18]. Given that aldosterone influences glucose, we excluded glucose from the primary mediation analysis of aldosterone in the association of ICH with incident T2D.
To examine the role of aldosterone as a potential mediator in the pathway between ICH metrics and incident diabetes, mediation analyses were performed using the “mediation” R package [31]. First, a linear regression model was used to assess the cross-sectional association between the exposure (ICH) and mediator (Aldosterone) which was used as the first argument of the mediate function (model.m, class=”lm”). Next, accelerated failure time models with a Weibull distribution were used instead of Cox proportional hazard models for the survival analyses to obtain estimated mean effects for incident diabetes. This model was used as the second argument of the mediate function (model.y, class=”survreg”). The hazard ratios from this model approximate those from the Cox proportional-hazards regression as described previously [31]. The R package “mediation” uses continuous or dichotomous treatment effects for the treat argument, thus, ICH metrics were categorized as 0–2 and ≥ 3 ICH score category. Finally, statistical significance of Average Causal Mediated Effects, Average Direct Effects, and Proportion Mediated were assessed by quasi-Bayesian approximation with 1000 Monte Carlo draws. Importantly, because blood glucose and BP are thought to be directly affected by aldosterone levels, both were excluded from the mediation analysis [[17], [32], [34]]. The proportion of ≥ 3 ICH category participants calculated without BP and glucose (15.3%) approximated that in the original set with ≥ 4 out of 7 ICH metrics (16.5%). In order to examine potential mediation effects of glucose and BP, we performed additional mediational analysis using dichotomous metrics treating glucose and BP as mediators in the pathway between aldosterone and diabetes. Given that the associations may vary by age, sex, and eGFR, we tested for effect modification by inserting an interaction term in the models and used the likelihood ratio test. To confirm the robustness of the findings, sensitivity analyses were performed with ICH categorized 0–1 (referent group), 2 and 3+ ICH metrics. Statistical significance was defined as two-sided p < 0.05 and p < 0.10 for interaction terms. Analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and R version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria) with mediation package.
3. Results
The characteristics of the participants included in this study across categories of ICH are presented in Table 1. Among 2791 adults (mean age 53 ± 12 years, 65% female), 2364 and 427 had 0–2 vs. ≥ 3 ICH components at baseline. Participants in ≥ 3 ICH category had higher levels of education, professional occupation status, renal function, along with lower levels of fasting glucose, systolic BP, BMI, and total cholesterol compared to participants in 0–2 ICH category at baseline (all p<0.01). Median serum aldosterone levels were 4.30 and 4.00 ng/dL in the 0–2 and ≥ 3 ICH categories, respectively (p = 0.053). The findings were similar with the 0–1, 2 and ≥ 3 ICH categorization (Supplemental Table S2). Over a median follow-up of 7.5 years, there were 447 and 50 incident diabetes cases in the 0–2 and ≥ 3 ICH categories, respectively.
Table 1.
Baseline Characteristics of Participants in the Jackson Heart Study by Ideal Cardiovascular Health Status 0–2 vs. ≥ 3.
| Variable Mean (SD)/n (%) |
All (n = 2791) |
0–2 ICH (n = 2364) | 3+ ICH (n = 427) | p-valuea |
|---|---|---|---|---|
| Age | 53 (12) | 54 (12) | 49 (13) | <0.001 |
| Sex | ||||
| Female | 1801 (64.5%) | 1549 (65.5%) | 252 (59%) | 0.0097 |
| Male | 990 (35.5%) | 815 (34.5%) | 175 (41%) | |
| Education | <0.001 | |||
| < high school | 366 (13.1%) | 335 (14.2%) | 31 (7.3%) | |
| ≥ high school | 2425 (86.9%) | 2029 (85.8%) | 396 (92.7%) | |
| Occupation | <0.001 | |||
| Management/Professional | 1133 (40.6%) | 912 (38.6%) | 221 (51.8%) | |
| Other | 1658 (59.4%) | 1452 (61.4%) | 206 (48.2%) | |
| Alcohol Use | <0.001 | |||
| No | 1430 (51.2%) | 1249 (52.8%) | 181 (42.4%) | |
| Yes | 1361 (48.8%) | 1115 (47.2%) | 246 (57.6%) | |
| Body Mass Index (kg/m2) | 31.2 (7.0) | 31.9 (6.9) | 27.7 (6.4) | <0.001 |
| Aldosterone (ng/dl)b | 4.2 [2.5, 6.9] | 4.3 [2.5, 7.1] | 4.0 [2.5, 6.3] | 0.0525 |
| Blood Pressure, mmHg | ||||
| Systolic Blood Pressure | 126 (16.0) | 126 (16) | 123 (15.6) | <0.001 |
| Diastolic Blood Pressure | 76 (8.5) | 76 (8.5) | 76 (8.6) | 0.1418 |
| Estimated Glomerular Filtration Ratec | 96.3 (19.7) | 95.7 (19.7) | 99.5 (19.6) | <0.001 |
| Fasting Plasma Glucose, mg/dL | 90 (8.9) | 91 (8.9) | 88 (8.3) | <0.001 |
| Total Cholesterol, mg/dL | 200 (38.6) | 204 (38.8) | 175 (25.9) | <0.001 |
| Ideal Cardiovascular Health Metrics | ||||
| Physical Activity | 605 (21.7%) | 309 (13.1%) | 296 (69.3%) | <0.001 |
| Blood Pressure | 675 (24.2%) | 509 (21.5%) | 166 (38.9%) | <0.001 |
| Cholesterol | 1314 (47.1%) | 921 (39%) | 393 (92%) | <0.001 |
| Glucose | 1534 (55%) | 1219 (51.6%) | 315 (73.8%) | <0.001 |
| Body Mass Index | 413 (14.8%) | 208 (8.8%) | 205 (48%) | <0.001 |
| Smoking | 2464 (88.3%) | 2045 (86.5%) | 419 (98.1%) | <0.001 |
| Nutrition | 22 (0.8%) | 7 (0.3%) | 15 (3.5%) | <0.001 |
p-value obtained using Students’ t-test for continuous variables and Chi-square test for categorical variables.
Median (IQR) and Wilcoxon rank sum test was used for aldosterone.
Estimated Glomerular Filtration Rate = Estimated glomerular filtration rate based on the Chronic Kidney Disease Epidemiology Collaboration (ml/min per 1.73 m2).
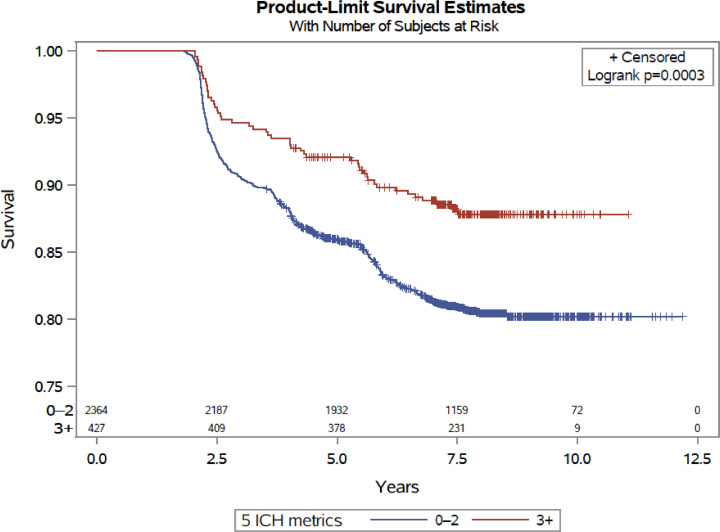
In Table 2, baseline attainment of ≥ 3 vs 0–2 ICH components was associated with a 37% lower risk of diabetes (HR 0.63, 95%CI 0.47, 0.84; p = 0.002). In the Kaplan-Meier curve, the risk of T2D was consistently lower in the ≥ 3 ICH category compared to 0–2 category (p<0.001) (Fig. 2). In the mediation analysis, 6.98% of the effect of the five ICH metrics (BMI, total cholesterol, physical activity, diet, and smoking) on incident diabetes was mediated through aldosterone (p = 0.006).
Table 2.
The Association of Ideal Cardiovascular Health with Incident Diabetes and Aldosterone as a Mediator.
| Baseline Ideal Cardiovascular Health Metrics | Hazard Ratio (95% CI) | p-valuea |
|---|---|---|
| 0–2 | 1 (referent) | |
| ≥ 3 | 0.63 (0.47, 0.84) | 0.002 |
| Percentage mediated (%), (95% CI) | p-valueb | |
|---|---|---|
| Aldosterone as mediator | 6.98 (1.8, 18.0) | 0.006 |
Adjusted for age, sex, education, occupation, alcohol use, and estimated glomerular filtration rate.
p-value is obtained using Cox proportional hazards model.
p-value is obtained using mediation analysis
Interpretation:
- Baseline attainment of ≥ 3 vs 0–2 ICH components was associated with a 37% lower risk of diabetes over a median of 7.5 years (p = 0.002).
- 6.98% of the effect of the five ICH metrics on incident diabetes was mediated through aldosterone (p = 0.006).
Fig. 2.
Kaplan-Meier Plot for 0–2 vs. 3+ Ideal Cardiovascular Health metrics and Incident Diabetes.
Incident diabetes curves for participants based on their baseline ICH score category. Blue curve indicates risk of incident diabetes in 0–2 ICH score category and red curve is the risk of incident diabetes in ≥3 ICH category. The risk of incident diabetes is consistently lower in the ≥ 3 ICH category compared to 0–2 category.
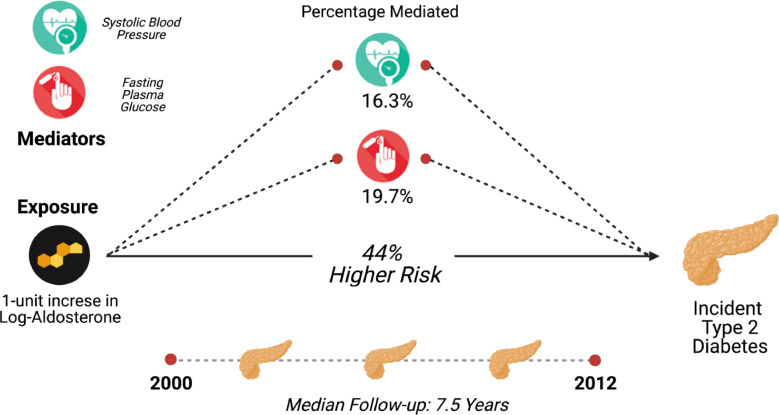
In Table 3, we explore the association of aldosterone with incident diabetes and the mediational role of BP and fasting glucose. A 1-unit increase in log-aldosterone was associated with a 44% higher risk of diabetes (HR 1.44, 95%CI 1.25–1.64) (Central Illustration). In analysis using aldosterone as exposure and BP or fasting glucose as the mediators, 16.3% (p<0.001) and 19.7% (p = 0.002) of the effect of aldosterone on incident diabetes was mediated through BP and fasting glucose, respectively. (Fig. 3 and Table 3).
Table 3.
The Association of Aldosterone with Incident Diabetes and the Mediational Role of Blood Pressure and Fasting Glucose.
| Hazard Ratio (95% CI) | p-valuea | |
|---|---|---|
| Per 1-unit increase in log-Aldosterone | 1.44 (1.25, 1.64) | <0.001 |
| Percentage mediated (%) | p-valueb | |
|---|---|---|
| Blood Pressure (Mediator) | 16.3 (7.0, 31.0) | <0.001 |
| Fasting Glucose (Mediator) | 19.7 (6.5, 34.0) | 0.002 |
Adjusted for age, sex, education, occupation, alcohol use and eGFR.
p-value is obtained using Cox proportional hazards model.
p-values are obtained using mediation analysis
Interpretation:
A 1-unit increase in log-aldosterone was associated with a 44% higher risk of diabetes (p<0.001). The interpretation with conversion to aldosterone is a that a 10% higher aldosterone was associated with 3.5% higher risk of diabetes. 16.3% (p<0.001) of the effect of aldosterone on incident diabetes is mediated through blood pressure and 19.7% (p = 0.002) is mediated through fasting glucose.
Central Illustration.
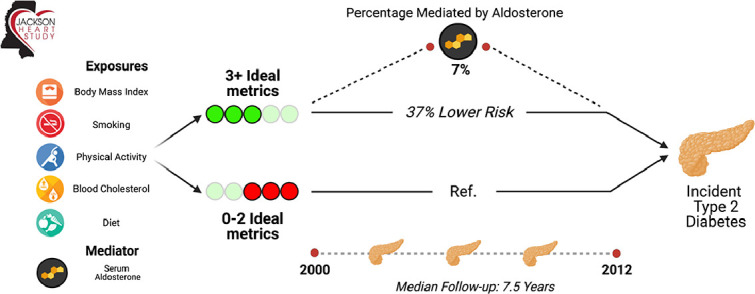
The Association of Ideal Cardiovascular Health Metrics (excluding BP and glucose) with Incident Diabetes Pathway with Aldosterone as a Mediator.
The 5 ideal cardiovascular health (ICH) metrics include weight, smoking status, diet, cholesterol and physical activity. Aldosterone mediates 6.98% of the effect in the risk attributed by ICH on incident diabetes. The figure was created with BioRender.com.
Fig. 3.
The Association of Aldosterone with Incident Diabetes Pathway with Blood Pressure and Glucose as mediators. Blood pressure and glucose mediate 16.3% and 19.7% of the effect in the risk attributed by aldosterone on incident diabetes, respectively. The figure was created with BioRender.com.
In Table 4, we found evidence of significant interactions by age, sex, and eGFR for the association of ≥ 3 vs 0–2 ICH components with incident diabetes. There was greater magnitude of the association in women vs. men. Using the medians for age and eGFR, there was greater magnitude of association in the groups with age greater than 53 years compared to less than or equal to 53 years, and eGFR less than or equal to 97 ml/min per 1.73 m2 compared to greater than 97 ml/min per 1.73 m2. For the mediation analyses, the mediation effect of aldosterone was larger for patients with eGFR below the median (p = 0.036), with no differences by age or sex (Table 5). Sensitivity analyses using a 3-level categorization of ICH metrics 0–1, 2 and ≥ 3 (Supplemental Table S3), showed similar findings to the 2-level categorization 0–2 vs. ≥ 3. Participants with 2 ICH metrics had a 20% lower risk of diabetes compared to participants with 0–1 ICH metrics (p = 0.017), aldosterone mediated 7.94% of the effect (p<0.001). Participants with ≥ 3 ICH metrics had a 44% lower risk of diabetes compared to participants with 0–1 ICH metrics (p<0.001), aldosterone mediated 8.22% of the effect (p<0.001).
Table 4.
The association of 3+ vs. 0–2 Ideal Cardiovascular Health Metrics with Incident Diabetes Stratified by Age, Sex, and Estimated Glomerular Filtration Rate.
| Hazard Ratios (95% CI) | Ideal vs non-ideal | p-value* |
|---|---|---|
| All participants | 0.63 (0.47, 0.84) | |
| Sex | 0.0058 | |
| Men (N = 990) | 0.90 (0.61, 1.33) | |
| Women (N = 1801) | 0.39 (0.25, 0.61) | |
| Age (median=53 years) | 0.0338 | |
| ≤median (N = 1389) | 0.80 (0.56, 1.15) | |
| >median (N = 1402) | 0.40 (0.24, 0.68) | |
| Estimated Glomerular Filtration Rate (median=97 ml/min per 1.73 m2) |
||
| ≤median (N = 1395) | 0.43 (0.27, 0.70) | 0.0625 |
| >median (N = 1396) | 0.77 (0.53, 1.12) |
p-value for the interactions.
Significant interactions existed for sex, age and estimated glomerular filtration rate in the association of ideal vs. non-ideal cardiovascular health with incident diabetes (all p<0.10). There was greater magnitude of the association in women vs. men, age greater than 53 years compared to less than or equal to 53 years and estimated glomerular filtration rate less than or equal to 97 ml/min per 1.73 m2 compared to greater than 97 ml/min per 1.73 m2. For instance, the 3+ ideal cardiovascular health metrics in women was associated with a 61% lower risk of incident diabetes compared those in the 0–2 group.
Table 5.
Percent Mediation of 3+ vs. 0–2 Ideal Cardiovascular Health Metrics by Aldosterone, Stratified by Age, Sex, and Estimated Glomerular Filtration Rate.
| Mediation analysis | % mediated | Estimated effects | p-value* | Estimated difference Men – Women or ≤median - >median |
||
|---|---|---|---|---|---|---|
| ADEa | ACMEb | ADE | ACME | |||
| All participants | 6.98 | 12.21 (3.87, 22.33) | 0.9 (0.23, 1.78) | 0.006 | ||
| Sex | ||||||
| Men (N = 990) | 10.7 | 1.46 (−6.84, 11.27) | 0.84 (−0.002, 2.13) | 0.644 | −29.65 (−53.82, −12.97) P = 0.02 |
−0.32 (−2.36, 1.88) p = 0.68 |
| Women (N = 1801) | 2.76 | 31.34 (12.55, 54.57) | 0.94 (−0.13, 2.46) | 0.1 | ||
| Age (median=53) | ||||||
| ≤median (N = 1389) | 11.7 | 4.98 (−4.84, 16.27) | 1 (0.05, 2.5) | 0.29 | −22.5 (−47.1, −3.39) P<0.001 |
0.38 (−1.79, 2.74) p = 0.72 |
| >median (N = 1402) | 1.67 | 26.99 (9.31, 50.72) | 0.47 (−0.64, 1.84) | 0.38 | ||
| Estimated Glomerular Filtration Rate (median=97 ml/min per 1.73 m2) | ||||||
| ≤median (N = 1395) | 6.17 | 22.57 (6.84, 42.63) | 1.53 (0.14, 3.41) | 0.036 | 14.06 (−2.67, 34.57) p = 0.14 |
2.05(−0.17. 4.5) p = 0.08 |
| >median (N = 1396) | 0.44 | 7.45 (−2.33, 19.02) | 0.08 (−0.39, 0.65) | 0.78 | ||
p-value for mediated effects.
Average Direct Effect.
Average Causal Mediation effect
Interpretation: Overall, 6.98% of the effect of ideal cardiovascular health (3+ vs. 0–2) on incident diabetes are estimated to be mediated by aldosterone (p = 0.006). Examining the interactions, the average direct effects were significantly different by sex and age groups (p = 0.020 and <0.001, respectively), but the average causal mediation effects were similar. The mediation effect of aldosterone is larger for patients with EGFR below the median (p = 0.036).
4. Discussion
In this novel analysis in a prospective cohort of AAs, this is the first report of the mediational role of aldosterone in the association of ICH with incident diabetes. Of the 37% lower risk of incident T2D over a median of 7.5 years among individuals with ≥ 3 ICH metrics at baseline compared to 0–2 ICH metrics, 7% of the effect was mediated by aldosterone. Thus, aldosterone represents one of the potential underlying physiological mediators that drive the impact of ICH on diabetes risk. Additionally, BP and glucose mediate 16% and 20% of the effect in the pathway of aldosterone on risk of incident T2D, respectively. These findings suggest that aldosterone is implicated in mediating the effect of ICH (physical activity, diet, cholesterol, BMI, smoking) on incident diabetes and aldosterone's effect on incident diabetes may be partially mediated by BP and glucose.
In recent years, aldosterone has gained significant attention as a contributor to various diseases including diabetes, CVD, hypertension, kidney disease, and obesity [16]. Aldosterone causes inflammatory and fibrotic changes in the heart, kidneys, vasculature and adipose tissue [35]. Lower attainment of ICH also impacts similar organs and disease states [36]. The RAAS system is targeted in cardiovascular and renal pathophysiological states; further insights into its role in metabolic disease may advance the development of new strategies to manage diabetes. We assessed the effects of aldosterone as mediator in the pathway for ICH and incident diabetes in this novel study. The risk of incident T2D was 33% lower in ≥3 ICH category compared to the 0–2 ICH category (Table 2). This is consistent with prior studies among AAs in JHS and across other ethnicities [7,8]. This emphasizes the protective role of ICH on the development of diabetes. In the conceptual model for the primary mediation analysis, BP and glucose were excluded due to the known effect of aldosterone increasing BP and altering glucose [3].
To our knowledge, this is the first study to explore mediating factors in the association between ICH and incident diabetes. Previously, a study of middle-aged Europeans found that the mediating effect of BMI, alcohol consumption, hypertension, triglycerides, HDL-cholesterol, physical activity, and smoking in the association between educational attainment and lower risk of incident diabetes ranged from 1.0% to 17.7% with BMI having the greatest effect [37]. Another study in the Framingham Offspring Study found that metabolic risk factors (waist circumference, triglycerides, HDL cholesterol, blood pressure, and blood glucose) and genetic risk scores mediated 11% and 9% of diabetes risk for individuals with a parental history of diabetes, respectively [38]. Thus, aldosterone mediating 7% of the effect of ICH attainment on incident diabetes (Central Illustration) is comparable to the mediation of established risk factors and further elucidates biological mechanisms in type 2 diabetes prevention.
Since aldosterone is known to alter BP and glucose levels (BP and glucose are downstream of aldosterone), we examined the mediation effects of BP and plasma glucose in the pathway of aldosterone and incident diabetes. Sixteen percent and 20% of the effect of aldosterone on incident T2D is mediated by BP and glucose, respectively (Fig. 3). Thus, aldosterone exerts part of its effect on incident T2D through altering BP and glucose. High blood pressure is known to induce microvascular and endothelial dysfunction, which are both tied to insulin resistance, as one pathway of increasing diabetes risk [39,40]. Aldosterone can decrease beta-cell function and impair insulin sensitivity as has been reviewed previously [3]. The observational findings presented here provide further evidence for human clinical studies with detailed metabolic assessment to further delineate the role of aldosterone in cardiometabolic disease.
4.1. Strengths and limitations
Strengths of our analysis include use of a large AA cohort with longitudinal data available on aldosterone, diabetes and ICH, comprehensive ascertainment of diabetes status (using A1C, glucose value, medications and self-reported physician diagnosis). This study is not without limitations. Given that the JHS is focused in southeastern USA, generalizability to all AAs is restricted. JHS does not include other racial/ethnic groups to allow for racial/ethnic comparisons. We were unable to include renin and other potential factors that would influence aldosterone and RAAS system due to inconsistency of the availability of data.
5. Conclusion
In conclusion, aldosterone partially mediated the association of ICH with incident diabetes among AAs. Targeting biological factors like aldosterone for primary prevention in addition to addressing primary prevention through lifestyle interventions in support of ICH may help to reduce the risk of diabetes. Further studies evaluating aldosterone and other biological factors in the development of T2D are needed for precision prevention strategies.
CRediT authorship contribution statement
Veena Kesireddy: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Visualization. Bjorn Kluwe: Methodology, Writing – original draft, Writing – review & editing, Visualization. Neal Pohlman: Conceptualization, Writing – review & editing, Visualization. Songzhu Zhao: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data curation, Writing – review & editing, Visualization. Yubo Tan: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data curation, Writing – review & editing, Visualization. David Kline: Conceptualization, Methodology, Data curation, Writing – review & editing, Supervision, Project administration. Guy Brock: Conceptualization, Methodology, Data curation, Writing – review & editing, Supervision, Project administration. James B. Odei: Conceptualization, Methodology, Data curation, Writing – review & editing, Supervision, Project administration. Valery S. Effoe: Conceptualization, Methodology, Writing – review & editing, Visualization. Justin B. Echouffo-Tcheugui: Conceptualization, Methodology, Writing – review & editing, Visualization. Rita R. Kalyani: Conceptualization, Methodology, Writing – review & editing, Visualization. Mario Sims: Conceptualization, Methodology, Writing – review & editing, Visualization. Herman A. Taylor: Conceptualization, Methodology, Writing – review & editing, Visualization. Morgana Mongraw-Chaffin: Conceptualization, Methodology, Writing – review & editing, Visualization. Ehimare Akhabue: Conceptualization, Methodology, Writing – review & editing, Visualization. Joshua J. Joseph: Conceptualization, Methodology, Investigation, Resources, Writing – original draft, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Disclosure statement
Nothing to Disclose
Acknowledgements
The Jackson Heart Study (JHS) is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I/HHSN26800001) and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute for Minority Health and Health Disparities (NIMHD). Preparation of this manuscript was supported by The Robert Wood Johnson Foundation (Harold Amos Medical Faculty Development Program ID# 76236) and the National Institute of Diabetes and Digestive and Kidney Diseases (K23DK117041, ⁎⁎⁎) of the National Institutes of Health and the Roessler Research Scholarship through the Ohio State College of Medicine (⁎⁎⁎). The authors also wish to thank the staff and participants of the JHS. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Footnotes
Fellowship supporting the writing of the paper: Veena Kesireddy, MD was supported by The Division of Endocrinology, Diabetes and Metabolism at The Ohio State University Wexner Medical Center.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2023.100466.
Appendix. Supplementary materials
References
- 1.Geiss L.S., Wang J., Cheng Y.J., et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980-2012. JAMA. 2014;312(12):1218. doi: 10.1001/jama.2014.11494. [DOI] [PubMed] [Google Scholar]
- 2.Spanakis E.K., Golden S.H. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep. 2013;13(6):814–823. doi: 10.1007/s11892-013-0421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joseph J.J., Echouffo-Tcheugui J.B., Kalyani R.R., et al. Aldosterone, renin, and diabetes mellitus in african americans: the Jackson heart study. J Clin Endocrinol Metab. 2016;101(4):1770–1778. doi: 10.1210/jc.2016-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joseph J.J., Echouffo-Tcheugui J.B., Kalyani R.R., Yeh H.C., Bertoni A.G., Effoe V.S., et al. Aldosterone, renin, cardiovascular events, and all-cause mortality among african americans: the Jackson heart study. JACC: Heart Fail. 2017;5(9):642–651. doi: 10.1016/j.jchf.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joseph J.J., Echouffo Tcheugui J.B., Effoe V.S., Hsueh W.A., Allison M.A., Golden S.H. Renin-angiotensin-aldosterone system, glucose metabolism and incident type 2 diabetes mellitus: MESA. J Am Heart Assoc. 2018;7(17) doi: 10.1161/JAHA.118.009890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lloyd-Jones D.M., Hong Y., Labarthe D., et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the american heart association's strategic impact goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 7.Joseph J.J., Echouffo-Tcheugui J.B., Carnethon M.R., et al. The association of ideal cardiovascular health with incident type 2 diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis. Diabetologia. 2016;59(9):1893–1903. doi: 10.1007/s00125-016-4003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Effoe V.S., Carnethon M.R., Echouffo-Tcheugui J.B., et al. The American Heart Association ideal cardiovascular health and incident type 2 diabetes mellitus among blacks: the Jackson Heart Study. J Am Heart Assoc. 2017;6(6) doi: 10.1161/JAHA.116.005008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joseph J.J., Bennett A., Echouffo Tcheugui, Effoe V.S., Odei J.B. Ideal cardiovascular health, glycaemic status and incident type 2 diabetes mellitus: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Diabetologia. 2019;62(3):426–437. doi: 10.1007/s00125-018-4792-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kesireddy V., Tan Y., Kline D., et al. The association of life's simple 7 with aldosterone among African Americans in the Jackson Heart Study. Nutrients. 2019;11(5):955. doi: 10.3390/nu11050955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen F., Liu J., Wang Y., et al. Aldosterone induces clonal β-cell failure through glucocorticoid receptor. Sci Rep. 2015;5(1):13215. doi: 10.1038/srep13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Underwood P.C., Adler G.K. The renin angiotensin aldosterone system and insulin resistance in humans. Curr Hypertens Rep. 2013;15(1):59–70. doi: 10.1007/s11906-012-0323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luther J.M. Effects of aldosterone on insulin sensitivity and secretion. Steroids. 2014;0:54–60. doi: 10.1016/j.steroids.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lastra-Lastra G., Sowers J.R., Restrepo-Erazo K., Manrique-Acevedo C., Lastra-González G. Role of aldosterone and angiotensin II in insulin resistance: an update. Clin Endocrinol (Oxf) 2009;71(1):1–6. doi: 10.1111/j.1365-2265.2008.03498.x. [DOI] [PubMed] [Google Scholar]
- 15.Taylor H.A., Jr, Wilson J.G., Jones D.W., et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. EthnDis. 2005;15(1049–510X):S6–17. (Print) [PubMed] [Google Scholar]
- 16.Sowers J.R., Whaley-Connell A., Epstein M. Narrative review: the emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Intern Med. 2009;150(11):776–783. doi: 10.7326/0003-4819-150-11-200906020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomaschitz A., Pilz S., Ritz E., Obermayer-Pietsch B., Pieber T.R. Aldosterone and arterial hypertension. Nat Rev Endocrinol. 2010;6(2):83–93. doi: 10.1038/nrendo.2009.263. [DOI] [PubMed] [Google Scholar]
- 18.Joseph J.J., Echouffo Tcheugui, Effoe V.S., Hsueh W.A., Allison M.A. Renin-angiotensin-aldosterone system, glucose metabolism and incident type 2 diabetes mellitus: MESA. J Am Heart Assoc. 2018;7(17) doi: 10.1161/JAHA.118.009890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carpenter M.A., Crow R., Steffes M., Rock W., Heilbraun J., Evans G., et al. Laboratory, reading center, and coordinating center data management methods in the Jackson heart study. Am J Med Sci. 2004;328(3):131–144. doi: 10.1097/00000441-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Baecke J.A., Burema J., Frijters J.E. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 21.Ainsworth B.E., Sternfeld B., Richardson M.T., Jackson K. Evaluation of the kaiser physical activity survey in women. Med Sci Sports Exerc. 2000;32(7):1327–1338. doi: 10.1097/00005768-200007000-00022. [DOI] [PubMed] [Google Scholar]
- 22.Tucker K.L., Maras J., Champagne C., et al. A regional food-frequency questionnaire for the US Mississippi Delta. Public Health Nutr. 2005;8(1):87–96. doi: 10.1079/PHN2005663. [DOI] [PubMed] [Google Scholar]
- 23.Carithers T., Dubbert P.M., Crook E., Davy B., Wyatt S.B., Bogle M.L., et al. Dietary assessment in african americans: methods used in the Jackson heart study. Ethn Dis. 2005;15(4 Suppl 6):46–55. [PubMed] [Google Scholar]
- 24.Joseph J.J., Echouffo-Tcheugui J.B., Talegawkar S.A., Effoe V.S., Okhomina V., Carnethon M.R., et al. Modifiable lifestyle risk factors and incident diabetes in african americans. Am J Prev Med. 2017;53(5):e165–e174. doi: 10.1016/j.amepre.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kesireddy V., Tan Y., Kline D., Brock G., Odei J.B., Kluwe B., et al. The association of life’s simple 7 with aldosterone among african americans in the Jackson heart study. Nutrients. 2019;11(5) doi: 10.3390/nu11050955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veromaa V., Kautiainen H., Juonala M., Rantanen A., Korhonen P.E. Self-rated health as an indicator of ideal cardiovascular health among working-aged women. Scand J Prim Health Care. 2017;35(4):322–328. doi: 10.1080/02813432.2017.1397299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spahillari A., Talegawkar S., Correa A., Carr J.J., Terry J.G., Lima J., et al. Ideal cardiovascular health, cardiovascular remodeling, and heart failure in blacks: the Jackson heart study. Circ Heart Fail. 2017;10(2) doi: 10.1161/CIRCHEARTFAILURE.116.003682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(1):S62–S67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang W., Young B.A., Fülöp T., de Boer, Boulware L.E. Effects of serum creatinine calibration on estimated renal function in african americans: the Jackson heart study. Am J Med Sci. 2015;349(5):379–384. doi: 10.1097/MAJ.0000000000000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hannemann A., Rettig R., Dittmann K., et al. Aldosterone and glomerular filtration - observations in the general population. BMC Nephrol. 2014;15(1) doi: 10.1186/1471-2369-15-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tingley D., Yamamoto T., Hirose K., Keele L., Imai K. mediation : R Package for causal mediation analysis. J Stat Softw. 2014;59(5) doi: 10.18637/jss.v059.i05. [DOI] [Google Scholar]
- 32.Corry D.B., Tuck M.L. The effect of aldosterone on glucose metabolism. Curr Hypertens Rep. 2003;5(2):106–109. doi: 10.1007/s11906-003-0065-2. [DOI] [PubMed] [Google Scholar]
- 33.Guo C., Ricchiuti V., Lian B.Q., et al. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-γ, and proinflammatory adipokines. Circulation. 2008;117(17):2253–2261. doi: 10.1161/CIRCULATIONAHA.107.748640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sowers J.R., Whaley-Connell A., Epstein M. Narrative review: the emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Intern Med. 2009;150(11):776–783. doi: 10.7326/0003-4819-150-11-200906020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown N.J. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat Rev Nephrol. 2013;9(8):459–469. doi: 10.1038/nrneph.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Younus A., Aneni E.C., Spatz E.S., et al. A systematic review of the prevalence and outcomes of ideal cardiovascular health in US and Non-US Populations. Mayo Clin Proc. 2016;91(5):649–670. doi: 10.1016/j.mayocp.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 37.Steele C.J., Schöttker B., Marshall A.H., et al. Education achievement and type 2 diabetes—What mediates the relationship in older adults? Data from the ESTHER study: a population-based cohort study. BMJ Open. 2017;7(4) doi: 10.1136/bmjopen-2016-013569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raghavan S., Porneala B., McKeown N., Fox C.S., Dupuis J., Meigs J.B. Metabolic factors and genetic risk mediate familial type 2 diabetes risk in the Framingham Heart Study. Diabetologia. 2015;58(5):988–996. doi: 10.1007/s00125-015-3498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feihl F., Liaudet L., Waeber B., Levy B.I. Hypertension: a disease of the microcirculation? Hypertension. 2006;48(6):1012–1017. doi: 10.1161/01.HYP.0000249510.20326.72. [DOI] [PubMed] [Google Scholar]
- 40.Meigs J.B., Hu F.B., Rifai N., Manson J.E. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA. 2004;291(16):1978–1986. doi: 10.1001/jama.291.16.1978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.