Significance
New therapeutics that combine efficacy with limited side effects and can be delivered noninvasively are needed to adequately treat patients with rheumatoid arthritis (RA) and other autoimmune diseases. Kv1.3 channel-expressing CCR7− effector memory T (TEM) lymphocytes are significant players in the pathogenesis of multiple autoimmune diseases, and blocking Kv1.3 reduces disease severity in rat models of RA and patients with plaque psoriasis. However, peptide therapeutics require repeated injections, reducing patient compliance. We used a bioengineered Lactobacillus reuteri as an oral delivery method of a Kv1.3 blocker for immunomodulation in rat models of atopic dermatitis and RA. This study demonstrates a novel approach for the noninvasive delivery of peptide-based therapeutics for the oral treatment of chronic inflammatory diseases.
Keywords: synthetic biology, Kv1.3 channel, drug delivery
Abstract
Engineered microbes for the delivery of biologics are a promising avenue for the treatment of various conditions such as chronic inflammatory disorders and metabolic disease. In this study, we developed a genetically engineered probiotic delivery system that delivers a peptide to the intestinal tract with high efficacy. We constructed an inducible system in the probiotic Lactobacillus reuteri to secrete the Kv1.3 potassium blocker ShK-235 (LrS235). We show that LrS235 culture supernatants block Kv1.3 currents and preferentially inhibit human T effector memory (TEM) lymphocyte proliferation in vitro. A single oral gavage of healthy rats with LrS235 resulted in sufficient functional ShK-235 in the circulation to reduce inflammation in a delayed-type hypersensitivity model of atopic dermatitis mediated by TEM cells. Furthermore, the daily oral gavage of LrS235 dramatically reduced clinical signs of disease and joint inflammation in rats with a model of rheumatoid arthritis without eliciting immunogenicity against ShK-235. This work demonstrates the efficacy of using the probiotic L. reuteri as a novel oral delivery platform for the peptide ShK-235 and provides an efficacious strategy to deliver other biologics with great translational potential.
Biologics now constitute a significant element of available medical treatments for various conditions such as chronic inflammatory disorders, cancer, and metabolic disease. Nearly 30% of all drugs approved by the U.S. Food and Drug Administration in 2015 to 2018 were biologics (1), yet the majority of biologics are administered via parenteral route because of poor oral bioavailability. Fear of needles, injection-associated infection and pain are responsible for skipping doses by patients, especially for those with chronic inflammatory diseases that often requires lifelong treatment. Rheumatoid arthritis (RA), one of the most common autoimmune diseases, mainly affects synovial joints but is also associated with a higher risk of cardiovascular, skeletal, and psychological disorders and carries a significant socioeconomic burden (2). Although current therapeutics have considerably improved the management of RA in the last decade, RA-induced reduction in lifespan has not improved and maybe even worsened (3). Current RA treatments include nonsteroidal anti-inflammatory drugs, corticosteroids, disease-modifying antirheumatic drugs, and biologic response modifiers such as antibodies targeting cytokines and their receptors (TNF-α or IL-1β, for example). These approaches focus on improving inflammation and pain control; however, they have little effect on the pathogenesis of RA and many increase the probability of infections or cancer (4). Many patients also experience significant side effects such as kidney or liver damage, leading 30 to 50% of patients to alter their treatment regimens (5, 6). Furthermore, the current biologics can be immunogenic and stimulate the generation of neutralizing antibodies after repeated drug administration (7, 8). Thus, new therapeutics that combines efficacy with limited side effects is needed to treat patients with RA adequately.
The pathogenic role of T lymphocytes in RA has been extensively studied. CCR7+ naïve and central memory T (TCM) cells are the predominant T lymphocyte populations in the circulation and lymphoid organs. In contrast, most T cells in the synovium and synovial fluid of patients with RA are CCR7− effector memory T (TEM) cells, making them a desirable therapeutic target for RA (9, 10). At rest, human and rat T lymphocytes express low levels of two K+ channels, Kv1.3 and KCa3.1, that regulate plasma membrane potential and the homeostasis of Ca2+, a crucial second messenger in T cell activation (11–13). Upon activation, CCR7− TEM cells up-regulate Kv1.3, while naïve and TCM cells up-regulate KCa3.1. Thus, TEM cells are exquisitely sensitive to inhibition by Kv1.3 blockers. On the contrary, naïve and TCM cells rely on KCa3.1 and escape Kv1.3 blockers. ShK-186 is a potent and selective peptide blocker of Kv1.3 that has been extensively tested in rats, non-human primates, healthy volunteers, and patients with a TEM cell-mediated autoimmune disease in a Phase 1A/B clinical trial (11, 12, 14, 15). The in vivo safety and efficacy of ShK-186 were demonstrated, like for other biologics, after injections. The delivery of Kv1.3-blocking peptides via the buccal mucosa showed that a transmucosal route of delivery is feasible, albeit with low efficacy (16).
Here, we report the bioengineering of a probiotic for the oral delivery of ShK-235. As ShK-186 cannot be produced recombinantly due to a non-amino acid adduct, we generated ShK-235 that can be produced recombinantly and retains the potency and selectivity of ShK-186 for Kv1.3 channels over related Kv1 channels (17). Lactobacillus reuteri is an indigenous bacteria of the human and vertebrate animal gastrointestinal (GI) tract. It is one of the lactic acid bacteria groups that has long been used as a cell factory in the food industry and is recognized as safe by the U.S. Food and Drug Administration (18). It has an excellent safety profile in infants, children, adults, and even in an immunosuppressed population (19). The strain L. reuteri ATCC PTA 6475 is a well-characterized probiotic that does not colonize but survives transit through the GI tract of humans and rodents (20). It primarily resides in the proximal GI tract but is also found in the urinary tract, skin, and breast milk (19). The benefits of L. reuteri include modulating host immune response, enhancing gut mucosal integrity, inhibiting bacteria translocation, and promoting nutrient absorption (19). With the genetic tools being developed to facilitate the engineering of L. reuteri 6475 genomes, interest in using L. reuteri as a bioengineered tool for the oral delivery of therapeutics has increased.
In this project, we bioengineered L. reuteri to generate LrS235 that secretes ShK-235. The secreted peptide is functional in blocking Kv1.3 channels and suppressing the activation of TEM lymphocytes in vitro. It crosses from the lumen of the GI tract into the circulation after the oral gavage of healthy rats and displays good bioavailability and pharmacokinetics. Treatment with LrS235 effectively reduces disease severity in a rat model of RA, including joint inflammation, cartilage destruction, and bone damage, without immunogenicity.
Results
Bioengineering of L. reuteri LJ01 to Secrete Functional ShK-235.
To generate a L. reuteri strain capable of secreting ShK-235, we codon-optimized the gene for ShK-235 for expression in L. reuteri, fused the modified usp45 signal peptide (21, 22) sequence to the 5′ end, and cloned it into the vector pSIP411 (23) resulting in plasmid pLL01. We have previously shown that the modified usp45, a signal peptide identified from Lactococcus lactis, is capable of high-level secretion of IL-22 from L. reuteri LJ01 (22). pSIP411 allows for the controlled expression of genes by the addition of a peptide pheromone that activates a promoter, based on a two-component regulatory system identified in Lactobacillus sakei (see SI Appendix, Fig. S2 A and B for details). The resulting bacterial strain containing the pLL01 was named LrS235 (L. reuteri ShK-235). As a control we used pSIP411 containing the gusA gene that encodes a β-glucoronidase (bacteria strain referred to as LrGusA thereafter). Induction of neither ShK-235 nor GusA secretion had any measurable effect on cells growth.
We assayed for the presence of active peptide by testing the ability of LrS235 supernatants to block Kv1.3 channels in mouse L929 fibroblast stably expressing mKv1.3 channels using whole-cell patch-clamp. LrS235 and LrGusA were grown to mid-exponential phase and induced for expression of ShK-235 or GusA, and supernatants were processed as described in the Materials and Methods. As an additional control, we used known concentrations of synthetic ShK-235 to plot a dose-response curve of the block of Kv1.3 (IC50 = 79 ± 21 pM; SI Appendix, Fig. S3 A and B). We next tested culture supernatants from LrS235 and LrGusA for Kv1.3 channel block and calculated the concentration of ShK-235 in supernatants using the dose-response curve. Supernatants from LrS235, but not those from LrGusA, blocked Kv1.3 currents (Fig. 1A). We calculated the mean concentration of ShK-235 in the supernatants from LrS235 to be ~450 pM (Fig. 1 A and B and SI Appendix, Fig. S3 A and B), well above the IC50 of ShK-235 for Kv1.3.
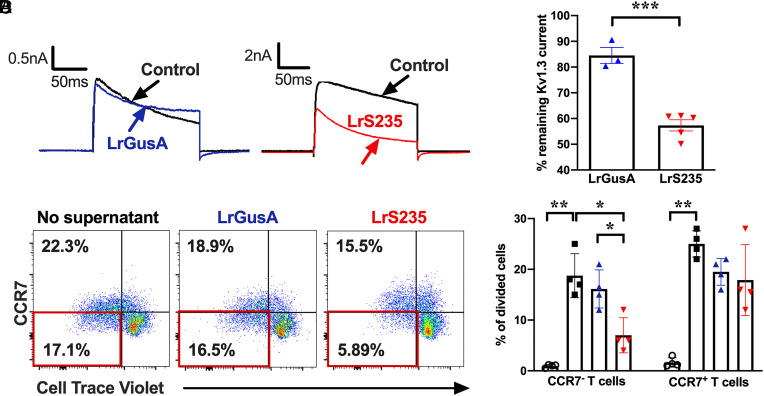
Fig. 1.
Supernatants from LrS235, but not from LrGusA, block Kv1.3 currents and inhibit the proliferation of human CCR7− TEM cells. (A) Representativewhole-cell recordings of L929 cells stably expressing mKv1.3 before (control) and after the addition of supernatants diluted 1/10 of LrGusA or LrS235. (B) Percentage of remaining mKv1.3 currents after addition of LrGusA ( ) or LrS235 (
) or LrS235 ( ) supernatants diluted 1/10. Mean ± SEM, each data point represents a different measurement. (C) Representative flow cytometry plots of CellTrace Violet dye dilution and CCR7 expression of CD3+ cells from human peripheral blood MNC stimulated for 7 d without any bacterial supernatants (Left) or in the presence of supernatants from LrGusA (Middle) or LrS235 (Right). (D) Percent of divided human CCR7− TEM and CCR7+ naïve/TCM cells in the absence of stimulation (
) supernatants diluted 1/10. Mean ± SEM, each data point represents a different measurement. (C) Representative flow cytometry plots of CellTrace Violet dye dilution and CCR7 expression of CD3+ cells from human peripheral blood MNC stimulated for 7 d without any bacterial supernatants (Left) or in the presence of supernatants from LrGusA (Middle) or LrS235 (Right). (D) Percent of divided human CCR7− TEM and CCR7+ naïve/TCM cells in the absence of stimulation ( ) and after anti-CD3 induced stimulation in the presence of Lr medium (■) or supernatants of LrGusA (
) and after anti-CD3 induced stimulation in the presence of Lr medium (■) or supernatants of LrGusA ( ) or LrS235 (
) or LrS235 ( ) diluted 1/100. Mean ± SEM, N = 4 different buffy coat donors. *P < 0.05, **P < 0.01.
) diluted 1/100. Mean ± SEM, N = 4 different buffy coat donors. *P < 0.05, **P < 0.01.
To further validate LrS235’s production of active ShK-235, we tested the ability of LrS235 supernatants to inhibit the proliferation of TEM cells. Incubation of supernatants from LrS235, but not from LrGusA, preferentially suppressed human CCR7− TEM cell proliferation by 63%, confirming the presence of biologically active and physiologically relevant ShK-235 peptide (Fig. 1 C and D).
Functional ShK-235 Is Detected in the Circulation of Healthy Rats following the Oral Administration of LrS235.
After validating that LrS235 produced and secreted functional ShK-235, we determined if oral gavage of the probiotic efficiently delivers functional ShK-235 to the circulation of rats. We first tested whether a compound of a molecular weight similar to that of ShK-235 can cross from the lumen of the GI tract into the circulation of healthy rats and rats with the collagen-induced arthritis (CIA) model of RA. The oral gavage of healthy rats or rats at the onset of CIA with 4 kDa dextran labeled with FITC showed that dextran could reach the circulation of both healthy and arthritic rats within 6 h, with a higher permeability in the latter (SI Appendix, Fig. S4).
We orally administered a single dose of 109 colony-forming units (cfu) of LrS235 or LrGusA to healthy rats by oral gavage. Six hours later, we collected sera and tested the ability of serum samples to block Kv1.3 channels by whole-cell patch-clamp. Sera from rats given LrS235, diluted 1/100, inhibited Kv1.3 currents by 24%, whereas sera from rats administered with LrGusA had no effect on Kv1.3 currents (Fig. 2 A and B). Based on the dose-response curve, the calculated concentration of LrS235 in the circulation 6 h after a single oral gavage is 7 nM, significantly higher than the IC50 for Kv1.3 block of 79 ± 21 pM (SI Appendix, Fig. S3 A and B). As controls, we encapsulated 0.3 mg ShK-235 peptide or unflavored gelatin powder into Torpac size 9 h gelatin capsules and enteric-coated these capsules with Acryl-EZE to target content delivery to the small intestine. Kv1.3 block was undetectable in both controls (Fig. 2 A and B).
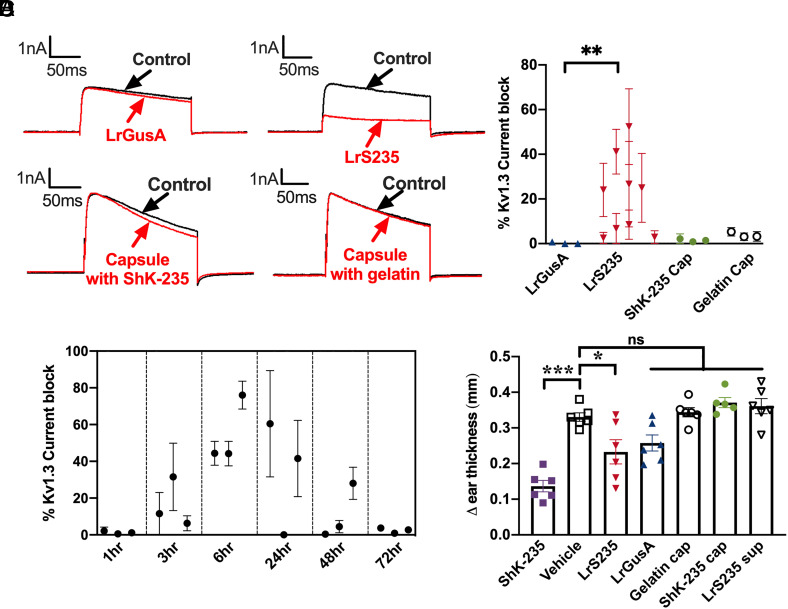
Fig. 2.
LrS235 secretes sufficient ShK-235 in the intestines for detection in the circulation of healthy rats. Healthy rats received an oral bolus of 1 × 109 cfu of LrGusA (▲) or LrS235 (▼), or an enteric-coated capsule filled with ShK-235 (●, 2 mg/kg body weight) or gelatin (o). Blood was drawn at different time points, and a single-cell patch-clamp was used to assess the ability of the serum to block Kv1.3 currents. A, Representative traces before (control) and after addition of serum diluted 1/100 from the 6-h time point. B, Current block of serum samples collected from rats at the 6-h time point. Each data point represents an individual rat. N = 3 to 4 measurements per rat. Serum dilution: 1/100. C, Current block of serum samples collected at the indicated time points. Each data point represents a rat. N = 3 to 4 measurements per rat. Serum dilution: 1/10. D, An active DTH reaction was induced against ovalbumin and rats received a single bolus of the following immediately before ear challenge: 1 × 109 cfu of LrGusA (▲) or LrS235 (▼) orally, an enteric-coated capsule filled with ShK-235 (●, 2 mg/kg body weight) or gelatin (o) orally, 1 mL of LrS235 culture supernatant orally (▽), or subcutaneous injection of 0.1 mg/kg synthetic ShK-235 (■) or vehicle (2). N = 6 rats per group (three males, three females). **P < 0.01, ***P < 0.001.
We next determined the pharmacokinetics of ShK-235 after oral delivery of LrS235 by providing LrS235 by single oral gavage (109 cfu) to healthy rats and measuring blockade of Kv1.3 channels by whole-cell patch-clamp. Serum samples were collected at various time points ranging from 1 to 72 h after the single bolus of LrS235. Peak levels of ShK-235 were detected 6 h after LrS235 delivery, with the range of functional activity from 3 h to 48 h postgavage (Fig. 2C). These results demonstrate bacteria-delivered peptides to the gut can enter the serum and remain functional.
We then assessed if LrS235 gavage could reduce a delayed-type hypersensitivity (DTH) reaction in the ear of rats, a local autoinflammation mediated by antigen-specific TEM lymphocytes (24–26). As expected, the oral gavage of LrS235 and the injection of ShK-235 significantly reduced inflammation by 30% and 58%, respectively, compared with the vehicle control (Fig. 2D). In contrast, treatment with LrGusA, capsules filled with ShK-235 or gelatin, and supernatants from the culture of the LrS235 had no significant effects on inflammation.
LrS235 Administration Stops Disease Progression and Bone and Joint Damage in CIA in Rats.
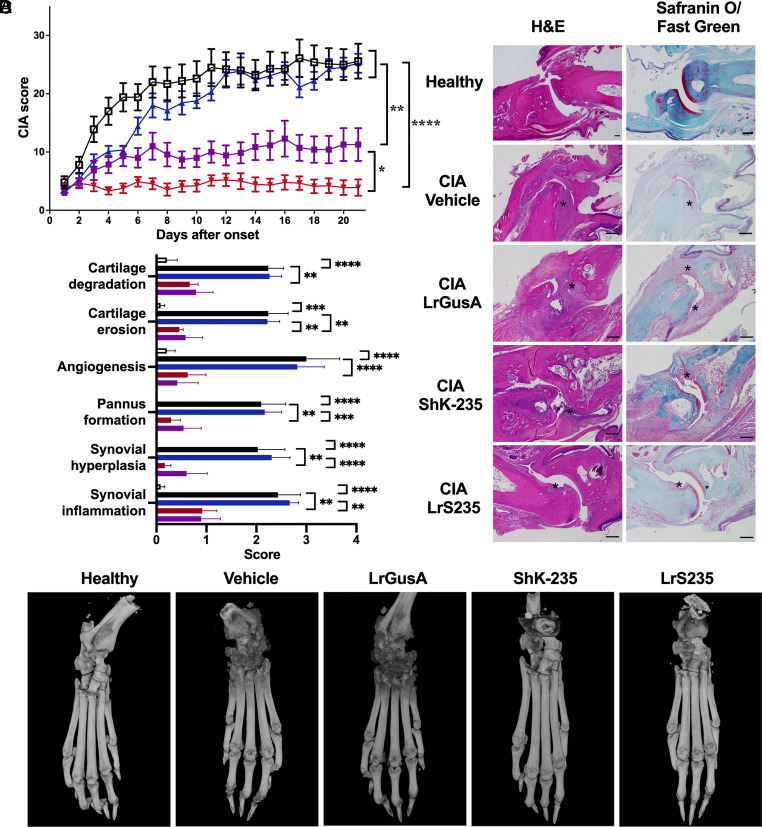
Since we detected functional ShK-235 in vitro and in the circulation of rats, we sought to assess its efficacy in a model of arthritis in Lewis rats induced by porcine collagen II. Four groups of rats were treated with P6N buffer vehicle or synthetic ShK-235 injections, or LrS235 or LrGusA by oral gavage, starting from the onset of clinical signs. All vehicle-treated animals developed severe arthritis with a mean score of 26 ± 3 (Fig. 3A). The administration of LrGusA did not affect overall disease severity (mean score 25 ± 2). In contrast, the injection of synthetic ShK-235 reduced the mean score by ~60% to 11 ± 3. The administration of LrS235 was even more effective with a mean score of only 4 ± 1, or an 84% reduction when compared to the vehicle control group. Histology performed on joints collected at the end of the in vivo trials showed severe cartilage degradation and erosion, angiogenesis, pannus formation, and synovial hyperplasia and inflammation in the CIA control rats (Fig. 3 B and C). LrGusA had no benefits in any of these parameters, whereas both synthetic ShK-235 and LrS235 significantly reduced all parameters, and no significant differences were seen between healthy controls and ShK-235 or LrS235 treated groups (SI Appendix, Table S1). Micro-CT imaging of hind limbs shows severe bone erosions in the CIA rats treated with vehicle or LrGusA and better-preserved bones in CIA rats treated with synthetic ShK-235 or LrS235 (Fig. 3D). The zoomed, axial, and pseudocoloral images are shown in SI Appendix, Fig. S5 and in Movie S1.
Fig. 3.
LrS235 stops disease progression and reduces bone and joint damage and inflammation in rats with CIA. A. Clinical scores of paw inflammation from rats with CIA treated with vehicle (2) or with 100 μg/kg ShK-235 ( ) every other day starting disease onset, 1 × 109 cfu LrGusA (
) every other day starting disease onset, 1 × 109 cfu LrGusA ( ) or LrS235 gavage daily (
) or LrS235 gavage daily ( ). B. Hematoxylin and eosin (Left) and safranin O/fast green (Right) staining and histology scoring (C) of joints from paws from CIA rats received different treatments. Original magnification, 10×, scale bars, 100 μm. Refer to “Histology and micro-CT” in the Materials and Methods for more details of the scoring system. D. Representative micro-CT of paws from CIA rats treated with vehicle, synthetic ShK-235 every other day, or oral gavage with LrGusA, LrS235 daily. Data presented as mean ± SEM. N = 7 to 10 rats per group. Asterisks indicate areas of cartilage erosions. *P < 0.05; **P < 0.01, ***P < 0.001, and ****P < 0.0001.
). B. Hematoxylin and eosin (Left) and safranin O/fast green (Right) staining and histology scoring (C) of joints from paws from CIA rats received different treatments. Original magnification, 10×, scale bars, 100 μm. Refer to “Histology and micro-CT” in the Materials and Methods for more details of the scoring system. D. Representative micro-CT of paws from CIA rats treated with vehicle, synthetic ShK-235 every other day, or oral gavage with LrGusA, LrS235 daily. Data presented as mean ± SEM. N = 7 to 10 rats per group. Asterisks indicate areas of cartilage erosions. *P < 0.05; **P < 0.01, ***P < 0.001, and ****P < 0.0001.
ShK-235 Produced by LrS235 Is Not Immunogenic.
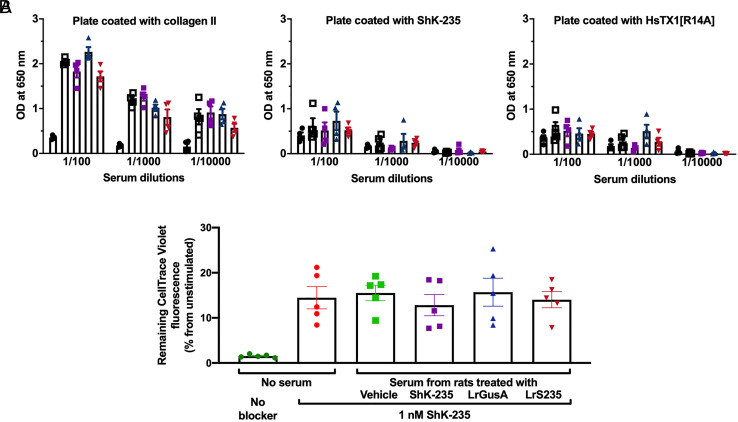
To test if ShK-235 produced by LrS235 is immunogenic, we assessed the sera of rats at the end of the 21-d CIA trial shown in Fig. 3 for anti-ShK-235 IgG by ELISA. Plates coated with collagen II and HsTX1[R14A] were used as controls. HsTX1[R14A] is another peptide blocker of Kv1.3 with no sequence or structural homology to ShK-235. As expected, we detected high titers of antibodies against collagen II in rats with CIA, but only low titers of anti-ShK-235 antibodies (Fig. 4A and SI Appendix, Fig. S6). Similar low reactivity was observed against HsTX1[R14A], suggesting that the signal detected against ShK-235 was non-specific. We also performed a neutralization assay with the same CIA rat serum samples, and no neutralization of ShK-235 was detected, showing the absence of ShK-235 neutralizing antibodies (Fig. 4B).
Fig. 4.
ShK-235, delivered via injection or LrS235, is not immunogenic A. ELISA plates were coated with 10 μg/mL of either porcine collagen II, ShK-235, or HsTX1[R14A]. Sera from rats with CIA treated with vehicle, ShK-235, LrGusA, or LrS235 were tested at tenfold dilution steps starting from a 1:100 dilution. The serum from non-immunized rats was used as a negative control. Each column represents the mean OD of four individual rat sera ± SEM. B. Data from different PBMC donors were normalized to the unstimulated cells with 100% CellTrace Violet. The higher dilution of CellTrace Violet indicates a higher division rate. Data are present as mean ± SEM.
To further investigate if long-term exposure of rats to LrS235 would elicit antibodies against ShK-235, we treated healthy rats daily with LrS235 or LrGusA for 8 wk, stopped treatment for 12 wk, and followed by additional daily gavages of either LrS235 or LrGusA for 1 wk. ELISA performed on blood samples collected either at the 8 wk or the 21 wk time points showed no IgG with specificity for ShK-235 was produced (SI Appendix, Fig. S7 A and B). This is expected as in the previous phase 1b trial for ShK-186, none of the patients who received ShK-186 by subcutaneous injection developed anti-peptide antibodies (15).
ShK-235 Does Not Block Other Potassium Channels of Importance in CIA Pathogenesis.
We had previously shown that ShK-235 exhibits a 2,250-fold selectivity for Kv1.3 over the closely related Kv1.1 channel and an even higher selectivity against Kv1.4 and Kv1.6 channels (17). T lymphocytes also express the KCa3.1 channel (a.k.a. IKCa), and block of this channel could explain some of the benefits of ShK-235 in DTH and CIA (12). In addition, joint-resident fibroblast-like synoviocytes express the KCa1.1 channel (a.k.a. BK, Maxi-K or Slo1), and blocking this channel stops disease progression in CIA (27). We therefore tested ShK-235 for its ability to block either KCa3.1 or KCa1.1. At a high concentration of 100 nM, ShK-235 inhibited less than 3% of either channels tested, showing a selectivity of ShK-235 of at least 1,000-fold (SI Appendix, Table S2).
Discussion
We have developed a bioengineered probiotic, LrS235, that secretes functional ShK-235 both in vitro and in vivo. Our engineering strategy enabled the production of an inducible peptide secretion system that fused ShK-235 to the modified usp45 secretion peptide, secretion of which can be induced upon the addition of the SppIP peptide pheromone. Oral gavage of the LrS235 daily starting at the onset of CIA in Lewis rats results in a reduction in disease severity. The protective effects of LrS235 in CIA are accompanied by a reduction in bone and joint damage with no immunogenicity.
Most peptide-based drugs require parenteral administration due to poor oral bioavailability (28). Those biologics are usually used for chronic diseases, and repetitive injections during the long-term disease course could reduce patient compliance and lead to poor outcomes. The convenience and widespread use of oral administration makes it the most desirable method in a clinical setting. Engineered probiotics can be used to deliver drugs or enzymes to treat metabolic disorders into the GI tract or tumors (29, 30). A genetically engineered strain of Escherichia coli Nissle was used to deliver the angiogenesis inhibitor tumstatin to treat murine melanoma (31). More recently, the Phe-metabolizing enzyme phenylalanine ammonia lyase and L-amino acid deaminase (LAAD) enzyme expressing Escherichia coli Nissle have completed human phase 1/2a clinical trials and yielded positive results in phenylketonuria patients (32), demonstrating the potential of using bacteria as a promising drug delivery method. However, in those successful experiments, tumstatin was delivered via intrapetitoneal injection of the bioengineered bacteria that were subsequently cleared by the immune system, and LAAD was effective directly in the GI tract. Here, we show that the engineered probiotic LrS235 can be given orally with a good bioavailability of the ShK-235 it produces in the circulation of the rats. In contrast, the oral administration of a high dose of synthetic ShK-235 packaged into enteric-coated capsules yielded no detectable ShK-235 in the circulation of the rats and no benefits in the DTH model and neither did the oral gavage of supernatants from the culture of LrS235 affect DTH. This is consistent with prior work showing low oral bioavailability of peptide and protein therapeutics (28). The bioavailability of venom-derived Kv1.3-blocking peptides through mucosal membranes has been demonstrated after buccal and pulmonary delivery (16, 33), suggesting the feasibility of delivery via the intestinal mucosa. A probiotic-based delivery presents several advantages as the daily ingestion of probiotics by humans is well established, and L. reuteri 6475 has already been tested in humans for research purposes and is also available as a commercially available supplement (Osfortis™). In addition, L. reuteri will continuously deliver ShK-235 throughout the GI tract, whereas delivery via capsule is pH-dependent and thus restricted to a one-time bolus to the distal intestine. Furthermore, L. reuteri has an affinity to mucus and will deliver the peptide in close proximity to the intestinal epithelium. Many bacteria produce extracellular vesicles that deliver bacterial products to the host’s circulation or tissues (34). More work is needed to determine whether such vesicles are involved in the remarkable bioavailability of ShK-235 following the oral delivery of LrS235.
We chose the modified signal peptide of Usp45 from L. lactis for the secretion of ShK-235 because it is cleaved from the peptide during secretion (35). The addition of large moieties to the N-terminus of ShK and its analogs prevents the peptide from accessing its binding site in the pore of the Kv1.3 tetramer and thus prevents channel block (36). The fact that the supernatants from LrS235, but not from LrGusA, block Kv1.3 channels and TEM cell proliferation suggests that the signal peptide is indeed cleaved from ShK-235.
Prior studies with injected blockers of the Kv1.3 channels, including ShK analogs, showed that a high efficacy in reducing the severity of CIA was accompanied by a reduction in DTH severity. Both injected synthetic ShK-235 and ShK-235 delivered orally via LrS235 reduced inflammation in both CIA and DTH, but LrS235 was more effective in CIA, and synthetic ShK-235 was more effective in DTH. Following subcutaneous injection, ShK and its analogs reach the circulation in less than 30 min (25). In contrast, the peak concentration of ShK-235 in the circulation of healthy rats is achieved 6 h after a single oral delivery of LrS235. After the DTH challenge with ovalbumin, ovalbumin-specific TEM cells enter the site of challenge and interact with local antigen-presenting cells within 3 h (24). We administered both synthetic ShK-235 and LrS235 at the time of challenge, giving sufficient time for the synthetic peptide to reach the circulation and the TEM cells before the start of the local inflammatory response but inflammation at the site of challenge had already begun by the time ShK-235 produced by LrS235 reached the circulation thus likely reducing the effectiveness of the treatment in such an acute model. Furthermore, other charged peptides with structures similar to that of ShK-235 accumulate in the joints following systemic administration, therefore increasing their local concentration at the site of inflammation in CIA (37, 38). This unique, and not fully understood, feature of these peptides likely also plays a role in the remarkable efficacy of ShK-235 in reducing disease severity in CIA.
LrS235 had significantly higher efficacy than the injected synthetic ShK-235 in reducing disease severity in CIA rats. At first glance, this could be explained by the administration frequency, LrS235 being given daily, while the synthetic peptide was injected every other day. However, we had tested Kv1.3 blocker administration at frequencies ranging from every 6 h to once a week and found that injections every other day provided a peak efficacy not improved by more frequent injections. This is likely due to the natural depot of the peptide at the site of subcutaneous injection, which leads to a slow release in the circulation (15). Another likely explanation is that the probiotic itself has anti-inflammatory effects. L. reuteri decreases intestinal inflammation in several mouse models (20, 39). In our CIA trials, L. reuteri producing GusA instead of ShK-235 reduced disease severity at the early stage of CIA, although significantly only on days 3 and 5 after the onset of clinical signs. Since RA is a chronic inflammatory disease, the probiotic and ShK-235 may synergistically suppress inflammation and improve the therapeutic effects of LrS235. While Kv1.3 blockers preferentially target TEM lymphocytes, L. reuteri products may target effectors of the innate immune system, such as macrophages and dendritic cells, thus showing a modest protective effect early in CIA progression. Since CIA induction involves incomplete Freund’s adjuvant that induces a strong inflammation, LrGusA may show more anti-inflammatory benefits in milder models of inflammation. In RA and its animal models, joint inflammation disturbs the balance between osteoclasts and osteoblasts by inhibiting osteoblast differentiation and augmenting osteoclast function (40). Indeed, probiotics have been shown to regulate bone health through secretion of peptides or lipids, modulation of the host immune system, modifying the gut microbiome, and influencing local pH (41–43). Finally, the intestinal microbiome is perturbed in RA and its animal models (44), and it is possible that the rescue of dysbiosis by LrS235 treatment accounts for the improved efficacy than synthetic ShK-235 injections. Further work is needed to validate this hypothesis.
We have used mKv1.3 (NCBI accession number NP_032444) to test ShK-235 by patch-clamp, hKv1.3 (NCBI accession number NP_002223) to test ShK-235 in functional assays in vitro, and rKv1.3 (NCBI accession number NP_062143) to test ShK-235 in vivo. All three are regularly used interchangeably when testing pore-blocking peptides as the alignment of all three amino acid sequences show complete homology in the pore region and all transmembrane domains between these species. The only differences between mKv1.3, hKv1.3, and rKv1.3 are in the intracellular N-terminus of the protein that is not implicated in peptide binding. Indeed, ShK has been tested by patch-clamp electrophysiology on mKv1.3, hKv1.3, and rKv1.3 with similar IC50s (45–47).
Many drugs, and especially biologics, are immunogenic, and the resulting neutralizing antibodies can eventually render the medications ineffective. ShK is highly homologous to a domain of MMP-23 (48), and thus resembles a self-peptide, and is likely recognized as such by the immune system. As a result, ShK and its analogs induce little to no immunogenicity in rats or humans (10, 15), and we have found no immunogenicity of ShK-235 delivered via LrS235 in either healthy or CIA rats, regardless of short or long-term treatment. In our immunogenicity ELISA, we used porcine collagen II as a positive control for detecting IgG as the rats had been immunized against this protein to induce CIA. There was no difference in anti-porcine collagen II IgG titers between the rats in the different treatment groups, and this suggests that the B lymphocytes producing these antibodies belong to the CD27−IgD− memory subset that relies on KCa3.1 rather than Kv1.3 channels for their function (49). The frequency of CD27-IgD- B cells is increased in patients with both early and established RA (50) and is likely the subset of B cells producing the IgGs detected in the rats with CIA.
Altogether, we have developed the engineered ShK-235 secreting probiotic LrS235 that treats the animal model of RA efficiently. Our findings provide an alternative delivery strategy for peptide-based drugs and suggest that such techniques and principles can be applied to a broader range of drugs and the treatment of chronic inflammatory diseases.
Materials and Methods
Construct Design for the Inducible Expression of ShK-235.
ShK-235 differs from ShK by a Q16K substitution, an I21M substitution, and the addition of an Ala to the C-terminus (SI Appendix, Fig. S1) (17). Codon optimized ShK-235 with modified signal peptide Usp45 was synthesized and ligated into NcoI-EcoRI digested pSIP411 generated pLL01 (SI Appendix, Fig. S2). The ShK-235 secretion plasmid pLL01 was enlarged in Escherichia coli 1,000 and was then electransformed into L. reuteri 647 competent cell resulting in ShK expression strain LrS235. All LrS235 or LrGusA used in this paper were induced with induction peptide unless otherwise stated.
Synthesis of ShK-235 and HsTX1[R14A].
Both Kv1.3-blocking peptides were previously described (17, 51). ShK-235 and HsTX1[R14A] were synthesized using an Fmoc-tBu solid-phase synthesis strategy. Each coupling was mediated with diisopropyl carbodiimide in the presence of hydroxybenzotriazole. All deprotections were accomplished with 20% piperidine in dimethyl formamide. Following synthesis of the linear chain, each peptide was cleaved and deprotected using trifluoroacetic acid (90%) with carbocation scavengers [triisopropyl silane, H2O, 3,6-dioxa-1,8-octanedithiol, and thioanisole, (2% of each v/v)] for 3 h at ambient temperature. The peptides were each precipitated into methyl t-butyl ether. The linear peptides were purified by reverse phase -high-performance liquid chromatography and subsequently oxidatively folded in the presence of glutathione in ammonium acetate buffered aqueous solution. The cyclized products were isolated by RP-HPLC, and fractions with a purity >95% by analytical high-performance liquid chromatography were subsequently pooled and lyophilized. Each peptide was found to have expected theoretic mass for the formation of three and four disulfide bonds, respectively, for ShK-235 and HsTX1[R14A]. Synthetic ShK-235 and HsTX1[R14A] were dissolved in P6N buffer (10 mM sodium phosphate, 0.8% NaCl, 0.05% polysorbate 20, pH 6.0) to prepare stocks at 1 mg/mL (15, 25).
Animals.
Male and female Lewis rats (7 to 8 wk old; Envigo) were group-housed and provided food and water ad libitum. All animals were housed in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International-accredited. All experiments involving rats were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine.
Cells and Cell Lines.
Buffy coats were purchased from the Gulf Coast Regional Blood Center, and MNC were enriched using Histopaque-1077 (Sigma-Aldrich). Mouse L929 fibroblasts stably expressing mKv1.3 channels (52) were a kind gift from Dr. K. George Chandy (University of California, Irvine) and were cultured in Dulbecco's Modified Eagle Medium (DMEM) + 10% fetal bovine serum (FBS) + 0.5 mg/mL G418 (EMD Chemicals). HEK 293 cells stably transfected with the KCa1.1 channel α subunit were a gift from Dr. Heike Wulff (University of California, Davis) and were cultured in DMEM medium + 10% FBS + 0.5 mg/mL G418. HEK 293 cells stably transfected with the KCa3.1 channel were a gift from Dr. Heike Wulff and were cultured in DMEM medium + 10% FBS + 10 µg/mL puromycin.
ShK-235 Production from L. reuteri for In Vitro Assays.
Inducible expression of ShK-235 in L. reuteri was performed based on previous expression conditions (22). Briefly, overnight cultures of LrS235 were grown in buffered de Man, Rogosa & Sharpe (MRS) broth with 8 µg/mL erythromycin at 37°C in anaerobic conditions. The next day, 400 µL of the overnight culture was added to 40 mL of buffered MRS with 8 µg/mL erythromycin in a 50 mL conical tube and incubated in a 37°C water bath. When the cultures reached an optical density at 600 nm (OD600) of 0.6 to 0.8, expression of ShK-235 was induced with 50 ng/mL of the pSIP411 induction peptide, and the culture was incubated for 5 h in a 37°C water bath. Then the cultures were centrifuged for 5 min at 4,800 g to pellet the cells, and the supernatant was removed and stored at −20°C. The supernatant was buffer exchanged from MRS to PBS using dialysis filtration. Briefly, supernatants were centrifuged in an Amicon Ultracel (Millipore) concentrator with a 3 KDa cutoff filter to reduce the volume by 80% and then resuspended to the original volume using sterile 1× PBS. This was repeated 5 to 6 times.
Preparation of Rat Sera for Patch-Clamp Electrophysiology.
Blood was withdrawn from animals by cardiocentesis under anesthesia (53) and allowed to clot at room temperature for 10 min. Serum was extracted by centrifugation at 300 g for 15 min at room temperature. The serum was then aliquoted and stored at −80°C until testing. No further purification was performed.
Patch-Clamp Electrophysiology.
To determine the concentration of ShK-235 in the LrS235 supernatants and rat sera, a dose-response of ShK-235 block of Kv1.3 was determined by adding known concentrations of the peptide to naïve rat serum and then testing by whole-cell patch-clamp of mouse L929 fibroblasts stably expressing mKv1.3 channels (52) using a Port-a-Patch automated patch-clamp system (Nanion), as described (17, 54). Culture supernatants and serum samples were then assessed with the same technique, and the dose-response curve was used to determine peptide concentration. The effects of 100 nM ShK-235 on KCa1.1 and KCa3.1 channels were assessed by manual whole-cell patch-clamp electrophysiology on HEK 293 cells stably expressing either channel using established protocols (55–57).
Human T Lymphocyte Proliferation Assays.
Peripheral blood MNC were loaded with 5 µM CellTrace Violet (Invitrogen) according to the manufacturer’s instructions (58) and incubated for 30 min with sterile-filtered supernatants from LrS235 or LrGusA, buffered to pH 7.4 and diluted 1/10 in tissue culture media, before the addition of anti-CD3 antibodies to stimulate T lymphocytes (clone OKT3, 1 ng/ml, 037-85, Thermo Fisher). Seven days later, cells were stained with anti-CD3 antibodies conjugated to phycoerythrin (BioLegend 300308, lot B209105) and anti-CCR7 antibodies conjugated to fluorescein (R&D Systems, FAB197F, lot LEU1615081), and the dilution of CellTrace Violet in CD3+CCR7− TEM cells and CD3+CCR7+ naive/TCM cells was measured by flow cytometry on a BD FACSCantoII as quantification of cell proliferation. Data were analyzed with FlowJo.
Encapsulation of ShK-235.
Torpac size 9 h gelatin capsules (Fairfield, NJ) were filled with either 0.3 mg synthetic ShK-235 or unflavored gelatin powder (Kraft Heinz) using the Torpac dosing kit. Each capsule was enteric-coated with Acryl-EZE® (Colorcon) prior to delivery via oral gavage to target content delivery to the small intestine.
Bioavailability and Pharmacokinetics of ShK-235 after Oral Delivery.
Male rats received a single oral gavage of either 1 × 109 cfus LrS235 or an enteric-coated capsule filled with ShK-235. Blood was collected via the saphenous vein at the different time points indicated in the figure, the last blood draw being a terminal cardiac puncture (59). Serum samples were assayed for Kv1.3 block by patch-clamp electrophysiology.
Induction and Monitoring of an Active DTH Reaction.
Male and female rats were immunized in the flanks with 200 μL of a 1:1 emulsion of ovalbumin (Sigma) in complete Freund’s adjuvant (Difco/Becton Dickinson, Franklin Lakes, NJ) (26). After 7 d, under isoflurane anesthesia, the rats were challenged with ovalbumin dissolved in saline in the pinna of one ear; the collateral ear was injected with saline (60). Rats received either a single subcutaneous injection of 0.1 mg/kg ShK-235 or P6N vehicle, a single oral gavage of 1 × 109 cfus LrS235 or LrGusA, oral gavage of 1 mL supernatant of LrS235, or a capsule filled with ShK-235 or gelatin immediately before the ear challenge. The DTH reaction was measured 24 h post-challenge as the thickness of the ear using a spring-loaded micrometer (Mitutoyo, Japan) and ear inflammation was determined by comparing the ear thickness of the ovalbumin-challenged with the saline-challenged ear from each rat (60). The animals were euthanized after the ear measurements.
Induction, Monitoring, Randomization, and Treatment of Rat CIA.
CIA was induced as described previously (54, 61). Briefly, female Lewis rats received a subcutaneous injection of 200 μL of a 1:1 emulsion of 2 mg/mL porcine type II collagen (20031, Chondrex, Redmond, WA) with incomplete Freund’s adjuvant at the base of the tail. After 7 d, rats were given a booster of 100 μL of collagen and adjuvant emulsion. Disease onset was defined as the development of at least one swollen or red paw joint. Clinical scores were determined daily by assigning one point for each swollen or red toe joint, two points for mildly swollen wrist or ankle joints, and five points for each severely swollen wrist or ankle, giving each rat a maximum possible score of 60. Upon disease onset, rats were treated every other day by the subcutaneous injection of P6N buffer vehicle or 0.1 mg/kg ShK-235, or the oral gavage of 1 × 109 cfus LrGusA or 1 × 109 cfus LrS235 daily. CIA is more severe in rats with early disease onset. To avoid biasing our results based on disease severity on the day, each rat developed signs of disease and accounting for differences in the time between immunization, and when a rat developed signs of illness, every rat that developed signs of disease on a given day was placed in a different treatment group to fill all groups in parallel.
Histology and Micro-CT.
Healthy rats and rats from the CIA trials were euthanized after 21 d of treatment, and their hind paws were collected and fixed in 10% buffered formalin. One paw from each rat was imaged in the Optical Imaging & Vital Microscopy Core at Baylor College of Medicine by Micro-CT using a Bruker SkyScan 1272 Scanner set at 13 µm resolution with no filtering, no averaging, and a rotation step of 0.3. Raw images were analyzed with CTvox (Bruker, MA, US). The other hind paw was decalcified, embedded in paraffin, and sectioned by the Pathology & Histology Core at Baylor College of Medicine. Slides were stained with either hematoxylin and eosin or safranin O/fast green and imaged at 4× magnification on a Nikon Ci-L bright-field microscope (Nikon Inc.) in the Integrated Microscopy Core at Baylor College of Medicine. Scoring of the slides was completed by an investigator blinded to treatment groups using a comprehensive histological scoring system as described elsewhere (54, 62), in which cartilage degradation, cartilage erosion, angiogenesis, pannus formation, synovial hyperplasia, and synovial inflammation were evaluated by the following criteria: synovial inflammation: five high-power magnification fields (HMF) were scored for the percentage of infiltrating mononuclear cells (MNC) as follows: 0 = absent, 1 = mild (1 to 10%), 2= moderate (11 to 50%), and 3 = severe (51 to 100%); cartilage erosion and degradation: 0 = absent, 1 = mild (1 to 10%), 2= moderate (11 to 50%), and 3 = severe (51 to 100%); synovial hyperplasia: 0 = absent, 1 = mild (5 to 10 layers), 2 = moderate (11 to 20 layers), and 3 = severe (>20 layers); angiogenesis: the number of vessels was counted in five HMF of synovial tissue, and the mean was used for analyses; extension of pannus formation was based on the reader’s impression (62).
Immunogenicity Assays in Rats with CIA.
High-protein binding 96 well microplates (3855, Thermo Fisher) were coated overnight with 10 µg/mL of either porcine collagen II (20031, Chondrex, Woodinville, WI), ShK-235, or HsTX1[R14A], dissolved in PBS. Non-specific binding sites were blocked with PBS + 5% skimmed milk and washed with PBS + 0.05% Tween 20 before adding serum from the CIA rats, diluted in PBS. After washes, anti-rat IgG antibodies conjugated to horseradish peroxidase (1 µg/mL; Pierce catalog 31471, lot UA280036) were added to all the wells. One-step TMB-ELISA (34028, Thermo Fisher) was used to detect absorbance on a plate reader at 650 nm.
Antibody Neutralization Assays.
To assay whether the serum of rats treated with synthetic ShK-235 or LrS235 contains neutralizing antibodies against ShK-235 and thus reduce the ability of ShK-235 from inhibiting the proliferation of CCR7− TEM cells, we pre-incubated synthetic 1 nM ShK-235 for 30 min with media supplemented with 10% of serum from the rats of the CIA trials or healthy, non-immunized and untreated rats. Human MNCs were loaded with CellTrace Violet according to the manufacturer’s instructions (58) and resuspended in the media supplemented with rat serum and synthetic ShK-235. After a 30 min incubation at 37°C, T lymphocytes were activated by the addition of anti-CD3 antibodies (clone OKT3, 1 ng/mL, 037-85, Thermo Fisher). Seven days later, cells were stained with anti-CD3 antibodies conjugated to phycoerythrin and anti-CCR7 antibodies conjugated to FITC, and the dilution of CellTrace Violet in CD3+CCR7− TEM cells was measured by flow cytometry on a BD FACSCantoII as quantification of cell proliferation. Data were analyzed with FlowJo.
Long-Term Immunogenicity Assays in Healthy Rats.
Healthy Lewis rats received a daily oral gavage of 1 × 109 cfus LrS235, 1 × 109 cfus-induced LrGusA, or vehicle for 8 wk. The rats were left untreated for 12 wk and then were again treated daily for 1 wk, reaching a total of 21 wk. Blood was drawn at the 8 wk and the 21 wk time points, and serum was tested for IgG against ShK-235 and HsTX1 [R14A] by ELISA, as described above.
Statistical Analysis.
Student’s t test, one-way ANOVA, and two-way ANOVA without correction were used to determine whether differences among the groups were statistically significant (P < 0.05). The CIA clinical scores analyses were completed using repeated measure one-way analysis of variance with Bonferroni post hoc test. Data were presented as mean ± SEM. All analyses were performed using GraphPad Prism.
Supplementary Material
Appendix 01 (PDF)
Representative video of pseudocolored micro-CT paws from CIA and control rats. 3D reconstructed and psedocolored paws from fig. S5. Red color corresponds to low density pixels, indicating area of bone erosion and cartilage damage, blue color corresponds to high density pixels, indicating high density bone.
Acknowledgments
This project was funded in part by a pilot grant from the Alkek Center for Metagenomics and Microbiome Research at Baylor College of Medicine (to C.B. and J.M.H.) and by Bridge Funding from Baylor College of Medicine (to C.B.). The work was supported by the Cytometry & Cell Sorting Core, the Pathology & Histology Core, the Optical Imaging & Vital Microscopy Core, and the Integrated Microscopy Core funded in part by the Cancer Prevention and Research Institute of Texas (RP180672, RP150578, RP1806721, and RP170719), the NIH (DK56338, CA125123, HG006348, and RR024574), the Dan L. Duncan Comprehensive Cancer Center, and the John S. Dunn Gulf Coast Consortium for Chemical Genomics.
Author contributions
Y.W., D.Z., L.C.O.-V., J.L.P., J.M.H., R.A.B., and C.B. designed research; Y.W., D.Z., J.L.P., J.M.H., R.A.B., and C.B. performed research; D.Z., L.C.O.-V., J.L.P., M.W.P., J.M.H., and R.A.B. contributed new reagents/analytic tools; Y.W., R.A.B., and C.B. analyzed data; and Y.W., D.Z., J.L.P., J.M.H., R.A.B., and C.B. wrote the paper.
Competing interest
The authors declare competing interest. The authors have organizational affiliations to disclose. R.A.B. is a cofounder of Mikrovia and PanaBio. The authors have patent filings to disclose. C.B. and M.W.P. are inventors on the patent protecting ShK-186, currently in clinical trials for the treatment of autoimmune diseases. C.B., J.M.H., and R.A.B. are inventors on a patent disclosure protecting LrS235.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Joseph M. Hyser, Email: jh126641@bcm.edu.
Robert A. Britton, Email: robert.britton@bcm.edu.
Christine Beeton, Email: beeton@bcm.edu.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Anselmo A. C., Gokarn Y., Mitragotri S., Non-invasive delivery strategies for biologics. Nat. Rev. Drug. Discov. 18, 19–40 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Smolen J. S., Aletaha D., McInnes I. B., Rheumatoid arthritis. Lancet 388, 2023–2038 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Voskuyl A. E., The heart and cardiovascular manifestations in rheumatoid arthritis. Rheumatology 45, 4–7 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Bilal J., et al. , Risk of infections and cancer in patients with rheumatologic diseases receiving interleukin inhibitors: A systematic review and meta-analysis. JAMA Netw. Open 2, e1913102–e1913102 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van den Bemt B. J., Zwikker H. E., van den Ende C. H., Medication adherence in patients with rheumatoid arthritis: A critical appraisal of the existing literature. Expert Rev. Clin. Immunol. 8, 337–351 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Fidder H. H., Singendonk M. M., van der Have M., Oldenburg B., van Oijen M. G., Low rates of adherence for tumor necrosis factor-α inhibitors in Crohn’s disease and rheumatoid arthritis: Results of a systematic review. World J. Gastroenterol. 19, 4344–4450 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strand V., Goncalves J., Isaacs J. D., Immunogenicity of biologic agents in rheumatology. Nat. Rev. Rheumatol. 17, 81–97 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Atiqi S., Hooijberg F., Loeff F. C., Rispens T., Wolbink G. J., Immunogenicity of TNF-inhibitors. Front. Immunol. 11, 312 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gattorno M., et al. , Phenotypic and functional characterisation of CCR7+ and CCR7- CD4+ memory T cells homing to the joints in juvenile idiopathic arthritis. Arthritis Res. Ther. 7, R256–R267 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beeton C., et al. , Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc. Natl. Acad. Sci. U.S.A. 103, 17414–17419 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beeton C., Pennington M. W., Norton R. S., Analogs of the sea anemone potassium channel blocker ShK for the treatment of autoimmune diseases. Inflamm. Allergy Drug Targets 10, 313–321 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chi V., et al. , Development of a sea anemone toxin as an immunomodulator for therapy of autoimmune diseases. Toxicon 59, 529–546 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandy K. G., Norton R. S., Peptide blockers of Kv1.3 channels in T cells as therapeutics for autoimmune diseases. Curr. Opin. Chem. Biol. 38, 97–107 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Munoz-Elias E. J., et al. , Dalazatide (ShK-186), a first-in-class blocker of Kv1.3 potassium channel on effector memory T cells: Safety, tolerability and proof of concept of immunomodulation in patients with active plaque psoriasis. Arthritis Rhe umatol. 67, 12 (2015). [Google Scholar]
- 15.Tarcha E. J., et al. , Safety and pharmacodynamics of dalazatide, a Kv1.3 channel inhibitor, in the treatment of plaque psoriasis: A randomized phase 1b trial. PLoS One 12, e0180762 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin L., et al. , Enabling noninvasive systemic delivery of the Kv1.3-blocking peptide HsTX1[R14A] via the Buccal Mucosa. J. Pharm. Sci. 105, 2173–2179 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Pennington M. W., et al. , Development of highly selective Kv1.3-blocking peptides based on the sea anemone peptide ShK. Mar. Drugs 13, 529–542 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.i. b. Food and Agriculture Organization of the United Nations, i. b. World Health Organization, Probiotics in food: Health and nutritional properties and guidelines for evaluation (Rome, World Health Organization: Food and Agriculture Organization of the United Nations, 2006, Italy, 2006). [Google Scholar]
- 19.Mu Q., Tavella V. J., Luo X. M., Role of Lactobacillus reuteri in human health and diseases. Front. Microbiol. 9, 757 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins F. L., et al. , Lactobacillus reuteri 6475 increases bone density in intact females only under an inflammatory setting. PLoS One 11, e0153180 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Asseldonk M., de Vos W. M., Simons G., Functional analysis of the Lactococcus lactis usp45 secretion signal in the secretion of a homologous proteinase and a heterologous alpha-amylase. Mol. Gen. Genet. 240, 428–434 (1993). [DOI] [PubMed] [Google Scholar]
- 22.Ortiz-Velez L., Goodwin A., Schaefer L., Britton R. A., Challenges and pitfalls in the engineering of human interleukin 22 (hIL-22) secreting Lactobacillus reuteri. Front. Bioeng. Biotechnol. 8, 543 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorvig E., Mathiesen G., Naterstad K., Eijsink V. G. H., Axelsson L., High-level, inducible gene expression in Lactobacillus sakei and Lactobacillus plantarum using versatile expression vectors. Microbiology 151, 2439–2449 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Matheu M., et al. , Imaging of effector memory T cells during a delayed-type hypersensitivity reaction and suppression by Kv1.3 channel block. Immunity 29, 602–614 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarcha E. J., et al. , Durable pharmacological responses from the peptide ShK-186, a specific Kv1.3 channel inhibitor that suppresses T cell mediators of autoimmune diseases. J. Pharmacol. Exp. Ther. 342, 642–653 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beeton C., Chandy K. G., Induction and monitoring of active delayed type hypersensitivity (DTH) in rats. J. Vis. Exp. 237 (2007), 10.3791/237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beeton C., KCa1.1 channels as therapeutic targets for rheumatoid arthritis. Expert Opin. Ther. Targets 21, 1077–1081 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caon T., Jin L., Simões C. M., Norton R. S., Nicolazzo J. A., Enhancing the buccal mucosal delivery of peptide and protein therapeutics. Pharm. Res. 32, 1–21 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Chen Y., et al. , Genetically engineered oncolytic bacteria as drug delivery systems for targeted cancer theranostics. Acta Biomater. 124, 72–87 (2021). [DOI] [PubMed] [Google Scholar]
- 30.Praveschotinunt P., et al. , Engineered Escherichia coli Nissle 1917 for the delivery of matrix-tethered therapeutic domains to the gut. Nat. Commun. 10, 5580 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He L., et al. , Escherichia coli Nissle 1917 engineered to express Tum-5 can restrain murine melanoma growth. Oncotarget 8, 85772–85782 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puurunen M. K., et al. , Safety and pharmacodynamics of an engineered Escherichia coli Nissle for the treatment of phenylketonuria: A first-in-human phase 1/2a study. Nat. Metab. 3, 1125–1132 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Jin L., et al. , Pulmonary delivery of the Kv1.3-blocking peptide HsTX1[R14A] for the treatment of autoimmune diseases. J. Pharm. Sci. 105, 650–656 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Bitto N. J., Kaparakis-Liaskos M., The therapeutic benefit of bacterial membrane vesicles. Int. J. Mol. Sci. 18, 1287 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng D. T., Sarkar C. A., Engineering signal peptides for enhanced protein secretion from Lactococcus lactis. Appl. Environ. Microbiol. 79, 347–356 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beeton C., et al. , A novel fluorescent toxin to detect and investigate Kv1.3 channel up-regulation in chronically activated T lymphocytes. J. Biol. Chem. 278, 9928–9937 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Ong S., et al. , Modulation of lymphocyte potassium channel KV1.3 by membrane-penetrating, joint-targeting immunomodulatory plant defensin. ACS Pharmacol. Transl. Sci. 3, 720–736 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergmann R., et al. , Distribution and kinetics of the Kv1.3-blocking peptide HsTX1[R14A] in experimental rats. Sci. Rep. 7, 3756 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCabe L. R., Irwin R., Schaefer L., Britton R. A., Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J. Cell Physiol. 228, 1793–1798 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shim J. H., Stavre Z., Gravallese E. M., Bone loss in rheumatoid arthritis: Basic mechanisms and clinical implications. Calcif. Tissue Int. 102, 533–546 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Britton R. A., et al. , Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J. Cell. Physiol. 229, 1822–1830 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins F. L., Rios-Arce N. D., Schepper J. D., Parameswaran N., McCabe L. R., The potential of probiotics as a therapy for osteoporosis. Microbiol. Spectr. 5 (2017), 10.1128/microbiolspec.BAD-0015-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quach D., Parameswaran N., McCabe L., Britton R. A., Characterizing how probiotic Lactobacillus reuteri 6475 and lactobacillic acid mediate suppression of osteoclast differentiation. Bone Rep. 11, 100227–100227 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaiss M. M., Joyce Wu H.-J., Mauro D., Schett G., Ciccia F., The gut–joint axis in rheumatoid arthritis. Nat. Rev. Rheumatol. 17, 224–237 (2021). [DOI] [PubMed] [Google Scholar]
- 45.Beeton C., et al. , Selective blockade of T lymphocyte K(+) channels ameliorates experimental autoimmune encephalomyelitis, a model for multiple sclerosis. Proc. Natl. Acad. Sci. U.S.A. 98, 13942–13947 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wulff H., et al. , The voltage-gated Kv1.3 K(+) channel in effector memory T cells as new target for MS. J. Clin. Invest. 111, 1703–1713 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang S. C., et al. , Expression and isotopic labelling of the potassium channel blocker ShK toxin as a thioredoxin fusion protein in bacteria. Toxicon 60, 840–850 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galea C. A.Nguyen H. M.George Chandy K.Smith B. J.Norton R. S., Domain structure and function of matrix metalloprotease 23 (MMP23): Role in potassium channel trafficking. Cell Mol. Life Sci. 71, 1191–1210 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wulff H., Knaus H. G., Pennington M., Chandy K. G., K+ channel expression during B cell differentiation: Implications for immunomodulation and autoimmunity. J. Immunol. 173, 776–786 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Moura R. A., et al. , B-cell phenotype and IgD-CD27- memory B cells are affected by TNF-inhibitors and tocilizumab treatment in rheumatoid arthritis. PloS One 12, e0182927–e0182927 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rashid M. H., et al. , A potent and Kv1.3-selective analogue of the scorpion toxin HsTX1 as a potential therapeutic for autoimmune diseases. Sci. Rep. 4, 4509 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grissmer S., et al. , Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Mol. Pharmacol. 45, 1227–1234 (1994). [PubMed] [Google Scholar]
- 53.Beeton C., Garcia A., Chandy K. G., Drawing blood from rats through the saphenous vein and by cardiac puncture. J. Vis. Exp. 266 (2007), 10.3791/266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanner M. R., et al. , KCa1.1 and Kv1.3 channels regulate the interactions between fibroblast-like synoviocytes and T lymphocytes during rheumatoid arthritis. Arthritis Res. Ther. 21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanner M. R., et al. , KCa1.1 inhibition attenuates fibroblast-like synoviocyte invasiveness and ameliorates rat models of rheumatoid arthritis. Arthritis. Rheum atol. 67, 96–106 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu X., et al. , KCa1.1 potassium channels regulate key proinflammatory and invasive properties of fibroblast-like synoviocytes in rheumatoid arthritis. J. Biol. Chem. 287, 4014–4022 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koshy S., et al. , Blocking KCa3.1 channels increases tumor cell killing by a subpopulation of human natural killer lymphocytes. PLoS One 8, e76740 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanner M. R., et al. , Prolonged immunomodulation in inflammatory arthritis using the selective Kv1.3 channel blocker HsTX1[R14A] and its PEGylated analog. Clin. Immunol. 180, 45–57 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beeton C., Chandy K. G., Drawing blood from the saphenous vein of rats and by cardiac puncture.J. Vis. Exp. 7, e266 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beeton C., Chandy K. G., Induction and monitoring of adoptive delayed-type hypersensitivity in rats. J. Vis. Exp. 325 (2007), 10.3791/325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanner M., et al. , Targeting KCa1.1 channels with a scorpion venom peptide for the therapy of rat models of rheumatoid arthritis. J. Pharmacol. Exp. Ther. 365, 227–236 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brenner M., et al. , Identification of two new arthritis severity loci that regulate levels of autoantibodies, interleukin-1β, and joint damage in pristane- and collagen-induced arthritis. Arthritis Rheum. 64, 1369–1378 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Representative video of pseudocolored micro-CT paws from CIA and control rats. 3D reconstructed and psedocolored paws from fig. S5. Red color corresponds to low density pixels, indicating area of bone erosion and cartilage damage, blue color corresponds to high density pixels, indicating high density bone.
Data Availability Statement
All study data are included in the article and/or SI Appendix.