Abstract
Objectives
The goal of this study was to analyze the clinical and magnetic resonance imaging (MRI) characteristics of autoimmune encephalitis (AE) and viral encephalitis (VE) at the initial stage of onset.
Methods
This study was a retrospective analysis of the clinical manifestations, laboratory tests, electroencephalogram examination, imaging examinations, and treatment outcomes of 24 VE patients and 20 AE patients.
Results
The onset age was significantly younger in the VE group than in the AE group, mainly occurring in adolescents (P < 0.05). The proportions of fever, headache, and vomiting were higher in the VE group than in the AE group (P < 0.05), and there were few manifestations of central hypoventilation. The incidence of abnormal myocardial enzymes was significantly higher in the VE group than in the AE group (P < 0.05). There was no significant difference in electroencephalogram test results between the VE and AE groups. Regarding magnetic resonance imaging (MRI), the proportion of single lesion involving a single lobe or multiple asymmetries involving the limbic system in the VE group was higher than that in the AE group (P < 0.05). The incidence of lesion enhancement in the VE group was higher than that in the AE group. Meanwhile, diffusion-weighted imaging sequence was more sensitive than T2 liquid-attenuated inversion recovery sequence in the detection, efficacy evaluation, and follow-up review of the AE and VE groups.
Conclusion
The onset age of VE is younger, and the clinical symptoms of AE and VE differ with statistical significance. MRI can objectively reflect the imaging characteristics of both groups. Combining early clinical manifestations with imaging manifestations can facilitate early diagnosis and treatment, and improve the prognosis.
Keywords: Autoimmune encephalitis, Clinical and imaging, Differential diagnosis, Viral encephalitis
الملخص
أهداف البحث
تحليل الخصائص السريرية وخصائص التصوير بالرنين المغناطيسي لالتهاب الدماغ المناعي الذاتي والتهاب الدماغ الفيروسي في المرحلة الأولى من ظهور المرض.
طرق البحث
التحليل الاستعادي للمظاهر السريرية، والاختبارات المعملية، وفحص مخطط كهربية الدماغ، وفحوصات التصوير ونتائج العلاج لـ ٢٤ حالة التهاب الدماغ الفيروسي و ٢٠ حالة التهاب الدماغ المناعي الذاتي.
النتائج
كان عمر بداية مجموعة التهاب الدماغ الفيروسي أصغر بكثير من مجموعة التهاب الدماغ المناعي الذاتي، وخاصة في المراهقين. كانت نسبة الحمى والصداع والقيء في مجموعة التهاب الدماغ الفيروسي أعلى من تلك الموجودة في مجموعة التهاب الدماغ المناعي الذاتي، وهناك مظاهر قليلة لنقص التهوية المركزية. كان معدل الإصابة غير الطبيعي لإنزيمات عضلة القلب في مجموعة التهاب الدماغ الفيروسي أعلى بكثير من تلك الموجودة في مجموعة التهاب الدماغ المناعي الذاتي. لم يكن هناك فرق كبير في مخطط كهربية الدماغ بين مجموعة التهاب الدماغ الفيروسي ومجموعة التهاب الدماغ المناعي الذاتي. بالنسبة لفحص التصوير، كانت نسبة الآفة المفردة التي تنطوي على فص واحد أو عدم تناسق متعدد في الجهاز الحوفي في مجموعة التهاب الدماغ الفيروسي أعلى من تلك الموجودة في مجموعة التهاب الدماغ المناعي الذاتي. كان معدل حدوث تحسين الآفة في مجموعة التهاب الدماغ الفيروسي أعلى من ذلك في مجموعة التهاب الدماغ المناعي الذاتي، وفي الوقت نفسه، كان تسلسل التصوير الموزون بالانتشار أكثر حساسية من تسلسل ترميم انعكاس التوهين السائل تي ٢ في الكشف وتقييم الفعالية ومراجعة المتابعة لمجموعة التهاب الدماغ المناعي الذاتي ومجموعة التهاب الدماغ الفيروسي.
الاستنتاجات
سن بداية التهاب الدماغ الفيروسي أصغر، والأعراض السريرية لالتهاب الدماغ المناعي الذاتي والتهاب الدماغ الفيروسي مختلفة مع دلالة إحصائية. يمكن أن يعكس التصوير بالرنين المغناطيسي بشكل موضوعي خصائص التصوير لكلا المجموعتين. يمكن أن يؤدي الجمع بين المظاهر السريرية المبكرة ومظاهر التصوير إلى التشخيص والعلاج المبكر، وتحسين الإنذار.
الكلمات المفتاحية: التهاب الدماغ المناعي الذاتي, التهاب الدماغ الفيروسي, مخطط كهربية الدماغ, التصوير بالرنين المغناطيسي, التشخيص التفريقي
Introduction
Autoimmune encephalitis (AE) refers to a kind of encephalitis mediated by an autoimmune mechanism. The pathogenesis is mediated by antibodies that attack neurotransmitters or protein receptors on the surface of neurons. AE includes many types, which are mostly induced by tumors and infection. The most common AE is N-methyl-d-aspartate receptor (NMDAR) encephalitis and autoimmune limbic encephalitis (LE), followed by anti-leucine-rich glioma-inactivated protein 1 encephalitis and anti-γ-aminobutyric acid B (GABABR) encephalitis. Although the clinical manifestations vary with the type of antibodies involved, the main common manifestations include mental and behavioral abnormalities, epileptic seizures, recent memory disorders, and other multifocal or diffuse brain damage.1,2
Viral encephalitis (VE) is an acute infectious disease of the central nervous system caused by virus infection. Herpes simplex virus is the most common virus, and other virus sources include enterovirus, West Nile virus, and varicella-zoster virus.3 VE and AE are both involved in central nervous system diseases such as subacute cognitive and behavioral disorders, consciousness, or seizures.4 VE also appears in the acute phase of seizures, similar to the symptoms of AE.5 Early diagnosis and treatment can greatly improve the prognosis of AE and VE patients. However, due to the overlap of clinical symptoms, and routine cerebrospinal fluid (CSF) examination indicators and imaging lesions involved in both diseases, the diagnosis of both diseases is often delayed and misdiagnosed. Therefore, identifying VE and AE in the early stage of this disease is particularly important.
The purpose of this retrospective comparative analysis was to explore and summarize the differences between VE and AE. To this end, clinical data, and results from laboratory tests, EEG examination, and imaging examinations were obtained from 24 patients with VE and 20 patients with AE at the First Affiliated Hospital of Nanchang University (Jiangxi, China). The findings from this study may help clinicians take effective intervention measures in a timely manner.
Materials and Methods
Study subjects
After strict inclusion and exclusion criteria, 20 patients with AE (9 males and 11 females, range 16–69 years, median age 42 years) admitted from March 2011 to November 2017 and 24 patients with VE (13 males and 11 females, range 178 years, median age 24.5 years) admitted from January 2011 to October 2017 were enrolled. The VE inclusion criteria were patients who were mostly diagnosed by virology and immunology. Some types of encephalitis viruses are difficult to identify by virus isolation or other laboratory tests, so their diagnosis depends heavily on the clinic. The AE inclusion criteria were: AE diagnosed according to the diagnostic criteria of NMDAR encephalitis and autoimmune LE published in 2016 by Lancet Neurol.6 Patients were excluded if they had: a history of previous stroke, seizure, head injury, neurological surgery, or other neurologic diseases; mental sickness; incomplete clinical information; or contraindica-tions for magnetic resonance imaging (MRI).
Laboratory examination
The CSF pressure of patients with AE or VE increases after disease onset, and the CSF protein and lymphocytes will increase to a certain extent, and biochemical indicators also change. In addition, the corresponding antibodies or viruses can also be detected. Therefore, the detection of CSF is the first choice for diagnosing or differentiating encephalitis. In this study, both groups of patients received CSF routine and CSF biochemical examination. CSF specimens were collected from all AE patients through lumbar puncture and sent to Wuhan Kangshengda Medical Laboratory Co. Ltd. (Wuhan, China). The patients were positive for CSF antibody.
As aforementioned, some types of VE are still difficult to identify by virus isolation or other laboratory tests, and their diagnosis depends largely on the clinic. Therefore, detecting myocardial enzymes and thyroid function may help physicians more easily distinguish VE from AE.
Different types of AE can be accompanied by different types of tumors. Anti-NMDAR encephalitis is often accompanied by teratoma, and more than half of patients with anti-GABABR encephalitis are diagnosed with lung cancer. Anti-LE encephalitis is often accompanied by breast, ovarian, and lung cancers.6, 7, 8 However, the relationship between VE and tumor markers has seldom been studied. Therefore, it is necessary to detect the tumor markers for VE.
Imaging test methods
Both groups of patients underwent head MRI conducted with the Siemens 3.0 T MRI scanner. The main sequences included cross-section T1-weighted imaging (T1WI), T2WI, diffusion-weighted imaging (DWI), and coronal T2 fluid-attenuated inversion recovery (T2-FLAIR) sequence, and the scanning layer thickness was 5 mm. T1WI-enhanced scans were obtained after intravenous injection of 0.1 mmoL/kg gadolinium-DTPA. The images were read by two senior professional image doctors independently.
EEG examination
Because patients with AE and VE have changes to their central nervous system, EEG examination of patients in the early stage of encephalitis can play an important role in prompting. Among the 20 patients in the AE group, 18 underwent EEG; and among the 24 patients in the VE group, 16 underwent EEG.
Statistical analyses
SPSS17.0 statistical software was used for analysis and processing. The χ2 test with continuity correction and the non-parametric test were used for two independent samples. The Fisher's exact test was used for data with a small theoretical value in the χ2 test. P < 0.05 was considered statistically significant.
Results
Clinical data
There were 12 (50%) children and adolescents (aged 1–18) in the VE group. Patients in the AE group were divided into the anti-NMDAR group with 12 patients (7 [58.3%] young women, range 16–23 years) and the LE group with 8 patients (young women, range 18–25 years) . VE and AE affected all ages, but the VE group had more adolescents, whereas the AE group comprised mainly young women in the anti-NMDAR group and the elderly in the LE group.
The early symptoms of the VE group were similar to those of the AE group. Fever, headache, epilepsy, and vomiting were the main symptoms in the VE group; and the main symptoms in the AE group were epilepsy, cognitive dysfunction, and abnormal mental behavior accompanied by central hypoventilation. The difference between the two groups was statistically significant (Table 1).
Table 1.
Identification of clinical symptoms in the two groups n (%).
| AE group (20 cases) | VE group (24 cases) | χ2value | P value | |
|---|---|---|---|---|
| Mental behavior abnormalities | 6 (30) | 7 (29.2) | >0.05 | |
| Epilepsy | 11 (55) | 11 (45.8) | >0.05 | |
| Cognitive dysfunction | 7 (35) | 6 (25) | >0.05 | |
| Irritability | 3 (15) | 4 (16.7) | >0.05 | |
| Disorder of consciousness | 4 (20) | 3 (12.5) | >0.05 | |
| Fever | 5 (25) | 19 (79.2) | 12.910 | <0.05 |
| Headache | 5 (25) | 15 (62.5) | 6.188 | <0.05 |
| Vomiting | 1 (5) | 7 (79.2) | 4.283 | <0.05 |
| Central hypoventilation | 4 (20) | 0 | 5.280 | <0.05 |
Laboratory examination (the first examination on admission was taken as the standard) in the VE group showed that 10 (41.7%) patients had elevated creatine kinase MB isoenzyme (CKMB) (≥24 U/L), of whom 2 patients reported critical values (≥48 U/L). There were three patients with increased CKMB in the AE group, accounting for 15% of the study population. The difference between the two groups was statistically significant (P < 0.05). The EEG test results of both groups were normal.
Thyroid function examination showed that three patients in the AE group and five patients in the VE group had significantly increased anti-thyroid peroxidase antibody. Among the 11 patients in the VE group, 6 (54.5%) were positive, mainly Ferr, carbohydrate 125 (CA125), CA153, and abnormally elevated neuron-specific enolase (NSE); malignant lymphoma was suspected in 1 patient. Among the 19 patients of the AE group, tumor markers were examined regularly, and 6 (31.6%) had elevated tumor markers, mainly Ferr, NSE, CA125, CA153, progastrin-releasing peptide, and CYFRA21G1 were abnormally increased. The pathology of one patient with anti-NMDAR encephalitis showed teratoma of the right ovary and one patient had suspected non-Hodgkin's lymphoma.
CSF analysis of the 24 patients in the VE group showed that 5 patients had significantly increased white blood cells, 11 patients had increased protein (1 with <0.5 g/L, 7 with 0.5–1.0 g/L, and 3 with >1.0 g/L). Among the 20 cases of CSF in the AE group, 6 patients had slightly increased white blood cells and 7 patients had increased protein (3 with <0.5 g/L, 2 with 0.5–1.0 g/L, and 2 with >1.0 g/L). The CSF level of the two groups was almost normal.
CSF antibody test, the AE group of 20 cases of NMDAR group of 12 patients, 8 cases (LE group [GABABR encephalitis in 5 cases, leucine-rich glioma inactivating protein 1 encephalitis in 2 cases, resistance to alpha amino G G3G G5G hydroxyl methyl G4G vision (evil) azole propionic acid receptor (anti G alpha GaminoG3GhydroxyG5GmethylG4Gisoxazolepropionicacidreceptor AMPAR) encephalitis in 1 case.
EGG examination showed abnormalities in all cases in both groups, one case of epileptiform discharge in the VE group, and five cases of epileptiform discharge in the AE group (Table 2).
Table 2.
General information on the patients and identification of the laboratory test results n (%).
| AE group | VE group | χ2 value | P value | |
|---|---|---|---|---|
| Age (0–18) | 3 (20) | 12 (24) | 5.948 | <0.05 |
| CKMB rise | 3 (20) | 10 (24) | 3.727 | <0.05 |
| Thyroid dysfunction | 3 (18) | 5 (11) | >0.05 | |
| Tumor marker-positive | 6 (19) | 6 (11) | >0.05 | |
| CSF-positive | ||||
| Leukocytosis | 6 (20) Slight rise |
5 (24) Significant increase |
>0.05 | |
| Protein increase | 7 (20) | 11 (24) | >0.05 | |
| Epileptiform discharge of ECG | 5 (18) | 1 (16) | >0.05 |
Treatment
Among the 20 cases of AE, 7 were misdiagnosed with VE combined with antiviral therapy in the early stage, leading to progressive aggravation of the patients' symptoms, and 3 were diagnosed with central system infection or encephalopathy in the early stage, without further identification of the cause of disease and delayed diagnosis. Twenty AE patients were treated with hormone and immunosuppressive therapy, and the symptoms were more slowly relieved and improved. Early diagnosis of the VE group was generally accurate, and the patients were treated with anti-viral and anti-infective therapies; cranial pressure lowering was carried out in parallel, and the symptoms were mostly alleviated and improved.
Results of brain MRI scan or enhancement
There were five MRI-positive cases in the AE group and 8 MRI-positive cases in the VE group. Both the AE group and VE group had white matter and gray matter to varying degrees, with a predominance of gray matter. Among them, white matter was dominant in two cases in the VE group. MRI signals in both groups were slightly longer T1 and T2 signals, DWI (b = 1000 s/mm2), T2-FLAIR sequences were high signals, and the apparent diffusion coefficient was slightly high or low signals, with no statistical significance. Among the five MRI-positive cases in the AE group, one case was misdiagnosed as VE, one case was only diagnosed with encephalitis, two cases were only descriptive and not qualitative, and one case was missed. Among the eight MRI positive cases in the VE group, two cases were diagnosed accurately, five cases were diagnosed as infectious diseases, and one case was only descriptive but not qualitative.
Lesions in the AE group and VE group were single or multiple, unilateral or bilateral, and the main areas involved areas were the medial frontal gyrus, cingulate gyrus, hippocampus, temporal lobe, insular lobe, and basal ganglia; however, the distribution of lesions differed in the two groups. In the AE group, five cases with multiple lesions were symmetrical near the midline, showing symmetrical involvement of the limbic system (hippocampus, temporal lobe, medial frontal lobe, insular lobe, and cingulate gyrus) (Figure 1). In the VE group, there were four cases with multiple lesions near the midline, but more extensive on one side, showing asymmetric involvement of the limbic system (hippocampus, temporal lobe, and insular lobe) (Figure 2). In the VE group, there were three cases with single lesions far from the midline, involving only the frontal lobe, parietal lobe, or temporal lobe (Figure 3). In the near midline, there was one case with symmetrical involvement. The hippocampus was most involved in both groups when multiple lesions occurred.
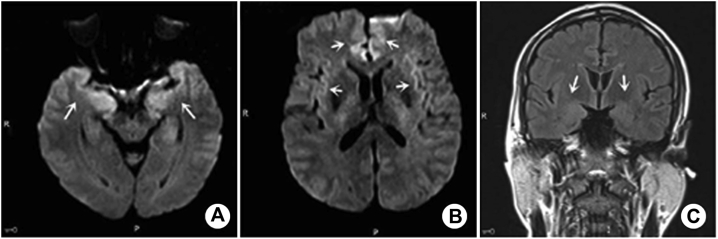
Figure 1.
A–C: Male, 52 years old, AE patient, recurrent limb convulsion for 20 days, CSF antibody against the GABAb receptor (1:10 dilution). MRI showed that DWI (b = 1000 s/mm2) of bilateral medial frontal gyrus, cingulate gyrus, hippocampus, and temporal lobe was abnormally high. Lesion characteristics: multiple symmetrical involvement of the limbic system. FLAIR sequence (C) showed that the lesions were not as sensitive as DWI.
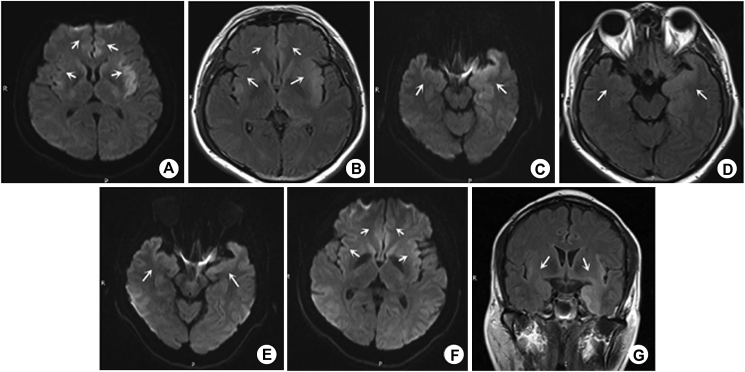
Figure 2.
A–G: Male, 45 years old, VE patient, fever with headache and dizziness for 5 days MRI showed that DWI (b = 1000 s/mm2) of bilateral medial frontal gyrus, cingulate gyrus, hippocampus, temporal lobe, and insular lobe was abnormally high signal, T2-FLAIR was slightly high signal (A–D), and the lesions involved the limbic system asymmetrically. After 1 month of treatment, the symptoms of the patients improved significantly. Review examination of MRI showed that (E–G): the original focus range had little change on the T2-FLAIR sequence, but basically disappeared on the DWI sequence. DWI is more sensitive than FLAIR sequence in the follow-up after treatment.
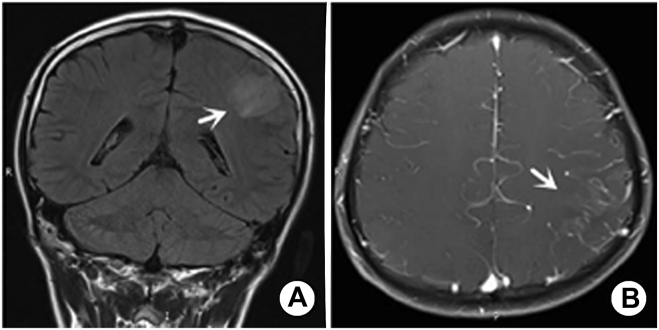
Figure 3.
A, B: Male, 14 years old, VE patient, paroxysmal convulsion for 10 days MRI showed that the left parietal gyrus was swollen and FLAIR sequence showed a slightly high signal. The lesion involved single lobe, and multiple vessels in the lesion area were enhanced by enhanced scanning.
Among the five cases of MRI abnormal signals in AE group, four cases were enhanced in parallel, and no enhancement was observed in the lesions. Among the eight cases of MRI abnormal signals in the VE group, seven cases were enhanced in parallel, three cases were enhanced in the lesions (mainly the increase of vascular gyrus), and one case had a hemorrhage signal (Table 3).
Table 3.
MRI identification of two groups of positive cases.
| AE (n = 5) |
VE (n = 8) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Distribution characteristics of focus | |||||||||||||
| Single | + | + | + | ||||||||||
| Multiple | + | + | + | + | + | + | + | + | + | + | |||
| One sided | + | + | + | ||||||||||
| On both sides | + | + | + | + | + | + | + | + | + | + | |||
| Gray matter dominated | + | + | + | + | + | + | + | + | + | + | + | ||
| White matter dominated | + | + | |||||||||||
| Near/far from the midline | Near | Near | Near | Near | Near | Far | Near | Near | Near | Far | Far | Near | Near |
| Whether symmetrical | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No | No | No | No | No |
| Lesion involvement | |||||||||||||
| Medial frontal gyrus | + | + | + | + | + | + | + | + | + | ||||
| Cingulate gyrus | + | + | + | + | + | + | + | + | + | ||||
| Hippocampus | + | + | + | + | + | + | + | + | + | + | |||
| Temporal lobe | + | + | + | + | + | + | + | + | + | ||||
| Insular lobe | + | + | + | + | + | + | + | + | + | ||||
| Basal ganglia region | + | + | + | + | |||||||||
| Frontal lobe | + | ||||||||||||
| Parietal lobe | + | ||||||||||||
| Lesion signal characteristics | |||||||||||||
| T2-FLAIR | 256.3 | 612.7 | 512.7 | 560.0 | 224.3 | 625.0 | 279.3 | 294.7 | 797.3 | 306.7 | 638.0 | 538.0 | 565.0 |
| DWI | 80.7 | 265.7 | 192.3 | 167.3 | 100.3 | 184.0 | 138.7 | 133.0 | 199.0 | 99.0 | 111.3 | 210.0 | 142.3 |
| Whether bleeding | Yes | ||||||||||||
| Whether to strengthen | No | No | No | No | No | No | Yes | Yes | Yes | No | No | ||
Note: the measurement of lesion signals on DWI and T2-FLAIR sequences follows the principle of avoiding artifacts, the FOV range is as large as possible (but within the lesion range), and the average value of multi-point measurements is taken; +, represents positive.
Discussion
VE is a common infectious disease of central nervous system, which is a localized or diffuse inflammation caused by a variety of viruses invading brain tissues. There are many kinds of VE virus, including enterovirus, Echovirus, Coxsackie virus, and measles, among which herpes simplex virus, adenovirus, and enterovirus are common pathogens.9 It is believed that patients with acute disseminated encephalomyelitis (ADEM) are diagnosed with VE. Nevertheless, ADEM is a rare disease, with an annual incidence of 0.2–0.8/100,00010,11; 80% of cases occur in children under 10 years of age,12 and 70–93% of patients have a history of infection or vaccination weeks before onset. Because the probability is very small, misdiagnosis can be ruled out.
AE is an autoimmune disease of the central nervous system, mediated primarily by humoral and/or cellular immunity due to an immune system disorder including anti-NMDAR encephalitis, LE, and other AE syndromes.
Clinical manifestation
Initial clinical manifestations of AE and VE are very similar, making it difficult to distinguish them clinically. The first symptoms of AE are mainly epilepsy, cognitive dysfunction and mental behavior abnormality, whereas the first symptoms of VE are fever, headache and vomiting, which are consistent with this study. This study found that the proportion of fever, headache, and vomiting as the first symptoms in the VE group was significantly higher than that in the AE group (P < 0.05). Therefore, suspected encephalitis patients with these symptoms should first consider viral encephalitis. Huang et al.13 reported that the first symptom of 28% of VE patients was epilepsy. In this study, 11 patients in the VE group had epilepsy as the first symptom, which also indicates that epilepsy cannot be used as a specific symptom to distinguish AE from VE. This study also found that about 20% of AE patients (all with anti-NMDAR encephalitis) presented with dyspnea and shortness of breath at disease onset, which were not present in the VE group (P < 0.05). This phenomenon is consistent with a previous study showing that 45% of patients with anti-NMDAR encephalitis can develop significant central hypoventilation, which may be related to the different states of epileptic persistence.14
There was a significant difference in age distribution between patients in the two groups in this study. Children and adolescents in the VE group were prone to the disease, which was of statistical significance (P < 0.05), whereas 60.0% of patients in AE group had anti-NMDAR encephalitis, mainly young women in the anti-NMDAR group and middle-aged and elderly in the LE group. These findings are consistent with the study by Kelley et al.,15 which showed that anti-NMDAR encephalitis is the most common type of AE, and is common in young women and children.
Laboratory and ECG examination
In this study, the occurrence rate of abnormal myocardial enzyme spectrum in the VE group was significantly higher than that in the AE group at the beginning of the disease (P < 0.05), but the corresponding ECG examination was generally normal. The myocardial enzyme spectrum can be used as an important index to diagnose VE children and evaluate the severity of the disease.16 We postulate that the mechanism of this phenomenon may be related to VE and viral myocarditis of the same pathogenic virus species, which invade the myocardium at the same time, causing transient damage.
This study found that the positive rate of thyroid antibody and the incidence of thyroid dysfunction were higher in the VE group than in the AE group. To date, no relevant studies have proven the relationship between anti-thyroid antibody and nerve cell surface antibody and VE. Anti-thyroid antibodies have been detected in patients with γ-amino butyric acid-a receptor (GABAAR) encephalitis.17 In this study, both groups showed a significant increase in anti-thyroid peroxidase antibody, indicating that positive anti-thyroid antibody is not a specific index of GABAAR encephalitis.
In this study, the positive rate of tumor markers in the VE group was higher than that in the AE group. At present, there is no relevant literature showing that VE is easily associated with tumors, which is a direction worth exploring. Forty-five percent of adult women with anti-NMDAR encephalitis have potential ovarian teratoma, and anti-AMPAR encephalitis is highly correlated with lung cancer, breast cancer, and thymoma.15 In this study, one patient with anti-NMDAR encephalitis showed ovarian teratoma on the right side, and there was one case of suspected lymphoma in the AE group and one case of suspected lymphoma in the VE group, although they were not confirmed by pathology. Although a tumor was not detected in most of the patients, clinician should still monitor serum tumor markers in patients, especially VE patients.
The number and protein content of CSF leukocytes were increased in the VE group and AE group, with a more obvious increase in the VE group. Positive CSF antibody is a necessary condition for the diagnosis of AE, and it is also an important auxiliary exclusion test for the diagnosis of VE.
In this study, there was no significant difference in EEG findings between the VE group and AE group, whereas the incidence of epileptiform discharge in the VE group was significantly lower than that in the AE group. Abnormal δ brush is a more specific EEG change of anti-NMDAR encephalitis.18 There were four cases with abnormal δ brush in the AE group and three cases with abnormal δ brush in the VE group, indicating that δ brush had no specificity in VE and AE. Of course, this may be due to the error caused by the inconsistency of the number of people receiving EEG examination in the sample.
Imaging findings
This study found that the distribution of lesions in the AE group and VE group was significantly different, with the VE group having involvement of a single lobe or multiple asymmetric lesions involving the limbic system, whereas the AE group involved multiple symmetrical lesions involving the limbic system. The proportion of single lesion involving single lobe or multiple asymmetric lesions involving limbic system in the VE group was significantly higher than that in the AE group. Therefore, patients with suspected encephalitis with symmetrical MRI findings should first consider autoimmune encephalitis. Both lesions mainly had gray matter, but white matter was predominant in the VE group. It is noteworthy that in recent years, it has been shown that AE can be secondary to VE (mainly herpes simplex encephalitis).19 In this study, four cases of VE and AE lesions were similar in scope and signal; however, whether this was related to the above findings remains to be further explored. AE and VE skull MRI showed slightly longer T1 and slightly longer T2 signal, and DWI and T2-FLAIR sequence showed a high signal. Some scholars have proposed that the signal of VE lesions on the DWI and T2-FLAIR sequence is higher than that of the AE lesions. This study showed that the signal of most cases on DWI was higher than that of T2-FLAIR sequence, but the measurement data were not statistically significant. The difference between the two signals may be related to the different proportion of cytotoxic edema and vascular water in the lesions. In addition, in this study, a total of six patients in the AE group and VE group underwent skull MRI reexamination after remission after treatment. It was found that there three cases of original lesions completely disappeared in each sequence, while the remaining three cases of lesions had little change or slight absorption in T2-FLAIR sequence. While two cases completely disappeared in the DWI sequence, one case obviously absorbed. We postulate that the DWI sequence is more sensitive than T2-FLAIR sequence in the evaluation of efficacy of AE and VE and follow-up review, which has important clinical significance. In this study, the incidence of lesion enhancement in the VE group was higher than that in the AE group, and the enhancement was mainly increased in the vascular gyrus, whereas no enhancement was found in the AE group.
At present, MRI is a routine method for finding lesions and evaluating lesion size and disease changes. It has an important application value for the diagnosis of AE and VE, the formulation of treatment plans, and the evaluation of efficacy. However, our study and others showed that the clinical symptoms and laboratory tests of AE and VE patients were consistent, while no abnormal signal was found on MRI, even though enhanced scanning was performed. Some studies have shown that F18 fluorodeoxyglucose positron emission tomography (18F-FDG-PET)/computed tomography (CT), PET-MRI, and functional MRI (fMRI) are of great value in diagnosing AE. Compared with the results of normal or non-specific MRI, 18F-FDG PET/CT can more sensitively detect intracranial abnormal functional metabolic regions in the diagnosis of AE.20 Susceptibility weighted imaging (SWI) can be used to detect microhemorrhage in patients with VE and can provide useful information for the formulation of a clinical treatment plan for the prognosis evaluation of VE,21 but AE has not been reported. The author believes that this is related to the pathogenesis of both diseases. VE is the direct destruction of tissues caused by virus invasion into brain parenchyma, and a large amount of neuronal necrosis and hemorrhage, which leads to the direct destruction of tissues and a large number of neuron necrosis and hemorrhage. AE is caused by autoantibody, which leads to the change of neuron membrane receptor, synaptic protein function, activation of inflammatory cytokine pathway, and less neuron necrosis and microbleeds. In this study, only three patients with AE were examined with SWI, and none were examined with 18F-FDG PET/CT, PET-MRI, or fMRI. We believe that a small part of the reason is that these tests are more expensive than the patient's family can afford, and the biggest reason is that clinicians do not know much about the value of these tests for the differential diagnosis of these two diseases.
Mathews et al.4 proposed that the enhanced FLAIR sequence could simultaneously show the enhancement of brain parenchyma and meningeal lesions, and overcome the shortcoming that conventional T1WI enhanced vascular enhancement could not be easily distinguished from meningeal enhancement. Therefore, we believe that the enhanced FLAIR sequence should be considered as a complementary sequence of T1WI enhancement and can be included as a routine test for the diagnosis of VE. In VE patients, it is mostly caused by RNA viruses led by the mumps virus.9 However, as many as 60% of presumed cases of VE remain unexplained because conventional laboratory techniques fail to detect infectious diseases.5
Treatment
Different from VE treatment methods, AE is mainly treated with immunosuppressive therapy.22 The prognosis is good. VE is mainly treated with antiviral, adjuvant immunotherapy, and symptomatic treatment in the early stage, and most patients can be cured by giving sufficient antiviral drugs within a few days. In this study, 35% of the patients in AE group were misdiagnosed with VE in the early stage, and 15% of them had delayed in diagnosis; all patients in the VE group were correctly diagnosed. We believe that VE is a common and frequently occurring disease of the central nervous system, but AE has only been known to everyone in recent years and studies have shown no specific efficacy.23 Clinicians and radiologists have a limited understanding of this disease, which is prone to misdiagnosis and delayed diagnosis. With the development of neuroimmunology, there are increasing subtypes of AE, and different types of AE have different pathogenic mechanisms and different clinical manifestations. Therefore, we should carefully identify their types, sum up the treatment experience, formulate corresponding treatment plans for each subtype, and improve the detection system of related antibodies.13
There are many similarities between AE and VE. AE may be related to VE,24 so it is very important to know the distinguishing points between them. This study found that AE and VE had statistical differences in clinical manifestations, imaging manifestations and some laboratory examinations, which are of great significance to guide clinical treatment. Clinicians should not regard suspected encephalitis patients as VE with preconceived notions. We should consider the possibility of AE. Special attention should be paid to the degree of fever and headache and whether the patient has central hypoventilation, and head MRI and related laboratory examination should be done in time. Early diagnosis and treatment are key to the good prognosis of two kinds of encephalitis. Reasonable use of valuable imaging techniques, combined with early clinical symptoms, signs, images and laboratory evidence, can make a more accurate differential diagnosis between AE and VE. In addition, it is necessary to emphasize the importance of clinical diagnosis before laboratory and imaging examination. It is expected that through the experience of clinicians, we can judge what type of encephalitis is and administer the appropriate treatment plan in time. Whether virus infection can induce AE and which type of virus infection is easy to cause AE will be the direction of our further research, which can alert clinicians at an early stage.
Source of funding
This study was supported by the National Natural Science Foundation of China (No. 81460329), Jiangxi Graduate Innovation Special Fund Project (No. YC2018-S109), and Health Commission of Jiangxi Science and Technology Plan (No. 20191040).
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
All patients provided written informed consent, and this study was approved by the Biomedical Ethics Committee of the First Affiliated Hospital of Nanchang University (2014 (037)).
Authors contributions
YMT conceived and designed the study, conducted the research, provided the research materials, and collected and organized data. ML analyzed and interpreted the data. LCH wrote the initial and final draft of the article, and provided logistic support. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Erazo T.R. Autoimmune encephalitis. Anti-NMDA receptor and new immunophenotypes. Medicina (B Aires) 2019;79(Suppl. 3):54–59. [PubMed] [Google Scholar]
- 2.Guasp M., Arino H., Dalmau J. Autoimmune encephalitis. Rev Neurol. 2018;66(s02):S1–S6. [PubMed] [Google Scholar]
- 3.Armangue T., Spatola M., Vlagea A., Mattozzi S., Cárceles-Cordon M., Martinez-Heras E., et al. Frequency, syndromes, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17(9):760–772. doi: 10.1016/S1474-4422(18)30244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collao-Parra J.P., Romero-Urra C., Delgado-Derio C. Autoimmune encephalitis. A review. Rev Med Chil. 2018;146(3):351–361. doi: 10.4067/s0034-98872018000300351. [DOI] [PubMed] [Google Scholar]
- 5.Misra U.K., Tan C.T., Kalita J. Viral encephalitis and epilepsy. Epilepsia. 2008;49(Suppl. 6):13–18. doi: 10.1111/j.1528-1167.2008.01751.x. [DOI] [PubMed] [Google Scholar]
- 6.Graus F., Titulaer M.J., Balu R., Benseler S., Bien C.G., Cellucci T T., et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391–404. doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan N. Anti-N-methyl-D-aspartate receptor-mediated encephalitis: recent advances in diagnosis and treatment in children. Curr Probl Pediatr Adolesc Health Care. 2016;46(2):58–61. doi: 10.1016/j.cppeds.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Newman M.P., Blum S., Wong R.C., Scott J.G., Prain K., Wilson R.J., et al. Autoimmune encephalitis. Intern Med J. 2016;46(2):148–157. doi: 10.1111/imj.12974. [DOI] [PubMed] [Google Scholar]
- 9.Venkatesan A., Murphy O.C. Viral encephalitis. Neurol Clin. 2018;36(4):705–724. doi: 10.1016/j.ncl.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Ayala P., Valle-Murillo M.A., Chávez-Barba O., Cabrera-Silva R.I., González-Hernández L.A., Amador-Lara F., et al. Acute disseminated encephalomyelitis: an unusual presentation of human immunodeficiency virus infection. Case Rep Infect Dis. 2020;2020 doi: 10.1155/2020/1020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matricardi S., Farello G., Savasta S., Verrotti A. Understanding childhood neuroimmune diseases of the central nervous system. Front Pediatr. 2019;7:511. doi: 10.3389/fped.2019.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malagon V.J. Demyelinizing diseases in children. Acute disseminated encephalomyelitis and multiple sclerosis. Medicina (B Aires) 2019;79(Suppl 3):66–70. [PubMed] [Google Scholar]
- 13.Huang Q., Wu Y., Qin R., Wei X., Ma M. Clinical characteristics and outcomes between children and adults with anti-N-methyl-D-aspartate receptor encephalitis. J Neurol. 2016;263(12):2446–2455. doi: 10.1007/s00415-016-8282-1. [DOI] [PubMed] [Google Scholar]
- 14.Lin K.L., Lin J.J. Neurocritical care for anti-NMDA receptor encephalitis. Biomed J. 2020;43(3):251–258. doi: 10.1016/j.bj.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelley B.P., Patel S.C., Marin H.L., Corrigan J.J., Mitsias P.D., Griffith B., et al. Autoimmune encephalitis: pathophysiology and imaging review of an overlooked diagnosis. Am J Neuroradiol. 2017;38(6):1070–1078. doi: 10.3174/ajnr.A5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan S., Gautam S.K., Singh M.P. Clinical and diagnostic evaluation in case of viral encephalitis. J Assoc Physicians India. 2020;68(1):58. [PubMed] [Google Scholar]
- 17.Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. 2014;13(3):276–286. doi: 10.1016/S1474-4422(13)70299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitt S.E., Pargeon K., Frechette E.S., Hirsch L.J., Dalmau J., Friedman D. Extreme delta brush: a unique EEG pattern in adults with anti-NMDA receptor encephalitis. Neurology. 2012;79(11):1094–1100. doi: 10.1212/WNL.0b013e3182698cd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bale J.J., Du Pasquier R. Relapse in herpes simplex virus encephalitis: it's not just about the virus. Neurology. 2015;85(20):1730–1731. doi: 10.1212/WNL.0000000000002132. [DOI] [PubMed] [Google Scholar]
- 20.Solnes L.B., Jones K.M., Rowe S.P., Pattanayak P., Nalluri A., Venkatesan A., et al. Diagnostic value of (18)F-FDG PET/CT versus MRI in the setting of antibody-specific autoimmune encephalitis. J Nucl Med. 2017;58(8):1307–1313. doi: 10.2967/jnumed.116.184333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X.J., Li C.T., Wang B., Zheng C.X., Wu L.B., Ma L.Z., et al. Microhemorrhage identified on 3.0 T MR susceptibility-weighted imaging for prognosis of viral encephalitis. J X Ray Sci Technol. 2018;26:1–8. doi: 10.3233/XST-17362. [DOI] [PubMed] [Google Scholar]
- 22.Shin Y.W., Lee S.T., Park K.I., Jung K.H., Jung K.Y., Lee S.K., et al. Treatment strategies for autoimmune encephalitis. Ther Adv Neurol Disord. 2018;11 doi: 10.1177/1756285617722347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lancaster E. The diagnosis and treatment of autoimmune encephalitis. J Clin Neurol. 2016;12(1):1–13. doi: 10.3988/jcn.2016.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linnoila J.J., Binnicker M.J., Majed M., Klein C.J., McKeon A. CSF herpes virus and autoantibody profiles in the evaluation of encephalitis. Neurol Neuroimmunol Neuroinflammation. 2016;3(4):e245. doi: 10.1212/NXI.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]