Abstract
We recently demonstrated that prothrombin kringle-2 (pKr-2) derived from blood-brain barrier (BBB) disruption could induce hippocampal neurodegeneration and object recognition impairment through neurotoxic inflammatory responses in the five familial Alzheimer's disease mutation (5XFAD) mice. In the present study, we aimed to determine whether pKr-2 induces microglial activation by stimulating toll-like receptor 4 (TLR4) upregulation and examine whether this response contributes to pKr-2-induced neuroinflammatory damage in the hippocampi of mice models. We observed that inflammatory responses induced by pKr-2 administration in the hippocampi of wild-type mice were significantly abrogated in TLR4-deficient mice (TLR4−/−), and caffeine supply or rivaroxaban treatment that inhibits the overexpression of hippocampal pKr-2 reduced TLR4 upregulation in 5XFAD mice, resulting in the inhibition of neuroinflammatory responses. Similar to the expression patterns of pKr-2, TLR4, and the TLR4 transcription factors, PU.1 and p-c-Jun, seen in the postmortem hippocampal tissues of Alzheimer's disease (AD) patients, our results additionally showed the influence of transcriptional regulation on TLR4 expression following pKr-2 expression in triggering the production of neurotoxic inflammatory mediators. Therefore, we conclude that pKr-2 may play a role in initiating upregulation of microglial TLR4, consequently inducing hippocampal neurodegeneration. Furthermore, the control of pKr-2-induced microglial TLR4 could be a useful therapeutic strategy against hippocampal neurodegeneration in AD.
Keywords: Prothrombin kringle-2, Alzheimer's disease, Toll-like receptor 4, Microglia, Hippocampus, Neuroinflammation, Neurodegeneration
Highlights
-
•
Increased levels of pKr-2, TLR4, and TLR4 transcription factors are seen in the hippocampi of AD patients and 5XFAD mice.
-
•
pKr-2 acts as a proinflammatory stimulus for microglial reactivity by stimulating TLR4 induction via PU.1 and c-Jun.
-
•
pKr-2-induced inflammatory responses and neurodegeneration are significantly abrogated in TLR4-deficient mice (TLR4−/−).
-
•
Caffeine or rivaroxaban treatment that inhibits hippocampal pKr-2 expression reduces TLR4 upregulation in 5XFAD mice.
1. Introduction
Alzheimer's disease (AD) is a common brain disorder characterized by hippocampal neurodegeneration, which plays a major role in cognitive impairment and memory loss (Burns and Iliffe, 2009; Cummings, 2004; Kawas, 2003; Querfurth and LaFerla, 2010). Although the etiology of AD is still unclear, accumulating evidence suggests that neuroinflammation mediated by glial activation is an important neurotoxic mechanism associated with AD initiation and progression (Heneka et al., 2015; Heppner et al., 2015; Kinney et al., 2018; Morales et al., 2014), with activated microglia being reported as crucial mediators of neurotoxic inflammatory processes (Baker et al., 2018; Heneka et al., 2015; Sarlus and Heneka, 2017). Thus, to determine whether neurotoxic inflammation is a definitive cause of AD, an examination of the key pathological mechanisms and endogenous molecules involved in initiating microglial activation is necessary.
Prothrombin kringle-2 (pKr-2) is a domain of prothrombin originating from its cleavage by active thrombin (Leem et al., 2016; Mann, 1976; Shin et al., 2015; Taneda et al., 1994). We have previously reported that the upregulation of pKr-2, which is not toxic to neurons by itself, can induce microglia-mediated neurotoxic inflammation, resulting in neurodegeneration in the nigrostriatal dopaminergic (DA) system in vivo (Kim et al., 2010, 2018; Leem et al., 2016; Shin et al., 2015). Moreover, we recently reported that pKr-2 upregulation was derived from blood-brain barrier (BBB) disruption in the hippocampus of the five familial AD (5XFAD) mouse model, while inhibition of its upregulation decreased hippocampal neurodegeneration and cognitive impairment in 5XFAD mice (Kim et al., 2022). Additionally, the protein levels of pKr-2 expression were significantly increased in postmortem brain tissues of patients with Parkinson's disease (PD) and AD when compared to that in age-matched controls, thus, suggesting a clinical correlation between pKr-2 overexpression and PD (Leem et al., 2016; Shin et al., 2015) and AD (Kim et al., 2022) pathogenesis.
Toll-like receptor 4 (TLR4) is a well-known innate immune receptor, associated with neuroinflammatory responses in the brain (Bachiller et al., 2018; Calvo-Rodriguez et al., 2020; Kettenmann et al., 2011) and regulated by transcription factors such as PU.1 and p-c-Jun (Gosselin et al., 2014, 2017; Gupta et al., 2009; Kierdorf et al., 2013; Tsatsanis et al., 2006; Waetzig et al., 2005). Although the pattern of TLR expression remains controversial, many reports consider microglia to be the key cells in TLR4-mediated neuroinflammatory responses, which may be involved in neurodegenerative diseases, such as AD and PD, via the production of neurotoxic and pro-inflammatory mediators, such as tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and inducible nitric oxide synthase (iNOS) (Bachiller et al., 2018; Calvo-Rodriguez et al., 2020; Fellner et al., 2013; Fiebich et al., 2018; Kettenmann et al., 2011). PU.1 has been identified as the crucial regulator of microglial growth, differentiation, and associated inflammatory responses (Pimenova et al., 2021), and controls TLR4 transcription start site positioning and TLR4 promoter usage by collaborating with its activator c-Jun (Lichtinger et al., 2007; Behre et al., 1999). Furthermore, c-Jun is critical during forced overexpression of TLR4 expression as it functions as a mediator of pro-inflammatory effects in microglia (Tsatsanis et al., 2006; Waetzig et al., 2005).
We previously found that microglial TLR4 upregulation following pKr-2 administration contributes to the degeneration of the nigrostriatal DA system in vivo (Leem et al., 2016; Shin et al., 2015) and recently demonstrated that pKr-2 derived from BBB disruption could induce hippocampal neurodegeneration and object recognition decline through neurotoxic inflammatory responses in animal models of AD (Kim et al., 2022). However, the molecular mechanism that regulates the initiation and intensity of pKr-2-mediated neurodegeneration and cognitive impairment remains unknown. In the present study, we have examined whether pKr-2 overexpression induces TLR4 upregulation via transcriptional regulation and whether pKr-2-mediated TLR4 upregulation contributes to neurotoxic inflammation involved in microglial activation in the hippocampi of adult mice. To this aim, we injected pKr-2 in the CA1 region of mouse hippocampus in a time-dependent manner and performed western blot analysis and immunofluorescence double staining. Cognitive impairment was recorded in WT and TLR4−/− mice using novel object and novel location recognition tests.
2. Materials and methods
2.1. Animals and ethics approval
Male C57BL/6J wild-type (WT) mice (8-week-old, weighing 20–22 g) were purchased from Daehan Biolink (Eumseong, Korea). Male TLR4−/− mice on a C57BL/6J background were provided by Prof. Sung Joong Lee (Seoul National University, Seoul, Korea). TLR4−/− genotype was confirmed by polymerase chain reaction using 5′-CGT GTA AAC CAG CCA GGT TTT GAA GGC-3′ (forward) and 5′-TGT TGC CCT TCA GTC ACA GAG ACT CTG-3′ (reverse) primer set. The polymerase chain reaction product was separated by electrophoresis in a 1.5% agarose gel, stained with RedSafe Nucleic Acid stain, and visualized under ultraviolet light.
Littermate WT mice and 5XFAD mice (as a model for AD) on a C57BL/6 background were provided by the Korea Brain Research Institute, Daegu, South Korea. The ratio of male and female mice was 3:2 to avoid gender bias in the results.
Animals were group housed in a controlled environment (22–23°C, 14/10 h light/dark cycle) and were fed ad libitum. All animal-related procedures were conducted in accordance with the Institutional Animal Care and Use Committee of Kyungpook National University (No. KNU 2016-0042, 2019-0002, and 2022-0018) guidelines and were consistent with the ethical guidelines of the National Institutes of Health. Additionally, all animal studies were conducted according to the ARRIVE guidelines (Kilkenny et al., 2010).
2.2. Human brain samples with AD and ethics approval
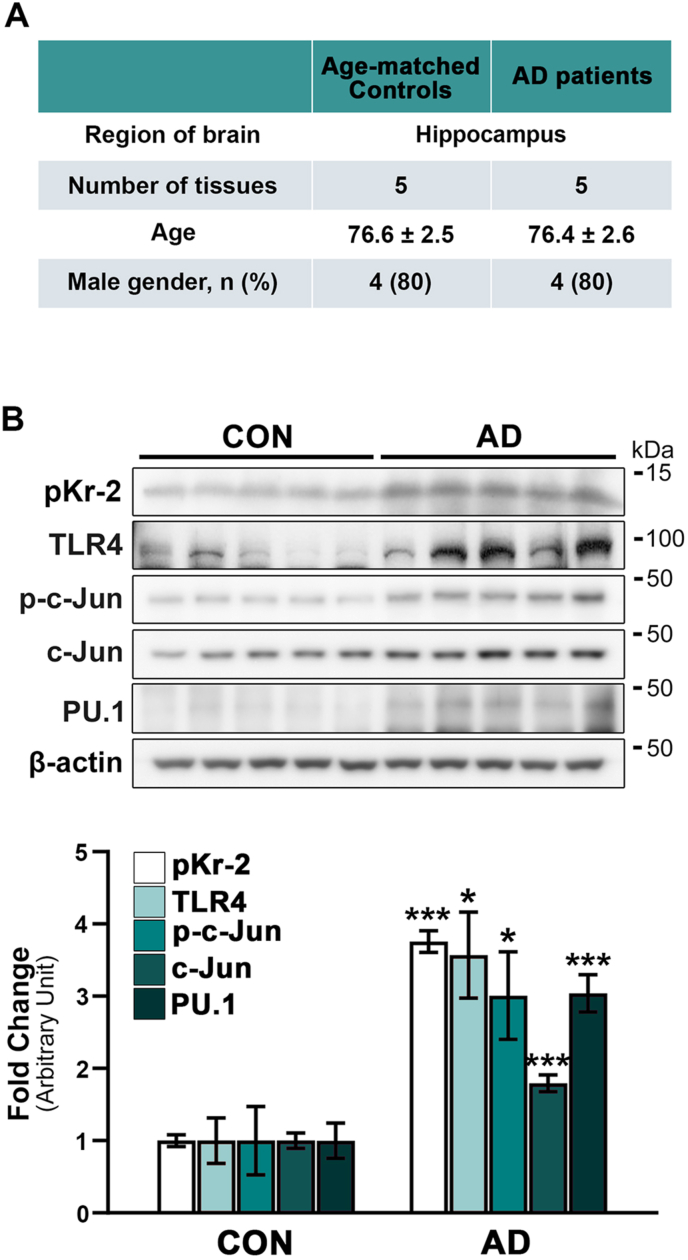
This study included ten postmortem human brain samples (8 men and 2 women) with a mean age of 76.6 ± 2.6 years, obtained from the Victorian Brain Bank Network. The present investigation is supported by The Florey Institute of Neuroscience and Mental Health and The Alfred and the Victorian Forensic Institute of Medicine and funded by Australia's National Health & Medical Research Council. Human tissue experiments were approved by the Bioethics Committee, Institutional Review Board, Kyungpook National University Industry Foundation (IRB Number: 2016–0011 and 2018-0207-1). Details of the human postmortem hippocampal tissues are shown in Fig. 1A.
Fig. 1.
Upregulation of pKr-2, TLR4, and TLR4 transcription factors in postmortem hippocampal tissues of patients with AD. (A) Details of the human postmortem hippocampal tissues are obtained from the Victorian Brain Bank Network. (B) Western blot analysis shows significantly increased protein levels of pKr-2, TLR4, p-c-Jun, c-Jun, and PU.1 in the postmortem hippocampal tissues of patients with AD compared to age-matched controls (CON). *p < 0.05 and ***p < 0.001 vs. CON (t-test; n = 5 for each group).
2.3. Materials
Materials were purchased from the following suppliers: pKr-2 (Hematologic Technologies Inc., Essex Junction, VT, USA), rivaroxaban (Selleckchem, Houston, TX, USA), and caffeine (Sigma, St. Louis, MO, USA). Antibodies were purchased as follows: anti-neuronal nuclei (NeuN) and rabbit anti-glial fibrillary acidic protein (GFAP) (Millipore, Billerica, MA, USA); goat anti-ionized calcium-binding adapter molecule 1 (Iba1) and rabbit anti-iNOS (Abcam, Cambridge, UK); rabbit/mouse anti-TLR4, rabbit anti- IL-1β, mouse anti-TNF-α, mouse anti-PU.1, mouse anti-phospho-c-Jun (p-c-Jun), and mouse anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA); rabbit anti-PU.1, rabbit anti-β-amyloid (Aβ), rabbit anti-p-c-Jun, and rabbit anti-c-Jun (Cell Signaling, Beverly, MA, USA); rabbit anti-Iba1 (Wako Pure Chemical Industries, Osaka, Japan); sheep anti-prothrombin fragment 2 (Fitzgerald, Acton, MA, USA); biotinylated anti-rabbit IgG, Texas Red-conjugated anti-rabbit/mouse IgG, fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG, and FITC-conjugated anti-goat IgG (Vector Laboratories, Burlingame, CA, USA); biotinylated anti-mouse IgG (Kirkegaard & Perry Laboratories Inc., Gaithersburg, MD, USA); FITC-conjugated anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Bar Harbor, ME, USA); horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (Enzo Life Sciences, Farmingdale, NY, USA); HRP-conjugated anti-mouse IgG (Thermo Fisher Scientific, Rockford, IL, USA); and HRP-conjugated anti-sheep IgG (GenWay Biotech Inc., San Diego, CA, USA). Sterile phosphate-buffered saline (PBS) was used as a vehicle to dissolve pKr-2.
2.4. Stereotaxic intrahippocampal pKr-2 injection
Mice were anesthetized with an intraperitoneal injection of a mixture of ketamine (115 mg/kg; Yuhan, Korea) and xylazine (23 mg/kg; Bayer Korea Ltd., Korea), and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA). A 10-μL Hamilton syringe (30 S needle) equipped with an automated syringe pump was used to perform stereotaxic injections of pKr-2 (24 μg in 2 μL PBS; 0.5 μL/min) on 5XFAD mice and respective controls either unilaterally (anterior-posterior [AP]: −2.0 mm, medial-lateral [ML]: −1.2 mm, dorsal-ventral [DV]: −1.5 mm) or bilaterally (AP: −2.0 mm, ML: ±1.2 mm, DV: −1.5 mm), relative to the bregma (Kim et al., 2018; Leem et al., 2016; Paxinos and Franklin, 2004; Shin et al., 2015), in the cornu ammonis (CA1) region of the hippocampus. The injection needle was left in place for another 5 min before being slowly withdrawn.
2.5. Immunostaining procedures
Brain tissues were prepared for immunostaining analysis as described previously (Jeon et al., 2020; Shin et al., 2015). The animals were transcardially perfused and fixed using paraformaldehyde. Animal brains were removed, frozen, and cut into 30-μm coronal sections using a cryostat microtome (Thermo Fisher Scientific).
For immunohistochemistry, free-floating brain sections were washed with ice-cold PBS and blocked using 0.5% bovine serum albumin in PBS. Sections were subsequently incubated for 48 h at 4°C with mouse anti-NeuN (1:500) and rabbit anti-Iba1 (1:2000) primary antibodies. After initial incubation with primary antibodies, brain sections were incubated with the secondary antibodies, biotinylated anti-mouse IgG (1:400) and anti-rabbit IgG (1:400), for 1 h at room temperature (RT). The signal following treatment with avidin-biotin reagent (Vectastain ABC kit) was detected by incubating the sections in 0.5 mg/mL 3,3′-diaminobenzidine (DAB; Sigma) in 0.1 M PB containing 0.015% H2O2. The stained sections were mounted on slides (Paul Marienfeld, Baden-Württemberg, Germany), and further analyzed under a bright-field microscope (Axio Imager, Carl Zeiss, Germany).
For immunofluorescence staining, washing and blocking were performed as indicated above. Sections were incubated at 4°C for 48 h with the following primary antibodies: rabbit anti-Iba1 (1:2000), goat anti-Iba1 (1:500), rabbit anti-GFAP (1:2000), mouse anti-TLR4 (1:500), mouse anti-PU.1 (1:500), mouse anti-p-c-Jun (1:500), mouse anti-TNF-α (1:500), rabbit anti-IL-1β (1:500), and rabbit anti-iNOS (1:500). Sections were then rinsed and incubated with fluorescence-conjugated secondary antibodies, including Texas Red-conjugated anti-rabbit IgG (1:400) or anti-mouse IgG (1:400) and FITC-conjugated anti-rabbit IgG (1:400), anti-mouse IgG (1:400), or anti-goat IgG (1:400) antibodies for 1 h at RT. Finally, the sections were washed and mounted using Vectashield mounting medium (Vector Laboratories). Each slide was examined and photographed using a fluorescence microscope (Carl Zeiss).
2.6. Caffeine and rivaroxaban treatment in 5XFAD mice
Caffeine was supplied as previously described by Kim et al. (2022). Briefly, 0.6 mg/mL of caffeine was dissolved in the drinking water of the mice. Considering that mice drink approximately 2.5 mL water/day, we administered a daily dose of 1.5 mg caffeine to each mouse daily for 7 months from the age of 2 months. To ensure that the caffeine remained thoroughly absorbed, the caffeinated water was changed every two days.
Rivaroxaban, an inhibitor of factor Xa (Perzborn et al., 2010; Verma and Brighton, 2009), was treated as previously described by Kim et al. (2022). 5XFAD mice received daily oral administration of rivaroxaban (2 mg/kg) dissolved in 1% ethanol for 3 months starting at the age of 6 months.
2.7. Western blot analysis
Western blotting was performed as described previously (Jeon et al., 2020; Shin et al., 2015). Mouse or human hippocampal tissues were homogenized and centrifuged at 14,000×g for 15 min at 4°C. The supernatant was transferred to a fresh tube, and protein concentrations were measured using a bicinchoninic acid assay kit (BCA assay, Bio-Rad Laboratories, Hercules, USA). A total of 50 μg of protein was electroblotted (Bio-Rad Laboratories) onto a polyvinylidene fluoride membrane (Millipore), which was subsequently incubated with primary antibodies specific for pKr-2 (1:500), TLR4 (1:1000), IL-1β (1:500), TNF-α (1:500), iNOS (1:1000), PU.1 (1:1000), p-c-Jun (1:1000), c-Jun (1:1000), Aβ (1:1000), and β-actin (1:1000). The membranes were then washed and incubated at RT for 1 h with HRP-conjugated anti-mouse IgG (1:4000), anti-sheep IgG (1:4000), and anti-rabbit IgG (1:4000) secondary antibodies. Protein complexes were visualized using enhanced chemiluminescence western blot detection reagents (GE Healthcare Life Sciences, Little Chalfont, UK). The signals were analyzed using a LAS-500 image analyzer (GE Healthcare Life Sciences). Relative band intensities were quantified using Multi-Gauge version 3.0 (Fuji Film, Tokyo, Japan), and the density of target proteins was normalized to that of the β-actin band for each sample.
2.8. Counting of hippocampal CA1 neurons
The hippocampal CA1 neurons were quantified as described previously, with some modifications (Jeon et al., 2020; Shin et al., 2015). Alternate sections were prepared from the coronal brain slices of each animal 1.7, 1.8, 2.0, and 2.1 mm posterior to the bregma. A rectangular box (1.5 × 0.5 mm) was centered over the CA1 cell layer beginning 0.5 mm lateral to the midline to ensure consistency in tissue sampling. Only neurons with visible nuclei in the CA1 cell layer were counted under a light microscope (Carl Zeiss) at × 200 magnification. A percentage of the contralateral control was used to measure the number of CA1 neurons in the ipsilateral hippocampus.
2.9. Object recognition test
Novel object recognition and object location recognition tests were conducted as described previously (Bevins and Besheer, 2006; Kim et al., 2022), with some modifications. Briefly, the mice were preadapted to an open-field testing arena (40 × 40 × 40 cm, white, opaque, acrylic open-field square box) for 10 min per day for three consecutive days prior to starting behavioral tests. The arena was cleaned with 70% ethyl alcohol between attempts. All behavioral tests were performed under low illumination light to minimize the stress levels of the animals.
In the object recognition test, the mice in both groups were allowed to explore two identical objects for 10 min, after which they were returned to their home cages for 24 h. During the test, the mice were exposed for 5 min to one familiar object and one novel object (a different shape and color) or one familiar object and one novel object (placed in a new position compared to earlier). A video camera was used to record the duration of the object exploration. Results of the object recognition test were analyzed using SMART 3.0 video tracking software (PanLab, Barcelona, Spain). To avoid observer bias, the behavioral experiments were scored with the help of a blinded experimenter who was completely unaware of the identity of the treatment group of mice.
2.10. Statistical analysis
All values are expressed as mean ± standard error of the mean. Differences between the two groups were analyzed using a t-test. Multiple comparisons between the groups were performed using one-way analysis of variance (ANOVA) or two-way ANOVA, followed by Tukey's post-hoc test. Western blot results were analyzed using one-way ANOVA, two-way ANOVA, and t-tests. Hippocampal cell counting results and behavioral test results were analyzed using two-way ANOVA. Genotype effect, treatment effect, and interaction effect were analyzed using two-way ANOVA and the results are provided as supplementary data in table 1. All statistical analyses were conducted using SigmaStat software (Version 14.0, Systat Software, San Leandro, CA, USA) and GraphPad Prism (Version 8.30, GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Increase in the levels of pKr-2, TLR4, and TLR4 transcription factors in AD patients
We previously reported on pKr-2 overexpression and microglial TLR4 upregulation in the substantia nigra and hippocampus of PD and AD brains, respectively (Kim et al., 2022; Leem et al., 2016; Shin et al., 2015), indicating a significant pathological correlation between pKr-2 and TLR4 upregulation in those diseases. However, it remains unclear whether pKr-2 overexpression contributes to TLR4 upregulation in microglia, resulting in neurodegeneration via microglial activation in the adult brain. In the present study, we investigated the protein levels of pKr-2 and TLR4, as well as those of p-c-Jun, c-Jun, and PU.1 as transcriptional regulators of TLR4, in the hippocampi of AD patients and age-matched controls using western blotting (Fig. 1A and B). Consistent with the increase in pKr-2 and TLR4 expression [*p < 0.05 and ***p < 0.001 vs. CON (Kim et al., 2022; Shin et al., 2015);], western blotting analysis showed a significant increase in the protein levels of both p-c-Jun and PU.1 in the hippocampi of AD patients compared to their age-matched controls (*p < 0.05 and ***p < 0.001 vs. CON).
3.2. Involvement of transcriptional activation in pKr-2-mediated upregulation of microglial TLR4 in the hippocampus of the adult brain
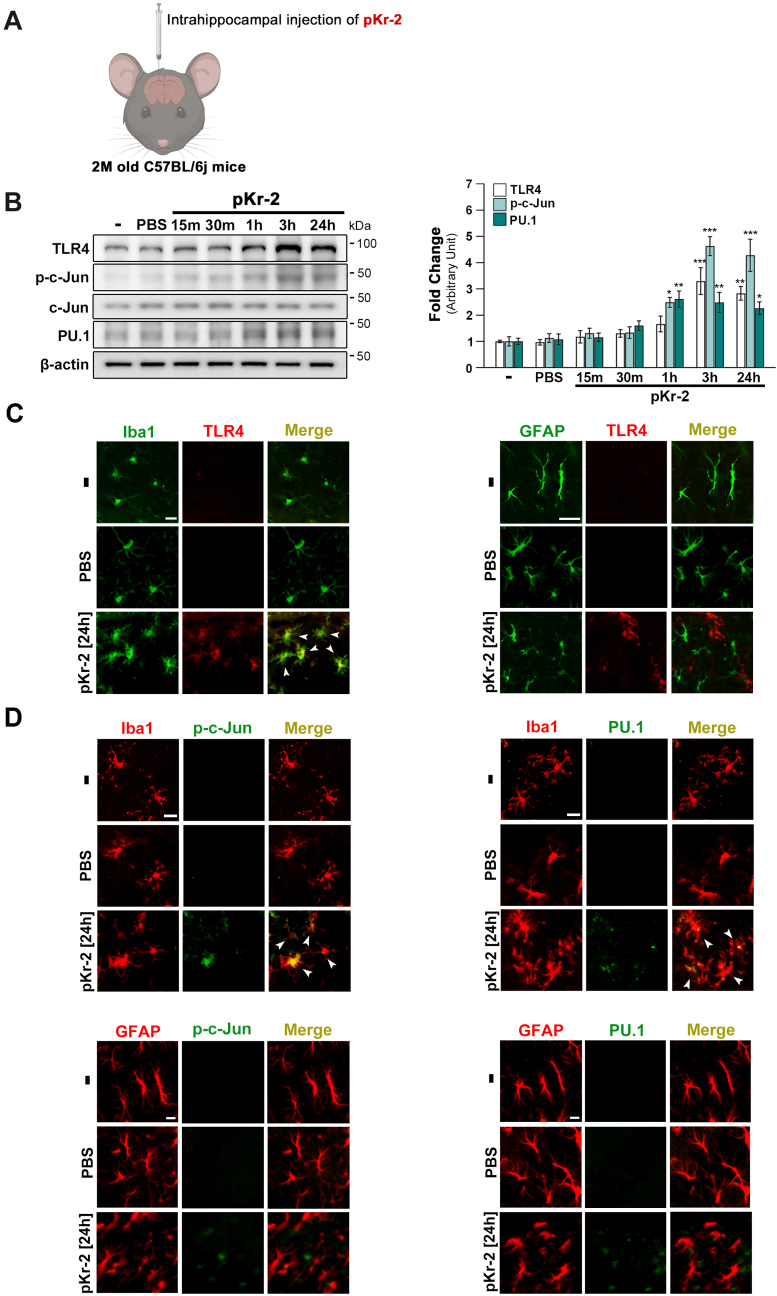
TLR4 is a crucial receptor for microglia-mediated neurotoxic inflammation, resulting in neurodegeneration in the adult brain (Calvo-Rodriguez et al., 2020; Fellner et al., 2013; Fiebich et al., 2018; Kettenmann et al., 2011; Lehnardt et al., 2002; Shin et al., 2015). To examine the transcriptional regulation in increasing TLR4 expression by pKr-2 overexpression, we examined the protein levels of p-c-Jun and PU.1 as transcriptional factors for TLR4 expression (Gosselin et al., 2014, 2017; Kierdorf et al., 2013; Tsatsanis et al., 2006; Waetzig et al., 2005) following an intrahippocampal injection of pKr-2 into mouse brain in a time-dependent manner (Fig. 2A and B). Results revealed a significant difference in p-c-Jun and PU.1 protein levels compared to non-treated controls after 1 h, 3 h, and 24 h post-pKr-2 injection into the hippocampi of mice (Fig. 2B; *p < 0.05, **p < 0.01, and ***p < 0.001 vs. intact controls). Similar trends in TLR4 levels were observed (Fig. 2B; **p < 0.01 and ***p < 0.001 vs. intact controls), indicating that there is a correlation between the upregulation of transcription factors and TLR4 following pKr-2 administration. Additionally, the increase in TLR4 expression following pKr-2 administration was primarily co-localized with Iba1-positive microglia but not with GFAP-positive astrocytes (Fig. 2C). Consistent with the increase in TLR4 expression, results obtained from immunostaining analysis revealed that the increased protein levels of transcription factors, p-c-Jun and PU.1, were mainly observed within Iba1-positive microglia, suggesting that TLR4 upregulation following pKr-2 administration could be influenced by these transcription factors (Fig. 2D).
Fig. 2.
Upregulation of TLR4 and its transcription factors in the hippocampi of pKr-2-treated mice. (A) Schematic representing the intrahippocampal injection in the mouse brain. Created with http://biorender.com. To investigate whether there are changes in the protein levels of TLR4 and its transcription factors at early time points after pKr-2 overexpression, we examined the protein levels of p-c-Jun and PU.1 as transcriptional factors for TLR4 expression following an intrahippocampal unilateral (right side) injection of pKr-2 in the male mouse brain in a time-dependent manner (15 m, 30 m, 1 h, 3 h and 24 h after pKr-2 injection). (B) Western blotting for TLR4, p-c-Jun, c-Jun, and PU.1 expression in the hippocampi at 15 min, 30 min, 1 h, 3 h, and 24 h following intrahippocampal injection of pKr-2. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. intact controls (one-way ANOVA with Tukey's post-hoc analysis; n = 5 for each group). (C) Immunofluorescence double staining for Iba1 (green) and TLR4 (red) or GFAP (green) and TLR4 (red) in the hippocampal CA1 region on day 1 following PBS or pKr-2 injection. White arrowheads indicate an increase in TLR4 expression co-localized within the microglia. Scale bar, 10 μm. (D) Immunofluorescence double staining for Iba1 (red) and p-c-Jun (green), or Iba1 and PU.1 (green), GFAP (red) and p-c-Jun (green), or GFAP (red) and PU.1 (green) in the hippocampal CA1 regions at 24 h after PBS or pKr-2 injection. Scale bar, 10 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. pKr-2-induced neuroinflammation and neurodegeneration are inhibited in TLR4−/− mice
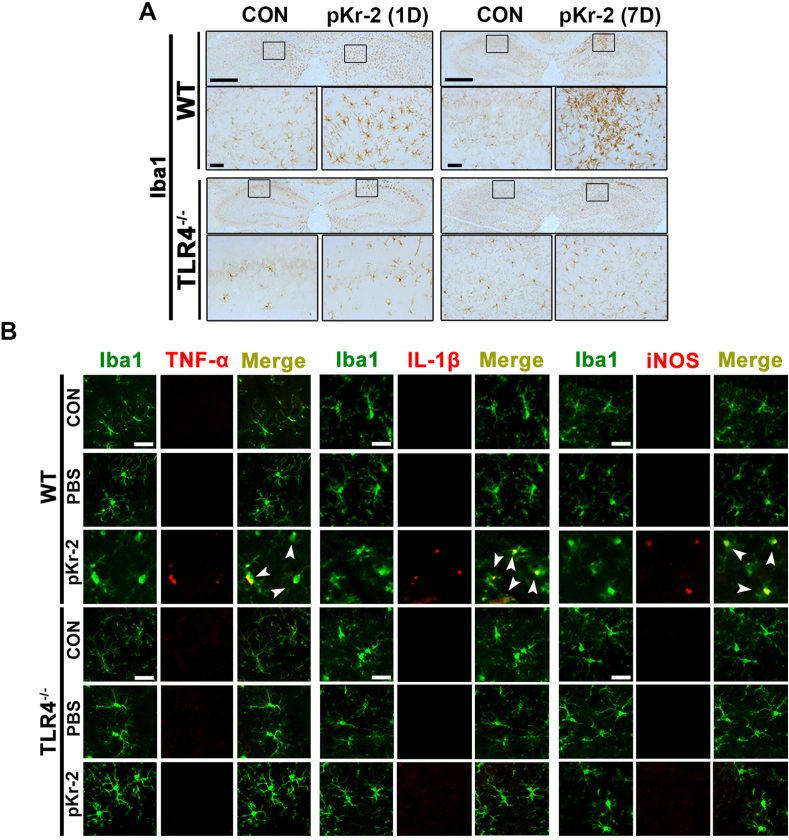
To further confirm the correlation of pKr-2-induced microglial activation and neurotoxicity with TLR4 induction, pKr-2 was unilaterally injected into the hippocampi of WT or TLR4−/− mice. TLR4−/− mice were confirmed using TLR4 genotyping and western blotting (Supplementary Fig. 1). To verify the possible link between TLR4 function and microglial activation, we assessed the extent of microglia in the hippocampus using immunostaining with an Iba1 antibody. In WT mice, activated microglia stained with anti-Iba1 were observed in the CA1 regions of the hippocampi at 1 and 7 days following pKr-2 administration; however, no morphological transformation of microglia was observed in the hippocampi of pKr-2-treated TLR4−/− mice (Fig. 3A). PBS injection did not alter the microglial morphology in the hippocampi of either WT or TLR4−/− mice compared to contralateral controls (Supplementary Fig. 2A). Moreover, hippocampal injection of pKr-2 in WT mice increased the expression levels of TNF-α, IL-1β, and iNOS in Iba1-positive microglia, as demonstrated by immunofluorescence staining performed on day 1 following pKr-2 administration; however, this increase was not observed in TLR4−/− mice (Fig. 3B).
Fig. 3.
Inhibition of pKr-2-induced microglial activation resulting in neuroinflammation in the hippocampi of TLR4−/−mice (A) Hippocampal sections obtained from male WT and TLR4−/− mice are immunostained with anti-Iba1 antibody (brown color) on 1 and 7 days following pKr-2 administration. The image in each rectangular box is magnified in the bottom panel. Scale bars, 500 and 50 μm, respectively. (B) Immunofluorescence double staining for Iba1 (green) and TNF-α (red), Iba1 (green) and IL-1β (red), and Iba1 (green) and iNOS (red) in the hippocampi of WT and TLR4−/− mice at 1 day following pKr-2 administration. White arrowheads indicate microglial cells co-localized with each inflammatory molecule. Scale bar, 20 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
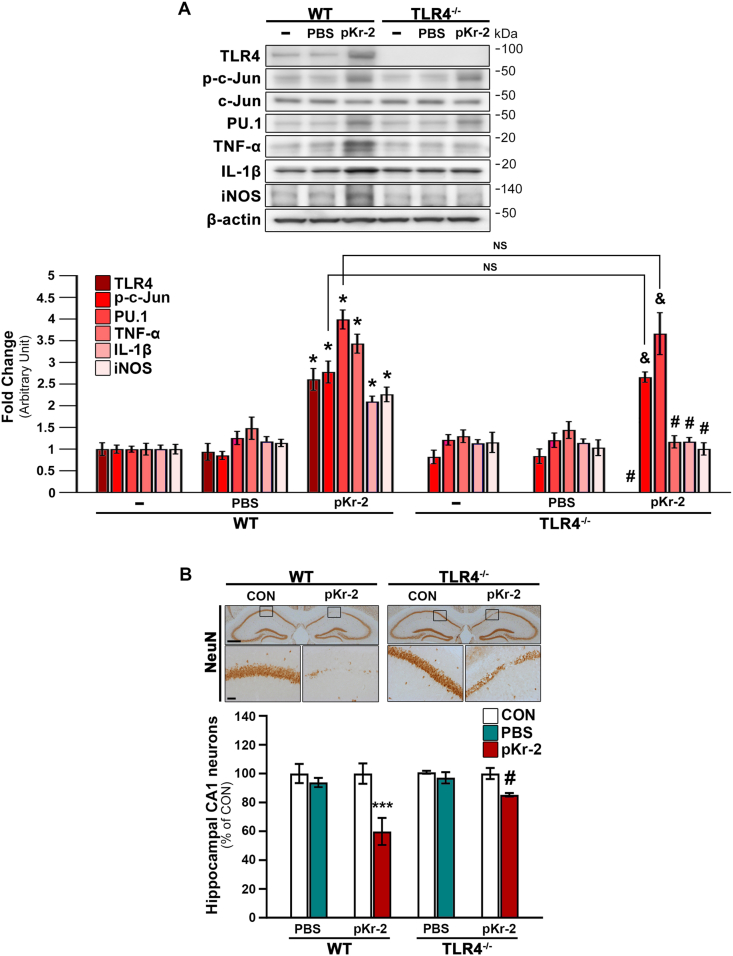
Western blotting demonstrated that pKr-2 administration significantly increased the protein levels of p-c-Jun, PU.1, and neurotoxic inflammatory molecules, such as TNF-α, IL-1β, and iNOS, in the hippocampi of pKr-2-treated WT mice compared to intact controls (Fig. 4A; *p < 0.001 vs. non-treated WT mice). We also confirmed changes in TLR4 expression in both pKr-2-treated WT and TLR4−/− groups (Fig. 4A; *p < 0.001 vs. non-treated WT mice; #p < 0.001 vs. pKr-2-treated WT mice). In addition, our results demonstrated that the increase in p-c-Jun and PU.1 protein levels following pKr-2 administration was observed in TLR4−/− mice (Fig. 4A; &p < 0.001 vs. non-treated TLR4−/− mice); moreover, there were no significant difference in p-c-Jun and PU.1 expression in the hippocampi of pKr-2-treated WT and TLR4−/− mice (Fig. 4A; NS, no significance), indicating that pKr-2 upregulation affected the increase in transcription factors for TLR4. However, the observed increase in the levels of inflammatory molecules, such as TNF-α, IL-1β, and iNOS, was significantly diminished in TLR4−/− mice (Fig. 4A; #p < 0.001 vs. pKr-2-treated WT mice). These results suggest that pKr-2 activates p-c-Jun and PU.1, which in turn positively regulates TLR4-triggered cytokine and inflammatory molecules produced in the hippocampus of the adult brain.
Fig. 4.
pKr-2 administration leads to neurotoxicity mediated by TLR4 induction in the hippocampus in vivo. To examine the protein levels of neuroinflammatory molecules at the early time point after pKr-2 overexpression, we checked 1 day after pKr-2 injection in the hippocampi of male WT and TLR4−/− mice. Moreover, to investigate the neurotoxicity caused by the pKr-2 injection, we checked the hippocampi of male WT and TLR4−/− mice, 7 days after the injection. (A) Western blot analysis of TLR4, p-c-Jun, PU.1, TNF-α, IL-1β, and iNOS protein levels in the hippocampus at day 1 following pKr-2 administration. *p < 0.001 vs. non-treated WT mice; &p < 0.001 vs. non-treated TLR4−/− mice; #p < 0.001 vs. pKr-2-treated WT mice; NS, no significance (two-way ANOVA with Tukey's post-hoc analysis; n = 5 for each group). (B) Representative images of immunohistochemical staining for anti-NeuN at day 7 following pKr-2 administration. Each image within a rectangular box is magnified in the bottom panel. Scale bars, 500 and 50 μm, respectively. The histogram quantitatively demonstrates NeuN-positive neurons in the counting area of the ipsilateral injection side compared to those of the contralateral control side (CON). ***p < 0.001 vs. CON; #p < 0.05 vs. pKr-2-treated WT mice (two-way ANOVA with Tukey's post hoc analysis; n = 5 for each group).
Furthermore, we investigated the number of preserved hippocampal neurons stained with anti-NeuN in the hippocampal CA1 regions of WT and TLR4−/− mice at 7 days following pKr-2 administration. Consistent with the findings of inhibition of pKr-2-induced microglial activation in TLR4−/− mice, pKr-2 administration induced the loss of hippocampal CA1 neurons in WT mice; however, the neuronal loss was not evident in TLR4−/− mice (Fig. 4B; ***p < 0.001 vs. CON; #p < 0.05 vs. pKr-2-treated WT mice). PBS-treated WT and TLR4−/− mice showed no signs of neurotoxicity (Supplementary Fig. 2B). Thus, our results suggest that pKr-2-induced microglial TLR4 may be a major mediator of pKr-2-induced neuroinflammation and neurotoxicity in the adult brain.
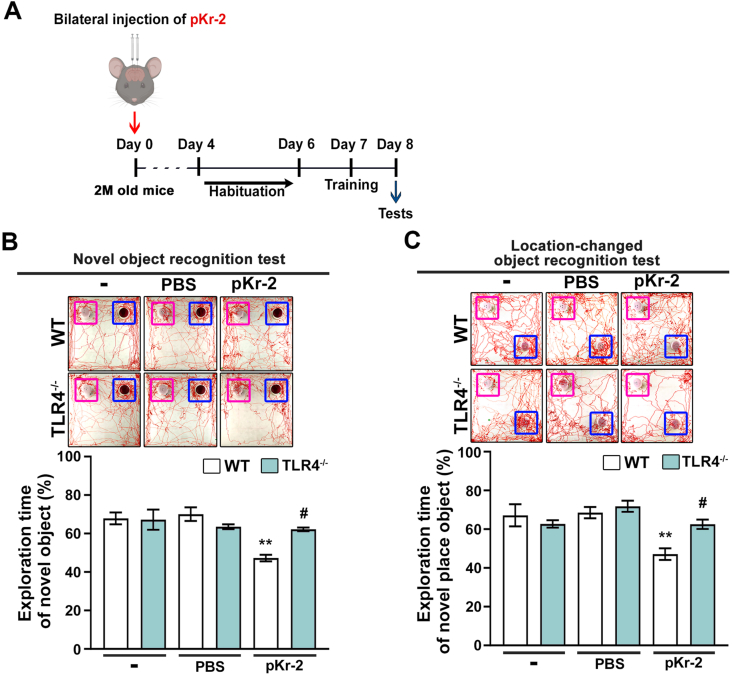
3.4. pKr-2 administration causes object cognitive impairment in WT mice but not in TLR4−/− mice
We investigated the differences in pKr-2-induced object cognitive impairment between WT and TLR4−/− mice treated with bilateral injections of pKr-2 in the CA1 region of the hippocampus of mice (Fig. 5A). The mice were subjected to two behavioral tests for object recognition, namely, novel object recognition and object location recognition (Kim et al., 2022), as depicted in the experimental schematic (Fig. 5A). In the novel object recognition test, the exploration time for the novel object (at 24 h after exploring two identical objects) was significantly shorter in pKr-2-injected WT mice than in non-treated control mice (Fig. 5B; **p < 0.01 vs. non-treated WT mice); however, the observed decline in recognition memory following pKr-2 administration in WT mice was significantly preserved in pKr-2-injected TLR4−/− mice (Fig. 5B; #p < 0.05 vs. pKr-2-injected WT mice). Similarly, in the object location recognition test, the exploration time for the location-changed object (at 24 h after exploring two identical objects) was significantly shorter in pKr-2-injected WT mice than in non-treated WT mice (Fig. 5C; **p < 0.01 vs. non-treated WT mice). However, pKr-2-injected TLR4−/− mice did not exhibit significant object recognition impairment as observed in pKr-2-injected WT mice (Fig. 5C; #p < 0.05 vs. pKr-2-injected WT mice).
Fig. 5.
Intrahippocampal administration of pKr-2 induces cognitive impairment in WT mice. (A) Behavioral tests (Novel object recognition test, Location-changed object recognition test) for assessing object recognition are conducted on day 8 following bilateral injections of pKr-2 into the hippocampal CA1 regions of male WT and TLR4−/− mice. (B) Novel object recognition tests (familiar object: purple square; novel object: blue square). Results are represented as the time ratio exploring the novel object (novel object/total object exploring time). **p < 0.01 vs. non-treated WT mice; #p < 0.05 vs. pKr-2-treated WT mice (two-way ANOVA with Tukey's post-hoc analysis; n = 8 for each group). (C) Object location recognition tests (familiar object: purple square; location-changed object: blue square). Results are represented as the time ratio exploring the location-changed object (location-changed object/total object exploring time). **p < 0.01 vs. non-treated WT mice; #p < 0.05 vs. pKr-2-treated WT mice (two-way ANOVA with Tukey's post-hoc analysis; n = 8 for each group). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
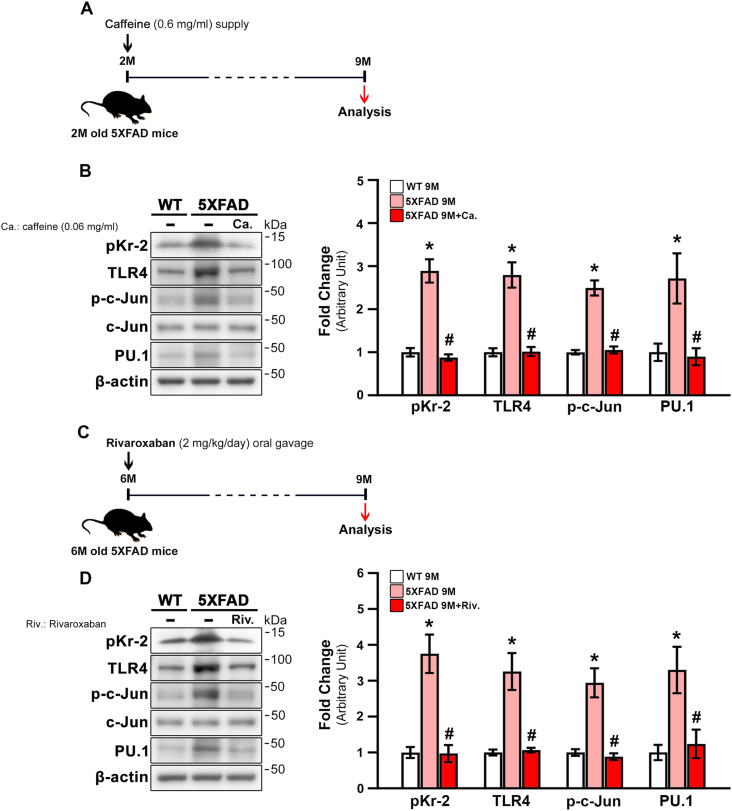
3.5. Inhibition of hippocampal pKr-2 expression reduces TLR4 upregulation in 5XFAD mice
To further investigate whether inhibition of pKr-2 reduces TLR4 and its transcriptional factors in the hippocampi of 5XFAD mice, we carried out the following two experiments: 1) 2-month-old 5XFAD mice were provided with caffeine water (0.6 mg/mL; Fig. 6A) for 7 months to enhance the BBB and prevent penetration of pKr-2 into the brain (Chen et al., 2008, 2010; Kim et al., 2022); and 2) 2-month-old 5XFAD mice received oral administration of rivaroxaban (2 mg/kg/day in 1% ethanol; Fig. 6C), an inhibitor of factor Xa (Perzborn et al., 2010; Verma and Brighton, 2009). Brain samples were collected when the mice were aged nine months to measure the protein levels of pKr-2, TLR4, and TLR4 transcription factors. The results shown in Fig. 6B revealed a significant increase in the protein levels of pKr-2 and TLR4 in the hippocampi of 9-month-old 5XFAD mice compared to WT mice (Fig. 6B; *p < 0.001 vs. 9-month-old WT mice). Additionally, p-c-Jun and PU.1 increased significantly in the hippocampi of 9-month-old 5XFAD mice (Fig. 6B; *p < 0.001 vs. 9-month-old WT mice). However, caffeine supply significantly attenuated the increased levels of pKr-2, TLR4, p-c-Jun, and PU.1 in the hippocampi of 5XFAD mice (Fig. 6B; #p < 0.001 vs. unsupplied 5XFAD mice). Similar to the results regarding caffeine supply, the increased protein levels of pKr-2, TLR4, and TLR4 transcription factors in 5XFAD mice were significantly decreased by oral administration of rivaroxaban for three months from the age of six months to 5XFAD mice (Fig. 6D; *p < 0.001 vs. 9-month-old WT mice; #p < 0.001 vs. non-treated 5XFAD mice). However, the protein levels of Aβ were not altered by either caffeine supply or rivaroxaban treatment (Supplementary Fig. 3; *p < 0.001 vs. 9-month-old WT mice) (Kim et al., 2022). Altogether, these results indicate that pKr-2 may play a crucial role in the induction of TLR4, which, in turn, is controlled by transcriptional activation.
Fig. 6.
Inhibition of pKr-2 expression by caffeine supply or rivaroxaban treatment decreases TLR4 transcription factors in the hippocampi of 5XFAD mice. (A) Experimental schematic demonstrating caffeine (0.6 mg/mL) supply. (B) Western blot analysis of pKr-2, TLR4, p-c-Jun, PU.1 protein levels in the hippocampi of male and female WT and 5XFAD mice aged seven months following caffeine supply. *p < 0.001 vs. WT mice; #p < 0.001 vs. unsupplied 5XFAD mice; (one-way ANOVA with Tukey's post-hoc analysis; n = 5 for each group). (C) Experimental schematic demonstrating rivaroxaban (2 mg/kg/day) oral treatment. (D) Western blot analysis of pKr-2, TLR4, p-c-Jun, PU.1 protein levels in the hippocampi of male and female WT and 5XFAD mice aged three months following rivaroxaban oral administration. *p < 0.001 vs. WT mice; #p < 0.001 vs. non-treated 5XFAD mice; (one-way ANOVA with Tukey's post hoc analysis; n = 5 for each group).
4. Discussion
Previous studies have established that microglia activated through the TLR4-mediated signaling pathway undergo phagocytic morphological changes in the brain, which result in the production of pro-inflammatory mediators, such as cytokines, chemokines, iNOS, and cyclooxygenase-2, ultimately resulting in neuronal damage, which may be crucial for AD initiation and progression (Choi et al., 2005; Heneka et al., 2015; Hensley, 2010; Heppner et al., 2015; Leem et al., 2016; Merlini et al., 2019; Shin et al., 2015). Recently, we demonstrated that pKr-2, a domain of prothrombin generated during its cleavage by active thrombin (Leem et al., 2016; Mann, 1976; Shin et al., 2015; Taneda et al., 1994), does not cause direct neurotoxicity (Kim et al., 2010) but can activate microglia, and the resulting production of neuroinflammatory cytokines from pKr-2 upregulation may contribute to neurodegeneration in the nigrostriatal DA system of the murine brain (Leem et al., 2016; Shin et al., 2015). In this study, we aimed to decipher whether the upregulation of pKr-2, which is seen in the hippocampi of AD patients, contributes to TLR4 upregulation in microglia, resulting in microglial activation and neurotoxic inflammation in the adult brain. Our results provide experimental evidence for the role of pKr-2 in microglial TLR4-mediated neuroinflammatory responses via the upregulation of transcription factors for TLR4 induction. Although an increase in protein levels of p-c-Jun and PU.1 as transcriptional factors for TLR4 expression was observed following pKr-2 administration in TLR4−/− mice, pKr-2-increased-neuroinflammatory molecules, such as TNF-α, IL-1β, and iNOS, in WT mice were significantly diminished in TLR4−/− mice (Fig. 4A). These results demonstrated that pKr-2-induced microglial activation in the hippocampus of the adult brain requires TLR4 expression. Hence, limiting pKr-2-induced microglial activation may be an effective therapeutic strategy for protecting hippocampal neurons in the adult brain. In addition, the control of pKr-2 upregulation by rivaroxaban or caffeine treatment in 5XFAD mice, which results in the protection of hippocampal neurons and cognitive function by impeding microglial activation (Kim et al., 2022), inhibited transcriptional activation of TLR4 expression (Fig. 6). Considered together with our previous data, the present results not only provide the first evidence of the critical role of TLR4 response in pKr-2-induced microglial activation but also provide new insight into the basic mechanisms involved in pKr-2-induced neuroinflammatory damage in the hippocampus of the adult brain. Moreover, since neuroinflammation contributes to the pathology and progression of a wide range of neurodegenerative disorders, these findings could explain the previously reported association between specific protein levels in blood and AD (Choi et al., 2005; Kim et al., 2022; Merlini et al., 2019).
Increased levels of both prothrombin and thrombin have been previously reported in the brains of patients with AD and PD (Arai et al., 2006; Ishida et al., 2006; Leem et al., 2016; Sokolova and Reiser, 2008). Additionally, pKr-2 expression is also upregulated in the brains of patients with neurodegenerative disorders (Kim et al., 2010; Leem et al., 2016; Mann, 1976; Shin et al., 2015; Taneda et al., 1994). We recently reported on the upregulation of pKr-2 in the hippocampus of 5XFAD mice and that inhibition of pKr-2 upregulation through BBB reinforcement and control of enzyme activity associated with its production could decrease neurotoxic symptoms in 5XFAD mice (Kim et al., 2022). However, we did not assess the potential pathways involved in pKr-2-mediated neurodegeneration and cognitive impairment. Based on previous reports, it is presumed that pKr-2 upregulation induces microglia-mediated neuroinflammation in the hippocampus (Chung et al., 2020); however, no report clearly describes the role of pKr-2 associated with the neuroinflammatory mechanisms that cause neurodegeneration in the hippocampus of an adult brain. Moreover, it remains unexplored whether an increase in pKr-2 expression is associated with the transcriptional regulation of microglial TLR4. TLR4 is the best-characterized inflammation inducer activated against various pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), and endogenous stimuli (Liew et al., 2005). As the immune system needs to constantly strike a balance between activation and inhibition to avoid harmful and unwarranted inflammatory responses, TLR signaling should be tightly regulated (Kumar, 2019). A crucial point of its control is at the level of gene transcription, which in turn is controlled by several transcription factors, as well as various transcriptional co-regulators and co-repressors (Medzhitov and Horng, 2009; Rosenfeld et al., 2006).
In previous studies, pKr-2 upregulation has been shown to strongly induce microglial TLR4 upregulation, resulting in neurodegeneration in the nigrostriatal DA system in vivo (Leem et al., 2016; Shin et al., 2015), thus, suggesting that pKr-2-induced TLR4 overexpression and the TLR4 signaling pathway may be important mechanisms for harmful microglial activation that contributes to neurodegeneration and cognitive impairment (Fellner et al., 2013; Jung et al., 2005; Reed-Geaghan et al., 2009). Here, we first determined whether the levels of TLR4 expression and TLR4-associated transcription factors, PU.1 and p-c-Jun, were increased in the brains of patients with AD; we measured their expression via western blotting in postmortem human hippocampal tissues obtained from control and AD brains (Fig. 1). Microglia are reported to recruit PU.1, an important lineage-determining transcription factor (LDTF), which mediates cell-type specific responses to inflammatory signals following the detection of pathogens or tissue damage (Davies et al., 2013; Medzhitov and Horng, 2009). Moreover, PU.1 binds to most enhancers in mouse and human microglia, thereby integrating cellular signaling pathways and emphasizing its crucial role in establishing the microglial enhancer landscape (Gosselin et al., 2014, 2017; Kierdorf et al., 2013). PU.1 deficiency also ablates microglia in mice (Kierdorf et al., 2013). Furthermore, c-Jun also plays a critical role during forced overexpression of TLR4, since they act as crucial mediators of pro-inflammatory responses in microglia (Tsatsanis et al., 2006; Waetzig et al., 2005). Additionally, the complex formation of PU.1 and c-Jun is essential for inflammatory gene expression (Gupta et al., 2009).
However, the existence of an endogenous molecules capable of inducing hippocampal neurodegeneration through microglial activation via TLR4 induction remained unclear. Here, we found that levels of TLR4 and TLR4-associated transcription factors as well as pKr-2 were elevated in the hippocampus of AD patients compared to their age-matched controls (Fig. 1), suggesting the possibility of a clinical correlation between the induction of microglial TLR4 expression and associated transcription factors following pKr-2 upregulation. We observed that an increase in TLR4 expression following pKr-2 administration was positively correlated with the expression levels of PU.1 and phosphorylation of c-Jun in the mouse hippocampus in a time-dependent manner, thus providing evidence for influencing transcriptional regulation of TLR4 expression following pKr-2 administration (Fig. 2). Although the pattern of TLR4 expression in the brain is still debated, microglia are considered as resident cells for TLR4-mediated neuroinflammatory responses (Kielian, 2006; Olson and Miller, 2004). Furthermore, several reports have also demonstrated TLR4 expression in various types of brain cells, including astrocytes, oligodendrocytes, and neurons (Bowman et al., 2003; Lehnardt et al., 2006; Tang et al., 2007). We observed that pKr-2 expression was co-localized within activated microglia in the present investigation, which was in line with the study findings of Lehnardt et al. (2002) demonstrating that TLR4 was mainly expressed in microglia (Fig. 2C). Moreover, increased PU.1 and p-c-Jun expression was mainly upregulated in Iba1-positive microglia in the hippocampus at 24 h following pKr-2 administration (Fig. 2D). Based on these collective findings, we can speculate an intriguing possibility that PU.1 and c-Jun may modulate each other's activity, and in the case of pKr-2, their activation might upregulate TLR4 signaling, thus orchestrates the neuroinflammatory responses. This is further corroborated by the results of the studies conducted by Behre et al. (1999), Joo et al. (2009), and Rehli et al. (2000), which showed that formation of a complex between PU.1 and c-Jun is essential for the expression of inflammatory genes including those encoding TLR4 and COX-2 (Behre et al., 1999; Joo et al., 2009; Rehli et al., 2000). Inactivation of c-Jun following treatment with JNK or p38 kinase inhibitors abolished this complex formation and suppressed PU.1 transcriptional activity (Joo et al., 2009).
Little evidence exists that indicates the role of endogenous molecules capable of inducing hippocampal neurodegeneration and impaired object cognition via neuroinflammatory responses through microglial activation without a direct neurotoxic effect (Choi et al., 2005; Chung et al., 2020; Kim et al., 2022; Merlini et al., 2019). Our experimental results revealed that pKr-2 administration induced a loss of NeuN expression in WT mice but not in TLR4−/− mice, and the increase in the protein levels of neuroinflammatory molecules caused by pKr-2 administration in WT mice was not observed in TLR4−/− mice (Fig. 3, Fig. 4). Our findings further indicated that pKr-2 upregulation contributes to cognitive impairment (Fig. 5) owing to neurodegeneration attributed to neurotoxic inflammatory responses in the hippocampus in vivo, which was not observed in TLR4−/− mice. Moreover, pKr-2-induced microglial-mediated neuroinflammation was dramatically attenuated in TLR4−/− mice compared to WT mice (Fig. 3, Fig. 4). These outcomes were consistent with studies conducted by Tang et al., Hyakkoku et al., and Lehnardt et al. (Hyakkoku et al., 2010; Lehnardt et al., 2002; Tang et al., 2007), indicating that knocking out or inhibiting TLR4 results in neuroprotective effects (Cui et al., 2020). Interestingly, transcription factors were increased when pKr-2 was injected into TLR4−/− mice; however, no significant increase was observed in the cytokines and pro-inflammatory marker levels (Fig. 4). These observations provide compelling evidence that activation of TLR4 expression by PU.1 and p-c-Jun is important for pKr-2 mediated neuroinflammation and subsequent neurodegeneration.
Our data are consistent with the findings of our previous study, which demonstrated that BBB dysfunction preservation via caffeine supply or by factor Xa inhibition associated with thrombin production by rivaroxaban can inhibit pKr-2 upregulation and reduce the corresponding neurotoxic conditions, such as the secretion of cytokines and pro-inflammatory markers, which in turn are responsible for hippocampal neurodegeneration and decline in object recognition in vivo (Kim et al., 2022). However, our study expands on previous work by demonstrating that TLR4 activation via pKr-2 depends on PU.1 and c-Jun activation during leakage of endogenous molecules from an impaired BBB. The results indicate that PU.1 and c-Jun activation by pKr-2 upregulation activates the TLR4 signaling pathway, which is supported by the observation that the effects of pKr-2 on the phosphorylation of c-Jun and PU.1 were reversed by inhibiting pKr-2 via caffeine and rivaroxaban administration (Fig. 6). These results were consistent with earlier observations in TLR4−/− mice, where after pKr-2 administration, an increase in transcription factor levels consistent with the reduction in microglia-mediated neuroinflammation was observed in the absence of the TLR4 gene (Fig. 4). Considered together with our previous studies, the present data indicate that pKr-2 activates TLR4 upregulation in microglia and is an important determinant of neuroinflammatory changes that occur in the hippocampus of AD brain.
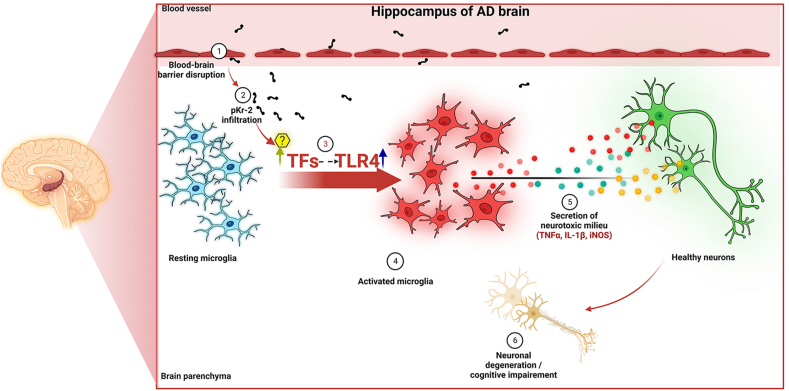
To determine whether this pathway operates the same way in the presence of specific inhibitors and to examine the significance of pKr-2 blockage or modulation of TLR4 in AD, further research is required. Furthermore, it is yet unknown whether pKr-2 requires binding for its activity. Henceforth, we cannot rule out the possibility that another mechanism may also be involved in pKr-2-induced neurotoxicity. However, our observation in the present study shows that the upregulation of pKr-2, which is a major endogenous activator of microglial activation, results in microglial TLR4 upregulation by activating the transcription factors PU.1 and c-Jun, which contributes to hippocampal neurodegeneration and object cognition impairment in adult mice, since activation of this pathway resulted in the production of neurotoxic molecules such as TNF-α, IL-1β, and iNOS form activated microglia (Fig. 7). Therefore, our current findings suggest that control of pKr-2-induced TLR4 upregulation in microglia could be crucial in preventing hippocampal neurodegeneration in AD.
Fig. 7.
Schematic representation of pKr-2-induced neurodegeneration in the hippocampus of the adult brain. Increased pKr-2 due to blood-brain barrier breakdown can lead to translocation of pKr-2 to the microglia, resulting in microglial activation via induction of microglial toll-like receptor 4 (TLR4) and its associated transcription factors (TFs). Furthermore, the activated microglia may induce hippocampal neurodegeneration by producing neuroinflammatory molecules. These observations suggest that pKr-2 upregulation is one of the major neuroinflammatory mechanisms associated with microglial activation leading to neurotoxic events in the hippocampus of the AD brain. This figure was created with http://biorender.com.
Online content
Supplementary data, figures, and figure legends are available in the online version of the paper.
Author contributions
S.K. and S.R.K. conceived and designed the experiments; C.S. and S.K. conducted the in vivo experiments and generated the figures; H.J.K., J.K, and S.R.K. supervised the analysis of the data obtained from human brain samples; S.K., M.S., and S.R.K. analyzed the in vivo experimental data; S.R.K. supervised the entire project. All authors contributed to the data analysis and preparation of the manuscript.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
This research was supported by a National Research Foundation of Korea (NRF) grant, funded by the Korean government (NRF-2020R1A2C2007954); and by Korea Health Industry Development Institute (HI14C1135 and HI21C1795) grants, also funded by the Korean government. We thank the Victorian Brain Bank Network for permission to use the human postmortem tissues.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2023.100593.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- Arai T., Miklossy J., Klegeris A., Guo J.P., McGeer P.L. Thrombin and prothrombin are expressed by neurons and glial cells and accumulate in neurofibrillary tangles in Alzheimer disease brain. J. Neuropathol. Exp. Neurol. 2006;65:19–25. doi: 10.1097/01.jnen.0000196133.74087.cb. [DOI] [PubMed] [Google Scholar]
- Bachiller S., Jimenez-Ferrer I., Paulus A., Yang Y., Swanberg M., Deierborg T., Boza-Serrano A. Microglia in neurological diseases: a road map to brain-disease dependent-inflammatory response. Front. Cell. Neurosci. 2018;12:488. doi: 10.3389/fncel.2018.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S.K., Chen Z.L., Norris E.H., Revenko A.S., MacLeod A.R., Strickland S. Blood-derived plasminogen drives brain inflammation and plaque deposition in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. U.S.A. 2018;115:E9687–E9696. doi: 10.1073/pnas.1811172115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behre G., Whitmarsh A.J., Coghlan M.P., Hoang T., Carpenter C.L., Zhang D.E., Davis R.J., Tenen D.G. c-Jun is a JNK-independent coactivator of the PU.1 transcription factor. J. Biol. Chem. 1999;274:4939–4946. doi: 10.1074/jbc.274.8.4939. [DOI] [PubMed] [Google Scholar]
- Bevins R.A., Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study 'recognition memory. Nat. Protoc. 2006;1:1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Bowman C.C., Rasley A., Tranguch S.L., Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43:281–291. doi: 10.1002/glia.10256. [DOI] [PubMed] [Google Scholar]
- Burns A., Iliffe S. Alzheimer's disease. BMJ. 2009;338:b158. doi: 10.1136/bmj.b158. [DOI] [PubMed] [Google Scholar]
- Calvo-Rodriguez M., Garcia-Rodriguez C., Villalobos C., Nunez L. Role of toll like receptor 4 in alzheimer's disease. Front. Immunol. 2020;11:1588. doi: 10.3389/fimmu.2020.01588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Gawryluk J.W., Wagener J.F., Ghribi O., Geiger J.D. Caffeine blocks disruption of blood brain barrier in a rabbit model of Alzheimer's disease. J. Neuroinflammation. 2008;5:12. doi: 10.1186/1742-2094-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Ghribi O., Geiger J.D. Caffeine protects against disruptions of the blood-brain barrier in animal models of Alzheimer's and Parkinson's diseases. J. Alzheimers Dis. 2010;20(Suppl. 1):S127–S141. doi: 10.3233/JAD-2010-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.H., Lee D.Y., Kim S.U., Jin B.K. Thrombin-induced oxidative stress contributes to the death of hippocampal neurons in vivo: role of microglial NADPH oxidase. J. Neurosci. 2005;25:4082–4090. doi: 10.1523/JNEUROSCI.4306-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y.C., Jeong J.Y., Jin B.K. Interleukin-4-mediated oxidative stress is harmful to hippocampal neurons of prothrombin kringle-2-lesioned rat in vivo. Antioxidants. 2020;9:1068. doi: 10.3390/antiox9111068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W., Sun C., Ma Y., Wang S., Wang X., Zhang Y. Inhibition of TLR4 induces M2 microglial polarization and provides neuroprotection via the NLRP3 inflammasome in alzheimer's disease. Front. Neurosci. 2020;14:444. doi: 10.3389/fnins.2020.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J.L. Alzheimer's disease. N. Engl. J. Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- Davies L.C., Jenkins S.J., Allen J.E., Taylor P.R. Tissue-resident macrophages. Nat. Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner L., Irschick R., Schanda K., Reindl M., Klimaschewski L., Poewe W., Wenning G.K., Stefanova N. Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia. Glia. 2013;61:349–360. doi: 10.1002/glia.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebich B.L., Batista C.R.A., Saliba S.W., Yousif N.M., de Oliveira A.C.P. Role of microglia TLRs in neurodegeneration. Front. Cell. Neurosci. 2018;12:329. doi: 10.3389/fncel.2018.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin D., Link V.M., Romanoski C.E., Fonseca G.J., Eichenfield D.Z., Spann N.J., Stender J.D., Chun H.B., Garner H., Geissmann F., Glass C.K. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 2014;159:1327–1340. doi: 10.1016/j.cell.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin D., Skola D., Coufal N.G., Holtman I.R., Schlachetzki J.C.M., Sajti E., Jaeger B.N., O'Connor C., Fitzpatrick C., Pasillas M.P., Pena M., Adair A., Gonda D.D., Levy M.L., Ransohoff R.M., Gage F.H., Glass C.K. An environment-dependent transcriptional network specifies human microglia identity. Science. 2017;356 doi: 10.1126/science.aal3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P., Gurudutta G.U., Saluja D., Tripathi R.P. PU.1 and partners: regulation of haematopoietic stem cell fate in normal and malignant haematopoiesis. J. Cell Mol. Med. 2009;13:4349–4363. doi: 10.1111/j.1582-4934.2009.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., Herrup K., Frautschy S.A., Finsen B., Brown G.C., Verkhratsky A., Yamanaka K., Koistinaho J., Latz E., Halle A., Petzold G.C., Town T., Morgan D., Shinohara M.L., Perry V.H., Holmes C., Bazan N.G., Brooks D.J., Hunot S., Joseph B., Deigendesch N., Garaschuk O., Boddeke E., Dinarello C.A., Breitner J.C., Cole G.M., Golenbock D.T., Kummer M.P. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley K. Neuroinflammation in Alzheimer's disease: mechanisms, pathologic consequences, and potential for therapeutic manipulation. J. Alzheimers Dis. 2010;21:1–14. doi: 10.3233/JAD-2010-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner F.L., Ransohoff R.M., Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- Hyakkoku K., Hamanaka J., Tsuruma K., Shimazawa M., Tanaka H., Uematsu S., Akira S., Inagaki N., Nagai H., Hara H. Toll-like receptor 4 (TLR4), but not TLR3 or TLR9, knock-out mice have neuroprotective effects against focal cerebral ischemia. Neuroscience. 2010;171:258–267. doi: 10.1016/j.neuroscience.2010.08.054. [DOI] [PubMed] [Google Scholar]
- Ishida Y., Nagai A., Kobayashi S., Kim S.U. Upregulation of protease-activated receptor-1 in astrocytes in Parkinson disease: astrocyte-mediated neuroprotection through increased levels of glutathione peroxidase. J. Neuropathol. Exp. Neurol. 2006;65:66–77. doi: 10.1097/01.jnen.0000195941.48033.eb. [DOI] [PubMed] [Google Scholar]
- Jeon M.T., Moon G.J., Kim S., Choi M., Oh Y.S., Kim D.W., Kim H.J., Lee K.J., Choe Y., Ha C.M., Jang I.S., Nakamura M., McLean C., Chung W.S., Shin W.H., Lee S.G., Kim S.R. Neurotrophic interactions between neurons and astrocytes following AAV1-Rheb(S16H) transduction in the hippocampus in vivo. Br. J. Pharmacol. 2020;177:668–686. doi: 10.1111/bph.14882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo M., Kwon M., Cho Y.J., Hu N., Pedchenko T.V., Sadikot R.T., Blackwell T.S., Christman J.W. Lipopolysaccharide-dependent interaction between PU.1 and c-Jun determines production of lipocalin-type prostaglandin D synthase and prostaglandin D2 in macrophages. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;296:L771–L779. doi: 10.1152/ajplung.90320.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D.Y., Lee H., Jung B.Y., Ock J., Lee M.S., Lee W.H., Suk K. TLR4, but not TLR2, signals autoregulatory apoptosis of cultured microglia: a critical role of IFN-beta as a decision maker. J. Immunol. 2005;174:6467–6476. doi: 10.4049/jimmunol.174.10.6467. [DOI] [PubMed] [Google Scholar]
- Kawas C.H. Clinical practice. Early Alzheimer's disease. N. Engl. J. Med. 2003;349:1056–1063. doi: 10.1056/NEJMcp022295. [DOI] [PubMed] [Google Scholar]
- Kettenmann H., Hanisch U.K., Noda M., Verkhratsky A. Physiology of microglia. Physiol. Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kielian T. Toll-like receptors in central nervous system glial inflammation and homeostasis. J. Neurosci. Res. 2006;83:711–730. doi: 10.1002/jnr.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierdorf K., Erny D., Goldmann T., Sander V., Schulz C., Perdiguero E.G., Wieghofer P., Heinrich A., Riemke P., Holscher C., Muller D.N., Luckow B., Brocker T., Debowski K., Fritz G., Opdenakker G., Diefenbach A., Biber K., Heikenwalder M., Geissmann F., Rosenbauer F., Prinz M. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 2013;16:273–280. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- Kilkenny C., Browne W., Cuthill I.C., Emerson M., Altman D.G., Group, N.C.R.R.G.W. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br. J. Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Moon G.J., Kim H.J., Kim D.G., Kim J., Nam Y., Sharma C., Leem E., Lee S., Kim K.S., Ha C.M., McLean C., Jin B.K., Shin W.H., Kim D.W., Oh Y.S., Hong C.W., Kim S.R. Control of hippocampal prothrombin kringle-2 (pKr-2) expression reduces neurotoxic symptoms in five familial Alzheimer's disease mice. Br. J. Pharmacol. 2022;179:998–1016. doi: 10.1111/bph.15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Moon G.J., Oh Y.S., Park J., Shin W.H., Jeong J.Y., Choi K.S., Jin B.K., Kholodilov N., Burke R.E., Kim H.J., Ha C.M., Lee S.G., Kim S.R. Protection of nigral dopaminergic neurons by AAV1 transduction with Rheb(S16H) against neurotoxic inflammation in vivo. Exp. Mol. Med. 2018;50:e440. doi: 10.1038/emm.2017.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.R., Chung E.S., Bok E., Baik H.H., Chung Y.C., Won S.Y., Joe E., Kim T.H., Kim S.S., Jin M.Y., Choi S.H., Jin B.K. Prothrombin kringle-2 induces death of mesencephalic dopaminergic neurons in vivo and in vitro via microglial activation. J. Neurosci. Res. 2010;88:1537–1548. doi: 10.1002/jnr.22318. [DOI] [PubMed] [Google Scholar]
- Kinney J.W., Bemiller S.M., Murtishaw A.S., Leisgang A.M., Salazar A.M., Lamb B.T. Inflammation as a central mechanism in Alzheimer's disease. Alzheimers Dement (N Y) 2018;4:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V. Toll-like receptors in the pathogenesis of neuroinflammation. J. Neuroimmunol. 2019;332:16–30. doi: 10.1016/j.jneuroim.2019.03.012. [DOI] [PubMed] [Google Scholar]
- Leem E., Jeong K.H., Won S.Y., Shin W.H., Kim S.R. Prothrombin kringle-2: a potential inflammatory pathogen in the parkinsonian dopaminergic system. Exp. Neurobiol. 2016;25:147–155. doi: 10.5607/en.2016.25.4.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S., Henneke P., Lien E., Kasper D.L., Volpe J.J., Bechmann I., Nitsch R., Weber J.R., Golenbock D.T., Vartanian T. A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J. Immunol. 2006;177:583–592. doi: 10.4049/jimmunol.177.1.583. [DOI] [PubMed] [Google Scholar]
- Lehnardt S., Lachance C., Patrizi S., Lefebvre S., Follett P.L., Jensen F.E., Rosenberg P.A., Volpe J.J., Vartanian T. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J. Neurosci. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtinger M., Ingram R., Hornef M., Bonifer C., Rehli M. Transcription factor PU.1 controls transcription start site positioning and alternative TLR4 promoter usage. J. Biol. Chem. 2007;282:26874–26883. doi: 10.1074/jbc.M703856200. [DOI] [PubMed] [Google Scholar]
- Liew F.Y., Xu D., Brint E.K., O'Neill L.A. Negative regulation of toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- Mann K.G. Prothrombin. Methods Enzymol. 1976;45:123–156. doi: 10.1016/s0076-6879(76)45016-4. [DOI] [PubMed] [Google Scholar]
- Medzhitov R., Horng T. Transcriptional control of the inflammatory response. Nat. Rev. Immunol. 2009;9:692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- Merlini M., Rafalski V.A., Rios Coronado P.E., Gill T.M., Ellisman M., Muthukumar G., Subramanian K.S., Ryu J.K., Syme C.A., Davalos D., Seeley W.W., Mucke L., Nelson R.B., Akassoglou K. Fibrinogen induces microglia-mediated spine elimination and cognitive impairment in an alzheimer's disease model. Neuron. 2019;101:1099–1108 e1096. doi: 10.1016/j.neuron.2019.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales I., Guzman-Martinez L., Cerda-Troncoso C., Farias G.A., Maccioni R.B. Neuroinflammation in the pathogenesis of Alzheimer's disease. A rational framework for the search of novel therapeutic approaches. Front. Cell. Neurosci. 2014;8:112. doi: 10.3389/fncel.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson J.K., Miller S.D. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J. Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Franklin K.B.J. second ed. Elsevier Academic Press; 2004. The Mouse Brain in Stereotaxic Coordinates, Compact. [Google Scholar]
- Perzborn E., Roehrig S., Straub A., Kubitza D., Mueck W., Laux V. Rivaroxaban: a new oral factor Xa inhibitor. Arterioscler. Thromb. Vasc. Biol. 2010;30:376–381. doi: 10.1161/ATVBAHA.110.202978. [DOI] [PubMed] [Google Scholar]
- Pimenova A.A., Herbinet M., Gupta I., Machlovi S.I., Bowles K.R., Marcora E., Goate A.M. Alzheimer's-associated PU.1 expression levels regulate microglial inflammatory response. Neurobiol. Dis. 2021;148 doi: 10.1016/j.nbd.2020.105217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querfurth H.W., LaFerla F.M. Alzheimer's disease. N. Engl. J. Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Reed-Geaghan E.G., Savage J.C., Landreth G.E. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J. Neurosci. 2009;29:11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehli M., Poltorak A., Schwarzfischer L., Krause S.W., Andreesen R., Beutler B. PU.1 and interferon consensus sequence-binding protein regulate the myeloid expression of the human Toll-like receptor 4 gene. J. Biol. Chem. 2000;275:9773–9781. doi: 10.1074/jbc.275.13.9773. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M.G., Lunyak V.V., Glass C.K. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20:1405–1428. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- Sarlus H., Heneka M.T. Microglia in Alzheimer's disease. J. Clin. Invest. 2017;127:3240–3249. doi: 10.1172/JCI90606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin W.H., Jeon M.T., Leem E., Won S.Y., Jeong K.H., Park S.J., McLean C., Lee S.J., Jin B.K., Jung U.J., Kim S.R. Induction of microglial toll-like receptor 4 by prothrombin kringle-2: a potential pathogenic mechanism in Parkinson's disease. Sci. Rep. 2015;5 doi: 10.1038/srep14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova E., Reiser G. Prothrombin/thrombin and the thrombin receptors PAR-1 and PAR-4 in the brain: localization, expression and participation in neurodegenerative diseases. Thromb. Haemostasis. 2008;100:576–581. [PubMed] [Google Scholar]
- Taneda H., Andoh K., Nishioka J., Takeya H., Suzuki K. Blood coagulation factor Xa interacts with a linear sequence of the kringle 2 domain of prothrombin. J. Biochem. 1994;116:589–597. doi: 10.1093/oxfordjournals.jbchem.a124565. [DOI] [PubMed] [Google Scholar]
- Tang S.C., Arumugam T.V., Xu X., Cheng A., Mughal M.R., Jo D.G., Lathia J.D., Siler D.A., Chigurupati S., Ouyang X., Magnus T., Camandola S., Mattson M.P. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc. Natl. Acad. Sci. U.S.A. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsatsanis C., Androulidaki A., Alissafi T., Charalampopoulos I., Dermitzaki E., Roger T., Gravanis A., Margioris A.N. Corticotropin-releasing factor and the urocortins induce the expression of TLR4 in macrophages via activation of the transcription factors PU.1 and AP-1. J. Immunol. 2006;176:1869–1877. doi: 10.4049/jimmunol.176.3.1869. [DOI] [PubMed] [Google Scholar]
- Verma A.K., Brighton T.A. The direct factor Xa inhibitor rivaroxaban. Med. J. Aust. 2009;190:379–383. doi: 10.5694/j.1326-5377.2009.tb02453.x. [DOI] [PubMed] [Google Scholar]
- Waetzig V., Czeloth K., Hidding U., Mielke K., Kanzow M., Brecht S., Goetz M., Lucius R., Herdegen T., Hanisch U.K. c-Jun N-terminal kinases (JNKs) mediate pro-inflammatory actions of microglia. Glia. 2005;50:235–246. doi: 10.1002/glia.20173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.