The circadian rhythms which govern the timing of our sleep–wake cycles synchronize most strongly with blue light. Because of this, blue light is enhanced in seasonal affective disorder therapeutic “happy” lights for morning use, and blue wavelengths are filtered from screens of many smart devices in the evening to reduce phase delays of sleep onset. It has been known for decades that visual photoreceptors are not required for the synchronization of circadian cycles to environmental light or “photoentrainment.” Both humans and mice which lack visual signals from dysfunctional or missing rods and cones continue to photoentrain and show other nonvisual light-driven phenomena, such as the pupillary light reflex (1, 2). As early as the 1920’s, Clyde Keeler identified a strain of mice with a mutation which rendered them visually blind but noted that their irises retained the ability to constrict when exposed to light (3, 4). It was not until 2002 that the responsible photoreceptor, named “melanopsin,” was identified in the mammalian retina outside of the visual rods and cones (5). Melanopsin responds most strongly to blue light (about 480 nm) and is expressed in a subset of retinal ganglion cells (RGCs) that send axons directly into the brain’s central circadian clock, the suprachiasmatic nucleus (6). These ganglion cells are called intrinsically photoreceptive RGCs (ipRGCs) (Fig. 1). In PNAS, St Hilaire et al. shed new light on the contribution of blue light-sensing cones into ipRGC pathways to regulate the circadian photoentrainment pathways in humans (7).
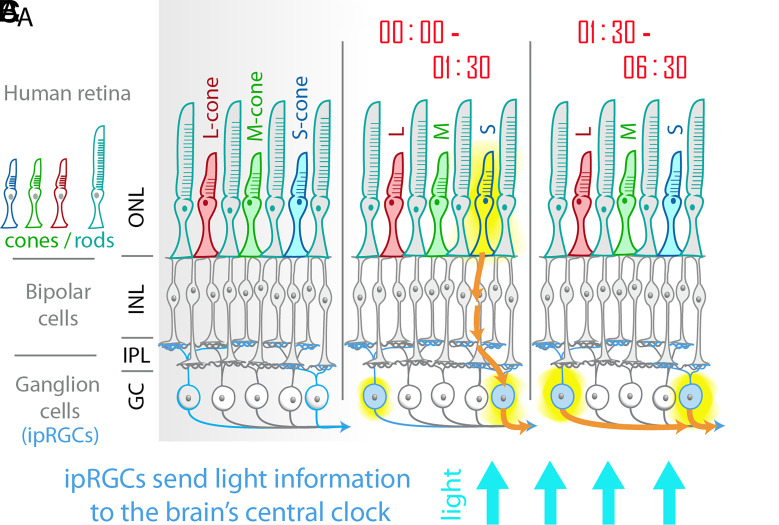
Fig. 1.
A) The human retina is made up of an outer layer of rod and cone photoreceptors (outer nuclear layer, ONL), a layer of bipolar cells (as well as horizontal cells and amacrine cells) in the inner nuclear layer, and a layer of RGCs. Cones and rods signal through bipolar cells which then make synaptic connections with RGCs at various levels of an IPL. In humans with trichromatic vision, there are three types of cones: long wavelength-sensitive (L-cone), middle-wavelength sensitive (M-cone), and short-wavelength sensitive (S-cone). RGCs are the cells which carry light information to the brain. Melanopsin-containing ipRGCs (represented in light blue) can be directly activated by light and send information to the brain’s central circadian clock. B) St Hilaire et al. show that in the early stages of a long-duration light exposure, cones (particularly S-cones) contribute significantly to the circadian response to light in human subjects. This is in addition to activation of ipRGCs by melanopsin. C) In prolonged exposure to blue light, cone activity subsides, and the response is dominated by melanopsin activation in the ipRGCs.
The retina utilizes redundant photoreceptors that drive nonvisual light effects such as circadian entrainment, pupillary light reflexes, and acute melatonin suppression. Mice lacking any one class of photoreceptor—either mice without melanopsin or mice without rod/cone signaling—still photoentrain (8, 9). Only animals lacking a combination of outer-layer photoreceptors (rods/cones) and melanopsin lose the ability to synchronize their behavior to light. Mice which have only cones or only rods are also able to photoentrain, although the lighting conditions must be specifically tailored to the remaining photoreceptor (10, 11). However, all rod and cone signals sufficient for circadian photoentrainment pass exclusively through the ipRGCs (12). Thus, multiple photoreceptors feed nonvisual photic information to the brain in a serial pathway.
The ipRGCs are peculiar compared to other RGCs in a number of ways. The dendrites of conventional RGCs make their terminal input connections with bipolar cells in discreet ON or OFF anatomical bands in the retina’s inner plexiform layer (IPL). As a general rule, the ganglion cells with synaptic connections closest to the ganglion cell layer respond when a light turns on (ON cells), and ganglion cells with dendritic arborizations terminating in the farther sublamina respond when a light turns off (OFF cells). While various classes of ipRGCs terminate in either or both of ON and OFF layers of the IPL, all intrinsic light responses are of the ON type—that is, action potentials are fired while a light is on (13). A further peculiarity of ipRGCs is their capacity for sustained firing of action potentials. While conventional ganglion cells quickly adapt and cease firing during prolonged light exposures, many ipRGCs will consistently produce action potentials under light exposures with durations as long as 10 h, and they continue to fire for minutes after the light has ceased (14, 15).
Much work on ipRGCs has been carried out in mice without rods and cones, or in experiments in which the outer photoreceptors have been pharmacologically silenced. In both primate and mouse retinas, cones contribute to the electric responses of various types of ipRGC (16–18). The upstream circuitry by which cones communicate with ipRGCs is still being uncovered. Pathways have been identified in primate retinas which allow for short-wavelength sensitive cones (S-cones) to give excitatory input to one class of ipRGC and inhibitory to another (19, 20). So, while melanopsin is the most proximal photoreceptor to the brain and gives only an ON signal, its message is shaped by the outer photoreceptors.
What does this mean functionally? What function do photoreceptors with overlapping spectral sensitivities uniquely contribute to a single circuit? One apparent difference between melanopsin and visual pigments is the rate of adaptation. In the pupillary light response, there are transient-fast responses mediated by visual photoreceptors and sustained-slow responses mediated by melanopsin activation (21, 22). It seems that a similar phenomenon is at play in the nonvisual light response of circadian photoentrainment. St Hilaire et al. now find similar transient vs. sustained functions of ipRGCs in human circadian phase shifting (7). Subjects were administered a 6.5-h light stimulation during a heroic 9-d temporal isolation. By establishing a baseline pattern of each of the 100 subjects’ melatonin rhythms, the authors were able to analyze changes throughout the light stimulation and during the following days. Circadian responses to various intensities of near-monochromatic light across the visible spectrum were compared, and they confirm that both acute melatonin suppression and circadian phase shifting are most sensitive to blue light. However, diving into the details, the authors were able to glean more information about the specific blue light that was functional at various stages of the light pulse. Melanopsin’s sensitivity peak is at approximately 480 nm, and S-cones’ at 439 nm: both are “blue” light photoreceptors. The authors fit curves which allowed for the representation of one or more photoreceptor class to the responses of the large cohort’s combined data. This revealed that different spectral sensitivities within the blue range were responsible for circadian phase shifting during the early stages compared with the later stages of the 6.5-h light pulse. In particular, S-cones contributed significantly in the first 1.5 h of light exposure, followed by a dominant melanopsin response for the remaining 5 h. The authors also observed a contribution of short- and middle- wavelength cones in the acute suppression of melatonin. Melatonin suppression may have a different sensitivity threshold than systemic phase shifts or be controlled by a slightly distinct circuit. The acute melatonin suppression was also dominated by melanopsin in the later stages of the prolonged light exposure.
“In PNAS, St Hilaire et al. shed new light on the contribution of blue light-sensing cones into ipRGC-pathways to regulate the circadian photoentrainment pathways in humans.”
It should be noted that the light exposures in this study were of continuous illumination and devoid of contrast. This was used to facilitate precise calibration of administered light. However, in natural visual scenes, our eyes are constantly scanning over areas of high contrast in changing levels of background illumination. In studies in mice, it has been shown that discontinuous light given as an on-off flicker produced larger circadian phase shifts than continuous stimuli, as the cones were not allowed to adapt (23). In the future, it will be interesting to learn whether this strategy holds true for the human circadian system as well. In the meantime, commercial therapeutic lights for personal circadian alignment have already begun to include discontinuous light paradigms designed to dynamically activate cones. While bright blue light activating melanopsin remains the standard method for early-morning activation of circadian light pathways, optimal stimulation of cones would allow for dimmer and smaller light sources. Future studies should delineate whether the ON and OFF pathways contribute equally to the fast-transient response of the circadian system to blue light, and this will allow further utilization of precise lighting to most efficiently activate (morning) or avoid (evening) the circadian resetting mechanism.
Acknowledgments
Author contributions
E.D.B. wrote the paper.
Competing interest
The author declares no competing interest.
Footnotes
See companion article, “The spectral sensitivity of human circadian phase resetting and melatonin suppression to light changes dynamically with light duration,” 10.1073/pnas.2205301119.
References
- 1.Czeisler C. A., et al. , Suppression of melatonin secretion in some blind patients by exposure to bright light. N Engl. J. Med. 332, 6–11 (1995), 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- 2.Freedman M. S., et al. , Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science 284, 502–504 (1999). [DOI] [PubMed] [Google Scholar]
- 3.Keeler C. E., Iris movements in blind mice. Am. J. Physiol. Legacy Content 81, 107–112 (1927), 10.1152/ajplegacy.1927.81.1.107. [DOI] [Google Scholar]
- 4.Keeler C. E., On the occurrence in the house mouse of mendelizing structural defect of the retina producing blindness. Proc. Natl. Acad. Sci. U.S.A. 12, 255–258 (1926), 10.1073/pnas.12.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Provencio I., Rollag M. D., Castrucci A. M., Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature 415, 493 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Berson D. M., Dunn F. A., Takao M., Phototransduction by retinal ganglion cells that set the circadian clock. Science 295, 1070–1073 (2002). [DOI] [PubMed] [Google Scholar]
- 7.St Hilaire M., et al. , The spectral sensitivity of human circadian resetting and melatonin suppression to light changes dynamically with light duration. Proc. Natl. Acad. Sci. U.S.A. 119, e2205301119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panda S., et al. , Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 298, 2213–2216 (2002), 10.1126/science.1076848298/5601/2213 [pii]. [DOI] [PubMed] [Google Scholar]
- 9.Hattar S., et al. , Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature 424, 76–81 (2003), 10.1038/nature01761 nature01761 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altimus C. M., et al. , Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat. Neurosci. 13, 1107–1112, nn.2617 10.1038/nn.2617 [pii] (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Diepen H. C., et al. , Distinct contribution of cone photoreceptor subtypes to the mammalian biological clock. Proc. Natl. Acad. Sci. U.S.A. 118, e2024500118 (2021), 10.1073/pnas.2024500118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guler A. D., et al. , Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature 453, 102–105 (2008), nature06829 [pii] 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao X., Stafford B. K., Godin A. L., King W. M., Wong K. Y., Photoresponse diversity among the five types of intrinsically photosensitive retinal ganglion cells. J. Physiol. 592, 1619–1636 (2014), 10.1113/jphysiol.2013.262782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong K. Y., A retinal ganglion cell that can signal irradiance continuously for 10 hours. J. Neurosci. 32, 11478–11485 (2012), 10.1523/JNEUROSCI.1423-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tu D. C., et al. , Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron 48, 987–999) (2005), S0896–6273(05)00891-3 [pii] 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 16.Dacey D. M., et al. , Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 433, 749–754 (2005), 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 17.Mure L. S., Vinberg F., Hanneken A., Panda S., Functional diversity of human intrinsically photosensitive retinal ganglion cells. Science 366, 1251–1255 (2019), 10.1126/science.aaz0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt T. M., Kofuji P., Differential cone pathway influence on intrinsically photosensitive retinal ganglion cell subtypes. J. Neurosci. 30, 16262–16271 (2010), 30/48/16262 [pii] 10.1523/JNEUROSCI.3656-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patterson S. S., Kuchenbecker J. A., Anderson J. R., Neitz M., Neitz J., A color vision circuit for non-image-forming vision in the primate retina. Curr. Biol. 30, 1269–1274.e1262 (2020), 10.1016/j.cub.2020.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patterson S. S., et al. , Another Blue-ON ganglion cell in the primate retina. Curr. Biol. 30, R1409–R1410 (2020), 10.1016/j.cub.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Y., et al. , Melanopsin-dependent persistence and photopotentiation of murine pupillary light responses. Invest. Ophthalmol. Vis. Sci. 48, 1268–1275 (2007), 48/3/1268 [pii] 10.1167/iovs.06-0925. [DOI] [PubMed] [Google Scholar]
- 22.Keenan W. T., et al. , A visual circuit uses complementary mechanisms to support transient and sustained pupil constriction. Elife 5, e15392 (2016), 10.7554/eLife.15392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lall G. S., et al. , Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron 66, 417–428 (2010), S0896-6273(10)00330-2 [pii] 10.1016/j.neuron.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]