Researchers are starting to decode the neural changes during sleep that underlie long-term memory
Our need for sleep is unavoidable. Shortchange yourself a few nights, and you’ll incur a debt that not only dulls the senses, but demands longer “recovery” sleep later on (1). Indeed, the brain has essential work to do while we slumber, as it synchronizes to slower, coordinated rhythms that ripple across its surface. Among the most crucial tasks on the agenda: creating lasting memories based on all that we experience while awake.
Scientists have suspected for more than a century that sleep improves recall of recently acquired information, and even performance on newly learned tasks. Decades of research have sought to connect large-scale patterns of brain activity during sleep to mechanisms of memory storage. Many of the findings suggest that sleep plays an active role in revisiting waking experiences and consolidating them into long-term memories (2).
But to better understand sleep’s role, researchers are taking a closer look at how individual neurons communicate, as well as what shapes information flow and encoding in long-term memory circuits. Studies are starting to identify molecular and cellular processes that tune the strength of the synapses in these circuits. Changes in synaptic strengths may underlie the brain’s ability to selectively transform and transfer some information from short-term experience to long-term storage, and forget all else. At the same time, evidence that some memories form better without sleep is leading neuroscientists to reconsider long-standing assumptions about sleep’s role in memory formation. Taken together, these studies are starting to change perceptions of the sleeping brain—and what it means to remember.
Getting the Gist
In cellular terms, memory is represented in the brain as collections of neurons that tend to fire together based on how they’re connected at synapses—the electrochemical connections between the axon of one neuron, which transmits information, and the dendrites of other neurons, which receive information. “We’re talking about patterns of connectivity that represent … the likelihood that that particular pathway will be activated and generate that particular experience or behavior or image,” says Sidarta Ribeiro, a neuroscientist at the Brain Institute of the Federal University of Rio Grande do Norte in Brazil. Recalling a memory can strengthen circuits by increasing the number of neurons or synapses; an inactive memory, on the other hand, can fade over time as synapses weaken or are lost.
It’s not a simple matter to gain insight into memory consolidation. Many studies of sleep and memory have relied on measuring neural activity from outside the skull with electroencephalography (EEG). EEG oscillations reflect the synchronized firing of large groups of neurons—thousands or perhaps even hundreds of thousands in the case of large-amplitude, low-frequency slow oscillations. It’s difficult to infer activity of specific neural circuits from EEG. The oscillations can be coarsely mapped to general brain regions, though, so researchers have used them to explore the neural processing needed to consolidate information from short-term memory networks, such as in the hippocampus, and move it into parts of the brain where it can be stored and accessed over longer periods of time.
“The [memory] at the beginning of the consolidation process is very much anchored in hippocampal networks, and in the end of this process, it primarily resides in neocortical networks,” says Jan Born, a neuroscientist at the University of Tübingen in Germany. Along the way, it undergoes a transformation that researchers are still working to understand.
New memories are rich with contextual clues such as the time, place, and sensory details of an experience; neuroscientists often refer to them as “episodic” at this stage. Over time, as memories get encoded in the cortex, many of those spatial and temporal details fade. What remain are the elements representing the essential core of the memory. When learning how to drive, for example, the movements needed to steer and brake are critical; the details of avoiding a specific car on a particular outing are not.
How the brain achieves this transformation, Born says, is still largely unknown. Some of it is likely driven by the brain’s limited storage capacity and a need to compress saved information. But to Born, such transformations suggest that sleep’s role in memory is not simply about passive storage. Rather, he envisions a more active process of consolidation that extracts key information and forms a generalized version of the overall memory that can later be accessed and applied to relevant situations. “We call it gist information or schema information,” Born says. With many specifics forgotten, “it’s kind of an abstracted version of the original memory.”
To Remember, We Forget
In fact, forgetting—through weakening or loss of synapses—seems to play a key role in the process of memory consolidation, especially during sleep.
“We had better forget most of what we do in a day,” says Chiara Cirelli, a neuroscientist at the University of Wisconsin–Madison. Remembering new information requires creating new neuronal connections or strengthening existing ones, and both incur more neuronal firing. But neuronal firing requires a lot of energy, notes Cirelli, and it simply isn’t sustainable to keep increasing levels of neural activity day after day. Eventually, synapses couldn’t get any stronger, and the brain wouldn’t encode new information. Effectively, without forgetting, neuronal circuits would saturate, making it impossible to learn.
What’s more, much of the incidental learning that takes place in a day helps us navigate our immediate environment but has little long-term adaptive value. “Tonight, if I asked you what you did today, you are very good in remembering all possible, extremely boring, details of what you did,” she says. “But then in a few days, they’re gone, because who cares?”
Cirelli believes that sleep is responsible for much of this culling. In the early 2000s, she and her longtime collaborator Giulio Tononi, also at UW–Madison, hypothesized that sleep causes a widespread weakening, or renormalization, of synapses (3). Dialing down neural activity across the board can clear out the clutter of less important synaptic connections formed during the day. In that way, forgetting the small stuff boosts the signal-to-noise ratio of stronger circuits and helps our brains hang on to important information. “Synaptic renormalization,” Cirelli says, “is crucial for the brain to keep working … in an efficient and sustainable way.”
This suggests that brain activity should drop over the course of sleep. And that’s indeed the case, as demonstrated by decreasing amplitudes of the major EEG oscillations seen during the early sleep stages. Using electron microscopy, Cirelli and Tononi have also measured a reduction in the size of individual synapses in mouse cortex after sleep, a trend associated with weaker connections between neurons (4, 5).
Learning may help counter such changes, however. Cirelli’s group tracked synapses on hundreds of individual dendritic spines in vivo in mice that were taught to run on a wheel with unevenly spaced rungs. The 10% of synapses with the largest learning-related changes during waking were least affected during sleep (6). “The neurons that have been firing a lot—and together—during wake do also the same during sleep,” Cirelli says. That coordinated activity, which creates strong synapses, may protect them against the mechanisms that pare down weaker synapses during sleep.
“The data out there is very messy about molecular changes occurring during sleep,” says Julie Seibt, a sleep researcher at the University of Surrey, UK.
There’s also evidence that some synapses are not only spared during downscaling, but strengthened. Wen-Biao Gan’s group at New York University found that the formation and persistence of some cortical synapses were actually boosted by non-REM sleep after a motor-learning task (7).
What these findings suggest, Cirelli says, is that sleep affords a general quieting that reduces the brain’s energy needs and makes room for the next day’s learning. At the same time, strong synapses and highly active circuits—such as those shaped by new learning—can stand out for consolidation.
From Oscillations to Synapses
In turn, this suggests that sleep-related cellular and molecular changes that tune the strength of synaptic connections are likely to be important for long-term memory formation. Many research efforts have focused on such changes associated with the different stages of sleep and types of EEG oscillations.
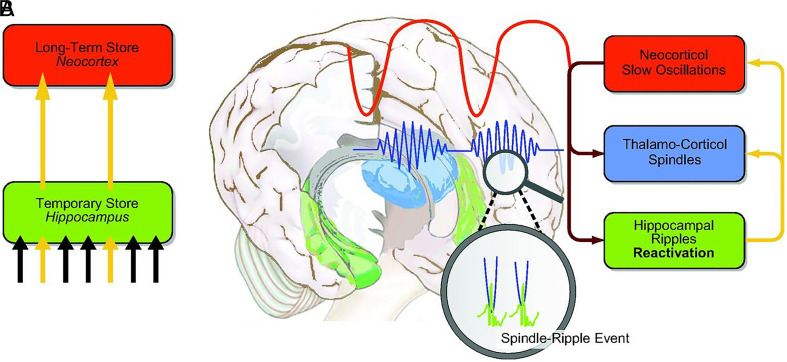
Sleep in mammals has distinct phases as characterized by specific EEG patterns. These include brain-wide slow oscillations (less than 1 Hz in frequency), sharp-wave ripples (100-300 Hz) in the hippocampus, and spindles (10-15 Hz), which are related to the firing of neurons in the circuits connecting the thalamus and the cortex. Upon onset of sleep, the brain enters a non-rapid eye movement (non-REM) phase. During non-REM sleep, slow oscillations sweep across large regions of the brain, punctuated by swells of spindles and bursts of sharp wave-ripples. A period of rapid eye movement (REM) sleep follows, with characteristic bursts of its namesake eye movements and low-amplitude theta oscillations around 4-8 Hz. The brain cycles through these phases throughout the sleep period, with slow-wave, non-REM sleep dominating the early hours and REM sleep the late hours.
The relative quiet of non-REM sleep may aid long-range brain communication and information processing, says Sara Mednick, a cognitive scientist at the University of California Irvine.
Although most research on sleep oscillations only looks for correlations, her research group has explored causal links between EEG activities in different brain areas. During most of non-REM sleep, coordination across the brain is weak. But Mednick’s group identified two narrow windows in the EEG signal when communication between brain regions was “off the charts” higher, she says: right before and right after the quietest part of the slow oscillation. These windows of greater coordination across long distances seem to represent peaks of information flow between the front and back of the brain; the greater the coordination, the better the performance on a memory test after sleep (8).
The result also means “there's something very important” about the relative lack of communication during most of non-REM sleep, Mednick says. It provides a quiet backdrop against which the slow oscillation-driven information flows stand out, possibly promoting memory consolidation.
There’s likely much more to it, though. Memory consolidation might also involve physiological changes induced by interactions among the major types of oscillations. To date, however, connecting EEG measures with specific cellular and subcellular changes has been very difficult.
Inspecting the Spindles
Increasingly, though, Seibt, Born, and others have identified an emerging role of sleep spindles. These oscillations have been associated with both stronger activity of localized cortical circuits and better performance on memory tasks, suggesting spindles could serve as a link between the two (9–11). Spindle activity correlates with an influx of calcium into cortical neurons, which may in turn trigger the molecular cascades needed to strengthen or tune synapses (9, 11).
Researchers are also taking early steps to link such neural activity to specific downstream effects. Ribeiro, for instance, is searching for protein changes associated with spindle activity. His team has identified dozens of protein modifications whose levels change in proportion to spindle-rich epochs during the transition from non-REM to REM sleep, including several connected to calcium flow and learning (12). Ribeiro is particularly intrigued by one protein called Cacna2d1, which is downregulated in the hippocampus, but not the cortex, at the end of non-REM slow wave sleep. That pattern could provide a basis for a memory to disengage the hippocampus as it starts to engage the cortex, Ribeiro says.
Although the associations are only correlative so far, Ribeiro suggests they support a model in which spindles at the end of slow wave sleep kick off a series of events that unfold in memory circuits over the next few hours: calcium influx triggers changes to proteins that in turn activate genetic and molecular cascades important in tuning synaptic strength. He likens the spindles to relay runners carrying information like a baton that they hand off from neural activity in non-REM sleep to molecular cascades in REM sleep. As a result, the remembered experience gets hard-coded into the synaptic structures of cortical neurons so the memory can persist even when it’s not being accessed regularly.
This model could also help explain distinct roles of different stages of sleep in memory formation. Non-REM sleep has been shown to be very important for consolidation of declarative memories—those based on recall of information—while REM sleep seems to play a larger part in procedural or task-based memories. Ribeiro suggests this may relate to the degree of synaptic change required. For declarative memories, most of the foundational learning has already taken place; remembering a new fact likely requires only small changes in synaptic strengths. By contrast, procedural memories require a massive amount of synaptic change. “If you want to learn how to ride a bike, or how to play capoeira … it's not like learning a new name,” Ribeiro says. “It’s weeks, months, years of work. And so it seems like REM sleep is really, really necessary to do this long-term persistent synaptic change.”
Looking Beyond Sleep
Understanding the molecular changes that underpin memory consolidation during sleep may also help clarify other factors at play in long-term memory formation. Already, researchers are reporting some unexpected findings that complicate the sleep-memory connection.
Born’s newest research is showing that some memory consolidation does not depend on sleep. In one experiment, rats were shown an unfamiliar object and then were either allowed to sleep or kept awake. The researchers then tested the rats on their recall of the object a week later. As expected based on prior experiments, the rats showed better recall after sleep than after wakefulness when tested in the same environment. But to Born’s surprise, when rats were tested in a new place, the animals that stayed awake after the original learning session performed better than those that had slept. They did so even when researchers blocked activity in the hippocampus between the initial learning and the test. The upshot: There could be multiple modes of memory consolidation in the brain, some of which don’t involve the hippocampus (13).
Based on these results, Born thinks it’s time to take a broader view of how memories are formed, with sleep playing a key, but not exclusive, role. “Sleep is not for consolidating memories, in general,” says Born. “No—sleep induces a certain kind of consolidation, and thereby produces a certain kind of memory.” More detailed studies—including a more extensive understanding of the molecular players involved—may help identify the specific role for sleep in memory storage.
Such studies may also elucidate roles of other physical factors, such as strong emotions, stress, and trauma, which are known to influence memory formation. For example, Amita Seghal, a molecular biologist at the University of Pennsylvania, has found neural circuits in Drosophila that can drive memory consolidation while awake—but only when the flies are hungry (14). And Mednick is now looking into memory-related effects of the autonomic nervous system, which controls involuntary physiological processes, such as heart rate, breathing, and digestion. “What’s happening in the body during encoding is incredibly important for how the brain decides what memories [it stores],” she says. Her group has found evidence that measures of autonomic activity during sleep, in some cases, may reflect memory performance better than EEG signals alone (15).
By better understanding how the various phases of sleep and other bodily rhythms interact, researchers may also identify optimal patterns for supporting healthy memory consolidation. In her recent book, The Power of the Downstate, Mednick discusses the importance of recurring quiet periods, such as slow wave sleep, to replenish our energy and physical resources in preparation for more active states (16). The slow wave stage of non-REM sleep “is the most restorative sleep you can get,” she said at a recent talk, and since REM sleep begins around the same time every night regardless of when we hit the pillow, “you want to try to get to bed early—because it makes sure that you get enough slow wave sleep before the REM onset.”
Such findings could lend credence to the old adage, “Early to bed, early to rise …,” after all. Even so, it’s clear that targeted sleep interventions to improve memory are not yet within reach.
Born says that people “always want to hear” that sleep is very good for your memory. “Well, it's not that simple,” he adds. Rather, different brain states may give rise to different kinds of memories, and each of those brain states could have certain types of adaptive functions for the brain and the individual. The key next step, says Born, will be homing in on what exactly these brain states do (Figs. 1 and 2).
Fig. 1.
Over time, as memories get encoded in the cortex, many of those spatial and temporal details fade. What remain are the elements representing the essential core of the memory. Image credit: Dave Cutler.
Fig. 2.
This model suggests that memory consolidation during sleep entails a constant dialogue between hippocampus and the neocortex. Image credit: Reprinted with permission from Reference 2.
References
- 1.Kitamura S., et al. , Estimating individual optimal sleep duration and potential sleep debt. Sci. Rep. 6, 35812 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasch B., Born J., About sleep’s role in memory. Physiol. Rev. 93, 681–766 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tononi G., Cirelli C., Sleep and synaptic homeostasis: a hypothesis. Brain Res. Bull. 62, 143–150 (2003). [DOI] [PubMed] [Google Scholar]
- 4.de Vivo L., et al. , Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science 355, 507–510 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cirelli C., Tononi G., Effects of sleep and waking on the synaptic ultrastructure. Phil. Trans. R. Soc. B 375, 20190235 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyamoto D., et al. , Net decrease in spine-surface GluA1-containing AMPA receptors after post-learning sleep in the adult mouse cortex. Nat. Commun. 12, 2881 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang G., et al. , Sleep promotes branch-specific formation of dendritic spines after learning. Science 344, 1173–1178 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niknazar H., Malerba P., Mednick S. C., Slow oscillations promote long-range effective communication: The key for memory consolidation in a broken-down network. Proc. Natl. Acad. Sci. U.S.A. 119, e2122515119. (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seibt J., et al. , Cortical dendritic activity correlates with spindle-rich oscillations during sleep in rodents. Nat. Commun. 8, 684 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peyrache A., Siebt J., A mechanism for learning with sleep spindles. Phil. Trans. R. Soc. B 375, 20190235 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niethard N., Brodt S., Born J., Cell-type-specific dynamics of calcium activity in cortical circuits over the course of slow-wave sleep and rapid eye movement sleep. J. Neurosci. 41, 4212–4222 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Souza A. C., et al. , Experience-dependent phosphoproteomic changes in hippocampus and neocortex correlate with the abundance of spindle oscillations during the transition between SWS and REM sleep. Preprint.
- 13.Sawangjit A., et al. , Two distinct ways to form long-term object recognition memory during sleep and wakefulness. Proc. Natl. Acad. Sci. U.S.A. 119, e2203165119. (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chouhan N. S., et al. , Availability of food determines the need for sleep in memory consolidation. Nature 589, 582–585 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen P.-C., et al. , Understanding the roles of central and autonomic activity during sleep in the improvement of working memory and episodic memory. Proc. Natl. Acad. Sci. U.S.A. (in press). [DOI] [PMC free article] [PubMed]
- 16.S.C. Mednick, The Power of the Downstate (Hachette Go, 2022). [Google Scholar]