Highlights
-
•
Ultrasound pretreatment enhanced the phenolics content of mung bean sprouts.
-
•
Phenolics content and antioxidant capacity of mung bean sprouts positively correlated.
-
•
A total number of 608 metabolites were detected in sprouted mung beans.
-
•
More than 50 phenolic compounds were identified in sprouts of mung bean.
-
•
Sprouted mung bean digest showed high phenolics content and antioxidant capacity.
Abbreviations: M, Mung bean; MC48, Mung bean control germinated for 48 h; MG48, Mung bean GABA treatment germinated for 48 h; MU48, Mung bean ultrasonic treatment germinated for 48 h; M0, Mung bean ultrasonic and GABA treatment germinated for 0 h; M12, Mung bean ultrasonic and GABA treatment germinated for 12 h; M24, Mung bean ultrasonic and GABA treatment germinated for 24 h; M48, Mung bean ultrasonic and GABA treatment germinated for 48 h; M72, Mung bean ultrasonic and GABA treatment germinated for 72 h; M96, Mung bean ultrasonic and GABA treatment germinated for 96 h; GD, Gastric digestion; ID, Intestinal digestion; T-AOC, Total antioxidant capacity; ABTS, 2,2-Azino-bis-3-etilbenzotiazolin-6-sulfonic acid; DPPH, 2,2-diphenyl-1-picrylhydrazyl; FF, Free flavonoids; FP, Free polyphenols; RSC, Radical scavenging capacity; GABA, γ-aminobutyric acid
Keywords: Mung bean, Germination, Ultrasound, γ-Aminobutyric acid, Polyphenols, Antioxidant capacity
Abstract
Mung bean seeds were treated by a combination of ultrasound and γ-aminobutyric acid (GABA). Effect of these treatments on the free polyphenols content, antioxidant activity, and digestibility of mung bean sprouts was evaluated. Additionally, phenolic compounds were analyzed and identified using a metabolomics approach. The combined ultrasound and GABA treatments significantly enhanced the free polyphenols and flavonoids content (P < 0.05) of mung bean sprouts depending on sprouting duration. Besides, a positive correlation (P < 0.05) was found between the polyphenols content and in vitro antioxidant activity of mung bean sprouts. Moreover, a total number of 608 metabolites were detected, and 55 polyphenol compounds were identified, including flavonoids, isoflavones, phenols, and coumarins. Also, the KEGG metabolic pathway analysis revealed 10 metabolic pathways of phenols, including flavonoid, isoflavone, and phenylpropanoid biosynthesis. Powder of 48 h sprouted mung bean released polyphenols during simulated gastric digestion and possessed antioxidant activity.
1. Introduction
Mung beans are rich in digestible protein, amino acids, vitamins, minerals, and soluble dietary fiber [1]. Additionally, they contain biologically active substances such as peptides, polysaccharides, and polyphenols [2]. However, anti-nutritional substances in mung beans might reduce the available amount of nutrients [3]. Sprouting is a natural biochemical process that can sufficiently enhance nutritional qualities of whole grains and reduce or eliminate anti-nutritional components such as phytic acid [4]. Germination can also enhance nutrient absorption, taste, flavor and bioactive compounds content, and antioxidant capacity of grains [5]. During germination, the bound polyphenols substances are partially converted into a free state and new polyphenol substances are synthesized at the same time, increasing the content of polyphenols [6]. Polyphenols with hydroxyl structure, generally composed of hydroxycinnamic acid and hydroxybenzoic acid carbon, are important secondary metabolites in higher plants [7]. Polyphenols are important components in the plant antioxidant system [8], and they have certain effects on plant nutrient absorption, protein synthesis, and cytoskeleton construction [9]. Polyphenols, flavonoids, and lignin are all generated via the shikimate pathway and the phenylpropanoid pathway, while the biosynthetic precursors of polyphenols are typically taken from the intermediates of the glycolysis and pentose phosphate processes. Polyphenols are not naturally formed in the human body, but they were found to possess anti-inflammatory, anti-cancer, antibacterial, anti-infective, hypoglycemic, and cholesterol-lowering effects [10].
γ-Aminobutyric acid (GABA), a four-carbon non-protein oxyacid, is generally synthesized through glutamic acid decarboxylation reaction or polyamine degradation pathway [11]. It has a variety of physiological functions in plants, including adverse resistance, temporary nitrogen storage, stimulating hormone production, regulating growth and development, and signaling [12]. Studies have shown that GABA treatment can increase the polyphenols content of rice sprouts, which is attributed to the increase in the expression of key enzyme genes and proteins, as well as enhancement of enzymatic polyphenols synthesis [13]. Under both stress and non-stress conditions, exogenous GABA treatment increased endogenous GABA concentration and phenolic acid content in tomato plants [14]. Additionally, exogenous GABA significantly promoted the growth of germinated soybean, modulated the activity of phenylalanine ammonia-lyase (PAL), and induced the accumulation of polyphenols substances, as well as enhanced the activity of antioxidant enzymes and the free radical scavenging activity in vitro [15]. Ultrasound, which is defined as sound waves with frequencies between 20 and 100 kHz and is typically above the human audible range [16], has been investigated as a potential new processing technology to help seeds germinate more quickly and accumulate compounds like GABA and polyphenols that are good for the human health [17], [18], [19]. Plants produce a significant amount of ROS when exposed to adverse conditions such as ultrasound, low temperature, dryness, UV radiation and salt stress [20]. Enzyme genes that control phenolic acid metabolism activate and produce a significant amount of polyphenol compounds in plants as a defense against free radical-induced oxidative damage [21]. A significant area of agricultural biophysics, ultrasonic breeding has the advantages of affordability, high productivity, and environmental protection [22]. Ultrasound can break the dormancy of plant seeds, improve the germination rate, and shorten the germination time [23]. This is because the energy produced by the ultrasound can enhance the vitality of plant cells, such as the activity of amylase and peroxidase, promoting the mechanical division of cells and increasing the speed of division and growth. Besides, under the action of ultrasound, macromolecules such as carbohydrates and amino acids can generate some new compounds and participate in the process of growth and metabolism [24]. Furthermore, cavitation bubbles are created in the cell fluid when ultrasound activates the cells, and these bubbles vibrate, grow, shrink, and collapse. Therefore, the high pressure causes the cell membrane to rupture, increasing the permeability of the cell membrane. The cell membrane produces very small pores when the ultrasonic intensity is appropriate, which facilitates the diffusion and transmembrane transport of ions and metabolites, further triggering physiological and biochemical changes in cells and controlling gene expression. However, high-intensity ultrasound can cause damage to cell structures, inhibit cell proliferation, and induce apoptosis. Therefore, appropriate ultrasonic intensity and dose should be used for different cell types [25].
Currently, metabolomics is mainly divided into targeted metabolomics and untargeted metabolomics [26]. Untargeted metabolomics, also known as discovery metabolomics, is characterized by the simultaneous measurement of a large number of metabolites in biological samples. To the best of our knowledge, it is yet unknown how the pretreatment with ultrasonic and exogenous GABA affects the diverse metabolites, metabolic pathways, and antioxidant capacity of polyphenols throughout the growth and development of mung bean sprouts. In food quality testing and component characterization, metabolomics technology is a crucial tool for the discovery and identification of distinct metabolites. Extensive targeted metabolomic analysis has been reported to characterize polyphenol compounds in mung bean sprouts treated with sucrose [27]. However, studies performed to investigate the effects of GABA combined with ultrasonic on the composition and content of polyphenols in mung bean seeds during germination are limited. Therefore, this study aimed to comprehensively evaluate the combined effects of pretreatment by GABA with ultrasound on the content and composition of polyphenols, as well as the antioxidant capacity of mung bean seeds during germination. Changes in the content of free polyphenols and flavonoids, as well as antioxidant activity based on in vitro assays, were investigated during the germination of mung bean seeds. Untargeted metabolomics technology was employed to measure and identify free polyphenols and flavonoids. Additionally, the differential metabolites of polyphenols were screened, and the main metabolic pathways were analyzed at different germination durations. Furthermore, in vitro digestibility of mung bean seed pretreated by a combination of GABA with ultrasound and germinated for 48 h was evaluated. Based on this research, a generalized research scheme was formed. It is the pretreatment with ultrasound combined with exogenous GABA to enhance the active substance content in cereals, legumes and other seeds, the identification of metabolite species and metabolic pathways using untargeted metabolomics techniques. The antioxidant activity of the active substance enriched products was also assessed by in vitro digestibility.
2. Materials and methods
2.1. Materials
Total Antioxidant Kit, ABTS, DPPH, potassium persulfate, ethanol, formic acid, ammonium formate, and acetonitrile were purchased from Reagent Sigma-Aldrich (Shanghai, China). Folin-Ciocalteu reagent, rutin, sodium carbonate, aluminum chloride, sodium nitrite, γ-aminobutyric acid (GABA; Purity, 99 %; Molecular formula, C4H9NO2; Molecular weight, 103.12; Cas number, 56-12-2), gallic acid, and sodium hypochlorite were purchased from Shanghai Macklin Biochemical Technology Co., Ltd. Artificial saliva, gastric juice, and intestinal juice were purchased from Scientific Phygene. Other chemicals and reagents were of analytical grade.
2.2. Methods
2.2.1. Mung bean germination
Mung bean seeds were obtained from Beidahuang Grain Group Co., Ltd. Seeds (50.00 g) with fully granular shape and uniform size, without visible scratches or mechanical injuries were chosen for the experiments. The surface of the mung bean seeds was disinfected by immersion in 0.5 % (v/v) sodium hypochlorite solution for 15 min [28], and then rinsed with distilled water 3 times and drained. The treated mung bean seeds were placed in a 250 mL beaker, and 10 mmol/L GABA solution was added at a seed/liquid ratio of 1:3 (w/v). The samples were placed in an ultrasonic generator (KH-500GDV Thermostatic Ultrasonic Generator, Kunshan, China) at 20 °C with a power of 370 W for 40 min. Then transferred to a 30℃ water bath for 8 h [29]. The soaked mung beans were placed in the germination tray and 500 mL of GABA solution was placed at the bottom of the germination tray, and the seeds were covered with two layers of gauze. The samples were placed in an incubator (ZXMP-R1230, China) at 30 °C [30] and a humidity of 75 % for 12, 24, 48, 72 and 96 h, and the GABA medium was replaced every 12 h. The untreated sample was marked as M, and samples taken at 0 (at the end of soaking), 12, 24, 48, 72 and 96 h of germination were marked as M0, M12, M24, M48, M72 and M96, respectively. The control sample without ultrasound or GABA treatment, GABA-treated sample, and ultrasonic-treated sample were germinated for 48 h and marked as MC48, MG48 and MU48, respectively.
2.2.2. Drying and storage of mung bean sprouts
The mung bean sprouts were washed with distilled water to remove mucus, freeze-dried to constant weight, crushed for 3 times, packaged in vacuum bags, and stored in a freezer at −80 °C until use.
2.2.3. Mung bean sprouts extraction
Optimal extraction conditions of sprouts by ethanol were chosen based on a preliminary study. A sample of 20.0 g mung bean sprout powder was weighed into a 1000 mL conical flask with a stopper and connected with a water-cooling device. Aqueous ethanol solution (60 %, v/v) was added at a solid-to-liquid ratio of 1:35 (w/v) and mixed well. The mixture was stirred at a temperature of 40 °C for 2 h using a magnetic stirrer. The extract was obtained by vacuum filtration. Each sample was extracted 3 times in parallel and stored in a refrigerator at 4 °C for determination of free polyphenols, free flavonoids content, antioxidant activity, and untargeted metabolomics assays.
2.2.4. Determination of total polyphenols and free flavonoids
The free phenol content was determined by the Folin-Ciocalteu method as described by Li [31], and the standard curve equation of gallic acid was as follows: y = 0.15x + 0.059 (R2 = 0.9997). A sample of 1.0 mL extract was pipetted into a 25 mL test tube, 3.0 mL of Folin-ciocalteu reagent was added, shaken well, and allowed to stand for 30 s. Then, 6.0 mL of 12 % Na2CO3 solution was added, the mixture shaken well, and diluted to 25 mL. The mixture was then stored in the dark at 25 °C for 2 h, and the absorbance was measured at a wavelength of 765 nm. The calculation formula was as follows (1).
| (1) |
where X is the content of free polyphenols in the sample (mg GAE/g DW). c is the mass concentration of polyphenols in the test solution obtained according to the standard curve equation (μg/mL). v is the volume of the extraction solution (mL). n is the dilution factor. m is the mass of the sample used for extraction (g).
The content of free flavonoids was determined with rutin as standard [32], and the standard curve equation of rutin was as follows: y = 11.817x + 0.0362 (R2 = 0.9992). A sample of 3.0 mL extract was put into a 10 mL graduated test tube, and 0.3 mL of 5 % NaNO2 was added. The mixture was shaken well and allowed to react in the dark for 6 min, and 0.3 mL of 10 % Al(NO3)3 was added, mixed well, and allowed to react in the dark again for 6 min. Finally, 4 mL of 4 % NaOH solution was added and the mixture was diluted to 10 mL using 70 % ethanol solution, mix well, and allowed to react in the dark for 15 min. The absorbance was then measured at a wavelength of 510 nm and the free flavonoids content was calculated as follows:
| (2) |
where X is the free flavonoids content in the sample (mg RE/g DW); c is the free flavonoids concentration of the extract calculated according to the standard curve (mg/mL); v is the volume of the solution to be tested (mL); n is the dilution factor, and m is the mass of mung bean powder (g).
2.2.5. Antioxidant activity assays
-
(1)
Total antioxidant capacity
The total antioxidant capacity of mung bean sprouts extract was determined using a T-AOC kit. According to kit instructions, three experiments were performed in parallel, and the absorbance was measured at 520 nm (TU-1800 UV, China). Total antioxidant capacity was calculated as follows:
| (3) |
In the formula: OD1 is the absorbance value of the sample to be tested. OD2 is the absorbance value of the control sample. V1 is the weight of the reaction solution. V2 is the sampling amount.
-
(2)
DPPH radical scavenging capacity
A sample of 0.40 mL extract was mixed with 2.6 mL of DPPH in ethanol solution (0.1 mmol/L, w/v) and placed in the dark for 30 min. Then, the absorbance was measured at 517 nm using absolute ethanol as a blank [33]. The DPPH radical scavenging capacity was calculated as follows:
| (4) |
where A1 is the absorbance of 400 μL sample extract and 2.6 mL DPPH solution. A2 is the absorbance of 400 μL extract and 2.6 mL absolute ethanol. A0 is the absorbance of 400 μL absolute ethanol and 2.6 mL DPPH solution Control absorbance value of the solution.
-
(3)
ABTS radical scavenging capacity
A sample of 0.1 g ABTS and 0.029 g potassium persulfate powder was dissolved in deionized water to make 100 mL of ABTS free radical stock solution. The prepared solution was stored in a refrigerator at 4 °C for 12 h and then was diluted until the absorbance at 734 nm was 0.700 ± 0.020. A sample of 0.2 mL extract was put in a test tube 5.8 mL of ABTS solution was added, mixed well, and the mixture was allowed to react in the dark for 6 min. Then, the absorbance was measured at 734 nm [34]. The formula is (5).
| (5) |
In the formula: A1 is the absorbance value of the blank control group. A2 is the absorbance value of the sample solution measurement group.
2.2.6. Untargeted metabolomic analysis
The extract was centrifuged at 12,000 rpm g for 10 min at 4 °C, the supernatant was filtered through a 0.22 μm membrane, and the filtrate was added to the detection bottle for LC-MS detection. Chromatographic conditions were as follows: chromatographic column, a flow rate of 0.25 mL/min, column temperature of 40 °C, and injection volume of 2 μL. In positive ion mode, the mobile phases were 0.1 % formic acid acetonitrile (C) and 0.1 % formic acid water (D), and in negative ion mode, the mobile phases were acetonitrile (A) and 5 mM ammonium formate water (B) [35]. Mass spectrometry conditions were as follows: Thermo Orbitrap Exploris 120 mass detector (Thermo Fisher Scientific, USA), electrospray ionization source (ESI), and data were collected in positive and negative ion modes. The first-level full scan was performed with a resolution of 60,000, the first-level ion scanning range was m/z 100 ∼ 1000, and the second-level fragmentation was performed by HCD. The collision voltage was 30 %, and the second-level resolution was 15,000. The first 4 ions were fragmented. At the same time, dynamic exclusion was used to remove unnecessary MS/MS information [36].
2.2.7. In vitro simulated digestion
In vitro simulated digestion of mung bean sprout powder, including oral, gastric, and intestinal stages was performed as described by Minekus [37]. A sample of 20.0 g mung bean powder was made into a 15 % homogenate, placed in a boiling water bath for 15 min, and was then placed in an oscillator at 37 °C for 1 h. Distilled water was then added at 37 °C to 200 g, and a high-speed homogenizer was used to make a homogenate. A sample of 10 g homogenate was put into a 50 mL centrifuge tube, and simulated digestion in vitro was performed at 37 °C and 170 r/min in an air bath shaker. 2 mL of simulated artificial saliva was added and mixed well. For simulated oral digestion, the pH of the sample was adjusted to 1.5, and 3 mL of artificial gastric juice was added to simulate the gastric digestion stage for 2 h. Then, the pH of the digest was adjusted to 6.8 and 3 mL of artificial intestinal juice was added to simulate intestinal digestion for 3 h. Samples were taken at 1 and 2, and at 1, 2, and 3 h of gastric digestion and intestinal digestion, respectively. Measurement of free polyphenols and flavonoids release, as well as antioxidant activity, was performed as described in 2.2.4 and 2.2.5.
2.3. Statistical analysis
All experiments were carried out at least in triplicate, and the data were statistically analyzed and expressed as mean ± SD. Analysis of variance (ANOVA) and Duncan's multiple range test with a confidence interval of 95 % was performed using SAS software version 9.2 (SAS Institute, Inc., Cary, NC). Metabolic data were analyzed by Suzhou BioNovoGene Biomedical Technology Co., Ltd., China.
3. Results and discussion
3.1. Effect of treatments and germination on the polyphenols content
The treatment by GABA, ultrasound, or ultrasound combined with GABA treatments significantly influenced the content of free polyphenols and flavonoids in mung beans germinated for 48 h (Table 1). Mung bean germinated for 48 h with GABA or ultrasound treatment showed a higher free polyphenolic and falvonoids content than that of the ungerminated control and samples germinated without GABA and ultrasound treatment. Also, mung bean sample treated with a combination of GABA and ultrasound showed higher free phenolics and falvonoids content than that of sample treated with a single GABA or ultrasound treatment after the same duration of germination (48 h). Furthermore, sample subjected to ultrasound treatment showed a relatively higher polyphenols content compared to that of sample treated with GABA. The effect of GABA is to increase protein expression and enzyme activity, with the effect of increasing polyphenol contentid content [38]. On the other hand, ultrasonic makes the seed shell fragment and accelerates the hydration process [39], [40], leading to changes in the molecular structure and catalysis of enzymes, triggering the defense response system and enhancing the production of secondary metabolites such as the polyphenols [41]. Also, the cavitation and mechanical effects of ultrasonics enhance the permeability of cell membranes, promoting the diffusion and transmembrane transport of ions and metabolites [42]. Furthermore, the exogenous GABA can pass through the cell membrane and enter the cell, stimulate hormone production, regulate growth and development, and further cause intracellular physiological and biochemical changes, thereby regulating polyphenols metabolism pathway gene expression to achieve the enrichment effect. Some studies have found that under UV-B stress, exogenous GABA can increase the activity of flavonoid synthase and the expression of related genes, inducing the accumulation of flavonoids [43]. Therefore, the increase in polyphenols content of mung beans can be attributed to the combined effect of GABA, ultrasonic, and germination treatments.
Table 1.
Effects of different treatments on free phenolic, free flavonoid, and antioxidant capacity of mung bean.
| Samples | FF (mg RE/g DW) | FP (mg GAE/g DW) | T-AOC (U/mL) | ABTS RSC (%) | DPPH RSC (%) |
|---|---|---|---|---|---|
| M | 4.44 ± 0.03d | 3.43 ± 0.01e | 16.49 ± 0.47d | 59.04 ± 0.52d | 56.33 ± 1.16e |
| MC48 | 5.04 ± 0.04c | 4.63 ± 0.01d | 18.80 ± 0.33c | 64.37 ± 0.23c | 63.06 ± 2.32d |
| MG48 | 5.06 ± 0.07c | 5.57 ± 0.02c | 20.64 ± 0.91b | 70.67 ± 0.17b | 67.83 ± 1.42c |
| MU48 | 5.24 ± 0.04b | 5.76 ± 0.02b | 24.50 ± 0.68a | 71.42 ± 0.38b | 75.67 ± 1.35b |
| M48 | 5.84 ± 0.04a | 5.94 ± 0.02a | 26.19 ± 0.35a | 78.64 ± 1.47a | 78.97 ± 1.35a |
Data are expressed as mean ± standard deviation. Different subscript letters within the same column indicate significant differences (P < 0.05). M, untreated mung bean; MC48, mung bean germinated for 48 h without GABA or ultrasound treatments; MG48, mung bean germinated for 48 h with GABA treatment; MU48, mung bean germinated for 48 h with ultrasound pretreatment; M48, mung bean germinated for 48 h with GABA and ultrasound treatment. FF, free flavonoids; FP, free polyphenols.
3.2. Effect of treatments on antioxidant activity
Antioxidant activity of mung bean significantly affected by GABA, ultrasonic, and germination treatments (Table 1). The highest total antioxidant capacity, ABTS radical scavenging activity, and DPPH radical scavenging activity were found for mung bean subjected to a combination of GABA and ultrasound treatments, and germinated for 48 h. Also, the ultrasound-treated samples showed higher antioxidant activity than that of GABA-treated ones. Besides, mung bean samples subjected to single or combined GABA and ultrasound treatments showed higher antioxidant activity compared to that of the ungerminated control or mung bean germinated for 48 h without GABA and ultrasonic treatment. These results indicate that the antioxidant activity is generally consistent with the polyphenols content, and the enhancement in antioxidant activity can be mainly attributed to the increase in antioxidant content such as polyphenols after GABA, ultrasound, and germination treatments of mung bean. Additionally, the antioxidant capabilities of mung beans may be influenced by the presence of vitamins such as vitamins E and C as well as other antioxidants [44]. The contents of these substances increase by the germination, enhancing the antioxidant capacity [45]. Exogenous GABA can also reduce NaCl stress, raise the activity of the enzymes involved in the production of phenolic acids, and increase the accumulation of total phenolic content, all of which increase the antioxidant ability [46].
3.3. Effect of treatments on polyphenols metabolites
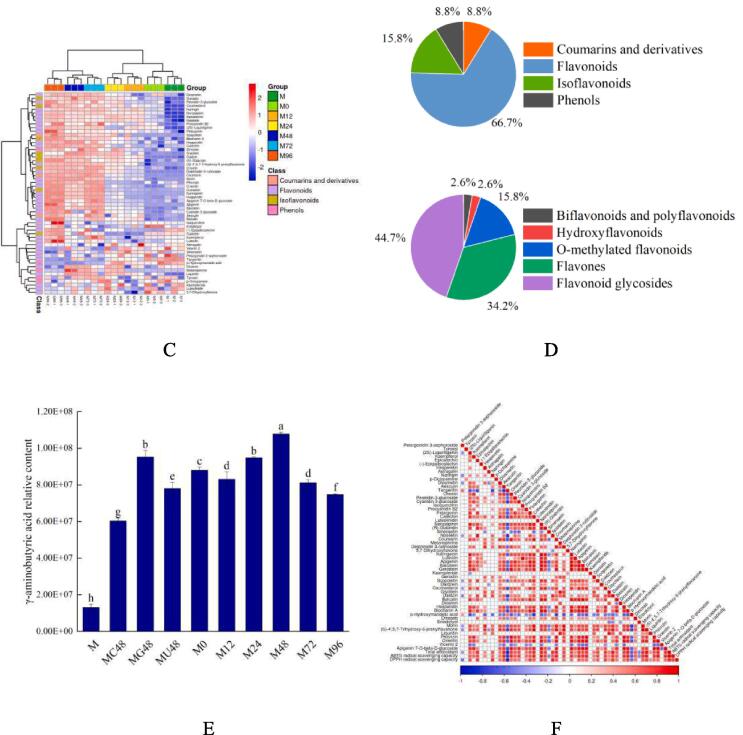
The polyphenols composition of mung beans significantly varied after GABA, ultrasound, and germination treatments. Using untargeted metabolomics technology, about 353 differences in metabolites were found for the treated mung bean (Fig. S1). A total of 41 differential metabolites of polyphenols were identified, accounting for 11.61 % of the total differential metabolites. The relative content is displayed by different colors in Fig. S1. The redder the color, the higher the expression level, and the bluer the color, the lower the expression level. The columns represent samples and the rows represent the metabolite names, and the cluster on the upper side of the figure is the differential metabolite clustering tree. The identified polyphenols are mainly coumarins and derivatives, flavonoids, isoflavones, and phenols. The main differential metabolites among different treatments were gingerol, hydroquinone, procyanidin B2, eupatilin, naringenin, esculin, nobiletin, salidroside, apigenin 7-O-beta-d-glucoside, and hesperetin 7-neohesperidoside.
3.4. Effect of germination duration on the polyphenols content and antioxidant activity
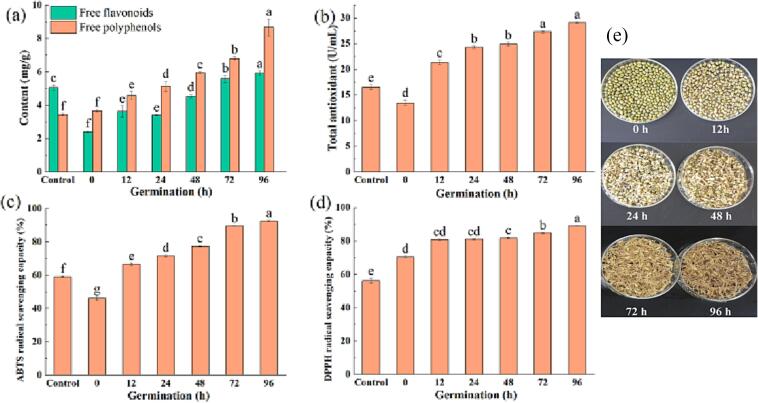
The free polyphenols content, free flavonoids content, total antioxidant capacity, ABTS free radical scavenging, and DPPH free radical scavenging of the ungerminated, soaked, and germinated mung bean seeds were determined, and the results are shown in Fig. 1A. Additionally, the morphological observation of mung bean sprouts at different durations of germination by ultrasound combined with GABA treatment is shown in Fig. 1B. Soaking of the mung bean seeds resulted in a decrease in the free polyphenols content, free flavonoids content, total antioxidant capacity, and ABTS scavenging activity compared to the untreated seeds (control). However, values of these parameters increased in the germinated seeds and as the germination duration prolonged. The decrease of free polyphenols content, free flavonoids content, and antioxidant indexes can be attributed to the mechanical and chemical effects of sound waves that destroy the cellular structure of mung bean seeds during the ultrasonic treatment process, promoting the release of free and intracellular polyphenols and flavonoids. The free polyphenols are soluble in water and easy to be lost, thereby affecting the antioxidant capacity. Also, it may be attributed to the degradation of free flavonoids by the higher ultrasonic power, resulting in a decrease in the content of flavonoids. In another study, it was found that the total polyphenol content decreased after soaking for 12 h, while increased by 41–76 % after germination for 48 h [47]. The untargeted metabolomics analysis results in this study verified the loss or degradation of flavonoids (Fig. 2A). On the other hand, the DPPH scavenging activity increased from 56.19 % to 70.49 % after soaking of the mung bean seeds (Fig. 1D), which may be attributed to that the exogenous GABA penetrated into the cells during soaking; therefore enhanced material metabolism and generated metabolites with strong DPPH free radical scavenging ability. Additionally, the exogenous GABA may be contributed to the DPPH scavenging ability of the soaked and germinated mung bean soaked. The DPPH scavenging activity further increased as germination of mung bean seeds was prolonged to up to 96 h.
Fig. 1.
The content of free polyphenols and free flavonoids (a); total antioxidant capacity (b); ABTS radical scavenging activity (c); and DPPH radical scavenging activity (d) of mung bean. The untreated mung bean (control); soaked mung bean (0); and mung bean germinated for 12, 24, 48, 72 and 96 h with GABA and ultrasound treatments.
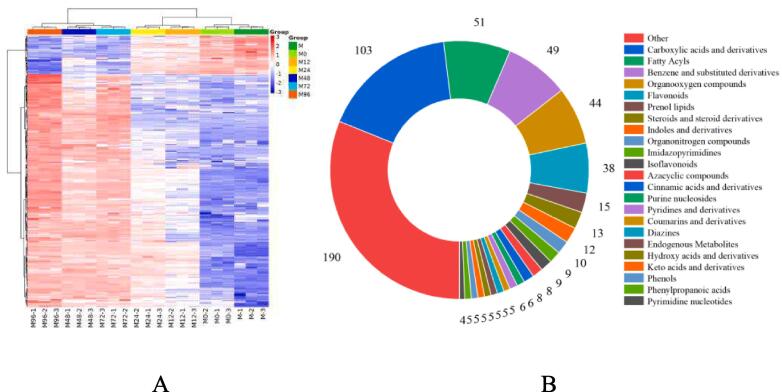
Fig. 2.
Total heat map (A and B), polyphenol heat map (C), component ratio chart (D), γ-aminobutyric relative content (E) and correlation heat map (F) of mung bean sprouts metabolites at different germination times. M, untreated mung bean; M0, soaked mung bean; M12, mung bean germinated for 12 h; M24, mung bean germinated for 24 h; M48, mung bean germinated for 48 h; M72, mung bean germinated for 72 h; M96, mung bean germinated for 96 h with GABA and ultrasound treatment.
It was reported that sprouting can significantly improve the metabolites of mung beans [48]. In another study, the total content of free flavonoids, free polyphenols, and total phenols gradually increased during germination [49]. In this study, the antioxidant activity increased as the free polyphenols and flavonoids contents increased during germination. Studies have shown that polyphenols and flavonoids are important antioxidants, and the antioxidant capacity is related to the concentration and composition of polyphenols [50]. This is consistent with a previous study that germination can increase the total phenolic content and the antioxidant capacity of mung bean seeds [51]. Therefore, untargeted metabolomics technology was used to further characterize the composition of mung bean sprout polyphenols, enriched by exogenous GABA combined with ultrasonic treatment.
3.5. Untargeted metabolomics characterization
Results of this study were evaluated and analyzed using PCA statistical analysis of QC samples and overall samples. On the principal component analysis (PCA) score graph, the QC samples were clustered together, indicating that the results were highly reliable and reproducible and the systematic error is within the controllable range (Fig. S2A). Additionally, the PCA method was used to analyze the overall distribution among all samples. PC1 and PC2 explained 53.70 % and 8.00 % of the total variance, respectively. Different germination durations were separated, and there was a clustering trend within the group (Fig. S2B). The analysis showed differences in metabolites of mung beans at different germination durations. The untargeted metabolomics results also showed differences in the content and composition of polyphenols during the germination of mung beans treated by ultrasound combined with GABA pretreatment (Tables S1, S2). These differences were caused by accumulation and metabolism of polyphenols metabolites during processing.
3.6. Differences in metabolites of mung bean sprouts
The ultrasonic pretreatment resulted in differences in the composition of metabolites in mung bean sprouts of different germination stages with exogenous GABA as a nutrient solution (Fig. 2, Table S1). A total of 608 metabolites were detected in mung bean sprouts (Fig. 2A), which were divided into 23 categories (Fig. 2B): 103 Carboxylic acids, 51 Fatty Acyls, 49 Benzene, 44 Organooxygen compounds, 38 Flavonoids, 15 Prenol lipids, 13 Steroids, 12 Indoles, 10 Organonitrogen compounds, 9 Imidazopyrimidines, 9 Isoflavonoids, 8 Azacyclic compounds, 8 Cinnamic acids, 6 Purine nucleosides, 6 Pyridines, 5 Coumarins, 5 Diazines, 5 Endogenous Metabolites, 5 Hydroxy acids, 5 Keto acids, 5 Phenols, 5 Phenylpropanoic acids, 4 Pyrimidine nucleotides, and 190 others. Specifically, the most abundant active substances were polyphenol metabolites, which contained 55 metabolites (Fig. 2C). The detected polyphenols compounds can be divided into 4 categories: Flavonoids, Isoflavonoids, Phenols, Coumarins, and derivatives. Among them, flavonoids are the most abundant, accounting for 66.70 % of the total polyphenols compounds, including Biflavonoids and polyflavonoids, Flavones, Flavonoid glycosides, Hydroxyflavonoids, O-methylated flavonoids (Fig. 2D, Fig. S3). It was reported that the content of polyphenols monomers increased as germination was prolonged [52]. These results indicate that the content and types of polyphenols compounds showed an upward trend with the extension of germination time after ultrasonic combined with GABA treatment. This means that mung bean sprouts are an excellent source of polyphenols compounds, which is consistent with previous studies [27]. Fig. 2E shows the GABA content in mung bean sprouts with different pretreatments and different germination times. It can be seen that the GABA content in mung beans was less than that in mung bean sprouts, and the GABA content increased significantly after germination. It can be seen from M0 that the ultrasonic treatment facilitated the uptake of GABA by mung beans during the soaking process. The decrease in GABA content at 12 h of germination compared to M0 may be due to the absence of newly synthesized GABA at the early stage of germination and the involvement of exogenous GABA in the metabolic processes such as polyphenols in mung bean sprouts, resulting in a decrease in content. With the extension of germination time GABA content showed a trend of first increasing and then decreasing, reaching the highest at 48 h of germination. With the enhancement of metabolism, the synthesis of GABA was activated. And after 48 h the consumption of GABA in mung bean sprouts dominated and the anabolism weakened.
3.7. Correlation between the polyphenols content and antioxidant capacity
The increase in antioxidant activity of mung bean seeds during germination is thought to be associated with the increase in their content of antioxidant compounds, such as vitamins, polyphenols acids, and flavonoids. Therefore, we analyzed the correlation between the content of polyphenols and antioxidant capacity (Fig. 2F, Table S4). The correlation analysis showed that there was a significant positive correlation between the content of 70.91 % polyphenols compounds and antioxidant activity (P < 0.05). For example, the correlations between Epicatechin, Aesculin, Catechin, Norizalpinin, Nobiletin, Coumarin, Morin, (S)-4′,5,7-Trihydroxy-6-prenylflavanone and total antioxidant were 0.814, 0.826, 0.804, 0.829, 0.801, 0.802, 0.811, 0.894, respectively. The correlations of Aesculin, Ononin, Catechin, Norizalpinin, Coumarin, Apigenin, Genistein, Hesperidin, Morin, Phlorizin, (S)-4′,5,7-Trihydroxy-6-prenylflavanone and ABTS were 0.855, 0.834, 0.849, 0.857, 0.863, 0.813, 0.841, 0.821, 0.830, 0.801 and 0.900, respectively. The correlations of Epicatechin, Naringin, Nobiletin, Daidzin, and DPPH scavenging activity were 0.809, 0.802, 0.860 and 0.818, respectively. There are also 29.09 % of polyphenols compounds that have negative or no correlation with total antioxidant, ABTS, or DPPH scavenging activity such as Tangeritin, p-Hydroxymandelic acid, Eriodictyol, Pelargonidin 3-sophoroside. From the perspective of relative content, the relative content of these polyphenols decreased or remained unchanged as germination was prolonged. Antioxidant activity is closely related to the total content of polyphenols, the chemical structure of polyphenols, and number and position of groups, which explain why some monomeric phenols have a negative or no correlation with antioxidant activity.
3.8. Differential metabolites of polyphenols
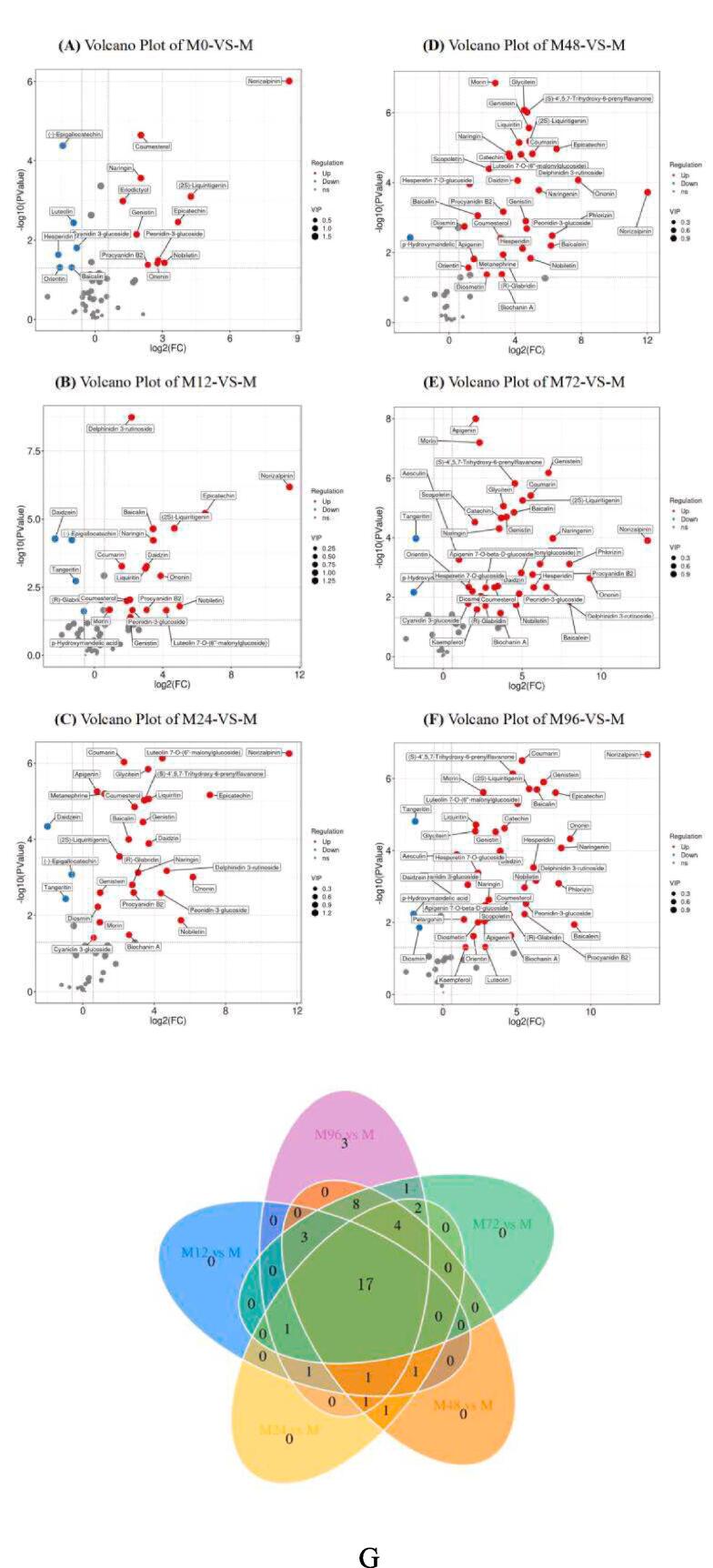
Differentially accumulated polyphenols compounds in mung bean sprouts of different germination durations were screened. The results are visualized in volcano plots (Fig. 3, Fig. S4). For the soaked mung bean (0 h, Fig. 3A), 104 and 101 metabolites were up-regulated and down-regulated, including 11 up-regulated and 6 down-regulated polyphenol compounds. However, 245 and 73 metabolites were up-regulated and down-regulated, including 18 up-regulated and 4 down-regulated polyphenol compounds in mung bean sprouts (Fig. 3B) at 12 h of germination. Moreover, when mung bean seeds were germinated for 24 h, 274 and 69 metabolites were up-regulated and down-regulated, including 26 up-regulated and 3 down-regulated polyphenol compounds (Fig. 3C). Furthermore, as the germination prolonged to 48 h, 312 and 66 metabolites were up-regulated and down-regulated, including 32 up-regulated and 1 down-regulated polyphenol compounds (Fig. 3D). Besides, 321 and 61 metabolites were up-regulated and down-regulated, including 34 up-regulated and 2 down-regulated polyphenol compounds in the mung bean sprouts of 72 h germination (Fig. 3E). Most differential metabolites were found for mung bean sprout of 96 h germination in which that 331 and 78 metabolites were up-regulated and down-regulated, including 38 up-regulated and 3 down-regulated polyphenol compounds.
Fig. 3.
Volcano plot and Venn plot depicting up- and down- accumulated polyphenols metabolites for each pairwise comparison of mung bean at different germination durations.
Venn diagram is a diagram showing the overlapping area of element sets, which can be used to count the number of common and unique differential metabolites in different alignment groups, and can visually display the similarity and overlap of differential metabolite compositions in different alignment groups. Fig. 3G (without M0 vs M) shows that there are 17 common polyphenol metabolites in mung bean sprouts at different germination durations, namely (2S)-Liquiritigenin, (R)-Glabridin, Apigenin, Baicalin, Coumarin, Coumesterol, Daidzin, Delphinidin 3-rutinoside, Epicatechin, Genistin, Morin, Naringin, Nobiletin, Norizalpinin, Ononin, Peonidin-3-glucoside, Procyanidin B2. Three polyphenol metabolites are unique to mung bean sprouts of 96 h germination, namely Astragalin, Pelargonin, and Luteolin. However, mung bean sprouts of 72 h germination shared Apigenin 7-O-beta-d-glucoside with mung bean sprouts of 96 h germination. Also, metanephrine was only found for mung bean sprouts of 24 h and 48 h germination.
3.9. Metabolic pathways of polyphenols
To explore the main metabolic pathways, differential polyphenol metabolites were annotated based on the KEGG database. The results showed that the main metabolic pathways of polyphenol metabolites in mung beans at different germination durations were flavonoid biosynthesis, isoflavonoid biosynthesis, biosynthesis of phenylpropanoids, flavone and flavonol biosynthesis, Anthocyanin biosynthesis, phenylpropanoid biosynthesis, tyrosine metabolism, neuroactive ligand-receptor interaction, biosynthesis of secondary metabolites, and metabolic pathways (Fig. 3A). Besides, differences were found in metabolites of the same pathway at different germination durations, the content was up-regulated or down-regulated, and the types of metabolites increased or decreased (Figs. 3B, S5). The most important metabolic pathways were flavonoid biosynthesis, isoflavones biosynthesis, and biosynthesis of phenylpropanoids. The main metabolic pathway during soaking was flavonoid biosynthesis, which may be due to that flavonoids are easily dispersed in the soaking solution, resulting in a decrease in the content, which is consistent with the results of the decrease in polyphenols substances and antioxidant activity in this study. The predominant metabolic pathway during germination is isoflavonoid biosynthesis, indicating that the metabolism and enrichment of isoflavones is a major contributor to polyphenols’ content and antioxidant activity. Taken together, these findings suggest that prolongation of germination is beneficial to the accumulation of total polyphenols after treatment with ultrasound in a combination with exogenous GABA (See Fig. 4).
Fig. 4.
KEGG pathway enrichment of differential metabolites.
3.10. In vitro digestibility of mung bean sprouts powder
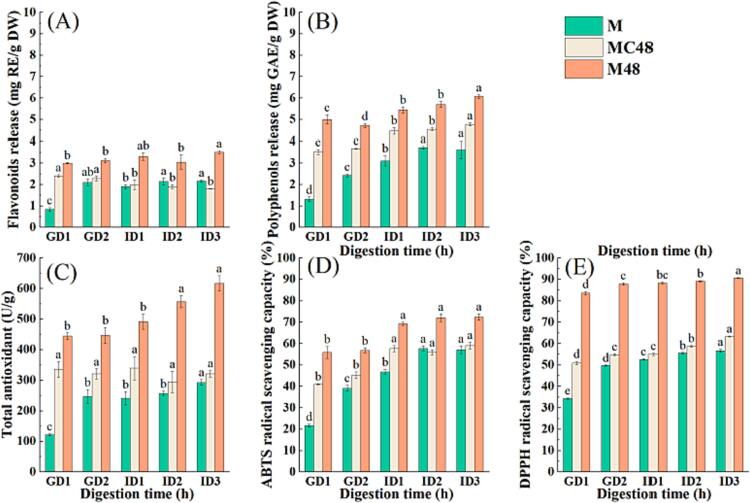
Some studies have found that 2 to 3 days of germination is appropriate for the large-scale preparation of mung bean sprouts [53]. Under the condition of maintaining palatability and good sanitation, the adverse effects of long-term germination on the sensory and nutritional characteristics of mung beans can be avoided. Powder of 48 h germinated mung bean sprouts were used for investigating the release rate and stability of polyphenols in the gastrointestinal digestion stage through simulated digestion in vitro. The release of polyphenols during in vitro digestion is shown in Fig. 5A-B. Polyphenols showed good stability during gastric and intestinal digestion, and the release of polyphenols gradually increased with the extension of digestion time [54]. This is beneficial in regulating blood sugar because polyphenols can bind to carbohydrate-digesting enzymes, inhibiting their activity [55]. At the end of gastric digestion, the free flavonoids and polyphenols released by the M48 sample were 3.11 mg RE/g DW, and 4.71 mg GAE/g DW; respectively. However, at the completion time of the in vitro gastrointestinal digestion, the release of free flavonoids and polyphenols by the M48 sample reached 3.50 mg RE/g DW and 6.07 mg GAE/g DW; respectively. The total release of the M48 sample was significantly higher than that of the M and MC48 samples. The increase in polyphenol content can be attributed to the hydrolysis of macromolecular substances during digestion, resulting in the release of polyphenols from polyphenol-protein, polyphenol-polysaccharide, and fiber-rich cell walls.
Fig. 5.
The release rate of free flavonoids (A); free polyphenols (B); and the total antioxidant capacity (C), ABTS scavenging activity (D), and DPPH scavenging activity(E) of untreated mung bean (M); and mung bean germinated for 48 h without GABA or ultrasound treatment (MC48), and mung bean germinated for 48 h with ultrasound combined with GABA treatments (M48).
Antioxidant activity, an effective tool for identifying potential health benefits, was assessed by TAOC, ABTS, and DPPH assays during simulated gastrointestinal digestion, as shown in Fig. 5C-E. These antioxidant indexes gradually increased with the increase of polyphenol release and showed a positive correlation trend with the polyphenols content. This is because polyphenols are natural antioxidants [56] and have a strong scavenging ability to free radicals. Oxidative stress plays a vital role in the occurrence and development of chronic diseases such as diabetes, Alzheimer's disease, and pigmentation. Studies have found that antioxidant active substances extracted from mung bean and mung bean sprouts have almost no side effects, indicating that green bean sprouts powder has great application potential in the field of functional food.
4. Conclusion
The free polyphenols and flavonoids content (P < 0.05) of mung bean sprouts was found to be enhanced by combined ultrasound and GABA treatments and depending on sprouting duration. Additionally, the polyphenols content positively correlated (P < 0.05) with the total antioxidant capacity, ABTS free radical scavenging, and DPPH free radical scavenging activity of mung bean sprouts. Moreover, 608 metabolites were detected by untargeted metabolomics analysis, and 55 polyphenol compounds were identified, including flavonoids, isoflavones, phenols, and coumarins. Also, 10 metabolic pathways of phenols, including flavonoid, Isoflavon, and phenylpropanoid biosynthesis identified. Furthermore, mung bean treated by ultrasound combined with GABA, and sprouted 48 h released a high amount of polyphenol compounds during simulated gastrointestinal digestion and possessed significant antioxidant activity (P < 0.05). The obtained results suggest that a combination of ultrasound and GABA treatments can be used as a promising technology for enhancing of bioactive compounds content of mung bean sprouts. Further research is needed to verify the potential health benefits of mung bean sprouts in animal models or human subjects.
CRediT authorship contribution statement
Lidong Wang: Data curation, Formal analysis, Investigation, Project administration. Xiaoqiang Li: Formal analysis, Software, Writing – original draft. Fei Gao: Software, Investigation, Writing – original draft. Ying Liu: Methodology, Investigation. Shuangjing Lang: Methodology, Project administration. Changyuan Wang: Validation, Resources. Dongjie Zhang: Funding acquisition, Methodology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors gratefully acknowledge the financial support provided by the National Key Research and Development Program of China (2021YFD2100902); the Major science and technology projects of Heilongjiang Province (2021ZX12B0203); the Major Science and Technology projects of Heilongjiang Province (2021ZX12B06); the Natural Science Foundation of Heilongjiang Province (LH2020C087); the Special projects for the central government to guide local scientific and technological development (DQKJJYD0001).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2023.106311.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.S.B. Partha, P. Aditya, G. Sanjeev, S. Kusum, T. Rakhi, P.S. Narendra, Physiological traits for shortening crop duration and improving productivity of greengram (vigna radiata l. wilczek) under high temperature, Front. Plant Sci. 10 (2019) 1508-1508, http://doi.org/10.3389/fpls.2019.01508. [DOI] [PMC free article] [PubMed]
- 2.D. Hou, Y. Laraib, Y. Xue, Q. Shen, Mung bean (Vigna radiata L.): Bioactive polyphenols, polysaccharides, peptides, and health benefits, Nutrients. 11 (2019) 1238, http://doi.org/10.3390/nu11061238. [DOI] [PMC free article] [PubMed]
- 3.Sandberg A.S. Bioavailability of minerals in legumes. Br. J. Nutr. 2002;88:281–285. doi: 10.1079/BJN/2002718. [DOI] [PubMed] [Google Scholar]
- 4.Lotika B., Kiran B. Effect of household processing on the in vitro bioavailability of iron in mungbean (Vigna Radiata) Food Nutr. Bulletin. 2007;28:18–22. doi: 10.1177/156482650702800102. [DOI] [PubMed] [Google Scholar]
- 5.Nelson K., Stojanovs K., Vasiljevic T., Mathai M. Germinated grains: a superior whole grain functional food? Can. J. Physiol. Pharmacol. 2013;91:429–441. doi: 10.1139/cjpp-2012-0351. [DOI] [PubMed] [Google Scholar]
- 6.Sharma S., Saxena D.C., Riar C.S. Changes in the gaba and polyphenols contents of foxtail millet on germination and their relationship with in vitro antioxidant activity. Food Chem. 2018;245:863–870. doi: 10.1016/j.foodchem.2017.11.093. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy D.O. Polyphenols and the human brain: plant secondary metabolite ecologic roles and endogenous signaling functions drive benefits. Adv. Nutr. 2014;5:515–533. doi: 10.3945/an.114.006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ponder A., Kulik K., Hallmann E. Occurrence and determination of carotenoids and polyphenols in different paprika powders from organic and conventional production. Molecules. 2021;26:2980. doi: 10.3390/molecules26102980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobo V., Cisneros Z.L. Bioactive phenolics and polyphenols: current advances and future trends. Int. J. Mol. Sci. 2020;21:6142. doi: 10.3390/ijms21176142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.González B.E., Gómez S.M.P. Effect of phenolic compounds on human health. Nutrients. 2021;13:3922. doi: 10.3390/NU13113922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Q.Y., Ma S.Z., Zhang W.W., Yao K.B., Chen L.u., Zhao F., Zhuang Y.Q. Accumulating pathways of γ-aminobutyric acid during anaerobic and aerobic sequential incubations in fresh tea leaves. Food Chem. 2018;240:1081–1086. doi: 10.1016/j.foodchem.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Li W., Liu J.H., Umair A., Li G., Li Y.L., Lu W.J., Gao L., Han F.G., Hu J.G. Exogenous γ-aminobutyric acid (gaba) application improved early growth, net photosynthesis, and associated physio-biochemical events in maize. Front. Plant Sci. 2016;7:919. doi: 10.3389/fpls.2016.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheteiwy M.S., Shao H.B., Qi W.C., Hamoud Y.A., Shaghaleh H., Khan N.U., Yang R.P., Tang B.P. Gaba-alleviated oxidative injury induced by salinity, osmotic stress and their combination by regulating cellular and molecular signals in rice. Int. J. Mol. Sci. 2019;20:5709. doi: 10.3390/ijms20225709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.P. Kerchev, T. Meer, N. Sujeeth, A. Verlee, T. Gechev, Molecular priming as an approach to induce tolerance against abiotic and oxidative stresses in crop plants, Biotechnol. Adv. 40 (2019) 107503, http://doi.org/10.1016/j.biotechadv.2019.107503. [DOI] [PubMed]
- 15.Ding Y.X., Wang Y., Yao Y.Y., Li W.M., Wang M., Wang P., Yang R.Q. Effect of exogenous γ-aminobutyric acid on the accumulation of phenolics and antioxidant capacity in germinated soybean. Food Sci. 2021;42:72–78. doi: 10.7506/spkx1002-6630-20200614-189. [DOI] [Google Scholar]
- 16.Ding J.Z., Hou G.G., Dong M.Y., Xiong S.B., Zhao S.M., Feng H. Physicochemical properties of germinated dehulled rice flour and energy re-quirement in germination as affected by ultrasound treatment. Ultrason. Sonochem. 2018;41:484–491. doi: 10.1016/j.ultsonch.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Ampofo J.O., Ngadi M. Ultrasonic assisted phenolic elicitation and antioxidant potential of common bean (Phaseolus vulgaris) sprouts. Ultrason. Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2020.104974. [DOI] [PubMed] [Google Scholar]
- 18.Ding J.Z., Ulanov A.V., Dong M.Y., Yang T.W., Nemzer B.V., Xiong S.B., Zhao S.M., Feng H. Enhancement of gama-aminobutyric acid (GABA) and other health-related metabolites in germinated red rice (Oryza sativa L.) by ultrasonication. Ultrason. Sonochem. 2018;40:791–797. doi: 10.1016/j.ultsonch.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 19.J. Ding, J. Johnson, Y.F. Chu, H. Feng, Enhancement of γ-aminobutyric acid, avenanthramides, and other health-promoting metabolites in germinating oats (avena sativa l.) treated with and without power ultrasound, Food Chem. 283 (2019) 239-247, http://doi.org/10.1016/j.foodchem.2018.12.136. [DOI] [PubMed]
- 20.Y. Ma, GABA-Mediated Enrichment Mechanism of Barley Maltolic Acid under NaCl Stress, Nanjing Agricultural University. (2019) 55-58, http://doi.org/10.27244/d.cnki.gnjnu.2019.000128.
- 21.Randhir R., Kwon Y.I., Shetty K. Improved health-relevant functionality in dark germinated mucuna pruriens sprouts by elicitation with peptide and phytochemical elicitors. Bioresour. Technol. 2009;100:4507–4514. doi: 10.1016/j.biortech.2009.01.078. [DOI] [PubMed] [Google Scholar]
- 22.Bian Z.X., Wang J.M., Wang S.M. Optimization of flavonoid enrichment technology and germination of buckwheat seed by ultrasonic method. J. Anhui Polytech. Univ. 2018;33:7–13. doi: 10.3969/j.issn.2095-0977.2018.05.002. [DOI] [Google Scholar]
- 23.Naumenko N., Potoroko I., Kalinina I. Stimulation of antioxidant activity and γ-aminobutyric acid synthesis in germinated wheat grain Triticum aestivum L. by ultrasound: Increasing the nutritional value of the product. Ultrason. Sonochem. 2022;86:106000. doi: 10.1016/j.ultsonch.2022.106000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu M., Liu H.Z., Shi A.M., Liu L., Wang Q. Preparation of resveratrol-enriched and poor allergic protein peanut sprout from ultrasound treated peanut seeds. Ultrason. Sonochem. 2016;28:334–340. doi: 10.1016/j.ultsonch.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 25.J.Y. Bai, Study on the Permeability of Plant Seed Cell Membrane by Ultrasonic Radiation, Shaanxi Normal University. (2014) 26-38.
- 26.Lyu C.Y., Zhao X., Chen M., Zhao Y., Zha L., Wu Y. Gc–MS-based nontargeted and targeted metabolic profiling identifies changes in the lentinula edodes mycelial metabolome under high-temperature stress. Int. J. Mol. Sci. 2019;20:2330. doi: 10.3390/ijms20092330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.X.Y. Zhang, L. Huang, X.X. Yuan, C.C. Xue, X. Chen, Widely targeted metabolomics analysis characterizes the phenolic compounds profiles in mung bean sprouts under sucrose treatment, Food Chem. 395 (2022) 133601, http://doi.org/10.1016/J.FOODCHEM.2022.133601. [DOI] [PubMed]
- 28.Geng Y.H., Cheng C., Wu Y.H. Regluation of ultraviolet and heat treatments regluates on the main physio-biochemical and melatonin enrichment in mustard sprouts. J. Nucl. Agric. Sci. 2021;35:1162–1169. doi: 10.11869/j.issn.100-8551.2021.05.1162. [DOI] [Google Scholar]
- 29.Wu X.Y., Zeng Q.X., Tian J.H. Study on the soaking process of mung bean and its effect on the germination of mung bean seeds. Sci. Technol. Food Ind. 2004;25:104–105. doi: 10.3969/j.issn.1002-0306.2004.02.040. [DOI] [Google Scholar]
- 30.Huang L.R., Cai M.H., Zhong Y.H. Effects of germination temperature on the antioxidant compounds and antioxidant capacity of mung bean sprouts. J. Anhui Agric. Univ. 2011;38:31–34. doi: 10.13610/j.cnki.1672-352x.2011.01.016. [DOI] [Google Scholar]
- 31.Li J.X., Wang B.Y. Folin-Ciocalteu colorimetric determination of total polyphenols in mulberry fruits. Food Sci. 2009;30:292–295. doi: 10.3321/j.issn:1002-6630.2009.18.066. [DOI] [Google Scholar]
- 32.Gao L.W., Li X.R. Study on microwave-assisted extraction and anti-oxidation activity of flavonoids from purple sweet potato. J. Zhejiang Univ. (Sci. Ed.). 2009;36:571–574. doi: 10.3785/j.issn.1008-9497.2009.05.020. [DOI] [Google Scholar]
- 33.Chen C.W., Chi-Tang H.O. Antioxidant properties of polyphenols extracted from green and black teas. J. Food Lipids. 2010;2:35–46. doi: 10.1111/j.1745-4522.1995.tb00028.x. [DOI] [Google Scholar]
- 34.Adom K.K., Liu R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002;50:6182–6187. doi: 10.1021/jf0205099. [DOI] [PubMed] [Google Scholar]
- 35.Zelena E., Dunn W.B., Broadhurst D., Francis-McIntyre S., Carroll K.M., Begley P., O’Hagan S., Knowles J.D., Halsall A., Wilson I.D., Kell D.B. Development of a robust and repeatable UPLC? MS method for the long-term metabolomic study of human serum. Anal. Chem. 2009;81(4):1357–1364. doi: 10.1021/ac8019366. [DOI] [PubMed] [Google Scholar]
- 36.Want E.J., Masson P., Michopoulos F., Wilson I.D., Theodoridis G., Plumb R.S., Shockcor J., Loftus N., Holmes E., Nicholson J.K. Global metabolic profiling of animal and human tissues via uplc-ms. Nat. Protoc. 2013;8(1):17–32. doi: 10.1038/nprot.2012.135. [DOI] [PubMed] [Google Scholar]
- 37.Minekus M., Alminger M., Alvito P., Ballance S., Bohn T., Bourlieu C., Carrière F., Boutrou R., Corredig M., Dupont D., Dufour C., Egger L., Golding M., Karakaya S., Kirkhus B., Le Feunteun S., Lesmes U., Macierzanka A., Mackie A., Marze S., McClements D.J., Ménard O., Recio I., Santos C.N., Singh R.P., Vegarud G.E., Wickham M.S.J., Weitschies W., Brodkorb A. A standardised static in-vitro digestion method suitable for food-an international consensus. Food Funct. 2014;5(6):1113–1124. doi: 10.1039/c3fo60702j. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y.Y., Xie C., Wang P., Gu Z.X., Yang R.Q. GABA regulates phenolics accumulation in soybean sprouts under NaCl stress. Antioxidants. 2021;10:990. doi: 10.3390/ANTIOX10060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Estivi L., Brandolini A., Condezo-Hoyos L., Hidalgo A. Impact of low-frequency ultrasound technology on physical, chemical and technological properties of cereals and pseudocereals. Ultrason. Sonochem. 2022;86 doi: 10.1016/j.ultsonch.2022.106044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalita D., Jain S., Srivastava B., Goud V.V. Sono-hydro priming process (ultrasound modulated hydration): Modelling hydration kinetic during paddy germination. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miano A.C., Pereira J.D., Costa C.N., Augusto P.E.D. Enhancing mung bean hydration using the ultrasound technology: description of mechanisms and impact on its germination and main components. Sci. Rep. 2016;6:38996. doi: 10.1038/srep38996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nobuki K., Kengo O., Katsuyuki Y. Sonoporation by single-shot pulsed ultrasound with microbubbles adjacent to cells. Biophys. J. 2009;96:4866–4876. doi: 10.1016/j.bpj.2009.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gu M., Yang J., Tian X., Fang W., Xu J., Yin Y. Enhanced total flavonoid accumulation and alleviated growth inhibition of germinating soybeans by gaba under UV-B stress. RSC Adv. 2022;12:6619–6630. doi: 10.1039/D2RA00523A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dahiya P.K., Linnemann A.R., Boekel M.V., Khetarpaul N., Grewal R.B., Nout M. Mung bean: technological and nutritional potential. Crit. Rev. Food Sci. Nutr. 2015;55:670–688. doi: 10.1080/10408398.2012.671202. [DOI] [PubMed] [Google Scholar]
- 45.Małgorzata S., Michał Ś. Effect of ascorbic acid postharvest treatment on enzymatic browning, phenolics and antioxidant capacity of stored mung bean sprouts. Food Chem. 2018;239:1160. doi: 10.1016/j.foodchem.2017.07.067. [DOI] [PubMed] [Google Scholar]
- 46.Ma Y., Wang P., Wang M., Sun M., Gu Z.X., Yang R.Q. Gaba mediates phenolic compounds accumulation and the antioxidant system enhancement in germinated hulless barley under nacl stress. Food Chem. 2019;270:593–601. doi: 10.1016/j.foodchem.2018.07.092. [DOI] [PubMed] [Google Scholar]
- 47.Muhammed T., Shinde M., Junna L. Effect of soaking and germination on polyphenol content and polyphenol oxidase activity of mung bean (Phaseolus aureus L.) cultivars differing in seed color. Int. J. Food Prop. 2014;17:782–790. doi: 10.1080/10942912.2012.654702. [DOI] [Google Scholar]
- 48.Prokudina E.A., Havlíček L., Al-Maharik N., Lapčík O., Strnad M., Gruz J. Rapid UPLC–ESI–MS/MS method for the analysis of isoflavonoids and other phenylpropanoids. J. Food Compos. Anal. 2012;26:36–42. doi: 10.1016/j.jfca.2011.12.001. [DOI] [Google Scholar]
- 49.Burachat S., Thasanporn S., Michael R.A., Morgan D.K. Effect of acid pretreatment and the germination period on the composition and antioxidant activity of rice bean (Vigna umbellata) Food Chem. 2017;227:280–288. doi: 10.1016/j.foodchem.2017.01.103. [DOI] [PubMed] [Google Scholar]
- 50.Susan L., Christopher B. Watkins, Superficial scald, its etiology and control. Postharvest Biol. Technol. 2011;65:44–60. doi: 10.1016/j.postharvbio.2011.11.001. [DOI] [Google Scholar]
- 51.Tang D., Dong Y., Guo N., Li L., Ren H. Metabolomic analysis of the polyphenols in germinating mung beans (vigna radiata) seeds and sprouts. J. Sci. Food Agric. 2014;94:1639–1647. doi: 10.1002/jsfa.6471. [DOI] [PubMed] [Google Scholar]
- 52.Sen S.K., Chouhan D., Das D., Ghosh R., Mandal P. Improvisation of salinity stress response in mung bean through solid matrix priming with normal and nano-sized chitosan. Int. J. Biol. Macromol. 2020;145:108–123. doi: 10.1016/j.ijbiomac.2019.12.170. [DOI] [PubMed] [Google Scholar]
- 53.G. Kapravelou, M. Rosario, G. Perazzoli, S,G. Cristina, J. Llopis, S. Cantarero, Germination improves the polyphenolic profile and functional value of mung bean (vigna radiata l.), Antioxidants. 9 (2020) 764, http://doi.org/10.3390/antiox9080746. [DOI] [PMC free article] [PubMed]
- 54.Phan A.D.T., Williams B.A., Netzel G., Mikkelsen D., D'Arcy B.R., Gidley M.J. Independent fermentation and metabolism of dietary polyphenols associated with a plant cell wall model. Food Funct. 2020;11(3):2218–2230. doi: 10.1039/c9fo02987g. [DOI] [PubMed] [Google Scholar]
- 55.Xie J., Sun N., Huang H., Xie J., Chen Y., Hu X., Hu X., Dong R., Yu Q. Catabolism of polyphenols released from mung bean coat and its effects on gut microbiota during in vitro simulated digestion and colonic fermentation. Food Chem. 2022;396 doi: 10.1016/j.foodchem.2022.133719. [DOI] [PubMed] [Google Scholar]
- 56.Shen Y., Guan Y., Song X., He J., Xie Z., Zhang Y., Zhang H., Tang D. Polyphenols extract from lotus seedpod (Nelumbo nucifera Gaertn.): Phenolic compositions, antioxidant, and antiproliferative activities. Food Sci. Nutr. 2019;7(9):3062–3070. doi: 10.1002/fsn3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.