Abstract
This case-control study investigates the positivity rates of the most prevalent neuroglial surface antibodies during the COVID-19 pandemic.
Several reports suggest autoantibody-associated neurologic conditions, such as autoimmune encephalitis and myelin-oligodendrocyte glycoprotein (MOG) antibody–associated diseases, may be triggered by the COVID-19 pandemic and associated vaccinations.1,2 Conversely, public health measures, including lockdowns, might reduce the incidence of immune-mediated neurologic disorders through fewer antecedent infective agents.3 To understand real-world implications of COVID-19–instigated public health measures on the most common autoantibody-mediated neurologic conditions, we compared positivity rates of the most prevalent neuroglial surface autoantibodies between prepandemic (2019) and postpandemic (2020) time points. We hypothesized that lockdown measures may reduce the rates of neurologic autoantibody positivity.
Methods
Across 2019 and 2020, serum samples, excluding repeated samples from individual patients, were routinely tested in Oxford, UK, for autoantibodies against the 5 most requested neuroglial surface antigens: N-methyl-D-aspartate receptor (NMDAR), MOG, aquaporin-4 (AQP4), leucine-rich glioma-inactivated 1 (LGI1), and contactin-associated proteinlike 2 (CASPR2) (Figure, A). We assessed the proportion of positive test results in 90-day epochs, sliding by 15-day intervals, statistically comparing equivalent time windows in 2019 and 2020 (Fisher exact test with Benjamini-Hochberg multiple-test correction). Anonymized and deidentified service evaluation approval was granted by Oxford University Hospitals NHS Foundation Trust. Informed consent was not required owing to the use of deidentified information. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
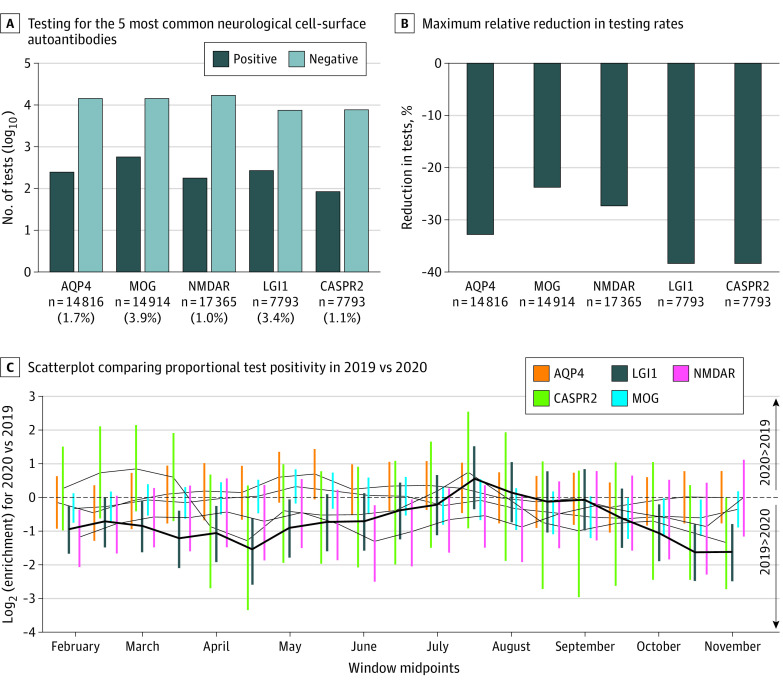
Figure. Autoantibody Positivity Rates Across 2019 vs 2020.
A, Autoantibody testing requests and positive results for the 5 most common neurologic cell-surface autoantibodies. B, The maximum relative reduction in testing rates between sliding 90-day windows in 2019 and 2020 for each autoantibody. C, Scatterplot comparing proportional test positivity in 2019 compared to 2020, using sliding 90-day windows. Negative log2 enrichment values indicate lower proportional positivity in 2020 relative to 2019. The size of points is proportional to the −log10 P value of each comparison. Lines connect adjacent points for each autoantibody. AQP4 indicates aquaporin-4; CASPR2, contactin-associated proteinlike 2; LGI1, leucine-rich glioma-inactivated 1; MOG, myelin-oligodendrocyte glycoprotein; NMDAR, N-methyl-D-aspartate receptor.
Results
This study included a total of 50 910 serum samples. Overall, across the autoantigens, there was a mean decrease of 32.2% in testing (range, 23.8%-38.4%) (Figure, B) within 90-day windows between March 31 and April 15 in 2020 vs 2019.
Of the 5 assessed autoantibodies, only the proportion of positive LGI1-antibody results was significantly reduced in the March to June period of 2020 (March 31 to June 29 window: false discovery rate = 0.002). This period overlapped closely with the first (March 26 to June 23, 2020) UK pandemic lockdown (Figure, C).
Discussion
Results of this case-control study suggest a selective reduction in LGI1-antibody positivity rates during the 2020 UK lockdown, compared with the same months in 2019. One explanation for these results is a reduction in infectious agent exposure(s), which may trigger this form of autoimmune encephalitis. In NMDAR-antibody encephalitis, a clear link with preceding herpes simplex virus encephalitis is known. Indeed, in NMDAR-antibody encephalitis and in MOG-antibody diseases, prodromal infectious symptoms are common.4 Yet, such direct or indirect infectious associations are rarely recognized in LGI1-antibody encephalitis, although a modest seasonal association has been reported once.5
Limitations of this analysis include the absence of clinical data (including time of symptom onset) from patients with autoantibodies and the lack of requested paired cerebrospinal fluid testing alongside more than 90% of sera. However, this analysis compares data across autoantibodies, thereby reducing the risk of onset-time biases, and it was our aim to examine serum positivity in diseases where the autoimmunization is likely to begin peripherally.6 Further, testing biases may have resulted from altered patient/clinician behaviors during the COVID-19 pandemic, despite data comparisons across different autoantibodies. This may be especially true for patients with LGI1 antibodies where older patients may be more prone to complete self-isolation or show greater reluctance to attend health care services, and limited (seizure-dominant) presentations may not immediately prompt medical appointments. Other potential limitations include not studying the vaccine rollout period and not directly studying seasonality by normalizing across sequential years; these can be addressed in future studies.
Overall, our data suggest that LGI1-antibody production may be incited by 1 or several organism(s), potentially including SARS-CoV-2, and future public health measures may selectively influence the incidence of this encephalitis. These data have implications for affected patients and ongoing multinational clinical trials seeking to recruit these patients.
Data Sharing Statement
References
- 1.Patone M, Handunnetthi L, Saatci D, et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat Med. 2021;27(12):2144-2153. doi: 10.1038/s41591-021-01556-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nabizadeh F, Balabandian M, Sodeifian F, Rezaei N, Rostami MR, Naser Moghadasi A. Autoimmune encephalitis associated with COVID-19: a systematic review. Mult Scler Relat Disord. 2022;62:103795. doi: 10.1016/j.msard.2022.103795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaseka ML, Ly M, Yea C, Longoni G, Yeh EA. Impact of COVID-19 public health measures on myelin oligodendrocyte glycoprotein IgG-associated disorders in children. Mult Scler Relat Disord. 2021;56:103286. doi: 10.1016/j.msard.2021.103286 [DOI] [PubMed] [Google Scholar]
- 4.Prüss H. Autoantibodies in neurological disease. Nat Rev Immunol. 2021;21(12):798-813. doi: 10.1038/s41577-021-00543-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai QL, Cai MT, Zheng Y, et al. Seasonal variation in autoimmune encephalitis: a multicenter retrospective study. J Neuroimmunol. 2021;359:577673. doi: 10.1016/j.jneuroim.2021.577673 [DOI] [PubMed] [Google Scholar]
- 6.Sun B, Ramberger M, O’Connor KC, Bashford-Rogers RJM, Irani SR. The B-cell immunobiology that underlies CNS autoantibody-mediated diseases. Nat Rev Neurol. 2020;16(9):481-492. doi: 10.1038/s41582-020-0381-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Sharing Statement