Abstract
In mid-2021, the Delta strain of SARS-CoV-2 caused the third wave of the COVID-19 pandemic. Huge efforts have been devoted to studying the effect of its mutations on the effectiveness of neutralizing antibodies. Much less attention was paid to the individual features of the presentation of its peptides by molecules of the major histocompatibility complex class I (MCHC-I). In this study, the correlation of the HLA-I genotype of patients under the age of 60 years with the severity of COVID-19 caused by the two most common variants of the SARS-CoV-2 Delta strain in the summer of 2021: AY.122 and B.1.617.2 was studied. Analysis of the severity of the course of COVID-19 revealed a more severe course of the disease caused by the AY.122 variant. Comparison of the mutation profile of the two most common variants of the Delta strain showed that that the G8R mutation in the NS8 protein makes the greatest contribution to the ability of MHC-I to present viral peptides. Given that the NS8 protein is able to suppress the maturation of MHC-I molecules, the appearance of a mutation in one of its immunogenic epitopes could make a significant contribution to the prevalence of the AY.122 variant in the Russian population.
Keywords: COVID-19, NS8, ORF8, HLA, MHC-I, AY.122, B.1.617.2
INTRODUCTION
In mid-2021, the SARS-CoV-2 coronavirus Delta strain caused the third wave of the COVID-19 pandemic in a number of countries around the world, including Russia [1]. The sharp increase in the incidence of the disease was associated with the high transmissibility of the new strain compared to the alpha variant [2]. The increase in transmissibility was largely due to an increase (up to 6 times) compared to the Alpha strain in the number of viral particles exhaled at the peak of infection by an infected person [3]. The Delta variant not only increased the risk of hospitalization for COVID-19 but also the risk of death in unvaccinated patients [4]. The Delta strain was actively mutating, which led to the formation of substrains with significantly different mutation profiles [1]. This circumstance is extremely important, because changes in the amino acid sequence of SARS-CoV-2 proteins significantly affect the ability of molecules of the major histocompatibility complex class I (MCHC-I) to present them on the surface of an infected cell [5].
MHC-I molecules are one of the key mediators of the first components in the development of a specific immune response to the SARS-CoV-2 virus. Active replication and release of the virus leads to the death of the infected cell and engulfing of its remains by dendritic cells and macrophages. After phagocytosis of the remains of dead cells and viral proteins contained in them, dendritic cells and macrophages migrate to regional lymph nodes and, using MHC-I molecules, present peptides of the SARS-CoV-2 virus to naive cytotoxic T cells. During presentation, cytotoxic T cells receive an activation signal and actively proliferate, forming a population of virus-specific cytotoxic CD8+ T cells (cT cells) [6]. Activated cT cells are able to recognize the cells infected with the SARS-CoV-2 virus. After finding a complex of the MHC-I molecule and the viral peptide on the surface of the infected cell, cT cells destroy it with the help of perforins and serine proteases [7], thereby interrupting virus reproduction.
There are three main types of MHC-I molecules encoded by the HLA-A, HLA-B, and HLA-C (human leukocyte antigen) genes. In the human genome, each gene can be represented in two variants (alleles) inherited from parents. There are dozens of variants of each allele in the population encoding MHC-I molecules with an individual ability to interact with foreign peptides. Individual combinations of MHC-I molecules significantly affect the severity of many infectious diseases [6].
Over more than two years of the COVID-19 pandemic, ample information on the features of the formation of T-cell immunity to COVID-19 has been accumulated [8]. However, the associations of the HLA genotype and the course of COVID-19 were mainly analyzed on the basis of the data related to the first wave of the pandemic and the initial strain of the virus. Furthermore, in the studies of the associations and severity of the course of COVID-19, the age of the recovered patients was practically not taken into account. It should be noted that age is a significant factor in the formation of the immune response to COVID-19 [9]. In particular, it was shown that, in people over 60 years old, the telomere length of naive T cells is significantly reduced, which leads to an almost tenfold decrease in their ability to divide after activation [10], and the repertoire of the T-cell receptor is reduced [11].
Previously, in a sample of patients of the first wave, we showed that the HLA-I genotype is a significant risk factor for severe COVID-19 only in patients under the age of 60 years [6]. In this work, we studied the correlation of the HLA-I genotype of patients under the age of 60 years with the severity of COVID-19 caused by the two most common variants of the SARS-CoV-2 Delta strain in the summer of 2021—AY.122 and B.1.617.2.
MATERIALS AND METHODS
The study included 45 patients hospitalized for COVID-19 in August 2021.
Smears from the nasopharynx and oropharynx were collected in test tubes with a transport medium. To test samples for the presence of SARS-CoV-2 RNA, various RT-PCR reagent kits and complete RNA isolation kits were used: POLYVIR SARS-CoV-2 Express (LITECH, Russia), AmpliPrime SARS-CoV-2 DUO/MagnoPrime FAST-R (NextBio, Russia), and SARS-CoV-2 CoV-2-Test (TestGen, Russia). Samples with Ct less than 30 were considered suitable for sequencing.
Libraries for sequencing were prepared using a panel of primers and barcodes (DiaSystems, Russia). The primer panel contained gene-specific parts from the ARTIC network database of the international consortium of researchers. Sequencing was performed on the Illumina MiSeq platform (Illumina) using the MiSeq 600 cycles v3 reagent kit running in read mode 2 × 250 bp. Sequence annotation was performed using Pango-lineage (version 4.1.2) [12] and NextStain [13] databases.
The HLA genotype was determined using the HLA-expert reagent kit (DNA-Technology, Russia) as described previously [6]. Analysis of the effect of mutations on the affinity of the interaction between SARS-CoV-2 peptides and MHC-I molecules was performed according to the T-CoV protocol [14]. SARS-CoV-2 substrain protein sequences were retrieved from the GISAID database [15]: AY.122 (EPI_ISL_895058) and B.1.617.2 (EPI_ISL_1315070).
Statistical analysis of the results was performed in the R environment using the Wilcoxon and Chi-square tests, as well as Fisher’s exact test.
RESULTS AND DISCUSSION
In August 2021, a sample of 45 patients hospitalized with moderate and severe COVID-19 was formed, HLA genotyping was performed, and the strain of the SARS-CoV-2 virus was determined. Characteristics of patients are summarized in Table 1. Sequencing of RNA isolated from nasopharyngeal swabs showed that patients were infected with AY.122 and B.1.617.2 variants of the SARS-CoV-2 Delta strain. According to the GISAID portal [15], the ratio of SARS-CoV-2 variants identified by us is typical for the Moscow region in August 2021 (odds ratio 2.2, p = 0.1, 95% confidence interval 0.8–5.4).
Table 1.
Characteristics of the sample of patients with COVID-19
| Index | Variant AY.122 | Option B.1.617.2 | p |
|---|---|---|---|
| n | 37 | 8 | |
| Age, median (25–75%) | 35 [32–46] | 45 [35–51.8] | 0.26 |
| Gender (male/female) | 10/27 | 1/7 | 0.65 |
| Severity | 0.04 | ||
| Medium | 14 | 8 | |
| Severe | 23 | 0 | |
| Maximum degree of lung damage on CT | 0.37 | ||
| CT-0 | 3 | 0 | |
| CT-1 | 10 | 4 | |
| CT-2 | 5 | 1 | |
| CT-3 | 9 | 0 | |
| CT-4 | 3 | 0 | |
| N.d. | 7 | 3 | |
| Outcome | 1 | ||
| Lethal | 1 | 0 | |
| Discharged | 36 | 8 | |
| Diabetes | 8 | 2 | 1 |
| Obesity | 7 | 1 | 1 |
| Hypertension | 8 | 2 | 1 |
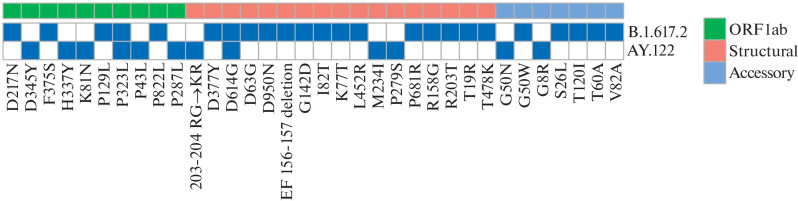
We established that the severity of the course of COVID-19 significantly differed for patients with AY.122 and B.1.617.2 variants (odds ratio undetermined, p = 0.4; 95% confidence interval 0.9 undetermined). We analyzed differences in the mutation profile of these SARS-CoV-2 variants (Fig. 1). In the AY.122 variant, 12 mutations were identified: six mutations in ORF1ab, two mutations in accessory proteins, and four mutations in structural proteins. The B.1.617.2 variant carries 24 mutations: five mutations in ORF1ab, five mutations in accessory proteins, and 14 mutations in structural proteins. It should be noted that two SARS-CoV-2 variants share only two mutations: D614G in the Spike protein and P323L in the RNA-dependent RNA polymerase from ORF1ab. Both of these mutations were fixed in the SARS-CoV-2 genome at the beginning of 2020 and are associated with the severity of COVID-19 [16]. Given that the vast majority of mutations in the virus variants are different, we analyzed their effect on the ability of MHC-I molecules to interact with the mutant peptides of each variant.
Fig. 1.
Mutation profile in SARS-CoV-2 variants AY.122 and B.1.617.2.
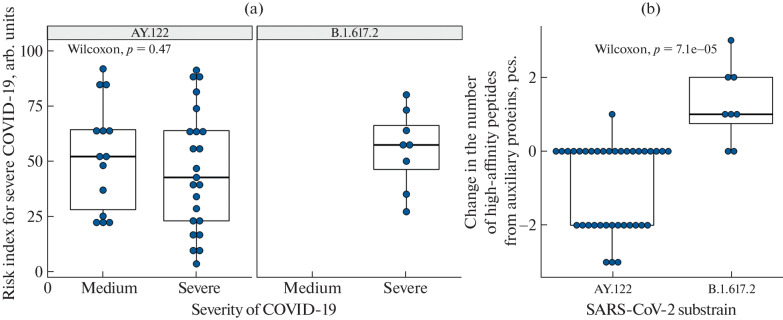
HLA genotyping of patients showed that the most frequent alleles in the sample were HLA-A*02:01 (frequency 0.26) and HLA-A*01:01 (frequency 0.25), which is typical for the population of the Moscow region [6]. The small sample size did not allow us to identify the correlation between the haplotypes and individual alleles and the severity of COVID-19 for the analyzed SARS-CoV-2 variants. However, the risk index for severe COVID-19 [6], which reflects the integral ability of an individual set of MHC-I molecules to present SARS-CoV-2 peptides, was assessed (Fig. 2a). Differences in the risk index between the patients who recovered from the AY.122 variant in moderate and severe forms were not significant (p = 0.47). However, a trend towards a higher risk index can be noted in patients with severe disease.
Fig. 2.
(a) Correlation of the risk index for severe course of COVID-19 with the actual course of the disease. (b) Effect of mutations in accessory proteins of AY.122 and B.1.617.2 variants on the affinity of their interaction with an individual set of MHC-I molecules.
Analysis of the effect of mutations on the number of viral peptides interacting with an individual set of MHC-I molecules with an affinity of less than 50 nM showed that the differences were significant for the auxiliary proteins of the virus (Fig. 2b). For example, in the patients infected with the AY.122 variant, the number of high-affinity peptides significantly decreased as compared to the patients infected with the B.1.617.2 variant (p < 0.01).
A significant decrease in the number of high-affinity peptides in the AY.122 variant is primarily associated with the G8R mutation in the NS8 protein. This mutation causes a decrease in the affinity of the interaction of the FLGIITTV and FLVFLGIITTV peptides with the MHC-I molecule encoded by the HLA-A*02:01 allele, which is the most frequent in Russian population. Previously, the immunogenicity of the FLGIITTV peptide was shown in patients that recovered from COVID-19 [17]. The fact that the NS8 protein (ORF8) is able to suppress the maturation of MHC-I molecules and their translocation to the surface of the infected cell is worth special attention [18, 19]. These circumstances could make a significant contribution to the prevalence of the AY.122 variant in the Russian population and the more severe course of COVID-19 caused by it as compared to other Delta strain variants. The proposed approach to the analysis of mutations at the level of SARS-CoV-2 variants seems to be very important, because SARS-CoV-2 continues to actively mutate.
CONCLUSIONS
1. Analysis of the correlation between the risk index for severe COVID-19, based on the affinity of the interaction of MHC-I molecules with virus peptides, and the actual course of the disease allows us to note a trend towards a higher risk index in patients with severe course of the disease.
2. Analysis of the effect of mutations in the accessory proteins of strains AY.122 and B.1.617.2 on the affinity of their interaction with an individual set of MHC-I molecules explains the prevalence of AY.122 in the Russian population and a more severe course of COVID-19 caused by this strain as compared to other Delta strains.
3. This study demonstrates additional opportunities for epidemiological monitoring of the SARS-CoV-2 coronavirus infection, which shows an obvious tendency to active spread and pronounced variability.
FUNDING
This work was performed as part of the implementation of the Moscow City Health Department Program “Scientific Support of the Capital’s Health Care for 2020–2022.”
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of interest. The authors declare that they have no conflicts of interest.
Statement of compliance with standards of research involving humans as subjects. The study protocol was approved by the Ethics Committee of Filatov City Clinical Hospital of Moscow Health Department. All patients signed an informed consent.
Footnotes
Translated by M. Batrukova
REFERENCES
- 1.Klink, G., Safina, K.R., Nabieva, E., et al., The rise and spread of the SARS-CoV-2 AY.122 lineage in Russia, Virus Evol., 2022, vol. 8, no. 1. [DOI] [PMC free article] [PubMed]
- 2.Callaway E. Delta coronavirus variant: scientists brace for impact. Nature. 2021;595:17–18. doi: 10.1038/d41586-021-01696-3. [DOI] [PubMed] [Google Scholar]
- 3.Earnest R., Uddin R., Matluk N. Comparative transmissibility of SARS-CoV-2 variants delta and alpha in New England, USA. Cell Rep. Med. 2022;3:100583. doi: 10.1016/j.xcrm.2022.100583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bast E., Tang F., Dahn J. Increased risk of hospitalisation and death with the delta variant in the USA. Lancet Infect. Dis. 2021;21:1629–1630. doi: 10.1016/S1473-3099(21)00685-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nersisyan S., Zhiyanov A., Zakharova M. Alterations in SARS-CoV-2 omicron and delta peptides presentation by HLA molecules. PeerJ. 2022;10:e13354. doi: 10.7717/peerj.13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shkurnikov M., Nersisyan S., Jankevic T. Association of HLA class I genotypes with severity of coronavirus disease-19. Front. Immunol. 2021;12:641900. doi: 10.3389/fimmu.2021.641900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wherry E.J., Ahmed R. Memory CD8 T-cell differentiation during viral infection. J. Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Titov, A., Shaykhutdinova, R., Shcherbakova, O.V., et al., Immunogenic epitope panel for accurate detection of non-cross-reactive T cell response to SARS-CoV-2, JCI Insight, 2022, vol. 7, no. 9. [DOI] [PMC free article] [PubMed]
- 9.Sanchez-Vazquez R., Guío-Carrión A., Zapatero-Gaviria A. Shorter telomere lengths in patients with severe COVID-19 disease. Aging. 2021;13:1–15. doi: 10.18632/aging.202463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson J.J., Susser E., Arbeev K.G. Telomere-length dependent T-cell clonal expansion: a model linking ageing to COVID-19 T-cell lymphopenia and mortality. eBioMedicine. 2022;78:103978. doi: 10.1016/j.ebiom.2022.103978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Britanova O., Putintseva E., Shugay M. Age-related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. J. Immunol. 2014;192:2689–2698. doi: 10.4049/jimmunol.1302064. [DOI] [PubMed] [Google Scholar]
- 12.O’Toole, A., Scher, E., Underwood, A., et al., Assignment of epidemiological lineages in an emerging pandemic using the pangolin tool, Virus Evol., 2021. [DOI] [PMC free article] [PubMed]
- 13.Hadfield J., Megill C., Bell S.M. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics. 2018;34:4121–4123. doi: 10.1093/bioinformatics/bty407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nersisyan S., Zhiyanov A., Shkurnikov M. T‑CoV: a comprehensive portal of hla-peptide interactions affected by SARS-CoV-2 mutations. Nucleic Acids Res. 2022;50:D883–D887. doi: 10.1093/nar/gkab701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elbe S., Buckland-Merrett G. Data, disease and diplomacy: GISAID’s innovative contribution to global health. Global Challenges. 2017;1:33–46. doi: 10.1002/gch2.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biswas S.K., Mudi S.R. Spike protein D614G and RdRp P323L: the SARS-CoV-2 mutations associated with severity of COVID-19. Genomics Inf. 2020;18:e44. doi: 10.5808/GI.2020.18.4.e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saini, S.K., Hersby, D.S., Tamhane, T., et al., SARS-CoV-2 genome-wide T cell epitope mapping reveals immunodominance and substantial CD8+ T cell activation in COVID-19 patients, Sci. Immunol., 2021, vol. 6, no. 58. [DOI] [PMC free article] [PubMed]
- 18.Zhang, Y., Chen, Y., Li, Y., et al., The ORF8 protein of SARS-CoV-2 mediates immune evasion through down-regulating MHC-I, Proc. Natl. Acad. Sci. U. S. A., 2021, vol. 118, no. 23. [DOI] [PMC free article] [PubMed]
- 19.Matsuoka K., Imahashi N., Ohno M. SARS-CoV-2 accessory protein ORF8 is secreted extracellularly as a glycoprotein homodimer. J. Biol. Chem. 2022;298:101724. doi: 10.1016/j.jbc.2022.101724. [DOI] [PMC free article] [PubMed] [Google Scholar]