Abstract
Purpose
Surgical site occurrences after transversus abdominis release in ventral hernia repair are still reported up to 15%. Evidence is rising that preoperative improvement of risk factors might contribute to optimal patient recovery. A reduction of complication rates up to 40% has been reported. The aim of this study was to determine whether prehabilitation has a favorable effect on the risk on wound and medical complications as well as on length of stay.
Methods
A retrospective cohort study was performed in a tertiary referral center for abdominal wall surgery. All patients undergoing ventral hernia repair discussed at multidisciplinary team (MDT) meetings between 2015 and 2019 were included. Patients referred for a preconditioning program by the MDT were compared to patients who were deemed fit for operative repair by the MDT, without such a program. Endpoints were patients, hernia, and procedure characteristics as well as length of hospital stay, wound and general complications.
Results
A total of 259 patients were included of which 126 received a preconditioning program. Baseline characteristics between the two groups were statistically significantly different as the prehabilitated group had higher median BMI (28 vs 30, p < 0.001), higher HbA1c (41 vs 48, p = 0.014), more smokers (4% vs 25%, p < 0.001) and higher HPW classes due to more patient factors (14% vs 48%, p < 0.001). There were no significant differences in intra-operative and postoperative outcome measures.
Conclusions
This study showed prehabilitation facilitates patients with relevant comorbidities achieving the same results as patients without those risk factors. The indication of a preconditioning program might be effective at the discretion of an MDT meeting. Further research could focus on the extent of such program to assess its value.
Keywords: Complex abdominal wall surgery, Prehabilitation, Multidisciplinary team meetings, Hernia, Postoperative outcomes, High-risk patients
Introduction
Incisional hernias following any kind of abdominal surgery remain an unremitting surgical challenge. This is most applicable in patients with large size (> 10 cm) hernias, recurrent hernias and hernias with a compromised surgical field, like an entero-atmospheric fistulas or an infected prosthetic mesh [1]. Recent studies report up to 33% of surgical site occurrences (SSO) after repair with open anterior component separation techniques of these complex abdominal wall hernias [2–5]. After posterior component separation techniques with a transversus abdominis release (TAR), SSOs occur still in up to 15% of patients [6].
Previous studies have demonstrated that SSOs occur mainly in patients having a high-risk characteristic at the time of surgery [7–9]. Numerous studies have unambiguously reported that preoperative smoking, obesity, or a low physical activity level influence incisional hernia repair negatively, in terms of SSO and recurrence [10–15]. Breaking the “vicious circle” of subsequential hernia repairs in a single patient, can be achieved by rigorously addressing these risk factors [3, 13]. Evidence is rising that preoperative modification of any of these risk factors, known as prehabilitation, increases patient recovery and deminishes complications [3, 16–21].
In 2018, Liang et al. completed the first randomized controlled trial on prehabilitation in ventral hernia repair. This study demonstrated that prehabilitated patients were more likely to be without complications after one month compared to non-prehabilitated patients (70% vs 48%, p = 0.015) [22]. Renshaw et al. described that patients prosecuting greater exercise frequency before surgery proved decreased risk of complications and readmission after ventral hernia repair [23, 24]. Delaying surgery and optimizing or improving the aforementioned risk factors may reduce complication rates by as much as 40% [15, 23, 25–27].
These results emphasize the potential effect of prehabilitation. It has even been suggested that prehabilitation of high-risk patients is as important as, if not more important than, the surgical technique itself [17, 28, 29].
The aim of this study was to evaluate whether prehabilitation of complex hernia patients with modifiable risk factors has a favorable effect on outcome in patients undergoing complex abdominal wall repair.
Methods
This retrospective cohort study was performed in a referral center for complex abdominal wall surgery. All consecutive patients surgically treated for complex abdominal wall hernias between December 2015 and December 2019 were included. Patients undergoing laparoscopic repair were excluded.
Abdominal wall hernias were defined complex if there was at least one of the following factors present: width > 10 cm, parastomal hernia, infected mesh, presence of a stoma, fistula or abscess, or loss of domain greater than 20% [30, 39]. Patients with at least one modifiable risk factor like body mass index (BMI) > 30 kg/m2, active nicotine abuses, diabetes mellitus (with HbA1c > 65), COPD (> Gold I), usage of immunosuppressive medication or MET score < 4, were also considered complex hernia patients.
All patients were discussed at least once in a multidisciplinary team meeting (MDT) by a surgeon, pulmonologist, cardiologist, anesthesiologist, and physiotherapist. Assays such as a CT-scan, an EKG, blood tests for hemoglobin, HbA1c and albumin were prosecuted beforehand. Hernias were anatomically graded by the EHS classification and HPW classification [11, 31].
During the MDT, a color code is allocated to each patient. Patients without any risk factors are allocated green and considered fit for surgery. Patients with at least one modifiable risk factor are allocated orange, and are eligible for surgery, only after successful prehabilitation. Patients with too many (or unmodifiable) risk factors are allocated red.
All orange patients were offered a preconditioning program, which was covered by patients’ insurance. Such a program compromised weight loss counseling, smoking cessation counseling, glycemic control by a specialized nurse, pulmonary preparation, and physiotherapy (Table 1). After prehabilitation, the patient was discussed again in the MDT. If prehabilitation was deemed successful by the MDT, the allocated color code shifted from orange to green.
Table 1.
Modifiable risk factors and prehabilitation interventions
| Risk factor | Defined by | Intervention | Achieved if |
|---|---|---|---|
| Smoking | ≥ 1 cigarette/day | Nicotine substitute | Quitted smoking |
| Quit smoking programme | ≥ 4 weeks prior to surgery | ||
| Morbid obesity | BMI ≥ 35 | Dietician | BMI ≤ 35 or |
| Physical activity | ≤ 5% weight loss | ||
| Bariatric surgery | |||
| Physical condition | MET score ≤ 4 | Physiotherapist | MET score > 4 |
| Sports physician | |||
| Diabetes, glycemic levels | HbA1c ≥ 65 | Diabetes nurse | HbA1c < 65 |
| Medication optimalization | |||
| Pulmonary condition | COPD II-IV | Consultation of pulmonologist | Optimal pulmonal condition |
| Other obstructive pulmonary diseases | Medication alteration | consented by pulmonologist | |
| Cardial condition | EKG abnormalities | Consultation of cardiologist | Optimal cardial condition |
| Medication alteration | consented by cardiologist |
Outcome after complex hernia repair was compared between two consecutive patient cohorts: green patients (without risk factors nor prehabilitation) versus orange patients (after successful prehabilitation). Endpoints were differences in baseline and intra-operative characteristics, and postoperative outcome (90-day complications such as SSO, SSI, SSE, pulmonary embolism, pneumonia, ileus/gastroparesis, or other systemic complications; length of hospital stay and readmission and reoperation).
Data were retrieved from a database in which every patient with a complex abdominal wall defect was registered prospectively since 2014. Differences between demographic groups of categorical data were tested using the Chi-squared or Fisher’s exact test. The summary statistic was the p value. The patient demographics were judged and continuous variables such as age, BMI and MET score were kept continuous, to prevent loss of data associated with categorizing. These variables were analyzed using an independent unpaired T test. A p value < 0.05 was considered statistically significant.
Results
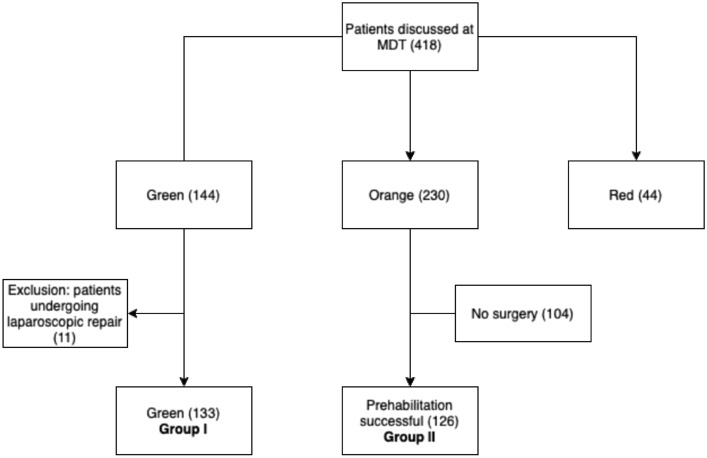
A total of 418 consecutive patients were discussed in the 4-year study period (Fig. 1). The MDT allocated 230 patients (55%) orange, 144 patients (34%) green and 44 patients (11%) red. Almost half (45%) of all primarily coded orange patients, did eventually not undergo surgery. Being unable to adequately finish prehabilitation was the most important reason. Other reasons to refrain from surgery were a concomitant disease requiring therapy, decrease of hernia-related complaints as a result of prehabilitation, or choosing another hospital. Eventually, 259 operated patients were included in this study: 133 primary green patients (group I), and 126 primarily orange patients after successful prehabilitation (group II).
Fig. 1.
Patient inclusions flowchart
Baseline characteristics between group I and II were different in BMI (median 28 versus 30, p < 0.001), HbA1c (mean 41 versus 48, p = 0.014) and the rate of active smokers (4% versus 25%, p < 0.001) (Table 2). After prehabilitation of orange patients, both BMI and nicotine abuse significantly decreased (Table 3). No differences in intra-operative conditions, like the rate of component separation techniques, were demonstrated (Table 4).
Table 2.
Preoperative characteristics of patients that underwent complex abdominal wall surgery
| Total | I (no risk factors) | II (prehabilitated patients) | p | ||
|---|---|---|---|---|---|
| n | 259 | 133 | 126 | ||
| Hernia factors | |||||
| Width < 10 cm (n,%) | 137 (53) | 77 (58) | 60 (48) | 0.254 | |
| Width 10–20 cm (n,%) | 109 (42) | 50 (38) | 59 (47) | ||
| Width > 20 cm (n,%) | 13 (5) | 6 (5) | 7 (6) | ||
| Recurrent hernia (n,%) | 79 (31) | 38 (29) | 41 (33) | 0.488 | |
| Patient factors | |||||
| Age (median, IQR) | 128 (54–68) | 63 (54–68) | 65 (57–72) | 0.052 | |
| Smoking (n,%) | 37 (14) | 5 (4) | 32 (25) | < 0.001 | |
| BMI (median, IQR) | 58 (25–29.5) | 28 (25–29.5) | 30 (27–33) | < 0.001 | |
| BMI > 30 (n,%) | 87 (34) | 22 (17) | 65 (52) | < 0.001 | |
| BMI > 35 (n,%) | 17 (7) | 3 (34) | 14 (11) | 0.004 | |
| Diabetes (n,%) | 29 (11) | 10 (8) | 19 (15) | 0.054 | |
| HbA1c (median, IQR) | 89 (34–54) | 41 (34–54) | 48 (42–57) | 0.014 | |
| HbA1c > 65 (n,%) | 3 (34) | 0 (0) | 3 (34) | 0.094 | |
| Immunosuppressives (n,%) | 14 (5) | 4 (3) | 10 (8) | 0.079 | |
| COPD (II-IV) (n,%) | 42 (16) | 17 (13) | 25 (20) | 0.123 | |
| Pulmonary preparation (n,%) | 62 (24) | 24 (18) | 38 (30) | 0.022 | |
| MET score (median, IQR) | 13 (6–8) | 7 (6–8) | 6 (5–7) | 0.004 | |
| MET score < 4 (n,%) | 20 (8) | 8 (6) | 12 (10) | 0.290 | |
| cP 0 (n,%) | 179 (69) | 114 (86) | 65 (52) | < 0.001 | |
| cP 1 (n,%) | 80 (31) | 19 (14) | 61 (48) | ||
| Wound factors | |||||
| cW 0 (n,%) | 212 (82) | 111 (83) | 101 (80) | 0.491 | |
| cW 1 (n,%) | 47 (18) | 22 (17) | 25 (20) | ||
| Previous wound infection (n,%) | 94 (36) | 44 (33) | 50 (40) | 0.270 | |
| HPW Classification | |||||
| HPW 1 (n,%) | 80 (31) | 54 (41) | 26 (21) | 0.003 | |
| HPW 2 (n,%) | 129 (50) | 54 (41) | 75 (60) | ||
| HPW 3 (n,%) | 42 (16) | 22 (17) | 20 (16) | ||
| HPW 4 (n,%) | 8 (3) | 3 (34) | 5 (4) | ||
BMI body mass index (kg/m2), COPD chronic obstructive pulmonary disease, MET metabolic equivalents, cW 1 contaminated field, HPW hernia, patient, wound
cP1: at least one patient risk factor present (BMI > 35, active smoker, use of immunosuppressives, diabetes)
cW1: CDC2-4 wound classifications (clean-contaminated, dirty-contaminated or dirty surgical field)
Statistically significant values are shown in bold
Table 3.
Effect of prehabilitation in patients with modifiable risk factors in 126 patients
| n = 126 | I Before | II After | p |
|---|---|---|---|
| Patient factors | |||
| Smoking (n,%) | 32 (25) | 16 (13) | 0.0103 |
| BMI (median, IQR) | 30 (27–33) | 29 (27–31) | < 0.001 |
| BMI > 30 (n,%) | 65 (52) | 48 (38) | 0.0312 |
| BMI > 35 (n,%) | 14 (11) | 7 (6) | 0.1106 |
| Diabetes (n,%) | 19 (15) | 19 (15) | 1.000 |
| HbA1c (median, IQR) | 48 (34–54) | 47.5 (42–55) | 0.065 |
| HbA1c > 65 (n,%) | 3 (1) | 1 (1) | 0.3134 |
| Immunosuppressives (n,%)* | 10 (8) | 5 (4) | 0.1831 |
| MET score < 4 (n,%) | 12 (10) | 5 (4) | 0.0787 |
*Patients who did not alter their regular immunosuppressives schedule were considered still using immunosuppressives
Statistically significant values are shown in bold
Table 4.
Perioperative characteristics of patients that underwent complex abdominal wall surgery
| Total | I (no risk factors) | II (prehabilitated patients) | p | |
|---|---|---|---|---|
| n | 259 | 133 | 126 | |
| Surgery time (mean, minutes) | 135.5 (90–168) | 132.9 (87–164) | 138.2 (92.5–173) | 0.244 |
| Type of myofascial release | ||||
| Retrorectus (n, %) | 132 (51) | 71 (53) | 61 (48) | 0.257 |
| Unilateral TAR (n, %) | 18 (7) | 6 (5) | 12 (10) | |
| Bilateral TAR (n, %) | 48 (19) | 21 (16) | 27 (21) | |
| eCST (n, %) | 56 (22) | 33 (25) | 23 (18) | |
| Ramirez (n, %) | 5 (34) | 2 (34) | 3 (34) | |
| Contamination of the surgical field (n, %) | 39 (15) | 22 (17) | 17 (13) | 0.493 |
| Infected mesh (n,%) | 21 (8) | 11 (8) | 10 (8) | 0.921 |
TAR transversus abdominis release, eCST endoscopic component separation technique
No significant differences in short-term complications were noted between the groups in the convalescence period (Table 5). The length of hospital stay was comparable between the groups: 6 days (p = 0.908).
Table 5.
Postoperative outcome measures
| Total | I (no risk factors) | II (prehabilitated patients) | p | |
|---|---|---|---|---|
| n | 259 | 133 | 126 | |
| SSO (n,%) | 87 (34) | 43 (32) | 44 (35) | 0.659 |
| Seroma I–II (n,%) | 22 (8) | 9 (7) | 13 (10) | 0.306 |
| Seroma III–IV (n,%) | 19 (7) | 10 (8) | 9 (7) | 0.908 |
| Hematoma I–II (n,%) | 28 (11) | 12 (9) | 16 (13) | 0.341 |
| Hematoma III–IV (n,%) | 8 (3) | 3 (2) | 5 (4) | 0.426 |
| SSE | 18 (7) | 6 (5) | 12 (10) | 0.113 |
| SSI | 31 (12) | 14 (11) | 17 (13) | 0.462 |
| SSOPI | 13 (5) | 6 (5) | 7 (6) | 0.700 |
| Systemic complications | ||||
| 0 (n,%) | 165 (64) | 81 (61) | 84 (67) | 0.190 |
| 1 (n,%) | 60 (23) | 31 (23) | 29 (23) | |
| 2 (n,%) | 17 (7) | 13 (10) | 4 (3) | |
| > 2 (n,%) | 17 (7) | 8 (6) | 9 (7) | |
| Airway infection (n,%) | 54 (21) | 28 (21) | 26 (21) | 0.934 |
| Pulmonary embolism (n,%) | 6 (34) | 5 (4) | 1 (34) | 0.113 |
| Gastrointestinal (n,%) | 24 (9) | 10 (8) | 14 (11) | 0.319 |
| Cardial (n,%) | 10 (4) | 7 (5) | 3 (34) | 0.229 |
| Other complications (n,%) | 45 (17) | 24 (18) | 21 (17) | 0.769 |
| Mortality (n,%) | 4 (2) | 3 (2) | 1 (1) | 0.340 |
| Length of stay (median, QR) | 6 (5–8) | 6 (5–8) | 6 (5–8) | 0.401 |
| Admission > 7 days (n,%) | 57 (22) | 27 (20) | 30 (24) | 0.496 |
| Reoperation (n,%) | 14 (5) | 4 (3) | 10 (8) | 0.079 |
| Readmission (n,%) | 12 (5) | 4 (3) | 8 (6) | 0.201 |
SSO surgical site occurrence, seroma/hematoma I–II no clinical significance, seroma/hematoma III–IV clinical significance- needing intervention, SSE surgical site event, SSI surgical site infection, SSOPI surgical site occurrence requiring procedural interventions
Discussion
This study demonstrated that outcome of complex ventral hernia repair in patients who underwent preoperative prehabilitation of modifiable risk factors was similar to patients without those risk factors. This finding is in line with studies reporting that prehabilitation might facilitate amelioration of the preoperative condition of patients undergoing complex abdominal wall repair [15, 17, 23, 27]. A recent systematic review performed by Jensen also concluded that smoking cessation and weight loss for obese patients led to reduced complication risks, as was seen in this study [28].
The conclusion of this study is based on patients operated before the COVID-19 pandemic, because during the pandemic patients were not able to prehabilitate accurately under supervision, nor could bariatric surgery be performed to correct morbid obesity, before definitive hernia repair. The conclusion is limited by the retrospective nature of this study and the fact that outcome of prehabilitated patients with risk factors could not be compared to non-prehabilitated patients with risk factors. In our cohort, all patients with modifiable risk factors were treated with prehabilitation.
The multidisciplinary team meeting provides comprehensive, patient-centered care and acts as a platform to discuss the optimal treatment strategy for a patient. Implementing this multidisciplinary team meeting to a complex hernia care pathway was promoted by several authors and even demonstrated improved outcomes after complex abdominal wall reconstruction [32–34]. Improved outcome may be a consequence of optimized patient selection by the MDT.
In our experience, the process of optimizing patient selection in the MDT passes a learning curve [8, 35]. Firstly, it was noticed that outcome could be improved by sticking tighter to the predetermined prehabilitation goals. In particular, the requirement to have a BMI < 30, and completely refrain from smoking, became, over time, an absolute prerequisite to be eligible for hernia repair. Improved adherence to the prehabilitation protocol, may have contributed to the good outcome in the high-risk patients. Secondly, the decision not to operate a complex hernia patient with (unmodifiable) risk factors is difficult. Formerly, these decisions were made by a surgeon in ‘splendid isolation’ [33]. By sharing in a team approach to care, these decisions became better substantiated. Subsequential analysis of these decisions improved the decision-making process in the MDT over time. Thirdly, increased attention for other risk factors developed [36]. Involving a geriatric physician in the multidisciplinary team meetings aids in addressing age-related risk factors [37]. Likewise, involving a psychiatrist, psychologist, or mental caretaker may decrease anxiety, medication usage, withdrawal symptoms or delirium in patient with mental diseases [38]. Finally, positive patient feedback, in combination with the results of this study, led to the continuation of the prehabilitation process. Noticeably, in some patients, increased exercises, a lower weight or stopped nicotine abuse (no more coughing) led to disappearance of hernia-related symptoms, which even dissolved their quest for repair [39].
To analyze the effects of prehabilitation, comparing centers that use a strict prehabilitation protocol, versus centers that do not use such a protocol, may shine light on this topic. However, as suggested in this study, the positive relation between prehabilitation and outcome may also be strongly influenced by the presence of an MDT with optimal patient selection. A lot of uncovered ground in this area of surgery is present, and further extensive research should be conducted to establish the best care pathway for this patient population.
While balancing patients’ demands and expectations, against the risk of surgery, the most difficult part of prehabilitation proves to be motivating and persuading the patient and preventing the surgeon from instant surgery. Prehabilitation is a promising tool to improve outcome in complex hernia patients. “First treat the patient, then treat the hernia”.
Conclusion
Prehabilitation of patients with modifiable risk factors may downstage complex hernia patients from high-risk to low-risk patients. Prehabilitation may have a favorable effect on outcome and the indication of such a preconditioning program might be at the discretion of a multidisciplinary team meeting. Future research could focus on the extent of such program to assess its value.
Declarations
Conflict of interest
There is no conflict of interest from any of the authors.
Ethical approval
Not necessary given the retrospective nature of the study. No changes were made in the original care pathway for patients.
Human and animal rights
The authors state that this research was conducted in accordance with the Helsinki Declaration as revised in 2008.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grove TN, Kontovounisios C, Montgomery A, Heniford BT, Windsor ACJ, Warren OJ, Europe Collaborative AWR. Perioperative optimization in complex abdominal wall hernias: delphi consensus statement. BJS open. 2021;5(5):zrab082. doi: 10.1093/bjsopen/zrab082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farooque F, Jacombs AS, Roussos E, Read JW, Dardano AN, Edye M, Ibrahim N. Preoperative abdominal muscle elongation with botulinum toxin A for complex incisional ventral hernia repair. ANZ J Surg. 2016;86(1–2):79–83. doi: 10.1111/ans.13258. [DOI] [PubMed] [Google Scholar]
- 3.Pereira JA, Montcusí B, López-Cano M, Hernández-Granados P, Fresno de Prado L. Risk factors for bad outcomes in incisional hernia repair: lessons learned from the national registry of incisional hernia (EVEREG). Factores de riesgo de mala evolución en la reparación de hernias incisionales: Lecciones aprendidas del Registro Nacional de Hernia Incisional (EVEREG) Cirugia espanola. 2018;96(7):436–442. doi: 10.1016/j.ciresp.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Berger RL, Li LT, Hicks SC, Davila JA, Kao LS, Liang MK. Development and validation of a risk-stratification score for surgical site occurrence and surgical site infection after open ventral hernia repair. J Am Coll Surg. 2013;217(6):974–982. doi: 10.1016/j.jamcollsurg.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Cornette B, De Bacquer D, Berrevoet F. Component separation technique for giant incisional hernia: a systematic review. Am J Surg. 2018;215(4):719–726. doi: 10.1016/j.amjsurg.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 6.Wegdam JA, Thoolen JMM, Nienhuijs SW, de Bouvy N, de VriesReilingh TS. Systematic review of transversus abdominis release in complex abdominal wall reconstruction. Hernia. 2019;23(1):5–15. doi: 10.1007/s10029-018-1870-5. [DOI] [PubMed] [Google Scholar]
- 7.Jacob B, Ramshaw B. The SAGES Manual of Hernia Repair. New York: Springer; 2013. [Google Scholar]
- 8.Howard R, Delaney L, Kilbourne AM, Kidwell KM, Smith S, Englesbe M, Dimick J, Telem D. Development and implementation of preoperative optimization for high-risk patients with abdominal wall hernia. JAMA Netw Open. 2021;4(5):e216836. doi: 10.1001/jamanetworkopen.2021.683610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knapp N, Breanna J, Robert M. Abdominal wall procedures: the benefits of prehabilitation. Plastic Aesthet Res. 2020;7:71. doi: 10.20517/2347-9264.2019.69. [DOI] [Google Scholar]
- 10.Huynh DTK, Ganem OM. Patient comorbidities complicating a hernia repair: the preoperative workup and postoperative planning. In: Jacob B, editor. The SAGES manual of hernia surgery. 2. Cham, Switzerland: Springer; 2018. pp. 109–123. [Google Scholar]
- 11.Novitsky YW. Classification of hernias. In: Novitsky YW, editor. Hernia surgery current principles. 1. Springer; 2016. [Google Scholar]
- 12.Kanters AE, Krpata DM, Blatnik JA, Novitsky YM, Rosen MJ. Modified hernia grading scale to stratify surgical site occurrence after open ventral hernia repairs. J Am Coll Surg. 2012;215(6):787–793. doi: 10.1016/j.jamcollsurg.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Rhemtulla IA, Hsu JY, Broach RB, Mauch JT, Serletti JM, DeMatteo RP, Fischer JP. The incisional hernia epidemic: evaluation of outcomes, recurrence, and expenses using the healthcare cost and utilization project (HCUP) datasets. Hernia. 2021;25(6):1667–1675. doi: 10.1007/s10029-021-02405-9. [DOI] [PubMed] [Google Scholar]
- 14.Howard R, Thompson M, Fan Z, Englesbe M, Dimick JB, Telem DA. Costs associated with modifiable risk factors in ventral and incisional hernia repair. JAMA Netw Open. 2019;2(11):e1916330. doi: 10.1001/jamanetworkopen.2019.16330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howard R, Yin YS, McCandless L, Wang S, Englesbe M, Machado-Aranda D. Taking control of your surgery: impact of a prehabilitation program on major abdominal surgery. J Am Coll Surg. 2019;228(1):72–80. doi: 10.1016/j.jamcollsurg.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holihan JL, Alawadi Z, Martindale RG, Roth JS, Wray CJ, Ko TC, Kao LS, Liang MK. Adverse events after ventral hernia repair: the vicious cycle of complications. J Am Coll Surg. 2015;221(2):478–485. doi: 10.1016/j.jamcollsurg.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 17.Joslyn NA, Esmonde NO, Martindale RG, Hansen J, Khansa I, Janis JE. Evidence-based strategies for the prehabilitation of the abdominal wall reconstruction patient. Plast Reconstr Surg. 2018;142(3 Suppl):21S–29S. doi: 10.1097/PRS.0000000000004835. [DOI] [PubMed] [Google Scholar]
- 18.Liang MK, Holihan JL, Itani K, Alawadi ZM, Gonzalez JR, Askenasy EP, Ballecer C, Chong HS, Goldblatt MI, Greenberg JA, Harvin JA, Keith JN, Martindale RG, Orenstein S, Richmond B, Roth JS, Szotek P, Towfigh S, Tsuda S, Vaziri K, Berger DH. Ventral hernia management: expert consensus guided by systematic review. Ann Surg. 2017;265(1):80–89. doi: 10.1097/SLA.0000000000001701. [DOI] [PubMed] [Google Scholar]
- 19.Macedo FIB, Mittal VK. Does enhanced recovery pathways affect outcomes in open ventral hernia repair? Hernia. 2017;21(5):817–818. doi: 10.1007/s10029-016-1553-z. [DOI] [PubMed] [Google Scholar]
- 20.Park H, de Virgilio C, Kim DY, Shover AL, Moazzez A. Effects of smoking and different BMI cutoff points on surgical site infection after elective open ventral hernia repair. Hernia. 2021;25(2):337–343. doi: 10.1007/s10029-020-02190-x. [DOI] [PubMed] [Google Scholar]
- 21.Slim K, Standaert D. Enhanced recovery after surgical repair of incisional hernias. Hernia. 2020;24(1):3–8. doi: 10.1007/s10029-019-01992-y. [DOI] [PubMed] [Google Scholar]
- 22.Liang MK, Bernardi K, Holihan JL, Cherla DV, Escamilla R, Lew DF, Berger DH, Ko TC, Kao LS. Modifying risks in ventral hernia patients with prehabilitation: a randomized controlled trial. Ann Surg. 2018;268(4):674–680. doi: 10.1097/SLA.0000000000002961. [DOI] [PubMed] [Google Scholar]
- 23.Renshaw SM, Poulose BK, Gupta A, Di Stasi S, Chaudhari A, Collins C. Preoperative exercise and outcomes after ventral hernia repair: making the case for prehabilitation in ventral hernia patients. Surgery. 2021;170(2):516–524. doi: 10.1016/j.surg.2021.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Moyer R, Ikert K, Long K, Marsh J. The value of preoperative exercise and education for patients undergoing total hip and knee arthroplasty: a systematic review and meta-analysis. JBJS Rev. 2017;5(12):e2. doi: 10.2106/JBJS.RVW.17.00015. [DOI] [PubMed] [Google Scholar]
- 25.Moran J, Guinan E, McCormick P, Larkin J, Mockler D, Hussey J, Moriarty J, Wilson F. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: a systematic review and meta-analysis. Surgery. 2016;160(5):1189–1201. doi: 10.1016/j.surg.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev. 2016;5(1):204. doi: 10.1186/s13643-016-0376-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Timmer AS, Claessen JJM, Boermeester MA. Risk factor-driven prehabilitation prior to abdominal wall reconstruction to improve postoperative outcome. A narrative review. J Abdom Wall Surg. 2022;1:10722. doi: 10.3389/jaws.2022.10722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen KK, East B, Jisova B, Cano ML, Cavallaro G, Jørgensen LN, Rodrigues V, Stabilini C, Wouters D, Berrevoet F. The European hernia society prehabilitation project: a systematic review of patient prehabilitation prior to ventral hernia surgery. Hernia. 2022;26(3):715–726. doi: 10.1007/s10029-022-02573-2. [DOI] [PubMed] [Google Scholar]
- 29.Molenaar CJL, Papen-Botterhuis NE, Herrle F, Slooter GD. Prehabilitation, making patients fit for surgery–a new frontier in perioperative care. Innov Surg Sci. 2019;4(4):132–138. doi: 10.1515/iss-2019-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al Sadairi AR, Durtette-Guzylack J, Renard A, Durot C, Thierry A, Kianmanesh R, Passot G, Renard Y. A simplified method to evaluate the loss of domain. Hernia. 2022;26(3):927–936. doi: 10.1007/s10029-021-02474-w. [DOI] [PubMed] [Google Scholar]
- 31.Muysoms FE, Miserez M, Berrevoet F, Campanelli G, Champault GG, Chelala E, Dietz UA, Eker HH, El Nakadi I, Hauters P, Hidalgo Pascual M, Hoeferlin A, Klinge U, Montgomery A, Simmermacher RK, Simons MP, Smietański M, Sommeling C, Tollens T, Vierendeels T, Kingsnorth A. Classification of primary and incisional abdominal wall hernias. Hernia. 2009;13(4):407–414. doi: 10.1007/s10029-009-0518-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kollias V, Reid J, Udayasiri D, Granger J, Karatassas A, Hensman I, Maddern G. Towards a complete cycle of care: a multidisciplinary pathway to improve outcomes in complex abdominal wall hernia repair. ANZ J Surg. 2022;92(9):2025–2036. doi: 10.1111/ans.17765. [DOI] [PubMed] [Google Scholar]
- 33.Muirhead LJ, Shaw AV, Kontovounisios C, Warren OJ. Establishing a robust multidisciplinary team process in complex abdominal wall reconstruction within a colorectal surgical unit. Tech Coloproctol. 2019;23(4):379–383. doi: 10.1007/s10151-019-01965-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlosser KA, Arnold MR, Kao AM, Augenstein VA, Heniford BT. Building a multidisciplinary hospital-based abdominal wall reconstruction program: nuts and bolts. Plast Reconstr Surg. 2018;142(3 Suppl):201S–208S. doi: 10.1097/PRS.0000000000004879. [DOI] [PubMed] [Google Scholar]
- 35.Wegdam JA, de Jong DLC, de VriesReilingh TS, Schipper EE, Bouvy ND, Nienhuijs SW. Assessing textbook outcome after implementation of transversus abdominis release in a regional hospital. J Abdom Wall Surg. 2022;1:10517. doi: 10.3389/jaws.2022.10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamilton J, Kushner B, Holden S, Holden T. Age-related risk factors in ventral hernia repairs: a review and call to action. J Surg Res. 2021;266:180–191. doi: 10.1016/j.jss.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caglià P, Tracia A, Borzì L, Amodeo L, Tracia L, Veroux M, Amodeo C. Incisional hernia in the elderly: risk factors and clinical considerations. Int J Surg (London, England) 2014;12(Suppl 2):S164–S169. doi: 10.1016/j.ijsu.2014.08.357. [DOI] [PubMed] [Google Scholar]
- 38.Ramshaw B. Applying systems and complexity science to real patient care. J Eval Clin Pract. 2020;26(5):1559–1563. doi: 10.1111/jep.13442. [DOI] [PubMed] [Google Scholar]
- 39.Slater NJ, Montgomery A, Berrevoet F, Carbonell AM, Chang A, Franklin M, Kercher KW, Lammers BJ, Parra-Davilla E, Roll S, Towfigh S, van Geffen E, Conze J, van Goor H. Criteria for definition of a complex abdominal wall hernia. Hernia. 2014;18(1):7–17. doi: 10.1007/s10029-013-1168-6. [DOI] [PubMed] [Google Scholar]