Abstract
The first Pd-catalyzed [5 + 2] rollover annulation of 1-benzylpyrazoles with alkynes to assemble 10H-benzo[e]pyrazolo[1,5-a]azepines (tricyclic 2-benzazepines) has been developed. The rollover annulation implies a twofold C–H activation of aryl and heteroaryl Csp2–H bonds (C–H/C–H) of 1-benzylpyrazoles (five-atom partners) and alkynes to give the [5 + 2] annulated compounds.
2-Benzazepines, in particular their hetero-fused tricyclic derivatives, are privileged structures present in a wide number of compounds with a diverse range of relevant biological activities, including Aurora kinase A,1 bromodomain,2 and acetylcholinesterase3 inhibitory properties as well as antihepatitis C drugs4 (Figure 1).
Figure 1.
Biologically active tricyclic 2-benzazepines.
The remarkable biological activity of the 2-benzazepine scaffolds5 and the synthetic appeal of assembling benzo-fused seven-membered N-heterocyclic rings has stimulated rich synthetic creativity throughout the years. These synthetic approaches range from classical condensations,6 cyclizations,7 and metal-catalyzed cycloadditions with imines8 and nitriles9 to the promising Pd-catalyzed intramolecular C–H heteroarylations10 and intermolecular carbopalladations11 that allow rapid assembly of hetero-fused tricyclic derivatives. In recent years, more sustainable approaches based on intermolecular metal-catalyzed cycloadditions involving the direct activation of C–H bonds (oxidative annulations) have strongly emerged to build up medium-sized heterocycles.12 Thus, for 2-benzazepin(on)es, Glorius, Matsunaga/Yoshino, and Cui independently developed a convergent Rh-catalyzed [4 + 3] cycloaddition between benzamides and α,β-unsaturated carbonyls13 or vinylcarbenoids14 (Scheme 1a). Besides, Kim developed a Rh-catalyzed [4 + 3] cycloaddition between N-allyl benzylamines and allyl derivatives (Scheme 1a).15 On the other hand, Carretero exploited a Pd-catalyzed [6 + 1] cycloaddition of γ-arylpropylamine derivatives with CO (Scheme 1b).16 These annulations involve initial Csp2–H activation followed by condensation or amidation reactions or, alternatively, CH/NH functionalizations. More recently, Mascareñas and Gulías described the first assembly of 2-benzazepines in an interesting formal [5 + 2] annulation process involving the activation of Csp3–H bonds (Scheme 1c).17 Being aware of the capacity of pyrazoles to participate in metal-catalyzed C–H functionalizations18 via rollover processes,19 we herein report the first examples of efficient Pd-catalyzed [5 + 2] rollover annulations involving 1-benzylpyrazoles (five-atom partners) 1 with alkynes (two-carbon partners) 2 to afford tricyclic pyrazolo-2-benzazepines 3 in good to excellent yields (Scheme 1d). This rollover annulation implies an unusual twofold C–H activation of aryl and heteroaryl Csp2–H bonds (C–H/C–H), compared to the more typical annulation involving C–H/N–H activations (Scheme 1).
Scheme 1. Metal-Catalyzed Oxidative Annulations to Form 2-Benzazepines.
We started our investigation by testing the reactivity between 1-benzylpyrazole (1a) and diphenylacetylene (2a) as model partners under the known Miura’s rollover conditions18 for 1-phenylpyrazole (Table 1, entries 1–3). Unfortunately, the reaction did not proceed with either Cu(OAc)2 or AgOAc as the oxidant or xylene (150 °C) or toluene (100 °C) as the solvent. As the structure of 1a contains a more flexible tetrahedrical Csp3 carbon compared to 1-phenylpyrazole, we thought that the formation of square-planar complexes might be more appropriate for catalytic C–H activation. Indeed, the reaction with Pd(OAc)2 as the catalyst and Cu(OAc)2 as the oxidant in MeCN gave the desired [5 + 2] rollover annulation product, tricyclic 2-benzazepine 3aa,19 although in a low 20% yield (Table 1, entry 4). Using O2 as the oxidant or classical palladium/benzoquinone oxidative combinations in DMF gave slightly better yields of 3aa (Table 1, entries 5 and 6). Typical metal oxidants like Cu(OAc)2 and AgOAc in DMF gave moderate yields of 3aa (Table 1, entries 7 and 8). Interestingly, using AgOAc and PivOH (1 equiv) as an additive, to favor a presumable CMD process,20 led to 3aa in a fairly good 75% yield (Table 1, entry 9).21 To our delight, when the amount of PivOH was increased to 5 equiv, 3aa was obtained in an excellent 80% isolated yield (Table 1, entry 10).22 Under these conditions but using other solvents (e.g., toluene, DCE, MeCN, dioxane, t-AmOH, NMP, HFIP, etc.) at various temperatures gave poorer results.23 To evaluate the practicality of this novel protocol, a scaled-up reaction was performed, leading to 3aa in fairly good yield even with a reduced amount of catalyst (Table 1, entry 11). The structure of compound 3aa was elucidated by X-ray diffraction analysis.
Table 1. Optimization of the Reaction Conditionsa.
| entry | cat. | oxidant | solventb | T (°C) | yield (%)c |
|---|---|---|---|---|---|
| 1d | [RhCp*Cl2]2 | Cu(OAc)2·H2O Na2CO3 | m-xyl | 150 | SM |
| 2d | [RhCp*Cl2]2 | AgOAc | m-xyl | 150 | SM |
| 3d | [RhCp*Cl2]2 | AgOAc | tol | 100 | SM |
| 4 | Pd(OAc)2 | Cu(OAc)2 | MeCN | 105 | 20 |
| 5 | Pd(OAc)2 | O2/NaOAc | DMF | 120 | 20 |
| 6 | Pd(OAc)2 | BQ/AcOH | DMF | 120 | 33 |
| 7 | Pd(OAc)2 | Cu(OAc)2 | DMF | 120 | 50 |
| 8 | Pd(OAc)2 | AgOAc | DMF | 120 | 64 |
| 9 | Pd(OAc)2 | AgOAc + PivOH (1 equiv) | DMF | 120 | 75 |
| 10 | Pd(OAc)2 | AgOAc + PivOH (5 equiv) | DMF | 120 | 88 (80)e |
| 11f | Pd(OAc)2 | AgOAc + PivOH (5 equiv) | DMF | 120 | 68 |
Typical conditions: 1a (0.2 mmol, 1 equiv), 2a (0.3 mmol, 1.5 equiv), catalyst (10 mol %), oxidant (2.1 equiv), solvent (2.0 mL), air atmosphere, unless otherwise stated.
m-xyl, m-xylene; tol, toluene.
Determined by 1H NMR analysis vs 1,3,5-trimethoxybenzene. The number in parentheses is the isolated yield.
[RhCp*Cl2]2 (2.5 mol %).
At 90 °C, 3aa was isolated in 73% yield.
1a (3 mmol), Pd(OAc)2 (5 mol %).
Having established the optimal conditions (Table 1, entry 10), we next investigated the scope and limitations of both reaction partners (Scheme 2). Symmetrical aryl alkynes 2b–2l bearing an electron-donating group (Me, OMe) or an electron-withdrawing group (CF3, F, COOMe) at the para or meta position were well-tolerated and gave the corresponding pyrazolo-2-benzazepines 3ab–3al in moderate to good yields.25 Pleasingly, aryl alkynes bearing halogens (Br, F) or coordinating groups (CN) afforded the products 3ad, 3ag, and 3aj in relatively good yields. Unfortunately, dialkyl alkynes failed to react under the standard conditions.26 On the other hand, the unsymmetrical diaryl alkyne 2m bearing substituents with different electronic properties gave 3am in 56% yield as a 1.6:1 mixture of regioisomers. Conjugated alkynes such as methyl 3-phenylpropiolate (2n) and 1-phenylpropyne (2o) regioselectively gave the corresponding pyrazolo-2-benzazepines 3an and 3ao, albeit in relatively low yields.
Scheme 2. Scope of the Reaction.
PivOH (10 equiv).
PivOH (15 equiv).
Reaction conditions: 1 (1 equiv), 2 (1.5 equiv), DMF (0.1 M), 120 °C, 24 h, open to air. The ORTEP drawing of 3aa shows ellipsoids at the 30% contour probability level.
The electronic effects of aryl substituents in 1 were then analyzed. On the one hand, substrates with electron-withdrawing or electron-donating substituents at the meta or para position gave comparable results (3ba–3ka). Pleasingly, halogenated substituents were tolerated at both positions, which would enable their future functionalization (3da, 3ga, 3ha), as well as substitution in ortho position (3la, 3ma). On the other hand, substituents on the pyrazole ring were also allowed (3na). In addition, other substituted substrates such as naphthalenyl- and 1-benzhydrylpyrazoles also participated, giving the corresponding pyrazole-2-benzazepines 3oa and 3pa in fairly good yields.
To gain insight into the reaction mechanism, a series of stoichiometric and catalytic experiments were conducted. The dimeric six-membered cyclometalated Pd(II) complex 4a, which could be characterized by X-ray crystallography, was formed in 91% yield by heating 1a with 1 equiv of Pd(OAc)2 in DCM for 5 h (Scheme 3, eq 1).27 Unlike the catalytic conditions (Table 1, entry 8), the stoichiometric reaction between dimeric palladacycle 4a and alkyne 2a needed the presence of PivOH to give 3aa in 83% yield (Scheme 3, eq 2).28 Pleasingly, palladacycle 4a can act as a catalyst to give the target product 3aa in 84% yield under the optimized conditions (Scheme 3, eq 3). The competition between 1a and the deuterated analogue 1a-d5 showed a nonconclusive primary kinetic isotopic effect, suggesting that the first C–H bond activation might be the rate-determining step (Scheme 4, eq 4).
Scheme 3. Mechanistic Studies.
Scheme 4. Derivatizations of 3aa.
With all these experimental data on hand, density functional theory (DFT) calculations23 for the reaction of 1a with 2a catalyzed by 1/2Pd2(OAc)4 in the presence of 1/2(AgOAc)2 and AcOH in DMF were performed. According to Fang and co-workers,29 starting materials coordinated to mononuclear palladium species represent the most plausible structures of the initial reaction complex under catalytic conditions. We started our calculations from complex I, which is isoenergetic with the starting materials (Figure 2).30 After agostic interaction of the ortho hydrogen of the phenyl ring in intermediate II,30 C–H activation would take place through TSII–III (16.8 kcal mol–1) to give the six-membered palladacycle III lying at −7.4 kcal mol–1.31 Then 1,2-migratory insertion of the alkyne into the C–Pd bond occurs, most probably from PdII–AgI bimetallic species IV through TSIV–V at 12.2 kcal mol–1, after which N-decoordination gives V.32 Further decoordination of AgOAc to give VI(32) followed by a CMD process through TSVI–VII (8.8 kcal mol–1) affords palladacycle VII (rollover process). Recoordination of AgOAc to form VIII followed by reductive elimination through TSVIII–IX (7.5 kcal mol–1) would release 3aa from the Pd0–AgI bimetallic complex IX (ΔG° = −17.7 kcal mol–1).32 An alternative mechanism involving a Pd(IV) species to favor a reductive elimination step was discarded since a catalytic reaction in the presence of oxidants (PhI(OAc)2, PIFA, Oxone, NFSI) failed while a stoichiometric experiment with PdII(OAc)2 and PivOH in the absence of AgOAc gave 3aa in almost quantitative yield.23
Figure 2.
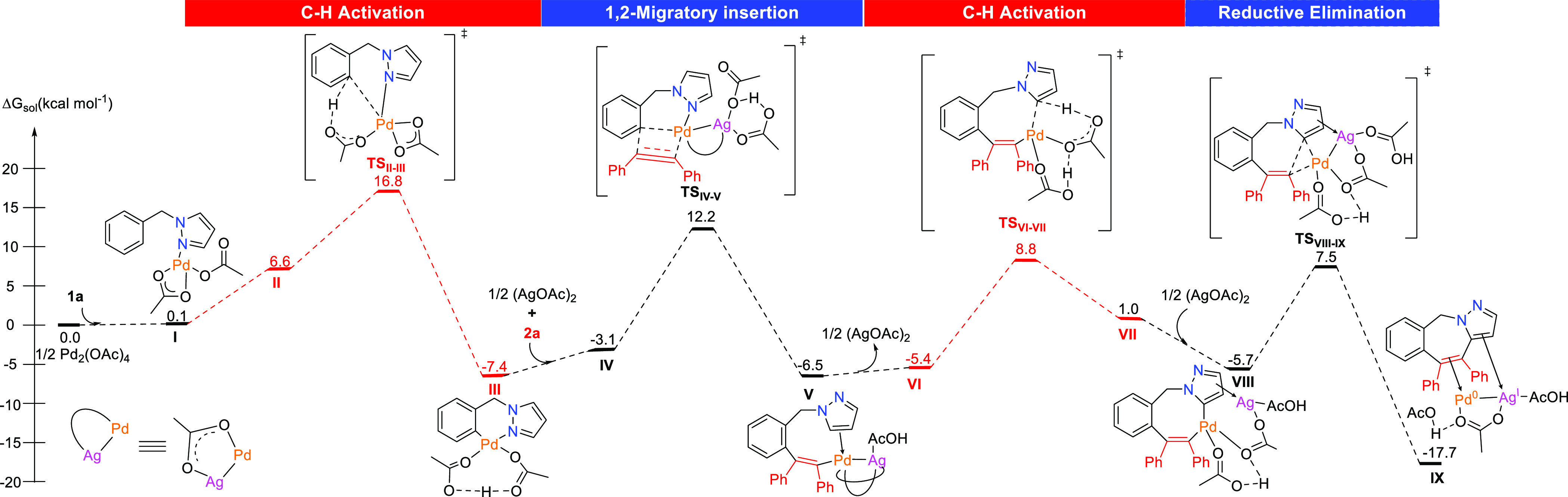
Free energy profile for the [5 + 2] rollover annulation of 1-benzylpyrazole (1a) with 1,2-diphenylacetylene (2a) catalyzed by monometallic PdII (in red) and bimetallic PdII–AgI (in black) species. Computational studies were performed at the B3LYP-D3/6-311++G(d,p)-cc-pVTZ-ppDMF(SMD)//B3LYP-D3/6-31G(d,p)-LANL2DZDMF(SMD) level. Energies are relative to 1/2Pd2(OAc)4 combined with those of the relevant substrates.
However, under stoichiometric conditions, formation of the binuclear Pd species 4a would be more plausible,29 which cannot undergo the 1,2-migratory insertion of the alkyne due to the high activation energy barrier (ΔG⧧ = 35.8 kcal mol–1) as experimentally observed.23 By using large amounts of an external ligand (PivOH or 1a; Scheme 3, eqs 2 and 3),23 the reaction would return to the mononuclear Pd catalytic cycle, which is able to afford the product 3aa.
Derivatizations of benzo[e]pyrazolo[1,5-a]azepine 3aa were then analyzed (Scheme 4). Electrophilic bromination with NBS at room temperature afforded 4-bromopyrazole derivative 5 in a fairly good yield (77%, a). Alkylation with methyl iodide gave rise to pyrazolium salt 6 in an excellent 97% yield (b). Interestingly, reduction of the pyrazole to the tetrahydro derivative 7 could be accomplished using NaBH4 in EtOH at 60 °C in 50% yield (c).33
In summary, we have developed a new Pd-catalyzed rollover annulation of 1-benzylpyrazoles with alkynes to obtain benzo[e]pyrazolo[1,5-a]azepines (tricyclic 2-benzazepines). The seven-membered azepine ring was built based upon a new [5 + 2] rollover annulation that implies a twofold C–H activation of aryl and heteroaryl Csp2–H bonds (C–H/C–H) of 1-benzylpyrazoles with alkynes. The pyrazole moiety of the tricyclic 2-benzazepines can be readily functionalized, which highlights the potential utility of our approach. Further applications are currently in progress in our laboratory and will be reported in due course.
Acknowledgments
We acknowledge financial support from MICINN (Project PID2020-118048GB-I00 and ORFEO–CINQA Network RED2018-102387-T), the Xunta de Galicia (Project ED431C 2022/27 and Centro Singular de Investigación de Galicia Accreditation 2019–2022, ED431G 2019/03), and the European Union (European Regional Development Fund). A.S.-L. thanks MICINN for a predoctoral contract.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.2c04300.
General experimental procedures, X-ray crystallographic data, and NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Beltran H.; Oromendia C.; Danila D. C.; Montgomery B.; Hoimes C.; Szmulewitz R. Z.; Vaishampayan U.; Armstrong A. J.; Stein M.; Pinski J.; Mosquera J. M.; Sailer V.; Bareja R.; Romanel A.; Gumpeni N.; Sboner A.; Dardenne E.; Puca L.; Prandi D.; Rubin M. A.; Scher H. I.; Rickman D. S.; Demichelis F.; Nanus D. M.; Ballman K. V.; Tagawa S. T. A phase II trial of the Aurora kinase A inhibitor alisertib for patients with castration-resistant and neuroendocrine prostate cancer: efficacy and biomarkers. Clin. Cancer Res. 2019, 25, 43–51. 10.1158/1078-0432.CCR-18-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht B. K.; Gehling V. S.; Hewitt M. C.; Vaswani R. G.; Cote A.; Leblanc Y.; Nasveschuk C. G.; Bellon S.; Bergeron L.; Campbell R.; Cantone N.; Cooper M. R.; Cummings R. T.; Jayaram H.; Joshi S.; Mertz J. A.; Neiss A.; Normant E.; O’Meara M.; Pardo E.; Poy F.; Sandy P.; Supko J.; Sims R. J.; Harmange J.-C.; Taylor A. M.; Audia J. E. Identification of a Benzoisoxazoloazepine Inhibitor (CPI-0610) of the Bromodomain and Extra-Terminal (BET) Family as a Candidate for Human Clinical Trials. J. Med. Chem. 2016, 59, 1330–1339. 10.1021/acs.jmedchem.5b01882. [DOI] [PubMed] [Google Scholar]
- Heinrich M.; Teoh H. L. Galanthamine from snowdrop-the development of a modern drug against Alzheimer’s disease from local Caucasian knowledge. J. Ethnopharmacol. 2004, 92, 147–162. 10.1016/j.jep.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Gentles R. G. Discovery of Beclabuvir: A Potent Allosteric Inhibitor of the Hepatitis C Virus Polymerase. Top. Med. Chem. 2019, 31, 193–228. 10.1007/7355_2018_38. [DOI] [Google Scholar]
- Zheng B. Z.; D’Andrea S. V.; Hanumegowda U.; Knipe J. O.; Mosure K.; Zhuo X.; Lemm J. A.; Liu M.; Rigat K. L.; Wang Y.-K.; Fang H.; Poronsky C.; Cutrone J.; Wu D.-R.; Arunachalam P. N.; Balapragalathan T. J.; Arumugam A.; Mathur A.; Meanwell N. A.; Gao M.; Roberts S. B.; Kadow J. F. Discovery of BMS-961955, an allosteric inhibitor of the hepatitis C virus NS5B polymerase. Bioorg. Med. Chem. Lett. 2017, 27, 3294–3300. 10.1016/j.bmcl.2017.06.024. [DOI] [PubMed] [Google Scholar]
- a Vieira T. O.; Alper H. An Efficient Three-Component One-Pot Approach to the Synthesis of 2,3,4,5-Tetrahydro-1H-2-benzazepines by Means of Rhodium-Catalyzed Hydroaminomethylation. Org. Lett. 2008, 10, 485–487. 10.1021/ol702933g. [DOI] [PubMed] [Google Scholar]; b Hasebein P.; Aulinger K.; Schepmann D.; Wünsch B. Heck reaction of ortho-substituted iodobenzenes with α,β-unsaturated nitriles as a key step in the synthesis of tetrahydro-2-benzazepines and hexahydro-3-benzazocines. Tetrahedron 2013, 69, 4552–4562. 10.1016/j.tet.2013.04.017. [DOI] [Google Scholar]; c Hasebein P.; Frehland B.; Lehmkuhl K.; Froehlich R.; Schepmann D.; Wuensch B. Synthesis and pharmacological evaluation of like- and unlike-configured tetrahydro-2-benzazepines with the α-substituted benzyl moiety in the 5-position. Org. Biomol. Chem. 2014, 12, 5407–5426. 10.1039/C4OB00510D. [DOI] [PubMed] [Google Scholar]; d Quick M. P.; Froehlich R.; Schepmann D.; Wuensch B. Asymmetric synthesis of 3-substituted tetrahydro-2-benzazepines. Org. Biomol. Chem. 2015, 13, 7265–7281. 10.1039/C5OB00731C. [DOI] [PubMed] [Google Scholar]
- a So M.; Kotake T.; Matsuura K.; Inui M.; Kamimura A. Concise Synthesis of 2-Benzazepine Derivatives and Their Biological Activity. J. Org. Chem. 2012, 77, 4017–4028. 10.1021/jo300380z. [DOI] [PubMed] [Google Scholar]; b Kamimura A.; Taguchi Y.; Omata Y.; Hagihara M. Convenient Synthesis of 2-Benzazepines via Radical Cyclization. J. Org. Chem. 2003, 68, 4996–4998. 10.1021/jo030052h. [DOI] [PubMed] [Google Scholar]
- Iqbal N.; Fiksdahl A. Gold(I)-Catalyzed Benz[c]azepin-4-ol Synthesis by Intermolecular [5 + 2] Cycloaddition. J. Org. Chem. 2013, 78, 7885–7895. 10.1021/jo401075n. [DOI] [PubMed] [Google Scholar]
- Inyutina A.; Dar’in D.; Kantin G.; Krasavin M. Tricyclic 2-benzazepines obtained via an unexpected cyclization involving nitrilium ylides. Org. Biomol. Chem. 2021, 19, 5068–5071. 10.1039/D1OB00773D. [DOI] [PubMed] [Google Scholar]
- Virelli M.; Moroni E.; Colombo G.; Fiengo L.; Porta A.; Ackermann L.; Zanoni G. Expedient Access to 2-Benzazepines by Palladium-Catalyzed C-H Activation: Identification of a Unique Hsp90 Inhibitor Scaffold. Chem. - Eur. J. 2018, 24, 16516–16520. 10.1002/chem.201804244. [DOI] [PubMed] [Google Scholar]
- Qureshi Z.; Kim J. Y.; Bruun T.; Lam H.; Lautens M. Cu/Pd-Catalyzed Synthesis of Fully Decorated Polycyclic Triazoles: Introducing C-H Functionalization to Multicomponent Multicatalytic Reactions ((MC)2R). ACS Catal. 2016, 6, 4946–4952. 10.1021/acscatal.6b00858. [DOI] [Google Scholar]
- a Velasco-Rubio Á.; Varela J. A.; Saá C. Recent Advances in Transition-Metal-Catalyzed Oxidative Annulations to Benzazepines and Benzodiazepines. Adv. Synth. Catal. 2020, 362, 4861–4875. 10.1002/adsc.202000808. [DOI] [Google Scholar]; b Gulías M.; Mascareñas J. L. Metal-Catalyzed Annulations through Activation and Cleavage of C-H Bonds. Angew. Chem., Int. Ed. 2016, 55, 11000–11019. 10.1002/anie.201511567. [DOI] [PubMed] [Google Scholar]; c Font M.; Gulías M.; Mascareñas J. L. Transition-Metal-Catalyzed Annulations Involving the Activation of C(sp3)-H Bonds. Angew. Chem., Int. Ed. 2022, 61, e202112848. 10.1002/anie.202112848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Shi Z.; Grohmann C.; Glorius F. Mild Rhodium(III)-Catalyzed Cyclization of Amides with α,β-Unsaturated Aldehydes and Ketones to Azepinones: Application to the Synthesis of the Homoprotoberberine Framework. Angew. Chem., Int. Ed. 2013, 52, 5393–5397. 10.1002/anie.201301426. [DOI] [PubMed] [Google Scholar]; b Kurihara T.; Kojima M.; Yoshino T.; Matsunaga S. Achiral Cp*Rh(III)/Chiral Lewis Base Cooperative Catalysis for Enantioselective Cyclization via C–H Activation. J. Am. Chem. Soc. 2022, 144, 7058–7065. 10.1021/jacs.2c01223. [DOI] [PubMed] [Google Scholar]
- Cui S.; Zhang Y.; Wang D.; Wu Q. Rh(III)-catalyzed C-H activation/[4 + 3] cycloaddition of benzamides and vinylcarbenoids: facile synthesis of azepinones. Chem. Sci. 2013, 4, 3912–3916. 10.1039/c3sc51777b. [DOI] [Google Scholar]
- Pandey A. K.; Han S. H.; Mishra N. K.; Kang D.; Lee S. H.; Chun R.; Hong S.; Park J. S.; Kim I. S. Synthesis of 2-Benzazepines from Benzylamines and MBH Adducts Under Rhodium(III) Catalysis via C(sp2)-H Functionalization. ACS Catal. 2018, 8, 742–746. 10.1021/acscatal.7b03812. [DOI] [Google Scholar]
- Martinez-Mingo M.; Rodriguez N.; Gomez Arrayas R.; Carretero J. C. Access to Benzazepinones by Pd-Catalyzed Remote C-H Carbonylation of γ-Arylpropylamine Derivatives. Org. Lett. 2019, 21, 4345–4349. 10.1021/acs.orglett.9b01523. [DOI] [PubMed] [Google Scholar]
- Vidal X.; Mascareñas J. L.; Gulías M. Assembly of Tetrahydroquinolines and 2-Benzazepines by Pd-Catalyzed Cycloadditions Involving the Activation of C(sp3)-H Bonds. Org. Lett. 2021, 23, 5323–5328. 10.1021/acs.orglett.1c01594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda N.; Hirano K.; Satoh T.; Shibata N.; Sato H.; Miura M. Rhodium-Catalyzed Oxidative 1:1, 1:2, and 1:4 Coupling Reactions of Phenylazoles with Internal Alkynes through the Regioselective Cleavages of Multiple C-H Bonds. J. Org. Chem. 2011, 76, 13–24. 10.1021/jo1021184. [DOI] [PubMed] [Google Scholar]
- a Yu J.; Lv W.; Cheng G. Palladium-Catalyzed Site-Selective C-H Arylation of 2,2′-Bipyridine-6-carboxamides via a Rollover Cyclometalation Pathway. Org. Lett. 2018, 20, 4732–4735. 10.1021/acs.orglett.8b01632. [DOI] [PubMed] [Google Scholar]; b Thenarukandiyil R.; Dutta C.; Choudhury J. Switching of Reaction Pathway from C-C Rollover to C-N Ring-Extension Annulation. Chem. - Eur. J. 2017, 23, 15529–15533. 10.1002/chem.201703687. [DOI] [PubMed] [Google Scholar]; c Zucca A.; Pilo M. I. Rollover cyclometalation as a valuable tool for regioselective C-H bond activation and functionalization. Molecules 2021, 26, 328. 10.3390/molecules26020328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.; Engle K. M.; Wang D.-H.; Yu J.-Q. Palladium(II)-Catalyzed C-H Activation/C-C Cross-Coupling Reactions: Versatility and Practicality. Angew. Chem., Int. Ed. 2009, 48, 5094–5115. 10.1002/anie.200806273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addition of AcOH (1 equiv) instead PivOH gave 3aa in 69% yield.
- The use of Pd(OPiv)2 as the catalyst (10 mol%) under the same reaction conditions gave 3aa in 80% NMR yield.
- See the Supporting Information for details.
- Ortho-substituted aryl alkynes failed to participate, probably because of steric hindrance.
- For other unsuccessful probes, see the Supporting Information.
- a Shi B.-F.; Maugel N.; Zhang Y.-H.; Yu J.-Q. Pd(II)-Catalyzed Enantioselective Activation of C(sp2)-H and C(sp3)-H Bonds Using Monoprotected Amino Acids as Chiral Ligands. Angew. Chem., Int. Ed. 2008, 47, 4882–4886. 10.1002/anie.200801030. [DOI] [PubMed] [Google Scholar]
- In the absence of PivOH, with or without AgOAc, no reaction was observed. See the Supporting Information for details.
- Zhang L.-L.; Zhang L.; Li S.-J.; Fang D.-C. DFT studies on the distinct mechanisms of C–H activation and oxidation reactions mediated by mononuclear- and binuclear-palladium. Dalton Trans. 2018, 47, 6102–6111. 10.1039/C8DT00236C. [DOI] [PubMed] [Google Scholar]
- For complete free energy profiles for monometallic PdII- and bimetallic PdII–AgI-catalyzed [5 + 2] rollover annulations, see the Supporting Information.
- C–H activation through a bimetallic PdII–AgI species cannot be ruled out since its transition state is isoenergetic with the monometallic species (see the Supporting Informantion for details). See:; a Anand M.; Sunoj R. B.; Schaefer H. F. III. Palladium–Silver Cooperativity in an Aryl Amination Reaction through C–H Functionalization. ACS Catal. 2016, 6, 696–708. 10.1021/acscatal.5b02639. [DOI] [Google Scholar]; b Davies D. L.; Macgregor S. A.; McMullin C. L. Computational Studies of Carboxylate-Assisted C–H Activation and Functionalization at Group 8–10 Transition Metal Centers. Chem. Rev. 2017, 117, 8649–8709. 10.1021/acs.chemrev.6b00839. [DOI] [PubMed] [Google Scholar]; c Fang L.; Saint-Denis T. G.; Taylor B. L. H.; Ahlquist S.; Hong K.; Liu S.; Han L.; Houk K. N.; Yu J.-Q. Experimental and Computational Development of a Conformationally Flexible Template for the meta-C–H Functionalization of Benzoic Acids. J. Am. Chem. Soc. 2017, 139, 10702–10714. 10.1021/jacs.7b03296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechanistic pathways involving both bimetallic PdII–AgI and monometallic PdII species were calculated, showing that the most favorable path depends on the elementary step considered. See the Supporting Information for details.
- Bañuelos L. A.; Cuadrado P.; González-Nogal A. M.; López-Solera I.; Pulido F. J.; Raithby P. R. The reduction of functionalized pyrazolium salts as a stereoselective route to functionalized pyrazolidines. Tetrahedron 1996, 52, 9193–9206. 10.1016/0040-4020(96)00470-X. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.









