Abstract

Biomaterials derived from brain extracellular matrix (ECM) have the potential to promote neural tissue regeneration by providing instructive cues that can direct cell survival, proliferation, and differentiation. This study focused on the development and characterization of microcarriers derived from decellularized brain tissue (DBT) as a platform for neural progenitor cell culture. First, a novel detergent-free decellularization protocol was established that effectively reduced the cellular content of porcine and rat brains, with a >97% decrease in the dsDNA content, while preserving collagens (COLs) and glycosaminoglycans (GAGs). Next, electrospraying methods were applied to generate ECM-derived microcarriers incorporating the porcine DBT that were stable without chemical cross-linking, along with control microcarriers fabricated from commercially sourced bovine tendon COL. The DBT microcarriers were structurally and biomechanically similar to the COL microcarriers, but compositionally distinct, containing a broader range of COL types and higher sulfated GAG content. Finally, we compared the growth, phenotype, and neurotrophic factor gene expression levels of rat brain-derived progenitor cells (BDPCs) cultured on the DBT or COL microcarriers within spinner flask bioreactors over 2 weeks. Both microcarrier types supported BDPC attachment and expansion, with immunofluorescence staining results suggesting that the culture conditions promoted BDPC differentiation toward the oligodendrocyte lineage, which may be favorable for cell therapies targeting remyelination. Overall, our findings support the further investigation of the ECM-derived microcarriers as a platform for neural cell derivation for applications in regenerative medicine.
Keywords: decellularization, brain, extracellular matrix, microcarriers, cell-instructive biomaterials, neural tissue engineering
Neurodegenerative diseases are a leading cause of disability worldwide and place a substantial economic burden on healthcare systems.1 Despite the presence of endogenous neural stem cells (NSCs), the central nervous system (CNS) has a limited capacity for self-repair that is further attenuated by the inhibitory microenvironment that develops following disease or injury.2 Recognizing the many limitations of conventional treatment approaches, there has been strong interest in stem cell therapies for neural regeneration. While much of the work to date has focused on the replacement of neurons,3 the regeneration of glial cells, such as oligodendrocytes, may also be beneficial, particularly for demyelinating diseases such as multiple sclerosis and spinal cord injuries (SCIs).4,5 Oligodendrocyte progenitor cells (OPCs) are the main regenerative cell population within the adult CNS and contribute to spontaneous remyelination.5 However, OPC activity diminishes with aging, reducing the brain’s regenerative capabilities in response to disease and injury, which has motivated the investigation of cell-based therapies.5 To date, two clinical trials have been performed evaluating the use of human embryonic stem cell (ESC)-derived OPCs to target SCI.6 A study recently completed in 2018 by Lineage Cell Therapeutics (NCT02302157) demonstrated that OPCs could be safely delivered to patients, with motor recovery observed in the upper extremities over a two-year follow-up period.7,8
Previous work has identified a population of brain-derived progenitor cells (BDPCs) that can be isolated and expanded from human brain biopsies taken during minimally invasive surgical procedures.9 These cells display robust expression of neural and oligodendrocyte progenitor markers including nestin and oligodendrocyte transcription factor 1 (Olig1).9 Based on RT-PCR analysis, BDPCs have also been shown to express neurotrophic factors (NTFs), including glial-derived neurotrophic factor (GDNF), cerebral dopamine neurotrophic factor (CDNF), and brain-derived neurotrophic factor (BDNF), which can have neuroprotective effects that may be favorable for therapeutic applications.10
As a step toward future clinical translation, this study focused on the development of novel culture platforms for BDPC expansion that would have the capacity to direct differentiation to help generate the many millions of mature cells that would be required for therapeutic use in humans. It is well recognized that the cellular microenvironment in vitro can alter the phenotype and function of stem and progenitor cells, influencing their proliferation and differentiation.11 As such, there has been increasing interest in the development and application of biomaterials that better recapitulate the native cellular niche, including mimicking the complex composition of the extracellular matrix (ECM) within tissues.12 In particular, decellularization is a strategy that can be used to isolate tissue-specific ECM, which can subsequently be applied as a starting material for fabricating a wide range of ECM-derived scaffold formats.13 Notably, the brain is challenging to decellularize due to its low ECM content,14 as well as its unique composition, containing large amounts of highly soluble glycosaminoglycans (GAGs) that are easily lost during processing.15 To date, the published brain decellularization protocols have been detergent-based, which is an effective method for cellular removal but can raise concerns with cytotoxicity and may contribute to the loss of bioactive ECM components including GAGs.16
Recognizing these limitations, our first objective was to establish a detergent-free method for decellularizing porcine brains and to characterize the resultant ECM. Porcine brains were selected as they are anatomically and physiologically similar to the human brain, and they provide a greater amount of ECM compared to rodent brains.17 We subsequently sought to apply the brain-derived ECM to develop a clinically translational 3D culture system for BDPC expansion. In particular, stirred bioreactors integrating microcarriers represent a promising alternative to 2D culture on tissue culture polystyrene (TCPS) substrates to support the proliferation of adherent stem or progenitor cell populations.18 Building from our previous work,19,20 we applied electrospraying methods to generate microcarriers composed exclusively of ECM that incorporated the porcine decellularized brain tissue (DBT) and were stable without chemical cross-linking. Within the literature, there is evidence to support that the incorporation of brain-derived ECM within culture platforms can increase neuronal migration and axon length, as well as promote neurite extension and the formation of neural networks within hydrogels.21−24 Furthermore, studies have shown that the neuronal differentiation of human NSCs can be augmented by culturing the cells on ECM-derived scaffolds sourced from CNS tissues relative to non-CNS sources, such as urinary bladder ECM.23,25 As such, we hypothesized that the incorporation of the DBT within the ECM-derived microcarriers would modulate the proliferation and differentiation of rat BDPCs cultured within spinner flask bioreactors as compared to non-tissue-specific control microcarriers fabricated from purified collagen (COL) that were structurally similar but compositionally distinct. In-depth in vitro testing confirmed that both microcarrier types supported BDPC expansion within the spinner flasks over 2 weeks and promoted their differentiation toward the oligodendrocyte lineage based on analysis of neural marker expression through immunofluorescence (IF) staining and RT-qPCR.
Results and Discussion
Porcine Brain Decellularization and Characterization
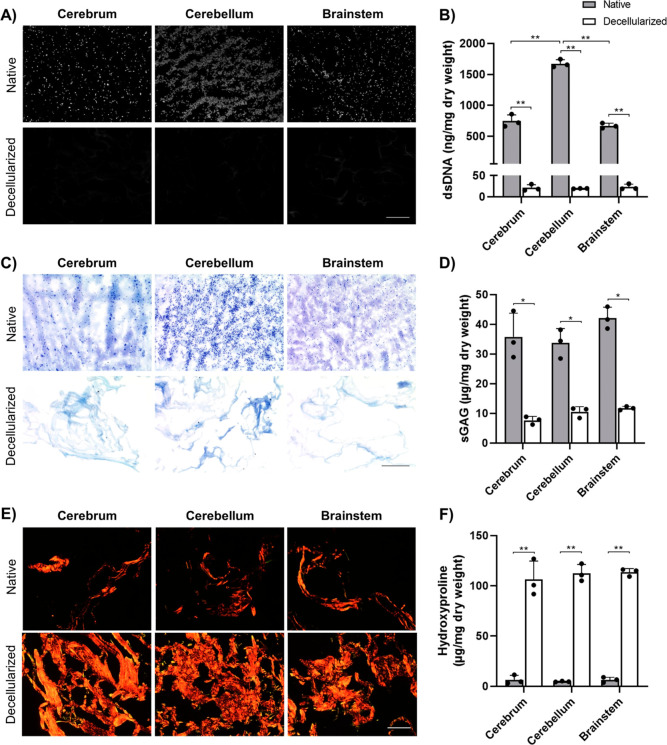
The first aim of this project was to develop a novel detergent-free brain decellularization protocol with the goal of effectively removing cellular content while preserving ECM components, including GAGs and COLs. Initially, a 5 day detergent-free adipose decellularization protocol previously established by our laboratory26 was tested and systematically altered to enhance double-stranded DNA (dsDNA) removal while retaining the GAG content. In brief, the finalized protocol involved three freeze–thaw cycles (−80 °C/37 °C) in a hypotonic buffer, followed by a 6 h enzymatic digestion in 0.25% trypsin–EDTA, a 16 h incubation in 4% ethanol supplemented with 0.1% peracetic acid, and a 4 h enzymatic digestion with DNase and RNase. To confirm the effectiveness of the new protocol and probe for compositional differences between the brain regions, pig cerebrum, cerebellum, and brainstem samples were separately decellularized and characterized. DAPI staining showed no detectable cell nuclei in any of the decellularized tissue samples (Figure 1A). Successful decellularization was confirmed by PicoGreen assay, which showed a significant reduction of >97% in the dsDNA content in all three regions (Figure 1B). Our results are comparable to published detergent-based brain decellularization protocols, which report dsDNA reduction ranging from ∼90%27,28 up to 99%.29 While our protocol was developed and tested with porcine brains, follow-up studies in our laboratory confirmed that it also effectively decellularized whole rat brains (Figure S1), supporting that it can be applied to decellularize tissues sourced from other species.
Figure 1.
New detergent-free protocol effectively decellularized porcine cortex, cerebellum, and brainstem. (A) Representative DAPI nuclear staining (gray) of native and decellularized pig brain samples showing no visible cell nuclei following decellularization. Scale bar = 200 μm. (B) Quantitative analysis of the dsDNA content by PicoGreen assay showing a significant reduction in dsDNA in the decellularized samples (n = 3 and N = 3; **p < 0.0001). (C) Representative toluidine blue staining of GAG (purple) showing retention of GAGs following decellularization. Scale bar = 200 μm. (D) Quantitative analysis of the sGAG content by DMMB assay showing 20–30% GAG retention in the decellularized samples (n = 3 and N = 3; *p < 0.001). (E) Representative picrosirius red staining of COL fibers (red/yellow) showing a dense network of thick and thin COL fibers in the decellularized tissues. Scale bar = 200 μm. (F) Quantitative analysis of the COL content by hydroxyproline assay showing a significant relative enrichment in the COL content following decellularization (n = 3 and N = 3; **p < 0.0001).
GAGs have been shown to play an important role in modulating the proliferation and differentiation of neural stem and progenitor cells.30−32 Due to their relative ease of extraction during tissue processing,33 the retention of GAGs was used as a key indicator of protocol effectiveness. Representative toluidine blue images showed an abundance of GAGs in the native tissues, with qualitatively lower levels retained in the decellularized samples, which had a more open porous structure (Figure 1C). Analysis of the sulfated GAG (sGAG) content in the DBT from all three regions via the dimethylmethylene blue (DMMB) assay showed between 20 and 30% retention of the sGAG content relative to native tissue samples (Figure 1D), which was a marked improvement from the ∼4% sGAG retention measured following the adipose decellularization protocol (data not shown). There were no significant differences in the GAG content in the DBT sourced from the various brain regions.
Most studies that have performed GAG quantification in both native and decellularized porcine brain tissue have reported slightly higher GAG retention than what was achieved with the current protocol.24,28,34 Both Seo et al.24 and Hong et al.34 followed a protocol initially developed by Crapo et al.21 involving treatment with the non-ionic detergent Triton X-100 and the ionic detergent sodium deoxycholate, reporting ∼40% retention of the sGAG content based on theDMMB assay. Notably, their total protocol duration was less than 24 h, which may have contributed to higher GAG retention.35 While the use of detergents in decellularization protocols has been associated with GAG loss, prolonged exposure to trypsin has also been shown to decrease the GAG content over time, particularly o-sulfated GAGs such as chondroitin sulfate.16,36 Moreover, although short-term exposure (<2 h) to low concentrations of peracetic acid has been suggested to have minimal effects on ECM components, including GAGs and COLs,37 other studies have indicated its use can be associated with growth factor removal and GAG dissociation.36,38 As such, in the future, it may be worth investigating whether GAGs and other soluble ECM components may be better preserved within the DBT if our protocol was optimized to reduce exposure to trypsin and peracetic acid, as well as reducing the overall processing time.
COL is the most abundant protein in most mammalian tissues, however, it makes up only a small portion of the native brain ECM.15 Visualization of picrosirius red staining by polarized light microscopy revealed dense networks of COL in the DBT samples, with a combination of thicker fibers (red/orange) and thinner fibers (yellow/green) (Figure 1E). The decellularized tissues appeared to have a relative enrichment of COL fibers compared to the native tissue samples, which was corroborated by the quantification of the COL content via hydroxyproline assay (Figure 1F). These findings are consistent with the work of Simsa et al., who also decellularized porcine brains,28 and are likely attributed to the loss of cells and other ECM components during decellularization. Similar to the DMMB assay results, no significant differences were observed in the hydroxyproline content between the three brain regions in both the native and decellularized samples.
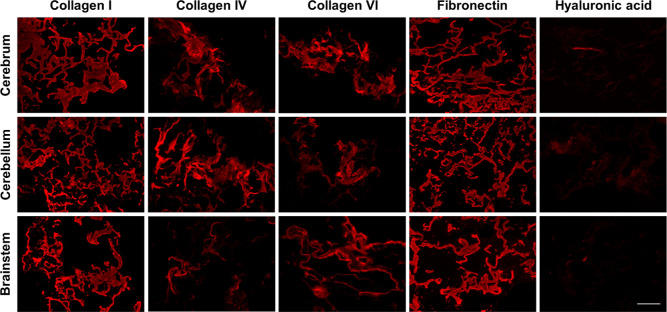
Further probing the ECM composition, IF staining confirmed the presence of COL I, COL IV, COL VI, fibronectin, laminin, hyaluronan (HA), keratan sulfate, and brevican within the native porcine brain samples (Figure S2). As expected, the native tissue samples were rich in GAGs and proteoglycans but contained relatively low amounts of COL. The cell-adhesive glycoproteins (laminin and fibronectin) were expressed throughout the tissues, with similar spatial distribution patterns to the COLs. There was some regional variability, with the cerebellum showing qualitatively higher expression of the COLs, laminin, and fibronectin as compared to the cerebrum and brainstem. Analysis of the DBT samples from all three brain regions showed that COL I, IV, and VI, along with fibronectin, were all retained at the end of processing (Figure 2). In contrast, there was minimal retention of HA (Figure 2), and keratan sulfate, brevican, and laminin were not detected in any of the DBT samples (data not shown). In general, our findings indicate that the decellularization process generated a COL-rich ECM, distinct from the more GAG-rich native brain ECM.15
Figure 2.
Immunohistochemical analyses confirmed the presence of various types of COL and fibronectin following decellularization. Representative staining showing the retention of multiple types of COL and fibronectin, with similar staining patterns in the decellularized porcine cerebrum, cerebellum, and brainstem samples (n = 3). Minimal amounts of hyaluronic acid were detected in the cerebrum and brainstem samples, and no laminin, keratan sulfate, or brevican were detected in any region. Scale bar = 200 μm.
While it would be expected that the loss of GAGs and laminin would alter scaffold bioactivity,39 bioscaffolds incorporating a diverse range of COL subtypes, mimicking the blend found within the native microenvironment, may also have favorable cell-instructive effects.40 COL I provides structural support to tissues, and its properties can aid in the fabrication of biomaterials that show both strength and resilience when applied in long-term culture.34,41 COL IV, a major component of the basal lamina, has been shown to regulate neurogenesis in Drosophila(42) and promote the neuronal differentiation of rat cortical progenitor cells in culture.43 Similarly, COL VI plays important structural and functional roles within the nervous system, including having neuroprotective effects in the brain following injury44 and in modulating the axonal growth of neurons.45 COL VI has also been indicated to inhibit apoptosis within the CNS.46 In addition, the preservation of fibronectin within the DBT may be favorable for promoting cell attachment to the bioscaffolds,15 and fibronectin has also been shown to stimulate the proliferation of rat astroglial cells in culture.47
Fabrication and Characterization of Brain-Derived Microcarriers
The second phase of this study focused on the development of novel ECM-derived microcarriers incorporating the DBT, along with control microcarriers fabricated from commercially available type I COL sourced from bovine tendon. Due to the low amount of ECM within the brain and the observed compositional similarity in the DBT generated from the three regions, we decided to process the brain as a whole, to maximize the yield of ECM available for microcarrier fabrication. Supporting this choice, Reginensi et al. cultured PC12 cells on ECM coatings derived from the cerebrum, cerebellum, and brainstem and found that the ECM source had no effect on cell viability and growth.35
To fabricate the microcarriers, we adapted methods we previously established using decellularized adipose tissue.19 Similar to other groups who have supplemented their brain-derived ECM with COL,22,24,34,48 we fabricated our DBT microcarriers with a 1:1 blend of DBT/COL to improve their mechanical properties and long-term stability in culture as preliminary testing indicated that microcarriers fabricated exclusively from DBT showed a loss of structural integrity following rehydration. In our approach, the COL was digested with α-amylase to generate a homogeneous suspension that was electrosprayed into liquid nitrogen, producing spherical beads composed exclusively of ECM that were stable following lyophilization and a controlled rehydration process without the need for chemical cross-linking or other additives that could impact the ECM bioactivity.19 The α-amylase digestion is postulated to increase solubility in acetic acid by cleaving carbohydrate groups within the telopeptide regions of COLs,49 while preserving their fibrillar structure that functions to stabilize the microcarriers through physical interactions and hydrogen bonding. In contrast to the COL, we found that the DBT could be solubilized directly into the acetic acid without the need for α-amylase digestion.
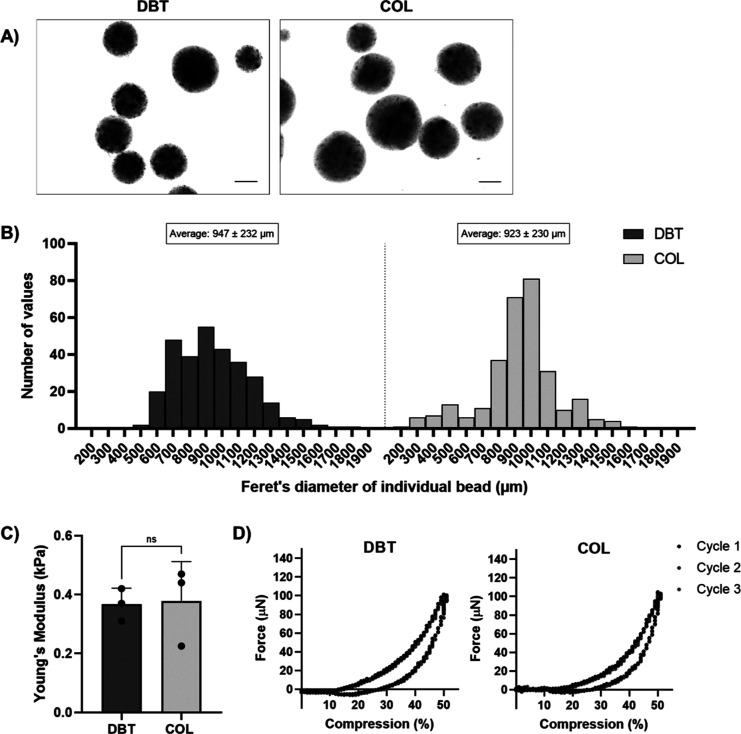
Brightfield imaging confirmed that the DBT and COL microcarriers had a spherical shape (Figure 3A) and were of a similar size range following rehydration in phosphate buffer saline (PBS), with an average diameter of 947 ± 232 and 923 ± 230 μm for the DBT and COL microcarriers, respectively (Figure 3B). Compressive testing performed using a CellScale MicroTester under simulated physiological conditions (PBS at 37 °C) revealed that both microcarrier types were similar in stiffness, with average Young’s moduli of 0.37 ± 0.06 kPa for the DBT microcarriers and 0.38 ± 0.13 kPa for the COL microcarriers (Figure 3C), ensuring that differences in the initial biomechanical properties would not confound our analysis of the effects of microcarrier composition on the BDPC response.50 Both microcarrier types were soft and compliant and returned to their original geometry after they were compressed to 50% of their original diameter repeatedly over six cycles (Figure 3D).
Figure 3.
DBT and COL microcarriers have similar shape, size, and stiffness. (A) Representative brightfield images of DBT and COL microcarriers showing their spherical shape. Scale bars = 400 μm. (B) Size distribution plots showing Feret’s diameter of the microcarriers following rehydration in PBS. Average diameter was not significantly different between the two groups when analyzed with an unpaired t test (n = 100 and N = 3). (C) Young’s moduli of the DBT and COL microcarriers, with no significant difference measured between the two groups (n = 6 and N = 3). (D) Representative force versus deformation curves for DBT and COL microcarriers. Data from three cycles are shown with compression at a strain rate of 0.01 s–1.
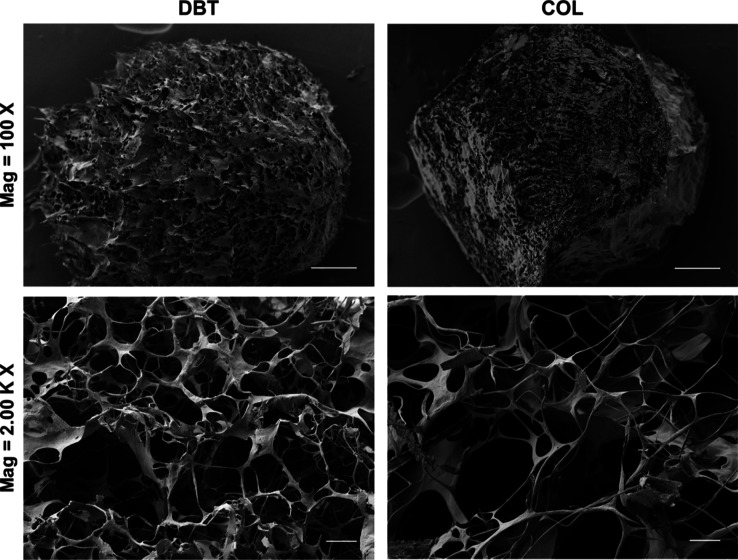
Scanning electron microscopy (SEM) was used to compare the ultrastructure of the DBT and COL microcarriers. Both microcarrier types incorporated sheetlike structures connected by a complex network of thick and thin fibers, forming a porous structure, with no obvious differences between the two groups (Figure 4).
Figure 4.
Representative SEM images visualizing the ECM ultrastructure of the DBT and COL microcarriers. Both microcarrier types had a porous and complex fibrous ultrastructure. Scale bars = 100 μm (top row), 5 μm (bottom row).
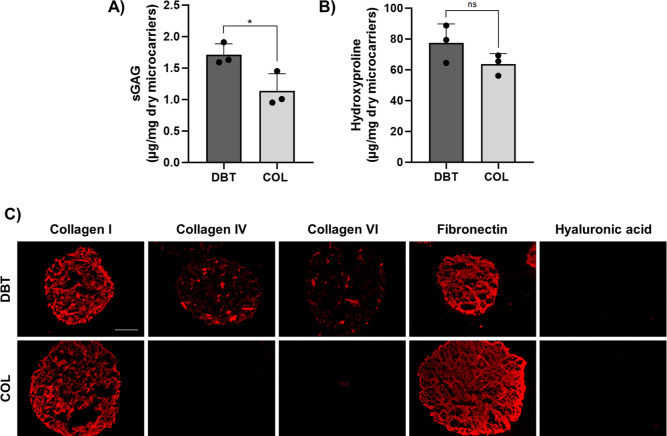
Analysis of the sGAG content with the DMMB assay and COL content with the hydroxyproline assay revealed that the DBT microcarriers contained significantly more sGAG and a similar amount of hydroxyproline as compared to the COL microcarriers (Figure 5A–C). Notably, the sGAG content was approximately five times lower in the DBT microcarriers as compared to the starting DBT source materials. GAG dissociation has been associated with exposure to low pH solutions,33 so this difference may be attributed at least in part to the processing in acetic acid required to enable electrospraying. IF analyses confirmed that the DBT microcarriers were more compositionally complex than the COL microcarriers (Figure 5C). While both microcarrier types showed strong positive staining for COL I and fibronectin, COL IV and COL VI were only detected in the DBT samples, while HA was not detected in either group.
Figure 5.
DBT microcarriers have a more complex composition than COL microcarriers. (A) Quantitative analysis of the sGAG content by DMMB assay showing that the DBT microcarriers contained significantly more sGAG (n = 3–6 and N = 3; *p < 0.05). (B) Quantitative analysis of the COL content, showing no significant difference in the hydroxyproline content between the DBT and COL microcarriers (n = 3 and N = 3). (C) Representative IF staining showing that the DBT microcarriers contained multiple types of COL and fibronectin, while only type I COL and fibronectin were detected in the COL microcarriers (n = 3 and N = 2). No hyaluronic acid was detected in either group. Scale bar = 200 μm.
Culture of Rat BDPCs on the DBT versus COL Microcarriers within Spinner Flask Bioreactors
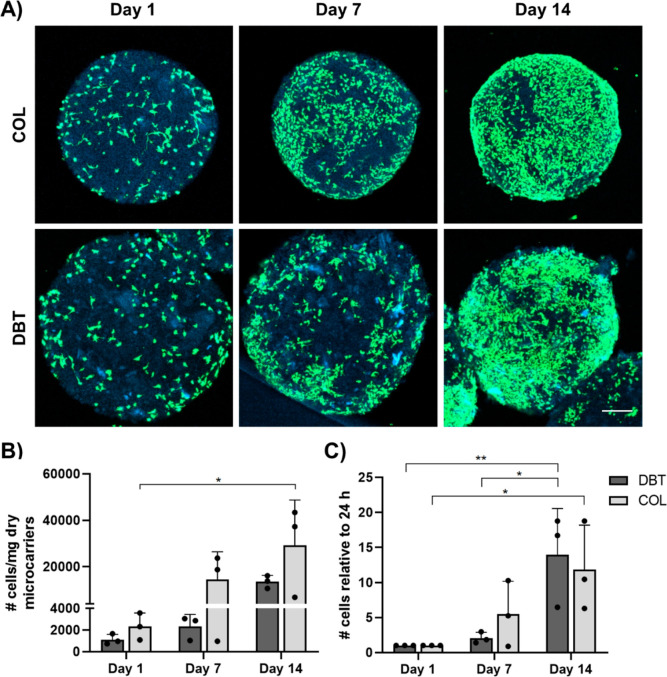
Following the characterization of the DBT and COL microcarriers, the subsequent studies focused on assessing their potential as a platform for the in vitro expansion of rat BDPCs within a low-shear spinner culture bioreactor system. Confocal imaging of LIVE/DEAD staining indicated that both microcarrier types supported BDPC attachment and growth over 14 days in culture within the spinner flasks at 20 rpm (Figure 6A). Quantification of the number of cells attached to the microcarriers by PicoGreen assay with a cell standard curve showed a significant increase between days 1 and 14 in the COL microcarrier group (Figure 6B). When the data were normalized to the cell density at day 1 to account for differences in initial cell attachment, the results indicated there was a similar significant population fold change from day 1 to day 14 in both groups, suggesting that cell growth was similar on the DBT and COL microcarriers (Figure 6C).
Figure 6.
ECM-derived microcarriers support the attachment and growth of BDPCs under dynamic culture. (A) Representative LIVE/DEAD staining visualized through confocal microscopy showing the presence of calcein+ live cells (green) on the surface of the microcarriers (blue), with a qualitative increase in the density observed over time. Scale bar = 200 μm. (B) dsDNA content was assessed by PicoGreen assay to quantify the cell content on the microcarriers at various timepoints. Cell density significantly increased between days 1 and 14 on the COL microcarriers (n = 3 and N = 3; *p < 0.05). (C) Cell density data normalized to day 1 showing a significant increase on the DBT and COL microcarriers between days 1 and 14, confirming that both platforms supported BDPC expansion (n = 3 and N = 3; *p < 0.05).
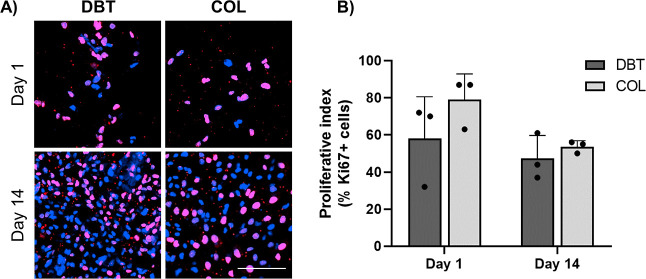
Cell proliferation on the microcarriers was assessed through IF staining for the proliferation marker Ki67 in samples collected on days 1 and 14, confirming that both microcarrier types were highly supportive of BDPC proliferation within the spinner flask bioreactors (Figure 7A). Manual counting using ImageJ revealed that an average of 58 ± 23% of the BDPCs on the DBT microcarriers and 79 ± 14% of the BDPCs on the COL microcarriers were Ki67+ on day 1, as compared to 47 ± 12% on the DBT and 54 ± 3% on the COL on day 14, with no significant differences between the groups (Figure 7B).
Figure 7.
BDPCs retain a high proliferative index over 2 weeks of culture under dynamic conditions on the DBT and COL microcarriers. (A) Representative Ki67 IF staining in rat BDPCs cultured dynamically on the DBT and COL microcarriers for 1 or 14 days within the spinner flask bioreactors, visualized through confocal microscopy. Scale bar = 100 μm. (B) Quantitative assessment of the proliferative index showing that a high percentage of the cells expressed Ki67 at both timepoints (n = 4–8 and N = 3).
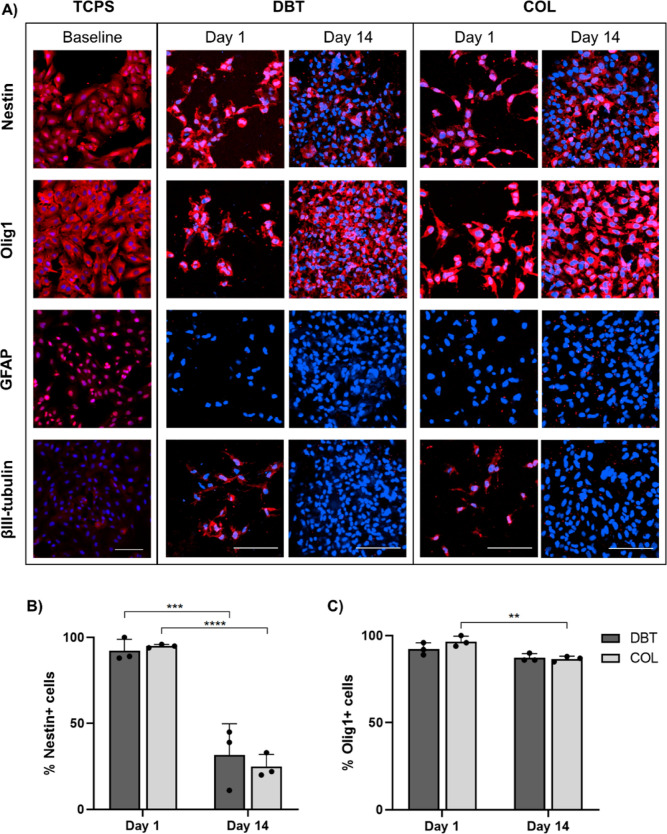
IF staining was also performed on microcarrier samples collected at days 1 and 14, along with baseline samples of the BDPCs cultured on TCPS, to assess the expression of the neural progenitor and lineage markers nestin, Olig1, glial fibrillary acidic protein (GFAP), and βIII-tubulin (Figure 8A). In the baseline samples, the BDPCs displayed robust expression of the neural progenitor marker nestin, oligodendrocyte marker Olig1, and radial glial/astrocyte marker GFAP, with minimal expression of the neuronal marker βIII-tubulin observed. At day 1, the BDPCs on both types of microcarriers expressed nestin and Olig1, but GFAP expression was no longer observed. Interestingly, cells in some regions of the microcarriers expressed βIII-tubulin after 1 day of culture within the spinner flask bioreactors, but no positive cells were detected in either microcarrier group at day 14. Manual counting using ImageJ software revealed a significant decrease in nestin+ cells on both the DBT and COL microcarriers following 2 weeks of culture (Figure 8B), with the 14 day results showing regional variability on both platforms (Figure S3). However, the cells cultured on both microcarriers retained robust expression of Olig1 at day 14, with a slight decrease in Olig1+ cells on the COL microcarriers.
Figure 8.
Dynamic culture of BDPCs on DBT and COL microcarriers modifies their expression of neural lineage markers at the protein level. (A) Representative IF staining visualized through confocal microscopy showing the presence and distribution of the neural progenitor marker nestin, oligodendrocyte marker Olig1, astrocyte marker GFAP, and neuronal marker βIII-tubulin in the rat BDPCs cultured over 2 weeks within the spinner flask bioreactors on the DBT and COL microcarriers (n = 3–5 and N = 3) relative to BDPCs cultured on TCPS as a baseline control. Prior to seeding, the BDPCs displayed robust expression of nestin, Olig1, GFAP, and βIII-tubulin. Following culture on both DBT and COL microcarriers, expression of GFAP and βIII-tubulin was not detected, while robust expression of Olig1 was observed, suggestive of differentiation toward an oligodendrocyte lineage. Scale bars = 100 μm. (B) Manual counting of nestin+ cells showing a significant decrease between days 1 and 14 on both DBT and COL microcarriers (n = 3–10 and N = 3; ***p < 0.001 and ****p < 0.0001). (C) Manual count of Olig1+ cells showing a slight decrease between days 1 and 14 on COL microcarriers, but no change was observed on DBT microcarriers (n = 3–7 and N = 3; **p < 0.005).
The positive expression of nestin and Olig1 observed in the BDPCs aligns with previous work that initially identified and characterized these cortex-derived cells.9 The predominant cell population in cultures derived from adult human brain tissues has been previously described as “glial-like” based on positive expression of GFAP.51 However, Perzelova et al. showed that a sub-population of these cells were also nestin+, indicating heterogeneity within these cultures.51 Since then, the isolation of glial progenitors from adult human cortex and subcortical regions,52,53 as well as rat and mouse cortex,54−56 has been repeatedly described. Most of these reports indicate a predisposition toward the oligodendrocyte lineage,54−57 while a few indicate the potential to give rise to both neurons and glia.52,53 The findings from the current study suggest that the BDPCs may represent a population of OPCs, which could have regenerative applications in remyelination strategies targeting SCI or multiple sclerosis.58
It is now recognized that the ECM plays a critical role in modulating cell behavior, including differentiation.11 The effects of brain-derived ECM on neural progenitor and stem cell differentiation have been studied by several groups, with a predominant focus on neuronal differentiation. For example, both Zhu et al. and Crapo et al. reported that the neuronal differentiation of human NSCs was augmented on scaffolds composed of brain-derived ECM compared to urinary bladder-derived ECM based on βIII-tubulin staining.23,25 Beachley et al. observed differentiation of mouse ESCs toward a neural progenitor phenotype following culture in a brain-derived ECM hydrogel supplemented with HA particles for 3 weeks, indicated by increased expression of nestin.59 Another study observed the maturation of human ESCs into brain organoids over 40 days in a brain-derived ECM hydrogel, indicated by the presence of both radial glial cells (SOX2+, Nestin+, and PAX6+) and mature neurons (βIII-tubulin+, DCX+, and MAP2+).28
To the best of our knowledge, the effects of the brain-derived ECM on the differentiation of glial progenitors such as OPCs have not yet been explored. Based on the IF analyses, the phenotype of the BDPCs was similar when cultured on the DBT and COL microcarriers. These findings may be consistent with the fact that both microcarrier types were enriched in type I COL and fibronectin and had similar structural and biomechanical properties. Fibronectin has been shown to promote OPC differentiation.60,61 More specifically, Hu et al. reported enhanced survival, proliferation, and differentiation of A2B5-expressing rat OPCs cultured on fibronectin coatings compared to those cultured on poly-d-lysine.60 In another study, Lourenço et al. found that culturing rat OPCs in proliferation medium in the presence of fibronectin promoted a bipolar morphology characteristic of oligodendrocyte precursors.61 Furthermore, differentiation of rat OPCs into oligodendrocytes has also been observed on type I COL microspheres.62 Taken together, these studies suggest that both type I COL and fibronectin may be important mediators of oligodendrocyte differentiation.
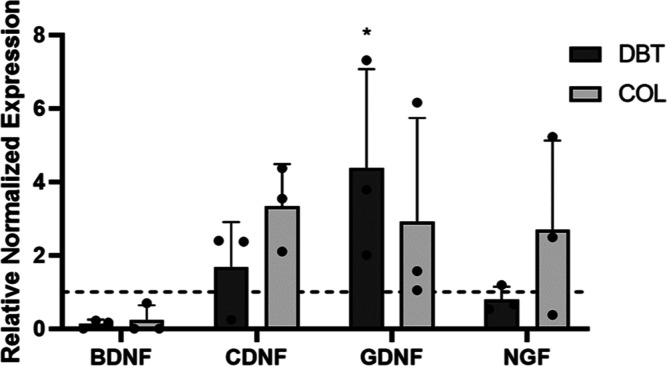
To investigate the potential effects of dynamic culture on the ECM-derived microcarriers on the expression of paracrine factors associated with neural cell types, RT-qPCR was performed to assess the gene expression levels of BDNF, CDNF, GDNF, and nerve growth factor (NGF) after 2 weeks of culture on the DBT or COL microcarriers relative to the baseline BDPCs cultured on TCPS (Figure 9). The delivery of NTFs to the brain either alone or in combination with cell-based therapies has shown promise in preclinical models, prompting further investigation in clinical trials.10 Thus, the ability to precondition cells to modulate their NTF secretion could prove beneficial for applications in neural regeneration. Culture specifically on the DBT microcarriers significantly increased GDNF gene expression levels in the BDPCs relative to the baseline samples. Notably, OPCs and newly formed oligodendrocytes have been shown to express GDNF,63 consistent with the interpretation that culturing the BDPCs on the microcarriers may help promote their differentiation toward an oligodendrocyte lineage. Interestingly, very low levels of expression of BDNF were observed following culture on the microcarriers. However, recent work has suggested that astrocytes and microglia are the main sources of BDNF production within glial cultures, rather than oligodendrocytes.63
Figure 9.
Gene expression of GDNF in the BDPCs was significantly enhanced on DBT microcarriers compared to culture on TCPS. Relative gene expression of the NTFs, BDNF, CDNF, GDNF, and NGF, in BDPCs cultured on DBT and COL microcarriers was analyzed. The hatched line indicates baseline levels in BDPCs cultured on TCPS. GDNF expression in BDPCs was significantly enhanced following culture on the DBT microcarriers relative to the baseline samples (n = 3 and N = 3; *p < 0.05).
Overall, we successfully established a detergent-free decellularization protocol for brain tissue and integrated the DBT within novel microcarriers composed exclusively of ECM that could support the attachment and growth of BDPCs under dynamic conditions within spinner flask bioreactors. This study represents important progress toward the development of cell-instructive platforms for progenitor cell expansion for applications in neural regeneration, as well as in understanding the effects of the ECM on BDPC differentiation. The in vitro studies showed a similar response with the DBT and COL microcarriers both promoting BDPC differentiation toward an oligodendrocyte lineage, although subtle differences in cell attachment and enhanced expression of GDNF in the DBT group support that the DBT did have bioactive effects. Notably, previous studies have indicated that the effects of the ECM on cell proliferation and differentiation may be concentration-dependent.21,23,24 Future studies building from this work should characterize the response with scaffolds fabricated with higher concentrations of DBT to see if there would be more significant differences between the groups, as well as comparing the effects of dynamic versus static culture conditions. In addition, the decellularization protocol and microcarrier fabrication methods resulted in a marked reduction in GAGs, which are known to regulate neural progenitor cell proliferation and differentiation.31 As such, it would be worthwhile to explore the supplementation of the microcarriers with GAGs such as HA, building from the work of Beachley et al. who showed positive effects of brain-derived ECM hydrogels supplemented with HA particles.59 Finally, it would be interesting to investigate the proliferation and differentiation of other cell populations, including human sources, on the microcarriers. For example, induced pluripotent stem cells, which are of great interest for cell-based strategies targeting a wide range of neurodegenerative diseases, would have broader differentiation potential than the BDPCs and may respond differently to the cues within the ECM.
Methods
Materials
All chemicals used were purchased from BioShop Canada Inc. (Burlington, ON, Canada) unless otherwise indicated.
Porcine Brain Tissue Collection and Preparation
Porcine brains were collected from freshly slaughtered adult pigs (∼6 months of age) at the Mount Brydges Abattoir (Mount Brydges, ON), transported to the laboratory on ice, and stored at −80 °C. To prepare for decellularization, the brains were thawed at room temperature in PBS (Wisent Inc., Saint-Jean-Baptiste, QC, Canada). In the initial studies developing the decellularization protocol, the brains were surgically dissected to isolate the cerebrum, cerebellum, and brainstem regions. For microcarrier fabrication, whole brains were processed. The tissues were minced into segments of less than 0.5 cm3 before further processing.
Porcine Brain Decellularization
All decellularization solutions were supplemented with 1% (v/v) antibiotic-antimycotic solution (Wisent Inc.) and 0.27 mM phenylmethylsulfonyl fluoride (PMSF), apart from the enzymatic digestion steps where the PMSF was excluded. All processing was performed with 100 mg starting mass of tissue in 100 mL of solution, under agitation on an orbital shaker at 120 rpm and 37 °C, unless otherwise indicated. The detergent-free brain decellularization protocol consisted of three freeze–thaw cycles (−80 °C to 37 °C) in a hypotonic buffer (pH 8.0) comprising 10 mM tris (hydroxymethyl)aminomethane (Tris) and 5 mM ethylenediaminetetraacetic acid (EDTA) in deionized H2O (dH2O), with complete solution changes between the freezing steps. Following this, the samples were incubated for 6 h in 50 mL of 0.25% trypsin–EDTA and rinsed three times for 10 min with Sorensen’s phosphate buffer rinsing solution (SPB rinse, containing 55 mM Na2HPO4 and 17 mM KH2PO4 in dH2O). The samples were then incubated for 16 h overnight under agitation at 100 rpm in 4% ethanol in dH2O supplemented with 0.1% peracetic acid, followed by three 10 min rinses with SPB rinse and a 4 h incubation in SPB digestion buffer comprising 55 mM Na2HPO4, 17 mM KH2PO4, and 4.9 mM MgSO4·7H2O in dH2O, supplemented with 15,000 U DNase type II from bovine pancreas (Sigma-Aldrich) and 12.5 mg of RNase type III from bovine pancreas (Sigma-Aldrich). Finally, the resultant DBT was rinsed three times for 10 min in SPB rinse, three times for 10 min in 70% ethanol, and three times for 10 min in dH2O and then transferred into 50 mL conical tubes, frozen at −80 °C in dH2O and lyophilized for 72 h.
Fabrication of DBT and Collagen-Based Microcarriers
Large batches of DBT were prepared by pooling brains from 8–9 pigs. Lyophilized DBT and commercially sourced COL from bovine Achilles tendon (Cat# C9879, Sigma-Aldrich) were milled following established methods.13 DBT suspensions were prepared by adding 50 mg/mL lyophilized DBT powder into 0.2 M acetic acid, and the samples were homogenized and incubated overnight at 37 °C and 120 rpm. The milled COL was digested with α-amylase following published methods19 and then similarly homogenized in 0.2 M acetic acid to generate a COL suspension. Prior to electrospraying, the DBT and COL suspensions were warmed to 37 °C in an incubator shaker and diluted to 35 mg/mL with 0.2 M acetic acid. To generate the DBT microcarriers, the DBT and COL suspensions were combined in a 1:1 ratio and homogenized. To generate the control COL microcarriers, the COL suspension was further diluted to 25 mg/mL. This concentration was selected based on preliminary testing, which indicated that the generated microcarriers would have similar Young’s moduli to control the initial biomechanical properties when assessing the effects of the ECM source on the cellular response. The suspensions were electrosprayed into liquid nitrogen following published methods,64 using a voltage of 14–15 kV and an extrusion rate of 35 mL/h. Following electrospraying, the frozen microcarriers were collected, lyophilized, and stored in absolute ethanol at 4 °C until further use. For subsequent characterization and culture studies, the microcarriers were gradually rehydrated through an ethanol series in PBS following published protocols.64
Histological Characterization of Decellularization
Native porcine brain and DBT samples were rinsed in dH2O, embedded in Optimal Cutting Temperature (OCT) Compound (Tissue-Tek, Sakura), and frozen on dry ice. The frozen samples were cryosectioned (6 μm thickness) using a Leica CM3050 S Cryostat (Germany) and stored at −20 °C. Sections were fixed in acetone at −20 °C for 10 min and stained with Fluoroshield Mounting Medium with DAPI (ab104139, Abcam) to visualize cell nuclei, toluidine blue to visualize GAGs, and picrosirius red to visualize COL, following standard protocols. Images were obtained using an EVOS FL fluorescence microscope (Thermo Fisher), an EVOS XL Core microscope (Thermo Fisher), and a Nikon Optiphot polarizing microscope (Nikon Instruments, United States), respectively.
Biochemical Characterization of ECM Composition
Biochemical assays were performed to characterize the ECM composition in the native brain, DBT, and microcarriers. Lyophilized native brain and DBT samples were finely minced, ground using a mortar and pestle in liquid nitrogen, and lyophilized. To prepare samples for PicoGreen assay, lyophilized tissue powder or rehydrated microcarriers were digested at 1 and 5 mg/mL concentrations, respectively, using the tissue lysis (ATL) buffer provided in the DNeasy Blood & Tissue Kit (Qiagen, Germany) overnight at 56 °C under agitation at 300 rpm. The samples were then sonicated using a Sonic Dismembrator model 100 (Thermo Fisher) and incubated for an additional hour, prior to thermal deactivation of the enzyme by incubation at 92 °C for 5 min. For the DMMB and hydroxyproline assays, samples were prepared, as described above using a 10 mM Tris–HCl, 1 mM EDTA (TE) buffer (pH 7.5) in place of the ATL buffer.
Quant-iT PicoGreen assay (Thermo Fisher Scientific Inc.) was performed according to the manufacturer’s protocols using a λ-DNA standard curve. In brief, 100 μL of each sample (diluted 1:2 in TE buffer) was combined with 100 μL of the Quant-iT reagent in technical triplicates, and the absorbance was read at 520 nm using a CLARIOstar microplate reader.
DMMB assay was used to quantify the sGAG content in the decellularized tissues relative to native brain controls, following published protocols, using a chondroitin sulfate standard curve.65 Briefly, 10 μL of each digested sample was combined with 200 μL of the DMMB reagent (0.016 mg/mL in 0.2% formic acid, pH 5.3) in technical triplicates, and the absorbance was read at 525 nm using the CLARIOstar microplate reader.
Hydroxyproline assay was used as a measure of the total COL content in the DBT and native tissue samples following published methods.65 In brief, 100 μL of each sample was hydrolyzed in 12 N HCl for 24 h at 110 °C and then neutralized with 5.7 N NaOH. In a 96-well plate, equal volumes of 0.05 N chloramine-T/20% 2-methoxyethanol (20 min incubation), 3.15 N perchloric acid (5 min incubation), and Ehrlich’s reagent (20 min incubation at 60 °C) were sequentially added to the digested samples in technical triplicates. Following a 5 min incubation at 4 °C and a 20 min incubation at room temperature, the absorbance was measured at 560 nm using the CLARIOstar microplate reader.
Immunofluorescence Analysis of the ECM Composition
IF analyses were performed to characterize the ECM composition within native brain, DBT, and unseeded microcarrier samples. In preparation, the samples were frozen in OCT and cryosectioned (6 μm thickness). Cryosections were fixed in acetone for 10 min at −20 °C, blocked with 10% goat serum in Tris-buffered saline (TBS) with 0.01% tween (TBST) for 1 h, and stained overnight at 4 °C with primary antibodies listed in Table 1. For hyaluronic acid staining, sections were blocked with avidin for 15 min, followed by biotin for 15 min prior to goat serum blocking. Primary and secondary antibodies were diluted in TBST with 2% BSA, which was also used in place of primary antibodies for no-primary control sections. Detection was carried out using an anti-rabbit secondary antibody conjugated to Alexa Fluor 594 (diluted 1:200, ab150080, Abcam), an anti-mouse secondary antibody conjugated to DyLight 650 (diluted 1:200, ab96882, Abcam), or a streptavidin-conjugated Alexa Fluor 555 (diluted 1:200, S32355, Thermo Fischer) for hyaluronic acid-binding protein. Images were acquired with an EVOS FL fluorescence microscope (Thermo Fisher).
Table 1. Primary Antibodies Used in IF Analysis of the ECM Composition.
| antibody | vendor | product no. | dilution |
|---|---|---|---|
| collagen I | Abcam | ab34710 | 1:100 |
| collagen IV | Abcam | ab6586 | 1:100 |
| collagen VI | Abcam | ab6588 | 1:300 |
| laminin | Abcam | ab11575 | 1:200 |
| fibronectin | Abcam | ab23750 | 1:150 |
| keratan sulfate | Santa Cruz Biotechnology | sc-73518 | 1:200 |
| brevican | Bio-Techne | MAB40091 | 1:50 |
| hyaluronic acid-binding protein | Sigma-Aldrich | H9910 | 1:100 |
Microcarrier Characterization
Microcarrier Size Distribution
Rehydrated DBT and COL microcarriers were imaged using an EVOS XL microscope (Life Technologies), and Feret’s diameter was measured using ImageJ to determine the size distribution.
Scanning Electron Microscopy
SEM imaging was performed to visualize the ECM ultrastructure in the DBT and COL microcarriers. Briefly, lyophilized microcarriers were coated with osmium and imaged with a LEO 1530 scanning electron microscope at an accelerating voltage of 20 kV and a working distance of 8.5 mm.
Mechanical Testing
Young’s moduli of the DBT and COL microcarriers were determined using a CellScale MicroTester system (Waterloo, ON, Canada) fitted with a 1.524 mm diameter cantilever. Following published protocols,64 the hydrated microcarriers were compressed to 50% of their initial diameter at a strain rate of 0.01 s–1 for three preconditioning cycles, with data collected from three consecutive cycles used to calculate Young’s moduli.
BDPC Isolation and Culture
BDPCs were isolated from rat cortical tissue using a protocol adapted from previously published work describing isolation from human brain biopsy samples.9 Briefly, fresh whole brains were obtained from 6 month old Fischer rats following euthanasia with carbon dioxide and decapitation. The outer membrane of the cortex was gently removed using sharp surgical scissors, and sections of cortex tissue were finely minced in a Petri dish in Dulbecco’s modified Eagle’s medium (DMEM) (Wisent Inc). The tissue was digested in 0.25% trypsin for 20 min at 37 °C and filtered through a 100 μm cell strainer, and the extracted cells were resuspended in complete medium [comprising DMEM supplemented with 10% fetal bovine serum (FBS) (Gibco), 1% penicillin/streptomycin, and 1% non-essential amino acids] and plated. The cells were cultured at 37 °C and 5% CO2, with medium changes every 2–3 days and passaged at 80–90% confluence. Passage 7 BDPCs were used for all in vitro culture experiments.
In Vitro Culture of BDPCs on Microcarriers
Spinner Flask Culture Studies
On the day before seeding, rehydrated DBT and COL microcarriers (100 mg dry weight/∼5.2 mg wet weight) were rinsed in 15 mL of complete medium, then resuspended in 25 mL of complete medium, transferred into 100 mL CELLSPIN flasks (INTEGRA Biosciences), and incubated overnight (37 °C, 5% CO2). For seeding, the BDPCs were trypsin-released and added to the spinner culture flasks in 25 mL of complete medium to achieve a density of 25,000 BDPCs/mg microcarriers (dry weight). To facilitate cell attachment, a 12 h intermittent stirring regimen previously established in the laboratory was performed.19 After 24 h, the medium was topped up to 100 mL, and the flasks were stirred continuously at 20 rpm for up to 2 weeks, with half media changes performed every 4–5 days.
Assessment of BDPC Attachment and Growth
BDPC attachment and growth on the DBT and COL microcarriers was assessed over 2 weeks, with triplicate samples taken at 24 h, 7 days, and 14 days. Background control samples of unseeded microcarriers from both groups were collected at day 0. At each timepoint, the samples were rinsed three times with PBS, frozen at −80 °C, and lyophilized overnight. To quantify cell growth on the microcarriers, dsDNA was extracted from the samples using the DNeasy Blood & Tissue Kit (Qiagen), following the manufacturer’s instructions. PicoGreen assay was then used to measure the total dsDNA content in each sample, as previously described. A known number of cells was used to generate a standard curve for each cell donor to report the data in terms of the cell number per mg of microcarriers. Cell-seeded microcarriers were also stained using LIVE/DEAD Viability/Cytotoxicity Assay (Cat# L3224, Invitrogen) and imaged on a Zeiss LSM800 confocal microscope (Germany).
Immunofluorescence Analysis of the BPDC Phenotype
For cell phenotype analysis, cell-seeded microcarrier samples collected at days 1 and 14 were fixed with 4% paraformaldehyde (PFA) for 24 h, rinsed three times with PBS, and stored in PBS at 4 °C until staining. Baseline BDPC samples were plated on glass coverslips, fixed with 4% PFA for 20 min, rinsed three times with PBS, and stored in PBS at 4 °C. Immediately prior to staining, all samples were permeabilized with 0.25% Triton X-100 in PBS for 15 min and blocked with 1% BSA in PBS +0.1% tween (PBST) for 1 h prior to staining overnight at 4 °C with primary antibodies listed in Table 2. Detection was carried out using an anti-mouse secondary conjugated to DyLight 650 (diluted 1:200, ab96882, Abcam) or an anti-rabbit secondary conjugated to Alexa Fluor 647 (diluted 1:500, ab150080, Abcam). Nuclei were counterstained with Hoechst 33258 (diluted 1:1000, H3569, Thermo Fischer Scientific Inc). Baseline BDPCs were mounted onto glass slides using Anti-Fade Fluoroshield Mounting Medium (ab104135, Abcam) and imaged on an EVOS FL fluorescence microscope (Thermo Fisher). For the cell-seeded microcarriers, images were acquired using the 25× water objective on a Zeiss LSM800 confocal microscope (Germany). The proliferative index was assessed by manual counting of Ki67+ cells using ImageJ. Manual counting was also performed to determine the percentage of cells expressing nestin and Olig1.
Table 2. Primary Antibodies Used in IF Analysis of BPDC Phenotype.
| antibody | vendor | product no. | dilution |
|---|---|---|---|
| Nestin | Proteintech | 66259 | 1:100 |
| βIII-tubulin | Promega | G7121 | 1:100 |
| Olig1 | Millipore Sigma | MAB5540 | 1:100 |
| Glial fibrillary acidic protein (GFAP) | Proteintech | 60190 | 1:100 |
| Ki67 | Abcam | ab16667 | 1:200 |
Gene Expression Analysis of Neurotrophic Factors
RT-qPCR was performed to analyze the gene expression levels of the NTFs, as listed in Table 3. Triplicate samples of the BDPC-seeded DBT and COL microcarriers were taken at day 14 for RNA extraction using PureZOL (Bio-Rad Laboratories). Baseline samples were also extracted from the BDPCs cultured on TCPS prior to microcarrier seeding. The microcarriers were mechanically dissociated through sonication using a model 100 Sonic Dismembrator (Fisher Scientific) in 1 s bursts. Total RNA was extracted using the Aurum Total RNA Fatty and Fibrous Tissue Kit (Bio-Rad), according to the manufacturer’s instructions. Complimentary DNA was synthesized from 500 ng of RNA using the iScript cDNA synthesis kit (Bio-Rad). For qPCR, samples were prepared using TaqMan Fast Advanced Master Mix (Thermo Fisher Scientific Inc.) with 5 ng cDNA/well. Gene expression was analyzed in using a BioRad CFX-384 system with the following amplification protocol: enzyme incubation at 50 °C for 2 min and activation at 95 °C for 10 s, followed by denaturation at 95 °C for 1 s and annealing and elongation at 60 °C for 20 s, repeated for 40 cycles. Transcript levels were analyzed using the ΔΔCT method, with normalization of each sample to the geometric mean of the housekeeping genes and using the BDPCs on TCPS as the calibrator. No reverse transcriptase and no template controls were included for all primer sets.
Table 3. Neurotrophic Genes Analyzed by RT-qPCR.
| gene | ThermoFisher Assay ID |
|---|---|
| glial cell-line derived neurotrophic factor (GDNF) | Rn01402432_m1 |
| brain-derived neurotrophic factor (BDNF) | Rn01484924_m1 |
| CDNF | Rn01765001_m1 |
| nerve growth factor (NGF) | Rn01533872_m1 |
| apolyubiquitin-C (UBC) | Rn01499642_m1 |
| ahypoxanthine-guanine phosphoribosyltransferase (HPRT1) | Rn01527840_m1 |
Indicates housekeeping gene.
Statistical Analyses
All numerical data are expressed as mean ± standard deviation (SD). All statistical analyses were performed using GraphPad Prism 8 (GraphPad Software, San Diego, CA) by unpaired t test or two-way ANOVA with Sidak or Tukey’s multiple comparison of the means. Differences of p < 0.05 were considered statistically significant.
Acknowledgments
Operational funding to support this study was provided by the Natural Sciences and Engineering Research Council (NSERC) of Canada (RGPIN-2017-04103) and a Collaborative Research Seed Grant from the Schulich School of Medicine and Dentistry at Western University. In addition, J.C.T. was supported through NSERC and OGS scholarships. The authors would like to acknowledge Todd Simpson and the Western Nanofabrication Facility for access to equipment and assistance with the SEM imaging, as well as Courtney Brooks for support with training.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.2c00232.
Rat brain decellularization; native porcine brain ECM staining; and regional nestin expression on microcarriers (PDF)
Author Contributions
This project was supported through operating grant funding awarded to L.E.F. and M.O.H. L.E.F. and J.C.T. were responsible for project conceptualization and design in collaboration with M.O.H. J.C.T. performed all experimental studies, with guidance on data analysis and interpretation provided by L.E.F. and M.O.H. J.C.T. and L.E.F. wrote the manuscript, with critical feedback provided by M.O.H.
The authors declare no competing financial interest.
Supplementary Material
References
- Gaskin J.; Gomes J.; Darshan S.; Krewski D. Burden of neurological conditions in Canada. Neurotoxicology 2017, 61, 2–10. 10.1016/j.neuro.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Horner P. J.; Gage F. H. Regenerating the damaged central nervous system. Nature 2000, 407, 963–970. 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- Mirahmadi M.; Rezanejadbardaji H.; Irfan-Maqsood M.; Mokhtari M. J.; Naderi-Meshkin H. Stem Cell Therapy for Neurodegenerative Diseases: Strategies for Regeneration against Degeneration. J. Genes Cells 2017, 3, 22. 10.15562/gnc.54. [DOI] [Google Scholar]
- Chamberlain K. A.; Nanescu S. E.; Psachoulia K.; Huang J. K. Oligodendrocyte regeneration: Its significance in myelin replacement and neuroprotection in multiple sclerosis. Neuropharmacology 2016, 110, 633–643. 10.1016/j.neuropharm.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan L.; Uyeda A.; Muramatsu R. Central nervous system regeneration: the roles of glial cells in the potential molecular mechanism underlying remyelination. Inflammation Regener. 2022, 42, 7. 10.1186/s41232-022-00193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic D.; Devito L.; Miere C.; Codognotto S. Human embryonic and induced pluripotent stem cells in clinical trials. Br. Med. Bull. 2015, 116, ldv045. 10.1093/bmb/ldv045. [DOI] [PubMed] [Google Scholar]
- Fessler R. G.; Ehsanian R.; Liu C. Y.; Steinberg G. K.; Jones L.; Lebkowski J. S.; Wirth E. D.; McKenna S. L. A phase 1/2a dose-escalation study of oligodendrocyte progenitor cells in individuals with subacute cervical spinal cord injury. J. Neurosurg. Spine 2022, 37, 812–820. 10.3171/2022.5.spine22167. [DOI] [PubMed] [Google Scholar]
- Hone I. C.Lineage Cell Therapeutics Provides Update on SCiStar Clinical Study and OPC1 Spinal Cord Injury Program, 2019.
- Xu H.; Belkacemi L.; Jog M.; Parrent A.; Hebb M. O. Neurotrophic factor expression in expandable cell populations from brain samples in living patients with Parkinson’s disease. FASEB J. 2013, 27, 4157–4168. 10.1096/fj.12-226555. [DOI] [PubMed] [Google Scholar]
- Staudt M. D.; Di Sebastiano A. R.; Xu H.; Jog M.; Schmid S.; Foster P.; Hebb M. O. Advances in neurotrophic factor and cell-based therapies for Parkinson’s disease: A mini-review. Gerontology 2016, 62, 371–380. 10.1159/000438701. [DOI] [PubMed] [Google Scholar]
- Robb K. P.; Shridhar A.; Flynn L. E. Decellularized Matrices As Cell-Instructive Scaffolds to Guide Tissue-Specific Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 3627–3643. 10.1021/acsbiomaterials.7b00619. [DOI] [PubMed] [Google Scholar]
- Bruggeman K. F.; Moriarty N.; Dowd E.; Nisbet D. R.; Parish C. L. Harnessing stem cells and biomaterials to promote neural repair. Br. J. Pharmacol. 2019, 176, 355–368. 10.1111/bph.14545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornmuller A.; Brown C. F. C.; Yu C.; Flynn L. E. Fabrication of extracellular matrix-derived foams and microcarriers as tissue-specific cell culture and delivery platforms. J. Visualized Exp. 2017, 2017, 55436. 10.3791/55436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C.; Syková E. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 1998, 21, 207–215. 10.1016/s0166-2236(98)01261-2. [DOI] [PubMed] [Google Scholar]
- Novak U.; Kaye A. H. Extracellular matrix and the brain: Components and function. J. Clin. Neurosci. 2000, 7, 280–290. 10.1054/jocn.1999.0212. [DOI] [PubMed] [Google Scholar]
- Gilpin A.; Yang Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. BioMed Res. Int. 2017, 2017, 9831534. 10.1155/2017/9831534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauleau P.; Lapouble E.; Val-Laillet D.; Malbert C. H. The pig model in brain imaging and neurosurgery. Animal 2009, 3, 1138–1151. 10.1017/S1751731109004649. [DOI] [PubMed] [Google Scholar]
- McKee C.; Chaudhry G. R. Advances and challenges in stem cell culture. Colloids Surf., B 2017, 159, 62–77. 10.1016/j.colsurfb.2017.07.051. [DOI] [PubMed] [Google Scholar]
- Yu C.; Kornmuller A.; Brown C.; Hoare T.; Flynn L. E. Decellularized adipose tissue microcarriers as a dynamic culture platform for human adipose-derived stem/stromal cell expansion. Biomaterials 2017, 120, 66–80. 10.1016/j.biomaterials.2016.12.017. [DOI] [PubMed] [Google Scholar]
- Kornmuller A.; Cooper T. T.; Jani A.; Lajoie G. A.; Flynn L. E. Probing the effects of matrix-derived microcarrier composition on human adipose-derived stromal cells cultured dynamically within spinner flask bioreactors. J. Biomed. Mater. Res., Part A 2023, 111, 415–434. 10.1002/jbm.a.37459. [DOI] [PubMed] [Google Scholar]
- Crapo P. M.; Medberry C. J.; Reing J. E.; Tottey S.; van der Merwe Y.; Jones K. E.; Badylak S. F. Biologic scaffolds composed of central nervous system extracellular matrix. Biomaterials 2012, 33, 3539–3547. 10.1016/j.biomaterials.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood D.; Chwalek K.; Stuntz E.; Pouli D.; Du C.; Tang-Schomer M.; Georgakoudi I.; Black L. D.; Kaplan D. L. Fetal Brain Extracellular Matrix Boosts Neuronal Network Formation in 3D Bioengineered Model of Cortical Brain Tissue. ACS Biomater. Sci. Eng. 2016, 2, 131–140. 10.1021/acsbiomaterials.5b00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T.; Tang Q.; Shen Y.; Tang H.; Chen L.; Zhu J. An acellular cerebellar biological scaffold: Preparation, characterization, biocompatibility and effects on neural stem cells. Brain Res. Bull. 2015, 113, 48–57. 10.1016/j.brainresbull.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Seo Y.; Jeong S.; Chung J. J.; Kim S. H.; Choi N.; Jung Y. Development of an Anisotropically Organized Brain dECM Hydrogel-Based 3D Neuronal Culture Platform for Recapitulating the Brain Microenvironment in Vivo. ACS Biomater. Sci. Eng. 2020, 6, 610–620. 10.1021/acsbiomaterials.9b01512. [DOI] [PubMed] [Google Scholar]
- Crapo P. M.; Tottey S.; Slivka P. F.; Badylak S. F. Effects of Biologic Scaffolds on Human Stem Cells and Implications for CNS Tissue Engineering. Tissue Eng., Part A 2014, 20, 313. 10.1089/ten.tea.2013.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn L. E. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials 2010, 31, 4715–4724. 10.1016/j.biomaterials.2010.02.046. [DOI] [PubMed] [Google Scholar]
- Baiguera S.; Del Gaudio C.; Lucatelli E.; Kuevda E.; Boieri M.; Mazzanti B.; Bianco A.; Macchiarini P. Electrospun gelatin scaffolds incorporating rat decellularized brain extracellular matrix for neural tissue engineering. Biomaterials 2014, 35, 1205–1214. 10.1016/j.biomaterials.2013.10.060. [DOI] [PubMed] [Google Scholar]
- Simsa R.; Rothenbücher T.; Gürbüz H.; Ghosheh N.; Emneus J.; Jenndahl L.; Kaplan D. L.; Bergh N.; Serrano A. M.; Fogelstrand P. Brain organoid formation on decellularized porcine brain ECM hydrogels. PLoS One 2021, 16, e0245685 10.1371/journal.pone.0245685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D.; Enright H. A.; Cadena J.; Peters S. K. G.; Sales A. P.; Osburn J. J.; Soscia D. A.; Kulp K. S.; Wheeler E. K.; Fischer N. O. Tissue-specific extracellular matrix accelerates the formation of neural networks and communities in a neuron-glia co-culture on a multi-electrode array. Sci. Rep. 2019, 9, 4159. 10.1038/s41598-019-40128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong A.; Kundu S.; Forsberg-Nilsson K. Heparan sulfate in the regulation of neural differentiation and glioma development. FEBS J. 2014, 281, 4993–5008. 10.1111/febs.13097. [DOI] [PubMed] [Google Scholar]
- Sirko S.; von Holst A.; Wizenmann A.; Götz M.; Faissner A. Chondroitin sulfate glycosaminoglycans control proliferation, radial glia cell differentiation and neurogenesis in neural stem/progenitor cells. Development 2007, 134, 2727–2738. 10.1242/dev.02871. [DOI] [PubMed] [Google Scholar]
- Preston M.; Sherman L. S. Neural stem cell niches: Roles for the hyaluronan-based extracellular matrix. Front. Biosci. 2011, 3, 1165. 10.2741/218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T.; Balestrini J. L.; Mendez J.; Calle E. A.; Zhao L.; Niklason L. E. Influence of pH on extracellular matrix preservation during lung decellularization. Tissue Eng., Part C 2014, 20, 1028–1036. 10.1089/ten.tec.2013.0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. Y.; Seo Y.; Davaa G.; Kim H. W.; Kim S. H.; Hyun J. K. Decellularized brain matrix enhances macrophage polarization and functional improvements in rat spinal cord injury. Acta Biomater. 2020, 101, 357–371. 10.1016/j.actbio.2019.11.012. [DOI] [PubMed] [Google Scholar]
- Reginensi D.; Ortiz D.; Pravia A.; Burillo A.; Morales F.; Morgan C.; Jimenez L.; Dave K. R.; Perez-Pinzon M. A.; Gittens R. A. Role of Region-Specific Brain Decellularized Extracellular Matrix on in Vitro Neuronal Maturation. Tissue Eng., Part A 2020, 26, 964–978. 10.1089/ten.tea.2019.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert T. W.; Sellaro T. L.; Badylak S. F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Crapo P. M.; Gilbert T. W.; Badylak S. F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane T. J.; Swinehart I. T.; Badylak S. F. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 2015, 84, 25–34. 10.1016/j.ymeth.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Silva J. C.; Carvalho M. S.; Han X.; Xia K.; Mikael P. E.; Cabral J. M. S.; Ferreira F. C.; Linhardt R. J. Compositional and structural analysis of glycosaminoglycans in cell-derived extracellular matrices. Glycoconjugate J. 2019, 36, 141–154. 10.1007/s10719-019-09858-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan S.; Gilmont R. R.; Bitar K. N. Neuroglial differentiation of adult enteric neuronal progenitor cells as a function of extracellular matrix composition. Biomaterials 2013, 34, 6649–6658. 10.1016/j.biomaterials.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoulders M. D.; Raines R. T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchiellini C.; Coulon J.; Le Parco Y. The function of type IV collagen during Drosophila muscle development. Mech. Dev. 1996, 58, 179–191. 10.1016/S0925-4773(96)00574-6. [DOI] [PubMed] [Google Scholar]
- Ali S. A.; Pappas I. S.; Parnavelas J. G. Collagen type IV promotes the differentiation of neuronal progenitors and inhibits astroglial differentiation in cortical cell cultures. Dev. Brain Res. 1998, 110, 31–38. 10.1016/S0165-3806(98)00091-1. [DOI] [PubMed] [Google Scholar]
- Gregorio I.; Braghetta P.; Bonaldo P.; Cescon M. Collagen VI in healthy and diseased nervous system. Dis. Models Mech. 2018, 11, dmm032946. 10.1242/dmm.032946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein P. J.; Higgins D.; Turner D. C.; Flier L. A.; Terranova V. P. The NC1 domain of type IV collagen promotes axonal growth in sympathetic neurons through interaction with the α1β1 integrin. J. Cell Biol. 1991, 113, 417–428. 10.1083/jcb.113.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cescon M.; Gattazzo F.; Chen P.; Bonaldo P. Collagen VI at a glance. J. Cell Sci. 2015, 128, 3525–3531. 10.1242/jcs.169748. [DOI] [PubMed] [Google Scholar]
- Goetschy J. F.; Ulrich G.; Aunis D.; Ciesielski-Treska J. Fibronectin and collagens modulate the proliferation and morphology of astroglial cells in culture. Int. J. Dev. Neurosci. 1987, 5, 63. 10.1016/0736-5748(87)90049-9. [DOI] [PubMed] [Google Scholar]
- Sood D.; Cairns D. M.; Dabbi J. M.; Ramakrishnan C.; Deisseroth K.; Black L. D.; Santaniello S.; Kaplan D. L. Functional maturation of human neural stem cells in a 3D bioengineered brain model enriched with fetal brain-derived matrix. Sci. Rep. 2019, 9, 17874. 10.1038/s41598-019-54248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven F. S. Nishihara Technique for the Solubilization of Collagen: Application to the Preparation of Soluble Collagens from Normal and Rheumatoid Connective Tissue. Ann. Rheum. Dis. 1964, 23, 300–301. 10.1136/ard.23.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha K.; Keung A. J.; Irwin E. F.; Li Y.; Little L.; Schaffer D. V.; Healy K. E. Substrate modulus directs neural stem cell behavior. Biophys. J. 2008, 95, 4426–4438. 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perzelova A.; Macikova I.; Tardy M.; Mraz P.; Bizik I.; Steno J. Subpopulation of nestin positive glial precursor cells occur in primary adult human brain cultures. Biologia 2007, 62, 633–640. 10.2478/s11756-007-0123-3. [DOI] [Google Scholar]
- Arsenijevic Y.; Villemure J. G.; Brunet J. F.; Bloch J. J.; Déglon N.; Kostic C.; Zurn A.; Aebischer P. Isolation of multipotent neural precursors residing in the cortex of the adult human brain. Exp. Neurol. 2001, 170, 48–62. 10.1006/exnr.2001.7691. [DOI] [PubMed] [Google Scholar]
- Nunes M. C.; Roy N. S.; Keyoung H. M.; Goodman R. R.; McKhann G.; Jiang L.; Kang J.; Nedergaard M.; Goldman S. A. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat. Med. 2003, 9, 439–447. 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- Dincman T. A.; Beare J. E.; Ohri S. S.; Whittemore S. R. Isolation of cortical mouse oligodendrocyte precursor cells. J. Neurosci. Methods 2012, 209, 219–226. 10.1016/j.jneumeth.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Rodríguez E. M.; Arenzana F. J.; Bribián A.; de Castro F. Protocol to isolate a large amount of functional oligodendrocyte precursor cells from the cerebral cortex of adult mice and humans. PLoS One 2013, 8, e81620 10.1371/journal.pone.0081620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Balasubramaniyan V.; Peng J.; Hurlock E. C.; Tallquist M.; Li J.; Lu Q. R. Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nat. Protoc. 2007, 2, 1044–1051. 10.1038/nprot.2007.149. [DOI] [PubMed] [Google Scholar]
- Roy N. S.; Wang S.; Harrison-Restelli C.; Benraiss A.; Fraser R. A. R.; Gravel M.; Braun P. E.; Goldman S. A. Identification, isolation, and promoter-defined separation of mitotic oligodendrocyte progenitor cells from the adult human subcortical white matter. J. Neurosci. 1999, 19, 9986–9995. 10.1523/jneurosci.19-22-09986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa N.; Takase H.; Josephine L.; Takahashi R.; Arai K. Clinical application of oligodendrocyte precursor cells for cell-based therapy. Brain Circ. 2016, 2, 121. 10.4103/2394-8108.192515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachley V.; Ma G.; Papadimitriou C.; Gibson M.; Corvelli M.; Elisseeff J. Extracellular matrix particle–glycosaminoglycan composite hydrogels for regenerative medicine applications. J. Biomed. Mater. Res., Part A 2017, 106, 147–159. 10.1002/jbm.a.36218. [DOI] [PubMed] [Google Scholar]
- Hu J.; Deng L.; Wang X.; Xu X. M. Effects of extracellular matrix molecules on the growth properties of oligodendrocyte progenitor cells in vitro. J. Neurosci. Res. 2009, 87, 2854–2862. 10.1002/jnr.22111. [DOI] [PubMed] [Google Scholar]
- Lourenço T.; Paes de Faria J.; Bippes C. A.; Maia J.; Lopes-da-Silva J. A.; Relvas J. B.; Grãos M. Modulation of oligodendrocyte differentiation and maturation by combined biochemical and mechanical cues. Sci. Rep. 2016, 6, 21563. 10.1038/srep21563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L.; Phan F.; Li Y. Collagen microsphere serving as a cell carrier supports oligodendrocyte progenitor cell growth and differentiation for neurite myelination in vitro. Stem Cell Res. Ther. 2013, 4, 109. 10.1186/scrt320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöyhönen S.; Er S.; Domanskyi A.; Airavaara M. Effects of neurotrophic factors in glial cells in the central nervous system: Expression and properties in neurodegeneration and injury. Front. Physiol. 2019, 10, 486. 10.3389/fphys.2019.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornmuller A.; Flynn L. E. Development and characterization of matrix-derived microcarriers from decellularized tissues using electrospraying techniques. J. Biomed. Mater. Res., Part A 2022, 110, 559–575. 10.1002/jbm.a.37306. [DOI] [PubMed] [Google Scholar]
- Morissette Martin P.; Grant A.; Hamilton D. W.; Flynn L. E. Matrix composition in 3-D collagenous bioscaffolds modulates the survival and angiogenic phenotype of human chronic wound dermal fibroblasts. Acta Biomater. 2019, 83, 199–210. 10.1016/j.actbio.2018.10.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.