Abstract
The aim of this study is to analyze and characterize the antimicrobial effect of cerium oxide nanoparticles (NP) synthesized using neem and ginger. Finely grounded neem and ginger powder were taken and mixed with distilled water. This mixture was then heated and filtered. Ammonium cerium nitrate dissolved in distilled water. Both the mixtures were mixed and stirred magnetically. A double-beam ultraviolet-visible spectrophotometer was used to monitor color changes. The extract was centrifuged at 8000 rpm for 15 min. The final pellet was powdered using a hot air oven at 70°C for 24 h. Visualization was done by transmission electron microscopy and spherical morphology was noted, with an average diameter of 5 nm, in aggregated form. The sample containing 100 mg of cerium oxide shows the most significant effect on the zone of inhibition of 11 mm of Staphylococcus aureus. The results obtained in the current study confirmed that CeO-NP possessed antioxidant and cytotoxic properties.
Keywords: Cerium, characterization, ginger, innovative, nanoparticles, neem
INTRODUCTION
Nanoscience and nanotechnology are emerging fields these days.[1] Nanotechnology involves materials that are made on a nanometer scale to gain characteristics and perform functions which cannot be achieved in another way.[2] The dimensions of the materials vary between 1 and 100 nanometers.[3] Optical absorption, electronic conductivity, chemical reactivity, and biocompatibility are some of the properties that undergo significant changes in nano-dimensions, in comparison with the macro-dimensions of the same material.[4]
The surface area and the number of atoms present on the surface increase extraordinarily, with the reduction in particle size, leading to a change in surface properties.[5,6] Nanotechnology currently remains in a preliminary phase ranging from basic research to industrial practice.[7] Further research in nanotechnology will benefit multiple industries.[7,8]
Cerium belongs to the lanthanide series and is a rare earth element. The crust of the earth is rich in this element, being the most abundant from the lanthanides, in spite of it being a rare earth material.[9,10,11] The ceria nanoparticles (NP), with their distinct structure, scavenge free radicals, thereby leading to cell longevity.[12,13] The coexistence of two oxidation states in cerium oxide NP greatly influences their antioxidant behavior.[14,15]
These NP can be used as antioxidants as well as antimicrobials. Cancer and metabolic syndrome are excessive reactive oxygen species diseases which can be treated.[16] The world is currently faced with multiple microorganisms that are multidrug resistant, which can be addressed by antimicrobial nature.[16,17,18]
The antimicrobial activity of neem and ginger is well researched and documented. The factors affecting the antimicrobial activity of a compound are composition, extraction method, volume of inoculum, concentration of the extract, type of pH media used, and the growth phase of the organism.[19]
Our research and knowledge have resulted in high-quality publications from our team.[20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38] Thus, the aim of this study is to analyze and characterize the antimicrobial effect of cerium oxide NP synthesized using neem and ginger.
MATERIALS AND METHODS
Preparation of neem and ginger extract
Finely grounded neem powder 0.5 g and ginger powder 0.5 g were taken and added to 100 mL of distilled water. This was then heated at 60°–80°C for 10 min. Once boiled, filtration of the mixture was done. The filtrate was then sealed and placed in the refrigerator [Figures 1 and 2].
Figure 1.

Prepared neem and ginger extract
Figure 2.

Filtrate
Preparation of cerium nanoparticle
About 0.274 g of ammonium cerium nitrate was weighed and then dissolved in 70 mL of distilled water. To this, 30 mL of filtered neem and ginger extract was incorporated. The final mixture was stirred magnetically for 2–3 days at 650–800 rpm. Uniform dispersion was thus obtained, which is a nanoparticle synthesis prerequisite. A double-beam ultraviolet-visible (UV-vis) spectrophotometer was used to detect color changes in the mixture. The final mixture was then centrifuged for 15 min at 8000 rpm. The obtained cerium oxide NP was then powdered at 70°C for 24 h in a hot air oven. It was preserved in an airtight vial to prevent contamination [Figure 3].
Figure 3.

Pellet and supernatant after centrifuge
Characterization of cerium nanoparticles
UV-vis spectrophotometer was used to measure the maximum absorbance of neem and ginger-mediated cerium oxide NP. The transmission electron microscopy analyzed the size and shape. The result showed that the CeO-NPs were spherical, and the average diameter was about 5 nm. They were also in aggregated form to some extent. Fourier-transform infrared spectroscopy analysis was done to identify the functional groups. Elemental analysis was done using an energy-dispersive X-ray detector.
Antimicrobial activity
Streptococcus mutans, Staphylococcus aureus, Enterococcus faecalis, and Candida albicans were the oral pathogens used to test the antimicrobial activity in this study, by measuring the zone of inhibition (ZI). Mueller–Hinton agar was used. The plates were prepared and sterilized for 45 min at 120 lbs. The media was then poured into the sterilized plates. It was then left stable for solidification. The wells were cut and swabbing was done with the test organisms. Cerium oxide NP of various concentrations was loaded. Incubation was done at 37°C for 24 h. The ZI was measured after the incubation time.
RESULTS
Antimicrobial activity
Cerium oxide samples of different concentrations (25, 50, and 100 mg of cerium oxide) were used to study the antimicrobial properties of cerium oxide. The sample containing 100 mg of Cerium oxide shows the most significant effect on the ZI of 11 mm of S. aureus. All the samples show a similar ZI of 9 mm for antimicrobial activity in all concentrations of cerium oxide NP. The green synthesized CeO-NPs showed significant antibacterial activity against Gram-positive bacteria (GPB) and Gram-negative bacteria (GNB). In addition, the antibody reaction was highest against E. faecalis at 40 mm. This was followed by S. mutans, S. aureus, and C. albicans at 28 mm, 22 mm, and 10 mm, respectively [Figures 4-8 and Table 1].
Figure 4.
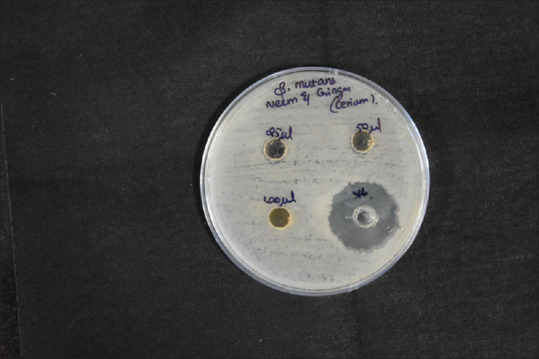
Disc diffusion for Streptococcus mutans
Figure 8.
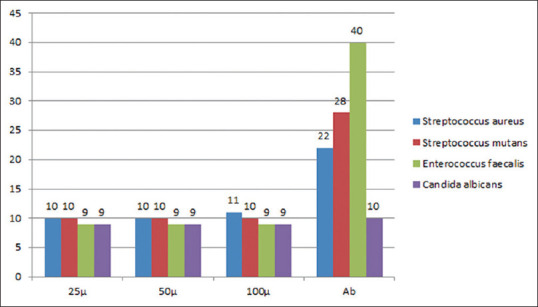
Figure depicting the zone of inhibition of Gram-positive and Gram-negative bacteria for various concentrations of cerium oxide nanoparticles
Table 1.
The zone of inhibition (mm) of Gram-positive and Gram-negative bacteria for various concentrations of cerium oxide nanoparticles
| 25 mg | 50 mg | 10 mg | Antibody | |
|---|---|---|---|---|
| Staphylococcus aureus | 10 | 10 | 11 | 22 |
| Streptococcus mutans | 10 | 10 | 10 | 25 |
| Enterococcus faecalis | 9 | 9 | 9 | 40 |
| Candida albicans | 9 | 9 | 9 | 10 |
Figure 5.
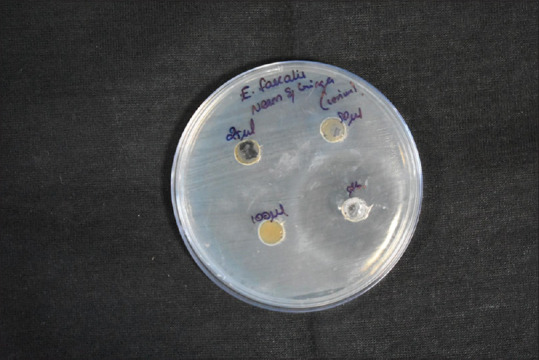
Disc diffusion for Enterococcus faecalis
Figure 6.
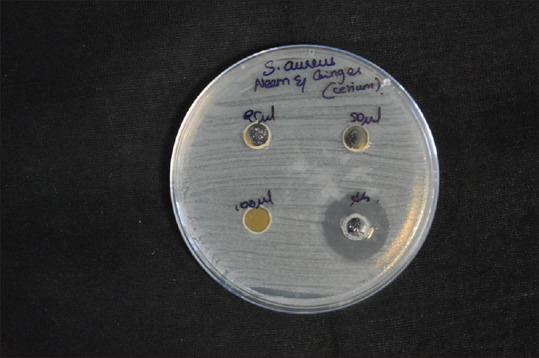
Disc diffusion for Staphylococcus aureus
Figure 7.
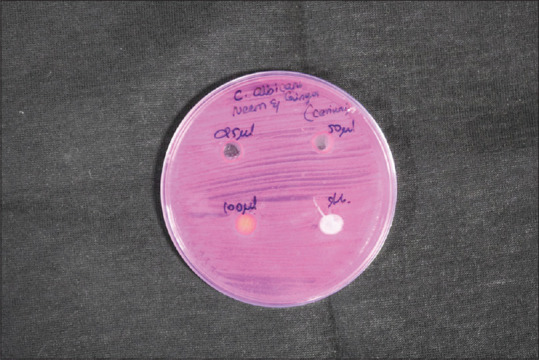
Disc diffusion for Candida albicans
DISCUSSION
The present study was done to explore the utility of NPs as a potential antibacterial agent. Since NP have less toxicity and are more heat resistant, they have multiple biological applications. Three different concentrations of cerium oxide samples were used in this study (25, 50, and 100 mg of cerium oxide). The sample containing 100 mg of cerium oxide shows the most significant effect on the ZI of 11 mm of S. aureus. All the samples show a similar ZI of 9 mm for antimicrobial activity in all concentrations of cerium oxide NP. The green synthesized CeO-NPs tested in antibacterial activity showed a significant effect on GPB and GNB. In addition, the antibody reaction was highest against E. faecalis at 40 mm. This was followed by S. mutans, S. aureus, and C. albicans at 28 mm, 22 mm, and 10 mm, respectively.
One study used a green method for the synthesis of CeO-NP using marjoram leaf extract.[39] A study on adult rat spinal cord neurons demonstrated the antioxidant and neuroprotective effect of CeO-NP.[40] The anti-inflammatory activity of this NP was similar to nitric oxide free radicals scavenging activity.[41] Fibroblasts of the skin were used to test the protective effects of these NPs and it was seen that the viability of the fibroblasts was not affected by exposure.[42] The results of this study were similar to those obtained from an antioxidant evaluation test where the synthesized CeO-NP protected the cells from oxidant-mediated apoptosis by preserving the antioxidant defense system of the cells.[43,44]
CONCLUSION
The cerium NP synthesized using neem and ginger extract by green method possessed both antioxidant and cytotoxic properties.
Financial support and sponsorship
The present study is funded by the:
Saveetha Institute of Medical and Technical Sciences
Saveetha Dental College and Hospitals
Saveetha University
Satyam Enterprises, Chennai.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors acknowledge Saveetha University for all the help and support.
REFERENCES
- 1.Kost B, Gonciarz W, Krupa A, Socka M, Rogala M, Biela T, et al. pH-tunable nanoparticles composed of copolymers of lactide and allyl-glycidyl ether with various functionalities for the efficient delivery of anti-cancer drugs. Colloids and Surfaces B: Biointerfaces. 2021;204:111801. doi: 10.1016/j.colsurfb.2021.111801. [DOI] [PubMed] [Google Scholar]
- 2.Nouailhat A. An introduction to nanoscience and nanotechnology. John Wiley & Sons; 2010. [Google Scholar]
- 3.Kushali R, Maiti S, Girija SAS, Jessy P. Evaluation of Microbial Leakage at Implant Abutment Interfact for Different Implant Systems: An In Vitro Study? J Long Term Eff Med Implants. 2022;32:87–93. doi: 10.1615/JLongTermEffMedImplants.2022038657. doi: 10.1615/JLongTermEffMedImplants. [DOI] [PubMed] [Google Scholar]
- 4.Kaiser M, Kurath M, Maasen S, Rehmann-Sutter C, editors. Springer Science & Business Media; 2009. Governing future technologies: nanotechnology and the rise of an assessment regime. [Google Scholar]
- 5.Wei F, Neal CJ, Sakthivel TS, Kean T, Seal S, Coathup MJ. Multi-functional cerium oxide nanoparticles regulate inflammation and enhance osteogenesis. Mater Sci Eng C Mater Biol Appl. 2021;124:112041. doi: 10.1016/j.msec.2021.112041. [DOI] [PubMed] [Google Scholar]
- 6.Yadav N, Singh S. Polyoxometalate-mediated vacancy-engineered cerium oxide nanoparticles exhibiting controlled biological enzyme-mimicking activities. Inorganic Chemistry. 2021;60:7475–89. doi: 10.1021/acs.inorgchem.1c00766. [DOI] [PubMed] [Google Scholar]
- 7.Casals G, Perramón M, Casals E, Portolés I, Fernández-Varo G, Morales-Ruiz M, et al. Cerium oxide nanoparticles: A new therapeutic tool in liver diseases. Antioxidants (Basel) 2021;10:660. doi: 10.3390/antiox10050660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miri A, Beiki H, Najafidoust A, Khatami M, Sarani M. Cerium oxide nanoparticles: Green synthesis using Banana peel, cytotoxic effect, UV protection and their photocatalytic activity. Bioprocess Biosyst Eng. 2021;44:1891–9. doi: 10.1007/s00449-021-02569-9. [DOI] [PubMed] [Google Scholar]
- 9.Goujon G, Baldim V, Roques C, Bia N, Seguin J, Palmier B, et al. Antioxidant activity and toxicity study of cerium oxide nanoparticles stabilized with innovative functional copolymers. Adv Healthc Mater. 2021;10:e2100059. doi: 10.1002/adhm.202100059. [DOI] [PubMed] [Google Scholar]
- 10.Mahato JK, Gupta SK. Exceptional adsorption of different spectral indices of natural organic matter (NOM) by using cerium oxide nanoparticles (CONPs) Environ Sci Pollut Res Int. 2021;28:45496–505. doi: 10.1007/s11356-021-13964-w. [DOI] [PubMed] [Google Scholar]
- 11.Asgharzadeh F, Hashemzadeh A, Rahmani F, Yaghoubi A, Nazari SE, Avan A, et al. Cerium oxide nanoparticles acts as a novel therapeutic agent for ulcerative colitis through anti-oxidative mechanism. Life Sci. 2021;278:119500. doi: 10.1016/j.lfs.2021.119500. [DOI] [PubMed] [Google Scholar]
- 12.Milenković I, Radotić K, Trifković J, Vujisić L, Beškoski VP. Screening of semi-volatile compounds in plants treated with coated cerium oxide nanoparticles by comprehensive two-dimensional gas chromatography. J Sep Sci. 2021;44:2260–8. doi: 10.1002/jssc.202100145. [DOI] [PubMed] [Google Scholar]
- 13.Chai WF, Tang KS. Protective potential of cerium oxide nanoparticles in diabetes mellitus. J Trace Elem Med Biol. 2021;66:126742. doi: 10.1016/j.jtemb.2021.126742. [DOI] [PubMed] [Google Scholar]
- 14.Sanders S, Golden TD. Functionalization of cerium oxide nanoparticles to influence hydrophobic properties. Langmuir. 2019;35:5841–7. doi: 10.1021/acs.langmuir.9b00201. [DOI] [PubMed] [Google Scholar]
- 15.Rzigalinski BA, Carfagna CS, Ehrich M. Cerium oxide nanoparticles in neuroprotection and considerations for efficacy and safety. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9:e1444. doi: 10.1002/wnan.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suri S. Synthesis, Structural and Magnetic Properties of Copper Doped Cerium Oxide Nanoparticles. West Virginia University; 2010. [Google Scholar]
- 17.Eriksson P. Cerium Oxide Nanoparticles and Gadolinium Integration: Synthesis, Characterization and Biomedical Applications. Linköping University Electronic Press; 2019. [Google Scholar]
- 18.Maiti S. Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University Chennai, Nadu T, India. Data on dental bite materials with stability and displacement under load [Internet] Bioinformation. 2020;16:1145–51. doi: 10.6026/973206300161145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh S, Kumar U, Gittess D, Sakthivel TS, Babu B, Seal S. Cerium oxide nanomaterial with dual antioxidative scavenging potential: Synthesis and characterization. J Biomater Appl. 2021;36:834–42. doi: 10.1177/08853282211013451. [DOI] [PubMed] [Google Scholar]
- 20.Abdul Wahab PU, Senthil Nathan P, Madhulaxmi M, Muthusekhar MR, Loong SC, Abhinav RP. Risk factors for post-operative infection following single piece osteotomy. J Maxillofac Oral Surg. 2017;16:328. doi: 10.1007/s12663-016-0983-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thanikodi S, Singaravelu DK, Devarajan C, Venkatraman V, Rathinavelu V. Teaching learning optimization and neural network for the effective prediction of heat transfer rates in tube heat exchangers. Thermal Science. 2020;24(1 Part B):575–81. [Google Scholar]
- 22.Subramaniam N, Muthukrishnan A. Oral mucositis and microbial colonization in oral cancer patients undergoing radiotherapy and chemotherapy: A prospective analysis in a tertiary care dental hospital. J Investig Clin Dent. 2019;10:e12454. doi: 10.1111/jicd.12454. [DOI] [PubMed] [Google Scholar]
- 23.Kumar SP, GIRIJA AS, Priyadharsini JV. Targeting NM23-H1-mediated inhibition of tumour metastasis in viral hepatitis with bioactive compounds from Ganoderma lucidum: A computational study. Indian Journal of Pharmaceutical Sciences. 2020;82:300–5. [Google Scholar]
- 24.Manickam A, Devarasan E, Manogaran G, Priyan MK, Varatharajan R, Hsu CH, et al. Score level based latent fingerprint enhancement and matching using SIFT feature. Multimedia Tools and Applications. 2019;78:3065–85. [Google Scholar]
- 25.Ravindiran M, Praveenkumar C. Status review and the future prospects of CZTS based solar cell–A novel approach on the device structure and material modeling for CZTS based photovoltaic device. Renewable and Sustainable Energy Reviews. 2018;94:317–29. [Google Scholar]
- 26.Vadivel JK, Govindarajan M, Somasundaram E, Muthukrishnan A. Mast cell expression in oral lichen planus: A systematic review. J Investig Clin Dent. 2019;10:e12457. doi: 10.1111/jicd.12457. [DOI] [PubMed] [Google Scholar]
- 27.Ma Y, Karunakaran T, Veeraraghavan VP, Mohan SK, Li S. Sesame inhibits cell proliferation and induces apoptosis through inhibition of STAT-3 translocation in thyroid cancer cell lines (FTC-133) Biotechnology and Bioprocess Engineering. 2019;24:646–52. [Google Scholar]
- 28.Mathivadani V, Smiline AS, Priyadharsini JV. Targeting Epstein-Barr virus nuclear antigen 1 (EBNA-1) with Murraya koengii bio-compounds: An in-silico approach. Acta Virol. 2020;64:93–9. doi: 10.4149/av_2020_111. [DOI] [PubMed] [Google Scholar]
- 29.Happy A, Soumya M, Venkat Kumar S, Rajeshkumar S, Sheba RD, Lakshmi T, et al. Phyto-assisted synthesis of zinc oxide nanoparticles using Cassia alata and its antibacterial activity against Escherichia coli. Biochem Biophys Rep. 2019;17:208–11. doi: 10.1016/j.bbrep.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prathibha KM, Johnson P, Ganesh M, Subhashini AS. Evaluation of salivary profile among adult type 2 diabetes mellitus patients in South India. J Clin Diagn Res. 2013;7:1592–5. doi: 10.7860/JCDR/2013/5749.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paramasivam A, Vijayashree Priyadharsini J. Novel insights into m6A modification in circular RNA and implications for immunity. Cell Mol Immunol. 2020;17:668–9. doi: 10.1038/s41423-020-0387-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponnanikajamideen M, Rajeshkumar S, Vanaja M, Annadurai G. In vivo type 2 diabetes and wound-healing effects of antioxidant gold nanoparticles synthesized using the insulin plant Chamaecostus cuspidatus in albino rats. Can J Diabetes. 2019;43:82–9.e6. doi: 10.1016/j.jcjd.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Vijayashree Priyadharsini J, Smiline Girija AS, Paramasivam A. In silico analysis of virulence genes in an emerging dental pathogen A. baumannii and related species. Arch Oral Biol. 2018;94:93–8. doi: 10.1016/j.archoralbio.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Anita R, Paramasivam A, Priyadharsini JV, Chitra S. The m6A readers YTHDF1 and YTHDF3 aberrations associated with metastasis and predict poor prognosis in breast cancer patients. Am J Cancer Res. 2020;10:2546–54. [PMC free article] [PubMed] [Google Scholar]
- 35.Ponnanna AA, Maiti S, Rai N, Jessy P. Three-dimensional-printed Malo bridge: Digital fixed prosthesis for the partially edentulous maxilla. Contemp Clin Dent. 2021;12:451–3. doi: 10.4103/ccd.ccd_456_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merchant A, Ganapathy DM, Maiti S. Effectiveness of local and topical anesthesia during gingival retraction: Anesthesia during cord packing. Brazilian Dental Science. 2022:25. [Google Scholar]
- 37.Agarwal S, Ashok V, Maiti S. Open- or closed-tray impression technique in implant prosthesis: A dentist's perspective. J Long Term Eff Med Implants. 2020;30:193–8. doi: 10.1615/JLongTermEffMedImplants.2020035933. [DOI] [PubMed] [Google Scholar]
- 38.Aparna J, Maiti S, Jessy P. Polyether ether ketone – As an alternative biomaterial for Metal Richmond crown-3-dimensional finite element analysis. J Conserv Dent. 2021;24:553–7. doi: 10.4103/jcd.jcd_638_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aseyd Nezhad S, Es-haghi A, Tabrizi MH. Green synthesis of cerium oxide nanoparticle using Origanum majorana L.leaf extract, its characterization and biological activities. Applied Organometallic Chemistry. 2020;34:e5314. [Google Scholar]
- 40.Das M, Patil S, Bhargava N, Kang JF, Riedel LM, Seal S, et al. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials. 2007;28:1918–25. doi: 10.1016/j.biomaterials.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dowding JM, Dosani T, Kumar A, Seal S, Self WT. Cerium oxide nanoparticles scavenge nitric oxide radical (˙NO) Chem Commun (Camb) 2012;48:4896–8. doi: 10.1039/c2cc30485f. [DOI] [PubMed] [Google Scholar]
- 42.Pezzini I, Marino A, Del Turco S, Nesti C, Doccini S, Cappello V, et al. Cerium oxide nanoparticles: The regenerative redox machine in bioenergetic imbalance. Nanomedicine (Lond) 2017;12:403–16. doi: 10.2217/nnm-2016-0342. [DOI] [PubMed] [Google Scholar]
- 43.Akhtar MJ, Ahamed M, Alhadlaq HA, Khan MA, Alrokayan SA. Glutathione replenishing potential of CeO₂ nanoparticles in human breast and fibrosarcoma cells. J Colloid Interface Sci. 2015;453:21–7. doi: 10.1016/j.jcis.2015.04.049. [DOI] [PubMed] [Google Scholar]
- 44.Bhushan B, Gopinath P. Antioxidant nanozyme: A facile synthesis and evaluation of the reactive oxygen species scavenging potential of nanoceria encapsulated albumin nanoparticles. J Mater Chem B. 2015;3:4843–52. doi: 10.1039/c5tb00572h. [DOI] [PubMed] [Google Scholar]


