Abstract
Kaempferia galanga rhizome (KGR) is a stemless, sweet-smelling, enduring, and rhizomatous monocotyledonous plant of the ginger family also called sand ginger or kencur. lt possesses many pharmacological effects and the goal of this study is to use several solvent solutions to determine the antioxidant and anti-diabetic potential of KGR. By observing KGR's alpha-glucosidase and alpha-amylase inhibitory activity, the in vitro diabetic activity was assessed. The in vitro cancer preventive action was evaluated by doing the 2,2-diphenyl-1-picrylhydrazyl test. The outcomes of the study showed increase in dose-dependent percentage of inhibition in α-amylase and α-glucosidase activity for both extract and standard ranging from 100 to 500 μg/ml. The current review proposes that the concentrate of KGR could be utilized for the treatment of diabetes mellitus as well with respect to dealing with the oxidative stress-related diseases.
Keywords: Alpha-amylase, alpha-glucosidase, Kaempferia galanga rhizome
INTRODUCTION
Kaempferia galanga rhizome (KGR) is a native spice which is local to India and found in different districts such as China, Bangladesh, Sri Lanka, Sudan, Myanmar, Indonesia, Vietnam, Laos, Sudan, Nigeria, Japan, and South Africa.[1] KGR has a bioactive compound called isopimarane and diterpenoids which is responsible for its anti-inflammatory effect.[2] KGR is an important functional food because of its ameliorative protective potential.[3] The antitumor potential of the polysaccharides of KGR can be studied by different structural characterization techniques[4] In the current study, ethanolic extract proved to give the maximum efficiency when compared to aqueous extract. Free radicals at very high levels are a constant threat to the body. They are inevitable by-products produced by the body.[5] The production of free radical in the body leads to oxidation-related stressors, damage to the genetic material, cause circulation of low-density cholesterol, and also lead to chronic diseases.[6] The leaves of KGR have CNS depressant properties and demonstrate its utilization as a customary medication[7] The anti-inflammatory effect of KGR is exerted because of the inhibition of cyclooxygenases 1 and 2.[8] Literature studies have revealed that the antioxidant and antidiabetic effect of KGR extract using different solvent systems was not scientifically documented earlier. Hence, the current study plans to create some scientific evidence. Modern medicine for oxidative stress is prone to many complications/side effects. Therefore, the search for herbal medicine is worldwide now.[9,10,11,12,13,14,15,16,17,18,19,20,21] Subsequently, the study proposed to find the antidiabetic activity of the KGR using different dissolvable fluids.
METHODOLOGY
The Reagents were obtained from Sigma Chemicals, Biolabs Promega, New England and Eurofins Genomics India Pvt Ltd.
Evaluation of the inhibitory effects of alpha-amylase on in vitro antidiabetic activity
The antidiabetic potential was observed using alpha-amylase inhibitory activity (Ademiluyi and Oboh, 2013).[22] A blend of reagents was taken in a 96 plate well. The blend contained ten microliter alpha-amylase, 50 pl phosphate cushion, and 20 pl of concentrations of plant extract. The blend was preincubated for 20 min at 37°C. Then, at that point, a substrate, on the addition of 20 pl of l% dissolvable starch and it was brooded further at 37° C for 30 min. Next 100 pl of the DNS disguising reagent was added to the above blend and kept for 10 min. At 540 nm the absorbance of the following blend was surveyed. It was evaluated with the assistance of a Multiplate Reader (Robonik). For standard, Acarbose at different focuses was utilized. Percentage inhibition was taken into consideration to express the results of the study.
Alpha-glucosidase inhibitory movement
Alpha-glucosidase inhibitory potential was determined with minor modification of technique of Shai et al., in 2011.[23] A mix containing the reagents were set in a 96-well plate. The reaction mix included 10 pl alpha-glucosidase (I U/ml), 50 pl phosphate support (100 mM, pH = fi. S), and 20 pl of moving centralizations of plant isolated (0.1 to 0.5 mg/ml) which was pre-brought forth for 15 min at 37°C. Then, as a substrate 20 pl P-NPG (5 mM) was added and on brood for a time of 20 min at 37°C. By the addition of 50 pl Na2 CO3 (0.1 M), the reaction was stopped. The absorbance of the mixture is measured at 405 nm. Acarbose was taken as a standard concentrate at various obsessions ranging from 0.1 to 0.5 mg/ml.. Each test was acted in sets of three. The percentage of percentage was taken up using the formulation 1-optical density of test divided by the optical density of control multiplied by percentage for 100.
2,2-diphenyl-1-picrylhydrazyl assay
Liyana Pathirana, Shahidi, and Kikuzaki and Nakatan, 1993, carried out the 2,2-diphenyl-1-picrylhydrazyl (DPPH)-free radical scavenging assay.[24] 200 μl of 0.1 μM solution of 2,2-diphenyl-1-picrylhydrazyl coordinated in solution of methanol was added to 100 μl of KGR compounds with a similar dilution concentration used in other assays. For 15 min, the resultant mixture was placed at room temperature without any light around. At 517 nm, absorbance was observed. The positive control was BHT. The experiment was done three times and value obtained was analyzed. The inhibition percentage of 2,2-diphenyl-1-picrylhydrazyl is taken up from the formulation as optical density of control subtracted optical density of sample divided by optical density of control and finally multiplying with percentage for 100.
Methods in statistics
Obtained data were thoroughly investigated in the method of one-way ANOVA. The Multiple Reach tests by Duncan have been opted. Then, P was accounted to < 0.05.
RESULTS
The findings of the study showed that there was a dose-related higher inhibition percentage in alpha-amylase concentration for both extract and standard ranging from 100 to 500 μg/ml. The maximum anti-diabetic activity both the extract and standard drug is observed at 500 μg/ml [Figure 1]. The findings of the study showed that the observation of dose-related higher inhibition in the inhibition of alpha-glucosidase activity for both extract and standard ranging from 100 to 500 μg/ml. The maximum anti-diabetic activity of both the extract and standard drug is observed at 500 μg//ml [Figure 2]. The findings of the study showed that there was a dose-dependent increase in the percentage of inhibition of DPPH concentration for both extract and standard ranging from 100 to 500 μg/ml. The maximum antioxidant activity by both the extract and standard drug is observed at 500 μg/ml [Figure 3].
Figure 1.
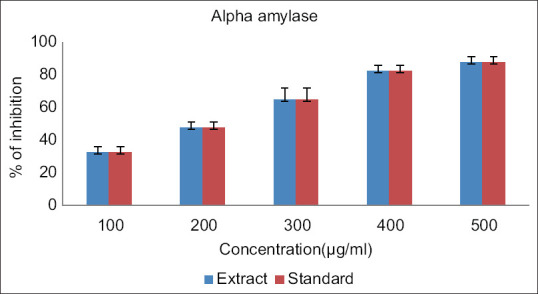
Alpha amylase inhibitory activity of Kaempferia galanga. The above reference chart addresses the relationship between level of alpha amylase with the groupings of ethanolic concentrate of KGR along with Acarbose. X hub addresses the groupings of ethanolic concentrates of KGR and standard from 100 μg for each ml to 500 μg for every ml. The Y hub addresses the rate of alpha amylase protein by both the concentrate and standard. The blue colour denotes the KGR extract while the red colour denotes the standard drug Acarbose. Every bar addresses the mean ± SD of 3 observations. Signficance P < 0.05. SD: Standard deviation, KGR: Kaempferia galanga rhizome
Figure 2.
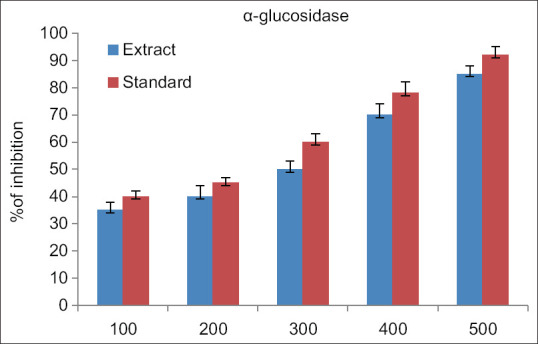
Alpha glucosidase inhibitory activity of Kaempferia galanga extract. The above reference chart addresses the relationship between level of alpha glucosidase with ethanolic concentrates of KGR along with Acarbose. X pivot addresses the ethanolic concentrates of KGR and standard from 100 μg for every ml to 500 μg for each ml. The Y pivot addresses the rate of alpha glucosidase by both the concentrate and standard. The blue color denotes the KGR extract while the red color denotes the standard drug Acarbose. Every bar addresses the mean with SD for 3 observations. P < 0.05. SD: Standard deviation, KGR: Kaempferia galanga rhizome
Figure 3.
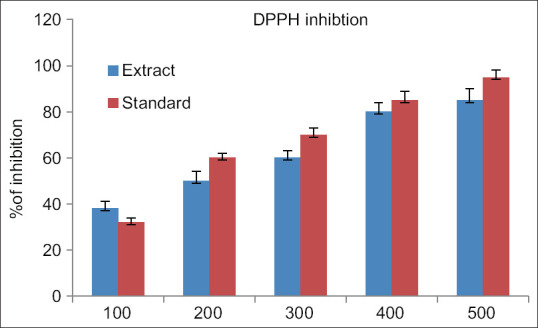
2,2 diphenyl 1 picrylhydrazyl radical inhibition of Kaempferia galanga extract. The above reference chart addresses the relationship between percentage inhibition of DPPH with ethanolic concentrates of KGR. X pivot addresses the concentrations of ethanolic extract of KGR and standard from 100 μg for each ml to 500 μg for every ml. The Y pivot addresses percentage inhibition of DPPH radical by both the extract and standard. Blue colour denotes the KGR extract while the red colour denotes the standard drug Vitamin C. The figure addresses the mean with the SD of 3 observations. P value is <0.05 is considered. SD: Standard deviation, KGR: Kaempferia galanga rhizome, DPPH: 2,2-diphenyl-1-picrylhydrazyl
DISCUSSION
In India, Kaempferia galanga is a vitally important plant that is frequently used to treat a wide range of illnesses. The rhizomes of Kaempferia galanga have been shown to contain a variety of bioactive phytochemicals, including flavonoids, esters, cyclic depsipeptide, polysaccharides, diarylheptanoids, terpenoids, glycoside, and phenolic acids. These phytochemicals are especially active biologically. The concentrate's amazing resistance to diabetic action was demonstrated by the presence of flavonoids. The anti-oxidant and anti-diabetic effects of KGR is attributed also to the presence of phenolic compounds as revealed by GCMS analysis supports the current finding.
The present study did not find any authentic information about the oxidant reducing power of KGR extract with that of the standard drug (Vitamin C). Despite our disclosures, a previous report by Narasinga et al. proposed that the KGR showed less anti-oxidant activity when compared to ascorbic acid.[25]
Similar to our study, a prior investigation into the anti-infective and anti-cancer properties of methanol induced concentrates of KGR (MGKR) revealed that the plant offered recognisable source of anti-cancer compounds.[26] Another survey supporting the concept that Kaempferia galanga L leaves has antioxidant and cytotoxicity effects. Along these lines, it maintains KGR use for various clinical purpose.[27]
Similar findings were discovered in a survey conducted by Yao et al. in the year 2013 that indicated certain phenolic combinations must be related to the presence of antioxidant activity of KGR.[28,29,30,31,32,33,34] The potential pharmacological effects caused by intake of KGR is attributed to the bioactive compounds such as phenols and flavonoids etc.[35] The survey's findings confirmed those of an earlier report, which also noted KGR's potential for antioxidant and antibacterial activity.[36] Regarding KGR's ability to combat diabetes, the current study prompted an in vitro evaluation and determined that KGR can be taken into consideration for treating diabetes-related confusion. Similar findings were observed in in vivo studies including Wistar rats and albino rats that revealed supportive document about the pharmacological profile of KGR.
CONCLUSION
The present analysis suggests that KGR had a dose-dependent ability to effectively scavenge free radicals. It has greater antioxidant action as a result. The fact that KGR extract effectively inhibited alpha amylase and alpha-glucosidase activity indicates that it may have anti-diabetic properties. Accordingly, the study implies that KGR may be used to treat diabetes mellitus as well as to control oxidative stress and the illnesses associated with it. KGR should also undergo in vivo tests to determine its anti-diabetic and antioxidant properties before being released onto the market as a potential anti-diabetic and antioxidant medication.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kumar A. Phytochemistry, pharmacological activities and uses of traditional medicinal plant Kaempferia galanga L.– An overview. J Ethnopharmacol. 2020;253:112667. doi: 10.1016/j.jep.2020.112667. [DOI] [PubMed] [Google Scholar]
- 2.Khuntia S, Sahoo D, Kar B, Sahoo S. Metabolic profiling of Kaempferia galanga leaf and rhizome extract using GC-MS. J Appl Adv Res. 2022;7:35–41. [Google Scholar]
- 3.Srivastava N, Mishra S, Iqbal H, Chanda D, Shanker K. Standardization of Kaempferia galanga L. Rhizome and vasorelaxation effect of its key metabolite ethyl p-methoxycinnamate. J Ethnopharmacol. 2021;271:113911. doi: 10.1016/j.jep.2021.113911. [DOI] [PubMed] [Google Scholar]
- 4.Das D, Jayaram Kumar K. Enhancing resilient property of Kaempferia galanga rhizome starch by succinylation. Int J Biol Macromol. 2019;124:1033–9. doi: 10.1016/j.ijbiomac.2018.11.182. [DOI] [PubMed] [Google Scholar]
- 5.Yang X, Ji H, Feng Y, Yu J, Liu A. Structural Characterization and Antitumor Activity of Polysaccharides from Kaempferia galanga L. Oxid Med Cell Longev. 2018;2018:9579262. doi: 10.1155/2018/9579262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridtitid W, Sae-Wong C, Reanmongkol W, Wongnawa M. Antinociceptive activity of the methanolic extract of Kaempferia galanga Linn.In experimental animals. J Ethnopharmacol. 2008;118:225–30. doi: 10.1016/j.jep.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Ali MS, Dash PR, Nasrin M. Study of sedative activity of different extracts of Kaempferia galanga in Swiss albino mice. BMC Complement Altern Med. 2015;15:158. doi: 10.1186/s12906-015-0670-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ariffin NS. Anti-inflammatory Effect of Kaempferia Galanga in Rats. Diss. Universiti Putra Malaysia. 2008 [Google Scholar]
- 9.Saraswathi I, Saikarthik J, Senthil Kumar K, Madhan Srinivasan K, Ardhanaari M, Gunapriya R. Impact of COVID-19 outbreak on the mental health status of undergraduate medical students in a COVID-19 treating medical college: A prospective longitudinal study. Peer J. 2020;8:e10164. doi: 10.7717/peerj.10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santhakumar P, Roy A, Mohanraj KG, Jayaraman S, Durairaj R. Ethanolic Extract of Capparis decidua fruit ameliorates methotrexate-induced hepatotoxicity by activating Nrf2/HO-1 and PPARγ mediated pathways. Ind J Pharm Educ. 2021;55:s265–74. [Google Scholar]
- 11.Nambi G, Kamal W, Es S, Joshi S, Trivedi P. Spinal manipulation plus laser therapy versus laser therapy alone in the treatment of chronic non-specific low back pain: A randomized controlled study. Eur J Phys Rehabil Med. 2018;54:880–9. doi: 10.23736/S1973-9087.18.05005-0. [DOI] [PubMed] [Google Scholar]
- 12.Egbuna C, Mishra AP, Goyal MR. 1st ed. Academic Press; 2020. Preparation of Phytopharmaceuticals for the Management of Disorders: The Development of Nutraceuticals and Traditional Medicine; pp. 96–115. ISBN: 9780128202845. [Google Scholar]
- 13.Kamath SM, Manjunath Kamath S, Jaison D, Rao SK, Sridhar K, Kasthuri N, et al. In vitro augmentation of chondrogenesis by Epigallocatechin gallate in primary Human chondrocytes - Sustained release model for cartilage regeneration. Journal of Drug Delivery Science and Technology. 2020;60:101992. Available from: https://doi.org/10.1016/j.jddst.2020.101992. [Google Scholar]
- 14.Barabadi H, Mojab F, Vahidi H, Marashi B, Talank N, Hosseini O, et al. Green synthesis, characterization, antibacterial and biofilm inhibitory activity of silver nanoparticles compared to commercial silver nanoparticles. Inorg Chem Commun. 2021;129:108647. [Google Scholar]
- 15.Bharath B, Perinbam K, Devanesan S, AlSalhi MS, Saravanan M. Evaluation of the anticancer potential of hexadecanoic acid from brown algae Turbinaria ornata on HT–29 colon cancer cells. J Mol Struct. 2021;1235:130229. [Google Scholar]
- 16.Gowhari Shabgah A, Ezzatifar F, Aravindhan S, Olegovna Zekiy A, Ahmadi M, Gheibihayat SM, et al. Shedding more light on the role of Midkine in hepatocellular carcinoma: New perspectives on diagnosis and therapy. IUBMB Life. 2021;73:659–69. doi: 10.1002/iub.2458. [DOI] [PubMed] [Google Scholar]
- 17.Sridharan G, Ramani P, Patankar S, Vijayaraghavan R. Evaluation of salivary metabolomics in oral leukoplakia and oral squamous cell carcinoma. J Oral Pathol Med. 2019;48:299–306. doi: 10.1111/jop.12835. [DOI] [PubMed] [Google Scholar]
- 18.Hannah R, Ramani P, Ramanathan A, JancyMerlin R, Gheena S, Ramasubramanian A, et al. CYP2 C9 polymorphism among patients with oral squamous cell carcinoma and its role in altering the metabolism of benzo[a] pyrene. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;130:306–12. doi: 10.1016/j.oooo.2020.06.021. [DOI] [PubMed] [Google Scholar]
- 19.PradeepChristopher J, Marimuthu T, Krithika C, Devadoss P, Kumar SM. Prevalence and measurement of anterior loop of the mandibular canal using CBCT: A cross sectional study. Clin Implant Dent Relat Res. 2018;20:531–4. doi: 10.1111/cid.12609. [DOI] [PubMed] [Google Scholar]
- 20.Wahab PU, Madhulaxmi M, Senthilnathan P, Muthusekhar MR, Vohra Y, Abhinav RP. Scalpel versus diathermy in wound healing after mucosal incisions: A split-mouth study. J Oral Maxillofac Surg. 2018;76:1160–4. doi: 10.1016/j.joms.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Mudigonda SK, Murugan S, Velavan K, Thulasiraman S, Krishna Kumar Raja VB. Non-suturing microvascular anastomosis in maxillofacial reconstruction – A comparative study. J Craniomaxillofac Surg. 2020;48:599–606. doi: 10.1016/j.jcms.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Ademiluyi AO, Oboh G. Soybean phenolic-rich extracts inhibit key-enzymes linked to type 2 diabetes (a-amylase and a-glucosidase) and hypertension (angiotensin I converting enzyme) in-vitro. Exp Toxicol Pathol. 2013;65:305–19. doi: 10.1016/j.etp.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Shai LJ, Magano SR, Lebelo SL, Mogale AM. Inhibitory effects of five medicinal plants on rat alpha-glucosidase: Comparison with their effects on yeast alpha-glucosidase. J Med Plant Res. 2011;5:2863–67. [Google Scholar]
- 24.Liyana-Pathirana CM, Shahidi F. Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L.) as affected by gastric pH conditions. J Agric Food Chem. 2005;53:2433–2440. doi: 10.1021/jf049320i. [DOI] [PubMed] [Google Scholar]
- 25.Rao N, Kaladhar D. Antioxidant and antimicrobial activities of rhizomeextracts of Kaempferia galanga. World J Pharma Pharma Sci. 2014;3:1180–9. [Google Scholar]
- 26.Ajay K. Phytochemistry, pharmacological activities and uses of Indian traditional medicinal plant Kaempferia galanga L.– An overview. Journal of Ethnopharmacology. 2020;253:112667. doi: 10.1016/j.jep.2020.112667. 10.1016/j.jep.2020.112667. [DOI] [PubMed] [Google Scholar]
- 27.Rahman I, Kabir T, Islam N, Muqaddim M, Sharmin S, Ullah MS, Uddin S. Investigation of Antioxidant and Cytotoxic Activities of Kaempferia galanga L. 2019;12:155–167. doi: 10.5958/0974-360X.2019.00365.2. [Google Scholar]
- 28.Fauziyah P.N, Sukandar E.Y, Ayuningtyas D.K. Combination effect of antituberculosis drugs and ethanolic extract of selected medicinal plants against multi-drug resistant Mycobacterium tuberculosis isolates. Sci Pharm. 2017;85:14. doi: 10.3390/scipharm85010014. doi: 10.3390/scipharm85010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. [Last accessed on 2021 Mar 13]. Available from: https://www.researchgate.net/profile/Md_Tanvir_Kabir/publication/329012378_Investigation_of_Antioxidant_and_Cytotoxic_Activities_of_Kaempferia_galanga_L/links/5cfa68c292851c874c553d4e/Investigation-of-Antioxidantand-Cytotoxic-Activities-of-Kaempferia-galanga-L.pdf.
- 30.Yao F, Huang Y, Wang Y, He X. Anti-inflammatory Diarylheptanoids and Phenolics from the Rhizomes of Kencur (Kaempferia Galanga L.) Ind Crops Prod. 2018;125:454–61. 10.1016/j.indcrop.2018.09.026. [Google Scholar]
- 31.Sani SA, Faik AA, Abdulla R, Kunasekaran S. Phytochemical, antioxidant and antibacterial activities of two kinds of Sabah Zingberaceae. J Phys Conf Ser. 2019;1358:012012. [Google Scholar]
- 32.Kokila S, Ragavan B. Effect of Kaempferia galanga rhizome extract on haematological parameters in streptozotocin induced diabetic Wistar rats. Int J Pharm Sci Drug Res. 2020;12:255–9. [Google Scholar]
- 33.Mustafa RA, Azizah AH, Suhaila M. Total Phenolic Compounds, Flavonoids, and Radical Scavenging Activity of 21 Selected Tropical Plants. Journal of food science. 2010;75:C28–35. doi: 10.1111/j.1750-3841.2009.01401.x. 10.1111/j.1750-3841.2009.01401.x. [DOI] [PubMed] [Google Scholar]
- 34.Hashiguchi A, San Thawtar M, Duangsodsri T, Kusano M, Kazuo N. Watanabe. Biofunctional properties and plant physiology of Kaempferia spp.: Status and trends. J Functl Foods. 2022;92:1–13. [Google Scholar]
- 35.Elshamy AI, Mohamed TA, Essa AF, Abd-ElGawad AM, Alqahtani AS, Shahat AA, et al. Recent Advances in Kaempferia Phytochemistry and Biological Activity: A Comprehensive Review. Nutrients. 2019;11:2396. doi: 10.3390/nu11102396. Published 2019 Oct 7. doi:10.3390/nu11102396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nonglang FP, Khale A, Bhan S. Phytochemical characterization of the ethanolic extract of Kaempferia galanga rhizome for anti-oxidant activities by HPTLC and GCMS. Futur J Pharm Sci. 2022;8 doi.org/10.1186/s43094-021-00394-1. [Google Scholar]


