Abstract
This research was aimed at observing how antibacterial strength, colour stability, and compressive strength of chitosan modified PMMA compare to non modified PMMA [polymethyl methacrylate]. The study consisted of 2 groups - chitosan modified PMMA was the test group while unmodified PMMA was the control group. Twenty-four specimens were prepared for each group. Compressive strength was evaluated using the Universal testing machine. The antimicrobial action against streptococcus mutans and lactobacillus was evaluated using the disc diffusion method. A reflectance spectrophotometer was used to measure the baseline colour following the CIE L*a*b* scheme. Following these experiments, the specimens were submerged in coffee and distilled water solutions [n = 8] for 15 days each. Color stability was measured by comparing the coordinates obtained pre and post the ageing method. Independent t test used to examine data on colour change and compressive strength. [α = 0.05] It was observed that the incorporation of chitosan into polymethylmethacrylate increases its compressive strength. This was statistically significant [P = 0.00]. Improved colour stability was also observed [P = 0.000]. Antimicrobial action against streptococcus mutans and lactobacillus was seen in the chitosan modified group. Chitosan incorporation provides a promising improvement in the properties of the polymethylmethacrylate however further research with invivo studies are required to come to a conclusion.
Keywords: Antimicrobial, chitosan, color stability, compressive strength, dental restoration, innovation, nanoparticle, polymethyl methacrylate, temporary
INTRODUCTION
Temporary restorations are a critical component of prosthetic clinical steps that safeguard the remaining tooth structure during dental operations.[1] They serve an important role until the luting of the final restoration.[2] They deliver strength, retention, and esthetics to the prepared teeth, all of which are critical for clinical success. Temporary restorations are most commonly manufactured using polymethyl methacrylate (PMMA), polyethyl methacrylate, and urethane dimethacrylate.[3,4] The desirability of any particular provisional restorative material is influenced by a number of factors such as fracture toughness, marginal accuracy, color stability, wear resistance, tissue compatibility, simplicity of manipulation, and affordability.
In recent times, nanotechnology has become a field of extensive research. The size of nanoparticles ranges from 1 to 100 nm. Because of the reduction in dimension to an atomic level, they have unique features. Chitosan is a nitrogenous, white, hard, inelastic polysaccharide formed by partial deacetylation of chitin.[5] Chitosan nanoparticles [ChNPs] integrate the features of chitosan along with the qualities of nanoparticles, such as the surface interface, compact size, and effects of its quantum size.[6] Owing to its antibacterial characteristics, nontoxicity, and biodegradability, it has a wide range of applications.[1] Because of the listed benefits, chitosan can be mixed with other biomaterials to enhance their biological and mechanical properties.[7] The use of chitosan materials in the dental world such as the alteration of dentifrices or dental luting agents has previously displayed promising results.[8] Any changes to the composition must enhance or at least keep the original material's esthetic qualities and surface characteristics from declining.[9] The goal of this research is to see how additives such as chitosan affect the strength, color stability, and antibacterial action of PMMA-based temporary restorations. The null hypothesis was that the compressive strength, color stability, and antimicrobial activity of chitosan-modified PMMA would have no statistically different mean values compared to control PMMA. Our research and knowledge have resulted in high-quality publications from our team.[10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35] This study will give knowledge about the material and its modification.
MATERIALS AND METHODS
Chitosan nanoparticle preparation
0.5 g of chitosan powder was mixed with 0.5 g of glacial acetic acid and 49 ml of distilled water. This solution was kept on a magnetic stirrer for constant mixing, till a clear solution was formed. Then, 4–5 drops of sodium tripolyphosphate were added. Characterization was done through transmission electron microscopy analysis [Figure 1].
Figure 1.
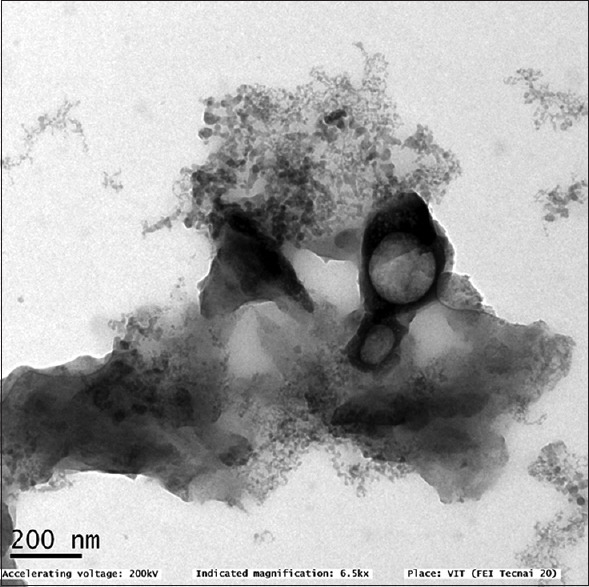
TEM image of chitosan nanoparticles. TEM: Transmission electron microscopy
Sample preparation
The ChNPs were directly incorporated into PMMA resin in the ratio of 1:2.5:1. For the antimicrobial test, ten specimens of each material measuring 1 cm × 1.5 cm were created, and samples of these materials were added in a metal mold, which was then coated with a polyester film and a glass slide, and pressed to eliminate extra materials and avoid the incorporation of air bubbles. The timer for the materials was set for a duration of 10 min. The specimens were kept at a temperature of 37°C and relative humidity of 100% for 24 h to guarantee full polymerization. After this period, the samples were polished on a polishing machine (APL-4) with 360-, 600-, and 1200-grit abrasive papers and completed with a solution containing diamond abrasives (6, 3, and 1 m) for a duration of 4 min each. Between and after the steps of polishing–finishing, the samples were rinsed with deionized water for 120 s using an ultrasonic cleaning device.
Color measurement
Color analysis of the specimens was carried out using the VITA Easyshade Advance digital spectrophotometer, following the (CIE L*a*b*) color scale at 10 a.m on a sunny day in sunlight when all other lights were switched off. All samples were analyzed on a single day.
Immersion in solution
Following the measurement, each specimen was immersed in containers containing coffee with a pH of 5.10 and distilled water (pH - 6.37) for 14 days at room temperature (37°C). Following the same process as before, new color measurements were taken after storage.
Color stability analysis
Following the new color measurements (3D color system), ΔE the aging treatments before and after were calculated (ΔE = [(ΔL*)2 + (Δa*)2 + (Δb*)2]). Depending on the material under study and the aging process, the mean values were determined.
Antimicrobial test
The antimicrobial activity tests of these samples were carried out using the disk diffusion method. Streptococcus mutans species and Lactobacillus species were taken and inoculated in the nutrient agar under room temperature. Once the samples were added to the nutrient agar in their respective concentration, they were placed in the incubator at a temperature of about 37°C for 24 h. After the incubation period, the samples were taken to observe for the zone of inhibition, which was then measured. The results were obtained and statistically analyzed [Figures 2 and 3].
Figure 2.

Zone of inhibition seen against Streptococcus mutans in the sample modified by chitosan nanoparticles
Figure 3.
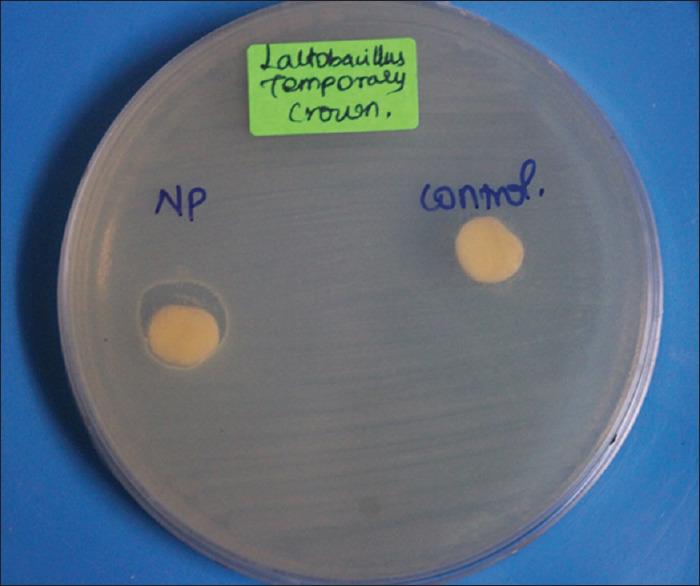
Zone of inhibition seen against Lactobacillus in the sample modified by chitosan nanoparticles
Measuring compressive strength
The specimens were made following the aforesaid process and then placed on top of the Instron universal testing machine's platform. A load of 10 kN load cells was applied (crosshead speed of 0.75 mm/min). The force that the sample could withstand before deformation started was measured in Newton and translated to MPa with the help of testing machine software [Figures 4 and 5].
Figure 4.
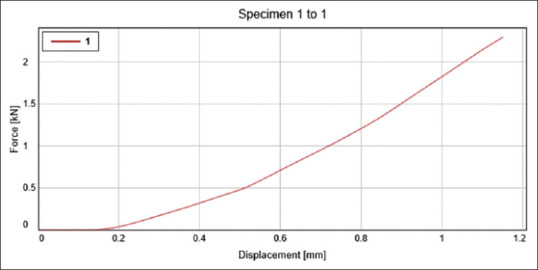
It depicts the displacement of the control group- sample 1 at a given force. Where the y-axis depicts the Force (kN) and x-axis depicts the displacement (mm)
Figure 5.
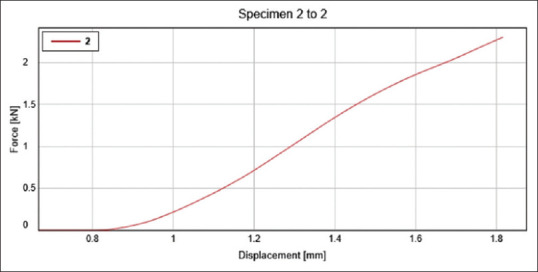
It depicts the displacement of the test group- sample 1 at a given force. Where the y-axis depicts the Force (kN) and x-axis depicts the displacement (mm)
Statistical tests
Descriptive statistical analysis was used to analyze the color stability and compressive strength of the chitosan-modified sample. The data were entered in the SPSS software (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY:IBM Corp.). An independent t-test was performed, and results were obtained in the form of tables and graphs.
RESULTS
Color stability
The result in Table 1 depicts that there was not any difference observed in the material poststorage in the distilled water solution (P > 0.05). However, the post being stored in the coffee solution, the material modified with chitosan displayed greater color stability when in comparison to the control group, both on day 1 and day 7. The results were statistically significant (P = 0.00).
Table 1.
Color stability of chitosan-modified sample is greater in coffee solution on both day 1 and day 7 when compared to the unmodified sample
| Solution | Groups | n | Mean | SD | P |
|---|---|---|---|---|---|
| Distilled water (Day 1) | Control | 10 | 0.1600 | 0.01491 | 0.13 |
| Chitosan | 10 | 0.1510 | 0.00994 | ||
| Coffee (Day 1) | Control | 10 | 2.1180 | 0.02821 | 0.00 |
| Chitosan | 10 | 1.6640 | 0.02011 | ||
| Distilled water (Day 15) | Control | 10 | 0.2600 | 0.01886 | 0.161 |
| Chitosan | 10 | 0.2490 | 0.01449 | ||
| Coffee (Day 15) | Control | 10 | 3.0500 | 0.15811 | 0.00 |
| Chitosan | 10 | 2.5280 | 0.01398 |
This result is statistically significant (P<0.05). SD: Standard deviation
Compressive strength
The compressive strength of the chitosan-modified group was greater than the control [Table 2]. The results were statistically significant (P = 0.00).
Table 2.
The compressive strength of the chitosan-modified sample was greater than the control group
| Group | n | Mean | SD | P | |
|---|---|---|---|---|---|
| Maximum force | Control | 10 | 2297.1840 | 1.44411 | 0.009 |
| Chitosan | 10 | 2298.6280 | 0.56478 | ||
| Compressive strength | Control | 10 | 29.2380 | 0.01751 | 0.00 |
| Chitosan | 10 | 29.2680 | 0.01317 |
SD: Standard deviation
Antimicrobial action
It was observed that the chitosan-modified group had an antimicrobial action against both Streptococcus and Lactobacillus. It formed a zone of inhibition against both bacteria, whereas the test group did not [Table 3].
Table 3.
Depicting the zone of inhibition formed
| Group | Zone of inhibition (mm) | |
|---|---|---|
|
| ||
| Streptococcus mutans | Lactobacillus species | |
| Chitosan-modified PMMA | 14 | 13 |
| Control group | 0 | 0 |
A zone of inhibition is observed against both Streptococcus mutans and Lactobacillus in the chitosan-modified group. No zone of inhibition is formed in the control group. PMMA: Polymethyl methacrylate
DISCUSSION
Provisional restorations greatly affect the success of the ongoing treatment. For a successful treatment, it is essential that they have adequate strength, good color stability to maintain esthetics, and prevent bacterial colonization.
Bacterial colonization on provisional prosthetic materials is higher than that on permanent prosthetic materials due to the high surface roughness and low marginal adaptation of provisional prosthetic materials.[36] Since polymethylmethacrylate crowns are porous, bacteria can colonize them. One way to encounter this issue was to cover up the PMMA surface with nanoparticle-based antimicrobial agents such as chitosan, however, the optimum solution is that the nanoparticles be contained in the polymer matrix, so that their release operates at the stage of biofilm formation. In the current study, it can be observed that the addition of ChNPs increased the antimicrobial action of the temporary crown on the tested microorganisms S. mutans and Lactobacillus. Chitosan has a bacteriostatic or bactericidal and anti-adhesion effect and can reduce biofilm formation. It also has higher penetration power than conventional antimicrobial agents due to its micro size.
The interaction of cationic chitosan with anionic cell surfaces increases membrane permeability and causes cellular material leakage, which could be the reason for chitosan's antibacterial mechanism. Chitosan may also interfere with the synthesis of mRNA and the embedding of proteins.[2]
During the function, the provisional fixed partial dentures are subjected to a variety of compressive, tensile, and shear stresses.[3,4] Accordingly, temporary crown material must have sufficient compressive strength to resist fracture to extend its life.[5] Hence, it is essential to improve its mechanical properties to achieve more crack-resistant restorations. In the current study, a statistically significant increase was seen in the compressive strength of temporary PMMA crowns postaddition of chitosan. The highly crystalline structure of chitosan along with its presence within the resin matrix most likely contributed to the increase in compressive strength. The fine dispersion of nanoparticles in the PMMA matrix may also have contributed to the increase in mechanical strength.[6] This was, however, different from the results obtained by a study conducted by.[7,8] In their study, they observed that the compressive strength of acrylic resin significantly decreased after the addition of 2% and 4% (w/w) ChNPs. They claimed that chitosan in acrylic resins acted as an impurity in the PMMA matrix, which reduced its flexural strength.[9] According to[9,10] chitosan may have a negative impact on polymerization conversion and result in an increase in the amount of residual monomer which acts as a plasticizer further decreasing compressive strength.
It is important that the inclusion of ChNP does not affect the esthetics of the restoration, hence color stability tests were performed. In the performed study, it was seen that the addition of chitosan increased the color stability of the temporary restorations in the sample with coffee on both days 1 and 7. The results were statistically significant. This could be due to the addition of methacrylate chitosan (low molecular weight) into the network of polymethyl methacrylate to produce stable materials. Similar findings were observed in a study conducted by.[7] They discovered that the chitosan-modified group exhibited better color stability after storage in distilled water as well as red wine.
CONCLUSION
The following findings can be drawn within the limitations of the current study:
Antimicrobial efficacy increased in the sample modified by ChNP against both S. mutans and Lactobacillus. The chitosan-modified sample demonstrated higher color stability and greater compressive strength than the nonmodified sample. Although the modification of PMMA with ChNP seems to be a promising temporary restorative material with good antimicrobial, color stability, and compressive strength properties, further studies with greater sample sizes are required to come to conclusive results.
Financial support and sponsorship
The present study is funded by the
Saveetha Institute of Medical and Technical Sciences
Saveetha Dental College and Hospitals
Saveetha University
JJ Trading Co. - Jamshedpur.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors acknowledge Saveetha University for all the help and support.
REFERENCES
- 1.Heinemann F, Grufferty B, Papavasiliou G, Dominiak M, García JJ, Trullenque-Eriksson A, et al. Immediate occluding definitive partial fixed prosthesis versus non-occluding provisional restorations – 4-month post-loading results from a pragmatic multicenter randomised controlled trial. Eur J Oral Implantol. 2016;9:47–56. [PubMed] [Google Scholar]
- 2.Burns DR, Beck DA, Nelson SK Committee on Research in Fixed Prosthodontics of the Academy of Fixed Prosthodontics. A review of selected dental literature on contemporary provisional fixed prosthodontic treatment: Report of the Committee on Research in Fixed Prosthodontics of the Academy of Fixed Prosthodontics. J Prosthet Dent. 2003;90:474–97. doi: 10.1016/s0022-3913(03)00259-2. [DOI] [PubMed] [Google Scholar]
- 3.Gough M. A review of temporary crowns and bridges. Dent Update. 1994;21:203–7. [PubMed] [Google Scholar]
- 4.Deshmukh M, Rajaraman V, Duraisamy R, Maiti S. Knowledge, awareness, and attitude of dentists toward use of denture adhesives in Tamil Nadu: A questionnaire survey. J Adv Pharm Technol Res. 2022;13(Suppl S1):243–8. doi: 10.4103/japtr.japtr_148_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badawy ME, Rabea EI. A biopolymer chitosan and its derivatives as promising antimicrobial agents against plant pathogens and their applications in crop protection. Int J Carbohydr Chem. 2011;2011:1–29. [Google Scholar]
- 6.Agarwal S, Ashok V, Maiti S, Agarwal V. Dentists’ Preference toward Fixed Versus Removable Implant Prosthesis on Edentulous Jaws to Improve Quality of Life? J Long Term Eff Med Implants. 2022;33:8389. doi: 10.1615/JLongTermEffMedImplants.2022038746. doi: 10.1615/JLongTermEffMedImplants. [DOI] [PubMed] [Google Scholar]
- 7.Sun Y, Yang Q, Wang H. Synthesis and characterization of nanodiamond reinforced chitosan for bone tissue engineering. J Funct Biomater. 2016;7:27. doi: 10.3390/jfb7030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Husain S, Al-Samadani KH, Najeeb S, Zafar MS, Khurshid Z, Zohaib S, et al. Chitosan biomaterials for current and potential dental applications. Materials (Basel) 2017;10:602. doi: 10.3390/ma10060602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perchyonok VT, Souza J, Küll MF, Suzuki TY, Maluly-Proni AT, Santos PH. Color stability and surface roughness of chitosan- and nanodiamond-modified bisacrylic resin. Braz Oral Res. 2019;33:e024. doi: 10.1590/1807-3107bor-2019.vol33.0024. [DOI] [PubMed] [Google Scholar]
- 10.Abdul Wahab PU, Senthil Nathan P, Madhulaxmi M, Muthusekhar MR, Loong SC, Abhinav RP. Risk factors for post-operative infection following single piece osteotomy. J Maxillofac Oral Surg. 2017;16:328–32. doi: 10.1007/s12663-016-0983-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subramaniam N, Muthukrishnan A. Oral mucositis and microbial colonization in oral cancer patients undergoing radiotherapy and chemotherapy: A prospective analysis in a tertiary care dental hospital. J Investig Clin Dent. 2019;10:e12454. doi: 10.1111/jicd.12454. [DOI] [PubMed] [Google Scholar]
- 12.Kumar SP, Girija AS, Priyadharsini JV. Targeting NM23-H1-mediated inhibition of tumour metastasis in viral hepatitis with bioactive compounds from Ganoderma lucidum: A computational study. Indian J Pharm Sci. 2020;82:300–5. [Google Scholar]
- 13.Ravindiran M, Praveenkumar C. Status review and the future prospects of CZTS based solar cell – A novel approach on the device structure and material modeling for CZTS based photovoltaic device. Renew Sustain Energy Rev. 2018;94:317–29. [Google Scholar]
- 14.Vadivel JK, Govindarajan M, Somasundaram E, Muthukrishnan A. Mast cell expression in oral lichen planus: A systematic review. J Investig Clin Dent. 2019;10:e12457. doi: 10.1111/jicd.12457. [DOI] [PubMed] [Google Scholar]
- 15.Mathivadani V, Smiline AS, Priyadharsini JV. Targeting Epstein-Barr virus nuclear antigen 1 (EBNA-1) with Murraya koengii bio-compounds: An in-silico approach. Acta Virol. 2020;64:93–9. doi: 10.4149/av_2020_111. [DOI] [PubMed] [Google Scholar]
- 16.Happy A, Soumya M, Venkat Kumar S, Rajeshkumar S, Sheba RD, Lakshmi T, et al. Phyto-assisted synthesis of zinc oxide nanoparticles using Cassia alata and its antibacterial activity against Escherichia coli. Biochem Biophys Rep. 2019;17:208–11. doi: 10.1016/j.bbrep.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prathibha KM, Johnson P, Ganesh M, Subhashini AS. Evaluation of salivary profile among adult type 2 diabetes mellitus patients in South India. J Clin Diagn Res. 2013;7:1592–5. doi: 10.7860/JCDR/2013/5749.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.aramasivam A, Vijayashree Priyadharsini J. Novel insights into m6A modification in circular RNA and implications for immunity. Cell Mol Immunol. 2020;17:668–9. doi: 10.1038/s41423-020-0387-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponnanikajamideen M, Rajeshkumar S, Vanaja M, Annadurai G. In vivo type 2 diabetes and wound-healing effects of antioxidant gold nanoparticles synthesized using the insulin plant chamaecostus cuspidatus in Albino rats. Can J Diabetes. 2019;43:82–9.e6. doi: 10.1016/j.jcjd.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Vijayashree Priyadharsini J, Smiline Girija AS, Paramasivam A. In silico analysis of virulence genes in an emerging dental pathogen A.baumannii and related species. Arch Oral Biol. 2018;94:93–8. doi: 10.1016/j.archoralbio.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Anita R, Paramasivam A, Priyadharsini JV, Chitra S. The m6A readers YTHDF1 and YTHDF3 aberrations associated with metastasis and predict poor prognosis in breast cancer patients. Am J Cancer Res. 2020;10:2546–54. [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwal S, Ashok V, Maiti S. Open- or closed-tray impression technique in implant prosthesis: A dentist's perspective. J Long Term Eff Med Implants. 2020;30:193–8. doi: 10.1615/JLongTermEffMedImplants.2020035933. [DOI] [PubMed] [Google Scholar]
- 23.Ponnanna AA, Maiti S, Rai N, Jessy P. Three-dimensional-printed Malo Bridge: Digital fixed prosthesis for the partially edentulous maxilla. Contemp Clin Dent. 2021;12:451–3. doi: 10.4103/ccd.ccd_456_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasabwala H, Maiti S, Ashok V, Sashank K. Data on dental bite materials with stability and displacement under load. Bioinformation. 2020;16:1145–51. doi: 10.6026/973206300161145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rupawat D, Maiti S, Nallaswamy D, Sivaswamy V. Aesthetic outcome of implants in the anterior zone after socket preservation and conventional implant placement: A retrospective study. J Long Term Eff Med Implants. 2020;30:233–9. doi: 10.1615/JLongTermEffMedImplants.2020035942. [DOI] [PubMed] [Google Scholar]
- 26.Merchant A, Ganapathy DM, Maiti S. Effectiveness of local and topical anesthesia during gingival retraction. Braz Dent Sci. 2022;25:E2591. [Google Scholar]
- 27.Aparna J, Maiti S, Jessy P. Polyether ether ketone – As an alternative biomaterial for Metal Richmond crown-3-dimensional finite element analysis. J Conserv Dent. 2021;24:553–7. doi: 10.4103/jcd.jcd_638_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merchant A, Maiti S, Ashok V, Ganapathy DM. Comparative analysis of different impression techniques in relation to single tooth impression. Bioinformation. 2020;16:1105–10. doi: 10.6026/973206300161105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwal S, Maiti S, Ashok V. Correlation of soft tissue biotype with pink aesthetic score in single full veneer crown. Bioinformation. 2020;16:1139–44. doi: 10.6026/973206300161139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kushali R, Maiti S, Girija SA, Jessy P. Evaluation of microbial leakage at implant abutment interfact for different implant systems: An in vitro study. J Long Term Eff Med Implants. 2022;32:87–93. doi: 10.1615/JLongTermEffMedImplants.2022038657. [DOI] [PubMed] [Google Scholar]
- 31.Gopal TM, Rohinikumar S, Thiyaneswaran N, Maiti S. Effect of submandibular fossa on implant length in the posterior mandibular region. J Long Term Eff Med Implants. 2020;30:219–26. doi: 10.1615/JLongTermEffMedImplants.2020035970. [DOI] [PubMed] [Google Scholar]
- 32.Tulsani M, Rohinikumar S, Maiti S, Nesappan T. Impact of level of crestal placement on marginal bone loss: A retrospective institutional study. J Long Term Eff Med Implants. 2020;30:227–32. doi: 10.1615/JLongTermEffMedImplants.2020035923. [DOI] [PubMed] [Google Scholar]
- 33.Sharmila R, Maiti S, Jessy P. Comparative analysis of abrasion resistance in relation to different temporary acrylic crown material using toothbrush simulator – An in vitro study. Int J Dent Oral Sci. 2021;08:2153–7. [Google Scholar]
- 34.Agarwal V, Maiti S. Prevalence of denture stomatitis and its assessment towards management – A retrospective study. ECB. 2022;11:23–9. [Google Scholar]
- 35.Agarwal V, Maiti S, Rajaraman V, Rajeshkumar S, Agarwal S, Ganapathy D. The cytotoxic effect of silver nanoparticles derived from amla fruit seed extract. J Coastal Life Med. 2022;10:280–5. [Google Scholar]
- 36.Buergers R, Rosentritt M, Handel G. Bacterial adhesion of Streptococcus mutans to provisional fixed prosthodontic material. J Prosthet Dent. 2007;98:461–9. doi: 10.1016/S0022-3913(07)60146-2. [DOI] [PubMed] [Google Scholar]


