Abstract
Background
The purpose of this study is to investigate the microbial patterns of periprosthetic joint infection (PJI) and fracture-related infection (FRI), and guide for the formulation of more accurate empirical antimicrobial regimens based on the differences in pathogen distribution.
Methods
A comparative analysis of pathogen distribution was conducted between 153 patients (76 with PJI and 77 with FRI). Predicted analyses against isolated pathogens from two cohorts were conducted to evaluate the best expected efficacy of empirical antimicrobial regimens (imipenem + vancomycin, ciprofloxacin + vancomycin, and piperacillin/tazobactam + vancomycin).
Results
Our study found significant differences in pathogen distribution between the PJI and FRI cohorts. Staphylococci (61.3% vs. 31.9%, p = 0.001) and Gram-negative bacilli (GNB, 26.7% vs. 56.4%, p < 0.001) were responsible for the majority of infections both in the PJI and FRI cohorts, and their distribution in the two cohorts showed a significant difference (p < 0.001). Multi-drug resistant organisms (MDRO) were more frequently detected in the FRI cohort (29.3% vs. 44.7%, p = 0.041), while methicillin-resistant coagulase-negative Staphylococci (MRCoNS, 26.7% vs. 8.5%, p = 0.002) and Canidia albicans (8.0% vs. 1.1%, p = 0.045) were more frequently detected in the PJI cohort. Enterobacter spp. and Acinetobacter baumannii were detected only in the FRI cohort (11.7% and 8.5%, respectively).
Conclusions
Staphylococci and GNB were responsible for the majority of infections in both PJI and FRI. Empirical antimicrobial therapy should focus on the coverage of Staphylococci in PJI and GNB in FRI, and infections caused by MDROs should be more vigilant in FRI, while the high incidence of MRCoNS in PJI should be noted, which could guide for the formulation of more accurate empirical antimicrobial regimens. Targeted therapy for FRI caused by A. baumannii and PJI caused by C. albicans needs to be further investigated. Our study reports significant differences in pathogen distribution between the two infections and provides clinical evidence for studies on the mechanism of implant-associated infection.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12891-023-06210-6.
Keywords: Pathogen distribution, Periprosthetic joint infection, Fracture-related infection, Microbial pattern, Empirical antimicrobial therapy
Introduction
Periprosthetic joint infection (PJI) and fracture-related infection (FRI) are both known as implant-associated infections and are devastating complications in arthroplasty and trauma surgery [1]. Unfortunately, patients with PJI or FRI typically require repeated surgical procedures, long-term antimicrobial therapy, and prolonged bone healing time, which may result in poor functional outcomes [2, 3]. PJI is currently the leading complication after arthroplasty, and the overall incidence remains 0.3–1.7% after THA and 0.8–1.9% after TKA [4]. FRI rates after internal fixation range from 1–2% in the case of closed fractures and up to 25–30% in the case of severe open injuries [5, 6]. As the number of arthroplasties and trauma surgeries increases year by year, prevention and treatment of implant-related infections are becoming increasingly important [7, 8].
To date, empirical antimicrobial therapy (EAT) for PJI with glycopeptide in combination with broad-spectrum β-lactams are currently recommended to cover common pathogens such as Gram-positive cocci and Gram-negative bacilli (GNB) [9–11]. Specific recommendations concerning the prevention and treatment of FRI have been proposed [12–14], since a consensus definition for FRI published in 2018 [15]. The differences in microbial epidemiology between PJI and FRI have only been reported once in Germany [12], and it was a single center study, which may lead to a local epidemiological bias. Due to different national, geographical and economic conditions, this single study cannot fully illustrate the differences in pathogen distribution between PJI and FRI in Asia. As a result, the conclusion of empirical antimicrobial regimens needs to be confirmed further to ensure reliability. The purpose of this study was mainly to investigate the microbial patterns of PJI and FRI, determine whether pathogen distribution in PJI and FRI shows differences in our institution, and provide guidance for the formulation of more accurate empirical antimicrobial regimens.
Materials and methods
Study design and patient identification
The study was approved by the Ethics Committee of Huashan Hospital, Fudan University (KY2022-803). Informed consent was obtained from all individual participants included in the study. A retrospective review of 153 patients (18 years old and above) treated for FRI or PJI within the period from 1 January 2016 to 28 February 2021 and performed at a single center was conducted. PJI was diagnosed according to the EBJIS definition of PJI, which was supported by MSIS and ESGIAI in 2021 [16]. FRI was diagnosed according to the latest consensus on the definition of FRI in 2018 [15]. Comparative analyses of pathogen distribution were performed between patients with FRI or PJI. Predicted analyses against isolated pathogens from PJI and FRI cohorts were conducted to evaluate the best-expected efficacy of empirical antimicrobial regimens.
Data collection
By reviewing case histories, information on patients with PJI and FRI was recorded, including sex, age, body mass index (BMI), comorbidities and infection sites. Comorbidities were evaluated by the Charlson Comorbidity Index (CCI) [17]. By reviewing microbiological reports, the results of pathogen detection and antimicrobial susceptibility testing were recorded. In addition, patients with culture-negative infections were included if the patient’s clinical symptoms and examination raised suspicion of PJI or FRI. Each pathogen was documented separately in cases of polymicrobial infection. Multi-drug resistant (MDR) was defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories [18].
Microbiological examination
Taken in at least four suspected tissues, two or more intraoperative deep tissue cultures or a combination of preoperative aspiration and intraoperative deep tissue cultures that yield the same organism may be considered definitive evidence of infection. Exceptionally, the growth of a virulent pathogen (e.g., S. aureus, Escherichia coli and Pseudomonas aeruginosa) in a single sample may also represent infection. If a single sample from multiple tissue cultures yields a pathogen considered a common contaminant (e.g., coagulase-negative Staphylococci, Propionibacterium acnes, and Corynebacterium spp.), we did not consider it as evidence of definitive infection, in which case it was evaluated in conjunction with other clinical evidence. Patients were excluded if less than four samples were taken.
Statistical analysis
Statistical analysis of the results was performed with STATA/SE 16.0 software (USA). Measurement data were statistically described as the mean, standard deviation, and 95% confidence interval (CI); counting data were described as the frequency. Normally distributed variables were analysed using the t-test. Categorical data are presented as proportions, which were analysed with the chi-square test or Fisher’s exact test. All statistical tests were bilateral, and a p-value below 0.05 was considered significant.
Results
Demographics
The PJI cohort comprised 76 patients, among whom 36 (47.4%) were male and 40 (52.6%) were female (Table 1). The mean age was 68.0 years (65.6–70.6), and the mean BMI was 23.8 ± 3.9 kg/m2. Most patients had comorbidities with a median CCI of 1 (range 0–4). PJI mainly occurred at the knee (28, 36.8%) and the hip (48, 63.2%). The mean delay from prosthesis implantation to the onset of infection was 28 days (range: 14 days-16 months). Seventy-seven patients were diagnosed with FRI in total. Overall, 45 (57.7%) of the patients were male and 32 (42.3%) were female. The mean age was 64.7 years (62.1–67.4), the mean BMI was 24.1 ± 3.7 kg/m2 and the median CCI was 1 (range 0–3). FRI mainly occurred at the tibia and/or fibula (33, 41.6%), femur (15, 19.5%), or ulna and/or radius (12, 15.6%). The mean delay from initial fracture treatment to onset of infection symptoms was 7 days (range: 3 days-19 days). In comparison, the groups did not differ significantly in sex, age, BMI, or CCI.
Table 1.
Baseline characteristics of the PJI and FRI cohorts
| Characteristic | PJI (n = 76) | FRI (n = 77) | p-value |
|---|---|---|---|
| Demographic data | |||
| Gender (male), n (%) | 36 (47.4%) | 45 (57.7%) | n.s |
| Age, years (95% CI) | 68.0 (65.6–70.6) | 64.7 (62.1–67.4) | n.s |
| BMI (kg/m2) | 23.8 ± 3.9 | 24.1 ± 3.7 | n.s |
| CCI (95% CI) | 1 (0–4) | 1 (0–3) | n.s |
|
Delay from prosthesis implantation/ trauma to infection, days (95% CI) |
28 days (14 days-16 months) | 7 days (3 days-19 days) | 0.000 |
| Sites, n (%) | |||
| Hip | 48 (63.2%) | 3 (3.9%) | |
| Knee | 28 (36.8%) | 8 (10.4%) | |
| Clavicle | 5 (6.5%) | ||
| Humerus | 9 (11.7%) | ||
| Ulna and/or radius | 12 (15.6%) | ||
| Femur | 15 (19.5%) | ||
| Tibia and/or fibula | 33 (41.6%) | ||
| Pedes | 11 (14.3%) | ||
| Microbiologic documentation | |||
| Multi-drug resistant, n (%) | 22 (29.3%) | 42 (44.7%) | 0.041 |
| Negative culture, n (%) | 26 (34.2%) | 18 (23.4%) | n.s |
| Polymicrobial infection, n (%) | 16 (21.1%) | 22 (28.6%) | n.s |
Microbiological analysis
MDR organisms (MDRO) were detected in our study, with 22 cases in the PJI cohort and 42 cases in the FRI cohort, resulting in a significant difference (29.3% vs. 44.7%, p = 0.041) (Table 1). Both the rate of negative cultures and polymicrobial infections showed no significant difference. Isolated microorganisms of both cohorts are presented in Table 2 and Fig. 1. We also show the isolated microorganisms of the early, delayed and late PJI and FRI respectively (Supplemental Tables 1 and 2).
Table 2.
Isolated microorganisms in positive culture
| Pathogens | PJI (n = 75) | FRI (n = 94) | p-value | ||
|---|---|---|---|---|---|
| Gram-positive cocci | 48 (64.0%) | 36 (38.3%) | 0.001 | ||
| 1 Staphylococci | 46 (61.3%) | 30 (31.9%) | 0.000 | ||
| 1.1 Staphylococcus aureus | 22 (29.3%) | 20 (21.3%) | n.s | ||
| 1.1.1 MRSA | 6 (8.0%) | 8 (8.5%) | n.s | ||
| 1.1.2 MSSA | 16 (21.3%) | 12 (12.8%) | n.s | ||
| 1.2 CoNS | 24 (32.0%) | 10 (10.6%) | 0.001 | ||
| 1.2.1 MRCoNS | 20 (26.7%) | 8 (8.5%) | 0.002 | ||
| 6 (8.0%) | MRSE | 5 (5.3%) | MRSE | n.s | |
| 5 (6.7%) | MRSHo | 1 (1.1%) | MRSHo | n.s | |
| 8 (10.7%) | MRSC | 0.001 | |||
| 1 (1.3%) | Staphylococcus warneri | n.s | |||
| 2 (2.1%) | MRSH | n.s | |||
| 1.2.2 MSCoNS | 4 (5.3%) | 2 (2.1%) | n.s | ||
| 4 (5.3%) | MSSE | 1 (1.1%) | MSSE | n.s | |
| 1 (1.1%) | MSSHo | n.s | |||
| 2 Streptococcus dysgalactiae | 0 | 1 (1.1%) | n.s | ||
| 3 Enterococcus spp. | 2 (2.7%) | 5 (5.3%) | n.s | ||
| 2 (2.7%) | Enterococcus faecalis | 3 (3.2%) | Enterococcus faecalis | n.s | |
| 2 (2.1%) | Enterococcus faecium | n.s | |||
| Gram-positive bacilli | 0 | 2 (2.1%) | n.s | ||
| 1 Corynebacterium | 0 | 1 (1.1%) | n.s | ||
| 2 Bacillus cereus | 0 | 1 (1.1%) | n.s | ||
| Gram-negative bacilli | 20 (26.7%) | 53 (56.4%) | 0.000 | ||
| 1 Enterobacteriaceae | 12 (16.0%) | 31 (33.0%) | 0.012 | ||
| 2 (2.7%) | Proteus mirabilis | 3 (3.2%) | Proteus mirabilis | n.s | |
| 6 (8.0%) | Klebsiella pneumoniae | 9 (9.6%) | Klebsiella pneumoniae | n.s | |
| 2 (2.7%) | Escherichia coli | 7 (7.4%) | Escherichia coli | n.s | |
| 2 (2.7%) | Salmonella enteritidis | n.s | |||
| 1 (1.1%) | Klebsiella oxytoca | n.s | |||
| 0 | Enterobacter | 11 (11.7%) | Enterobacter spp. | 0.001 | |
| 9 (9.6%) | Enterobacter cloacae | 0.005 | |||
| 2 (2.1%) | Enterobacter kobei | n.s | |||
| 2 Pseudomonas spp. | 8 (10.7%) | 8 (8.5%) | n.s | ||
| 4 (5.3%) | Pseudomonas aeruginosa | 7 (7.4%) | Pseudomonas aeruginosa | n.s | |
| 2 (2.7%) | Pseudomonas putida | 1 (1.1%) | Pseudomonas putida | n.s | |
| 2 (2.7%) | Pseudomonas mendocina | n.s | |||
| 3 Stenotrophomonas maltophilia | 0 | 3 (3.2%) | n.s | ||
| 4 Acinetobacter baumannii | 0 | 8 (8.5%) | 0.009 | ||
| 5 Serratia marcescens | 0 | 3 (3.2%) | n.s | ||
| Obligate anaerobe | 0 | 2 (2.1%) | n.s | ||
| 1 (1.1%) | Bacteroides fragilis | n.s | |||
| 1 (1.1%) | Prevotella | n.s | |||
| Canidia albicans | 6 (8.0%) | 1 (1.1%) | 0.045 | ||
| Mycobacterium tuberculosis | 1 (1.3%) | 0 | n.s | ||
MRSA Methicillin-resistant S. aureus, MSSA Methicillin-sensitive S. aureus, CoNS Coagulase-negative Staphylococci, MRCoNS Methicillin-resistant CoNS, MRSE Methicillin-resistant S. epidermidis, MRSHo Methicillin-resistant S. hominis, MRSH Methicillin-resistant S. haemolyticus, MRSC Methicillin-resistant S. capitis, MSCoNS Methicillin-sensitive coagulase-negative Staphylococci, MSSE Methicillin-sensitive S. epidermidis, MSSHo Methicillin-sensitive S. hominis
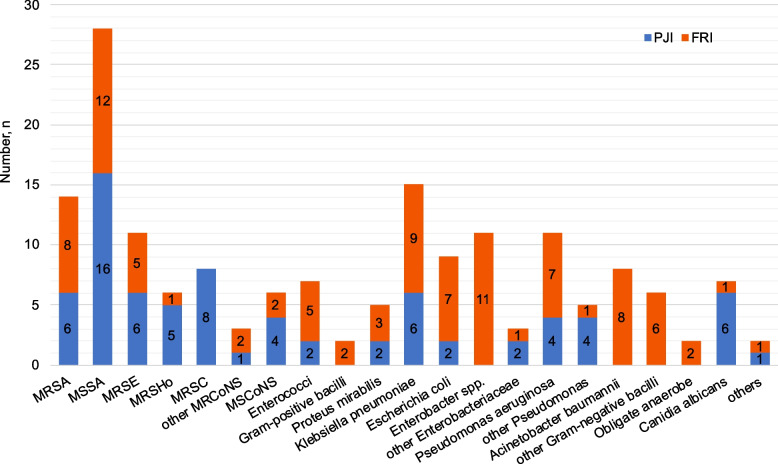
Fig. 1.
Comparison of isolated micro-organism amounts between PJI and FRI cohorts
In the PJI cohort, the most prevalent pathogens were Staphylococci (61.3%), which included coagulase-negative Staphylococci (CoNS) and S. aureus (32.0% and 29.3%, respectively). Methicillin-resistant CoNS (MRCoNS) accounted for 26.7%, and methicillin-sensitive S. aureus (MSSA) accounted for 21.3%, followed by methicillin-resistant Staphylococcus capitis (MRSC, 10.7%). GNB were the most frequently identified pathogens in the FRI cohort (56.4%), and Staphylococci accounted for 31.9%. The most prevalent GNB was Enterobacteriaceae (33.0%), including Enterobacter spp. (11.7%) and Klebsiella pneumoniae (9.6%).
Fisher’s exact test was used to compare every pathogen distribution between the two cohorts (p < 0.001). Staphylococci were more common in the PJI cohort than in the FRI cohort (61.3% vs. 31.9%, p < 0.001), among which MRCoNS (26.7%, p = 0.002) and Candida albicans (8.0%, p = 0.045) were more frequently detected in the PJI cohort. GNB was more common in the FRI cohort than PJI (56.4% vs. 26.7%, p < 0.001). In addition, Enterobacter cloacae and Acinetobacter baumannii were detected only in the FRI cohort (9.6% and 8.5%, respectively).
Empirical antimicrobial therapy
We noticed that Staphylococci and GNB were responsible for the majority of infections in both the PJI and FRI cohorts. Thus, Pearson’s chi-squared test was carried out and showed significant differences in the distribution of these two types of pathogens between the two cohorts (p < 0.001). We conducted predicted analyses against isolated pathogens from PJI and FRI cohorts except fungi and Mycobacterium tuberculosis to evaluate the expected efficacy of three combinations of antibiotics (imipenem + vancomycin, ciprofloxacin + vancomycin, and piperacillin/tazobactam + vancomycin). The combination of these three antibiotics was based on the preliminary analysis of the results of antimicrobial susceptibility testing (Supplemental Tables 3, 4, 5, and 6 and Figs. 2 and 3), combined with the reports confirmed in the previous literature [9–11, 13, 14, 19] and clinical experience obtained from consultation with the Department of Infectious Diseases of our hospital.
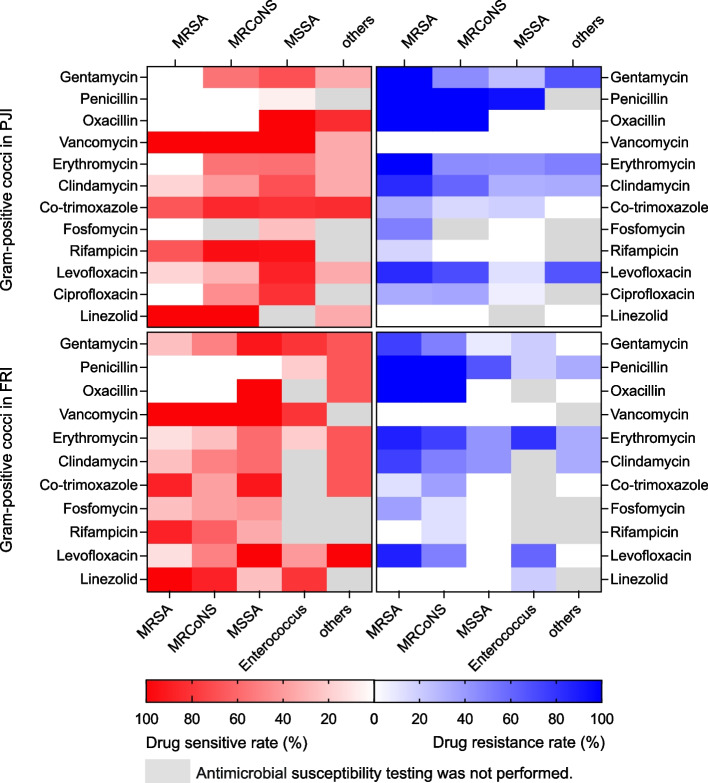
Fig. 2.
Heatmap showing drug resistance rates and sensitivity rates analysis of Gram-positive cocci in the PJI and FRI cohorts
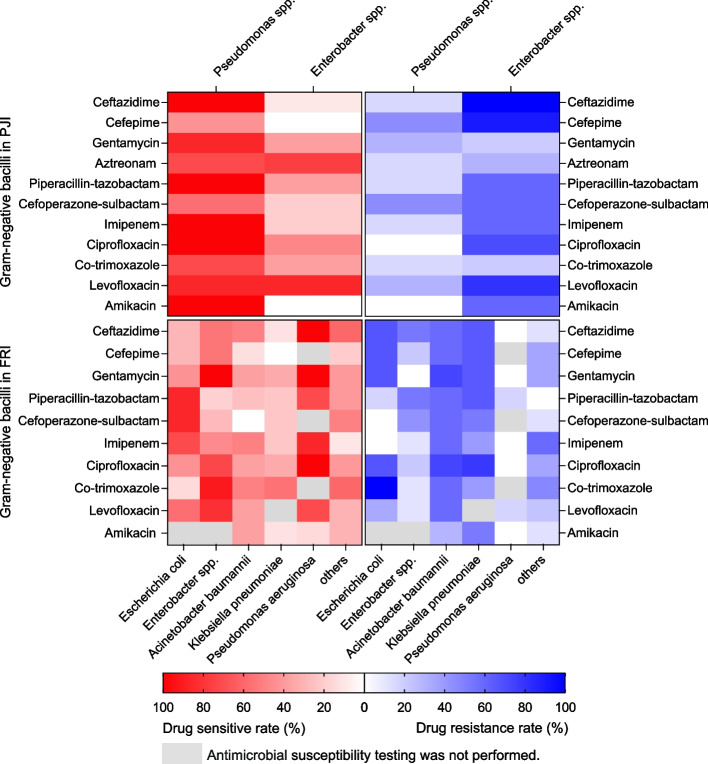
Fig. 3.
Heatmap showing drug resistance rates and sensitivity rates analysis of Gram-negative bacilli in the PJI and FRI cohorts
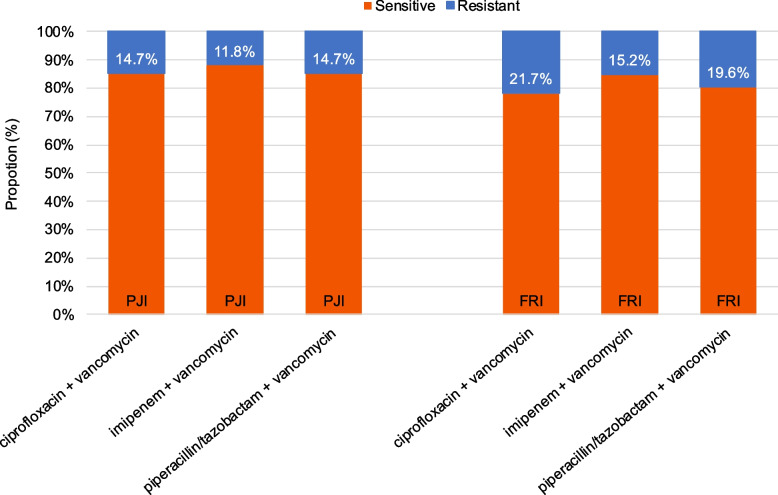
The combination of imipenem + vancomycin showed the broadest antibiotic coverage (88.2%) of isolated pathogens in the PJI cohort, followed by ciprofloxacin + vancomycin and piperacillin/tazobactam + vancomycin, which both achieved 85.3% antibiotic coverage (Fig. 4). In the FRI cohort, imipenem + vancomycin achieved 84.8% antibiotic coverage of isolated pathogens, followed by piperacillin/tazobactam + vancomycin, which covered 80.4%. A lower sensitivity rate was found for ciprofloxacin + vancomycin (78.3%).
Fig. 4.
Predicted efficacy of empirical antimicrobial regimens for the PJI and FRI cohorts
Discussion
Our study found significant differences in the pathogen distribution of PJI and FRI cohorts, and the most prevalent pathogens in the PJI cohort were Staphylococci, while GNB were the most frequently identified pathogens in the FRI cohort. Furthermore, the proportion of these two types of pathogens between the two cohorts showed a significant difference. In addition, MDROs were more frequently detected in the FRI cohort, while MRCoNS and C. albicans were more frequently detected in PJI.
Pathogen distribution
In this study, Gram-positive cocci, especially Staphylococci, were the main reason for PJI. A multicenter study analysed the leading pathogens causing PJI in China, and showed that the proportion of Gram-positive cocci was 65.7% [20]. Ares et al. [21] conducted a review of 14 studies showing that S. aureus and CoNS both accounted for 27.0% of PJI. Triffault-Fillit et al. [22] disclosed 28.6% CoNS in PJI cases, which is comparable to the 30.4% found in our study. The abovementioned previous literature has reported similar results, which are consistent with our study. In addition, the prevalence of PJI caused by drug-resistant pathogens and fungi is increasing.
Ravi et al. [23] found that the proportion of MRCoNS was 25.6% compared to 26.7% in our PJI cohort. Analysis of PJI microbiological patterns in a large cohort containing 997 patients with PJI and 180 (18.1%) of them were detected with MRCoNS [24]. Charalambous et al. [25] reported a poor rate of success in treating CoNS PJI, which was likely due to the interaction of inherent virulence through biofilm formation and decreased antibiotic efficacy. Regarding fungal infections, C. albicans were detected more frequently in the PJI cohort, with a prevalence of 8.0%. Candida periprosthetic joint infection (CPJI) is a rare, difficult-to-treat disease. Rarely, PJI may be caused by fungi with a global incidence of 1%, and Candida species are responsible for at least 80% of PJI cases [26, 27]. Up to 20% of fungal PJI cases are accompanied by bacterial infections [28]. We found C. albicansinfection in the PJI cohort, which might be related to the risk factors for fungal infection, including immunosuppression, systemic diseases, and prolonged antimicrobial therapy [29]. Candida species often grow as a biofilm adhering to bioprosthetic implants, including cement spacers [30], and this may contribute to the persistence and relapse of the infection [31, 32].
GNB were the most frequent pathogens in our FRI cohort. A multicenter study in Northeast China investigated 744 patients with FRI from 2011 to 2020, and 266 GNB (47.0%) were identified among the detected pathogens. Furthermore, S. aureus, S. epidermidis, Pseudomonas aeruginosa, Escherichia coli, K. pneumoniae, and Enterobacter cloacaewere similar to our study of the bacterial species and proportions [33]. Depypere et al. [34] reported that GNB was isolated in 27.8% of FRI cases, which is higher than the 14% generally reported in PJI studies. Nevertheless, some literature from Europe reported that the main pathogens of FRI were Staphylococci [12, 34–36]. A higher rate of GNB in our FRI cohort might be due to initial EAT in our institution mainly against Gram-positive bacteria. Another reason might be that the causes of open fractures in China are dominated by traffic accidents and factory mechanical injuries compared with developed countries.
Multi-drug resistant organisms
In our study, MDROs were detected more frequently in the FRI cohorts. A comparison of bacteria isolated from open fractures following debridement and subsequent infection concluded that 60.8% of postoperative infections were caused by MDROs [37]. Zhang et al. [38] reported that 546 strains of pathogens were detected in FRI patients, with only 105 strains (19.2%) of MDROs. The pathogenesis of FRI was more complex, and patients with FRI were more severely ill, resulting in more bacterial species and higher MDR infection rates in the FRI cohort.
Specific pathogens in each cohort
Enterobacter spp. and A. baumannii were only isolated in the FRI cohort. FRI within three weeks after surgery due to Enterobacter spp. occurred primarily in lower extremity fractures, especially hip fractures, because patients with these traumas tended to be elderly [39], undertook indwelling urinary catheters, required absolute bed rest, and might couple with inadequate perineal care. These characteristics result in infection caused by colonizing the microbiota of the local skin [40]. Yeramosu et al. [41] enrolled 248 patients who underwent operatively treated pilon fractures and declared that Enterobacter cloacae was one of the most common pathogens, accounting for 16.7%.
The prevalence of A. baumannii was 12.1%, which was only isolated in the FRI cohort. Hao et al. [37] analysed bacteria isolated from open fractures following debridement and subsequent infection and demonstrated that A. baumannii accounted for 49.3%, which indicated that A. baumannii was more likely to cause infections in patients with open fractures and severe trauma. Moreover, 87.5% of patients in whom A. baumannii was detected underwent severe trauma and therefore had a long duration in the ICU. This might be because A. baumannii is an opportunistic human pathogen that predominantly infects critically ill patients [42], so it was entirely concentrated in patients with FRI. In addition, Caricato et al. [43] concluded that the presence of long-term trans-skeletal traction was the only independent risk factor for A. baumannii infection (p = 0.04). However, we could not isolate Enterobacter spp. and A. baumannii in the PJI cohort.
Differences in mechanisms
To determine the reason for the differences in pathogen distribution between the two groups, we compared the mechanisms of these two infections. In the previous literature, PJI occurred through various mechanisms: first, direct seeding from external contaminants or contiguous spread with airborne organisms and those present on the patient’s skin [44]; second, haematogenous spread from other body sites; and third, recurrent infection [45]. FRI generally occurs exogenously due to the trauma itself (e.g., open fractures), during insertion of the fixation device, or disturbed wound healing or late soft tissue coverage in cases of open fractures. Haematogenous infections are rare [46, 47]. Therefore, the differences in mechanisms can partially explain the differences in the pathogen distribution of the two infections. However, the mechanism has not yet been elucidated thoroughly and remains to be further studied.
Empirical antimicrobial regimens
Previous guidelines in PJI recommend the use of an anti-Gram-positive agent such as vancomycin in combination with broad-spectrum β-lactams such as piperacillin-tazobactam and 3rd- or 4.th-generation cephalosporins to permit bone tissue penetration [9, 10]. Moran et al. [11] recommended the use of vancomycin combined with a carbapenem as the empirical therapy for PJI. Reported recommendations of empirical antimicrobial regimens used for FRI include a glycopeptide and an agent covering GNB [13]. A recent study recommends that meropenem + vancomycin, gentamicin + vancomycin, and co-amoxiclav + glycopeptide are the best therapeutic options for FRI, regardless of the onset of infection [35].
In our study, Staphylococci and GNB were responsible for the majority of infections in both the PJI and FRI cohorts. As a result, we concluded that EAT mainly aimed at these two types of pathogens in PJI and FRI consistently, which is in line with previous literature. The empirical antibiotic regimen for FRI is recommended for piperacillin/tazobactam + vancomycin. For PJI, ciprofloxacin + vancomycin and piperacillin/tazobactam + vancomycin exhibited the same antibiotic coverage, so both can be recommended. However, microbial epidemiology in PJI and FRI depends heavily on the center and the clinical situation [9], making it difficult to provide universal recommendations.
The current empirical antimicrobial regimens cover both Gram-positive cocci and GNB, but they are too broad to be accurate, which tends to result in adverse effects of antibiotic abuse. Although several studies have recommended the use of carbapenems for PJI and FRI, empirical antimicrobial regimens should avoid the containment of reserve antibiotics due to hitherto unknown effects on the development of multi-drug resistance [35]. Once there is evidence of pathogens and their sensitivity, patients should be treated with targeted and de-escalated antimicrobial therapy due to the risk of enhanced antimicrobial resistance by broad-spectrum antimicrobial combination [12, 48]. Local antibiotic carriers (gentamicin + vancomycin) are a feasible approach to avoid the side effects of systemic antimicrobial therapy [12, 49], especially for the treatment of PJI, as antibiotic coverage reached 91.2% in our data. According to previous studies, intraoperative application of implants or cement loaded with the combination of antibiotics is reasonable [50]; however, clinical efficacy needs to be further investigated [51, 52]. Our further research found that the pathogen distribution of Staphylococci and GNB showed significant differences between the two cohorts. Therefore, empirical antimicrobial regimens should focus on the coverage of Staphylococci in PJI and GNB in FRI, which could guide for the formulation of more accurate empirical antimicrobial regimens.
Limitations
A retrospective study of only one center and a relatively small cohort may result in a local epidemiological bias. Since the incidence of PJI is relatively low, a long study period is necessary to obtain a large patient cohort. An oft-cited reason for the situation in which antimicrobial susceptibility testing for certain antibiotics can be negative is the administration of antibiotics before treatment in our center and obtaining cultures. In addition, there are inherent differences in the pathogen distribution between implant-associated infections at different sites, mainly because of the specific colonizing bacteria.
Conclusions
Staphylococci and GNB were responsible for the majority of infections in both PJI and FRI, and the distribution of these two types of pathogens showed significant differences between the two cohorts. EAT should focus on the coverage of Staphylococci in PJI and GNB in FRI, and infections caused by MDROs should be more vigilant in FRI while the high incidence of MRCoNS in PJI should be noted, which could guide for the formulation of more accurate empirical antimicrobial regimens. Targeted therapy for FRI caused by A. baumannii and PJI caused by C. albicans needs to be further investigated. At present, there are few studies on the differences in pathogen distribution between PJI and FRI, and mechanistic investigations are rare. Our study reports pathogen differences between these two infections and provides clinical evidence for studies on the mechanism of infection.
Supplementary Information
Additional file 1: Supplemental table 1. Comparison of isolated microorganisms in early, delayed and late PJI.
Additional file 2: Supplemental table 2. Comparison of isolated microorganisms in early, delayed and late FRI.
Additional file 3: Supplemental table 3. Analysis of drug resistance rates and sensitivity rates of Gram-positive cocci in PJI.
Additional file 4: Supplemental table 4. Analysis of drug resistance rates and sensitivity rates of Gram-negative bacilli in PJI.
Additional file 5: Supplemental table 5. Analysis of drug resistance rates and sensitivity rates of Gram-positive cocci in FRI.
Additional file 6: Supplemental table 6. Analysis of drug resistance rates and sensitivity rates of Gram-negative bacilli in FRI.
Additional file 7: Supplemental Table 7. Isolated microorganisms in FRI after open fractures and closed fractures.
Acknowledgements
We acknowledge Dr Qi Zhou for her help with revision.
Abbreviations
- FRI
Fracture-related infection
- PJI
Periprosthetic joint infection
- GNB
Gram-negative bacilli
- MDR
Multi-drug resistant
- MDRO
Multi-drug resistant organism
- EAT
Empirical antimicrobial therapy
- THA
Total hip arthroplasty
- TKA
Total knee arthroplasty
- BMI
Body mass index
- CCI
Charlson Comorbidity Index
- SD
Standard deviation
- MRSA
Methicillin-resistant Staphylococcus aureus
- MSSA
Methicillin-sensitive Staphylococcus aureus
- CoNS
Coagulase-negative Staphylococci
- MRCoNS
Methicillin-resistant CoNS
- MRSE
Methicillin-resistant Staphylococcus epidermidis
- MRSHo
Methicillin-resistant Staphylococcus hominis
- MRSH
Methicillin-resistant Staphylococcus haemolyticus
- MRSC
Methicillin-resistant Staphylococcus capitis
- MSCoNS
Methicillin-sensitive CoNS
- MSSE
Methicillin-sensitive Staphylococcus epidermidis
- MSSHo
Methicillin-sensitive Staphylococcus hominis
Authors’ contributions
All authors contributed to the study conception and design. TM and JL analysed the data, prepared figures and tables and authored drafts of the paper. JM, XH and KC analysed the statistics and prepared figures and tables of this study. SW and YW reviewed drafts of the paper and approved the final draft. JX obtained grant funding. GH and GZ conceived the study, collected the data, reviewed drafts and approved the final draft.
Funding
This work was supported by grants from the Ministry of Science and Technology of the People’s Republic of China (2020YFC2002804).
Availability of data and materials
All data used and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Informed consent was obtained from all participants and approval for this study was given by the Ethics Committee and Institutional Review Board of Huashan Hospital, Fudan University (KY2022-803). All methods were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The data in this study were obtained from microbiological reports during treatment, and no human tissue samples were used.
Consent to publish
No applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tiancong Ma and Jinyang Lyu contributed equally to this work and shared first authors.
Contributor Information
Jun Xia, Email: hudbt17089@gmail.com.
Guanglei Zhao, Email: gzhao13@fudan.edu.cn.
Gangyong Huang, Email: cormierhuang@163.com.
References
- 1.Rupp M, et al. Terminology of bone and joint infection. Bone Joint Res. 2021;10(11):742–3. 10.1302/2046-3758.1011.Bjr-2021-0371. [DOI] [PMC free article] [PubMed]
- 2.Berkes M, et al. Maintenance of hardware after early postoperative infection following fracture internal fixation. J Bone Joint Surg Am. 2010;92(4):823–8. 10.2106/jbjs.I.00470. [DOI] [PubMed]
- 3.Papakostidis C, et al. Prevalence of complications of open tibial shaft fractures stratified as per the Gustilo-Anderson classification. Inj. 2011;42(12):1408-15. 10.1016/j.injury.2011.10.015. [DOI] [PubMed]
- 4.Bjerke-Kroll BT, et al. Periprosthetic joint infections treated with two-stage revision over 14 years: an evolving microbiology profile. J Arthroplasty. 2014;29(5):877–82. 10.1016/j.arth.2013.09.053. [DOI] [PubMed]
- 5.Vanvelk N, et al. Duration of Perioperative Antibiotic Prophylaxis in Open Fractures: A Systematic Review and Critical Appraisal. Antibiot (Basel). 2022;11(3):293. 10.3390/antibiotics11030293. [DOI] [PMC free article] [PubMed]
- 6.Metsemakers WJ, et al. Infection after fracture fixation: Current surgical and microbiological concepts. Inj. 2018;49(3):511–22. 10.1016/j.injury.2016.09.019. [DOI] [PubMed]
- 7.Kurtz S, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780–5. 10.2106/jbjs.F.00222. [DOI] [PubMed]
- 8.Rupp M, et al. Projections of primary TKA and THA in Germany From 2016 Through 2040. Clin Orthop Relat Res. 2020;478(7):1622–33. 10.1097/corr.0000000000001214. [DOI] [PMC free article] [PubMed]
- 9.Casenaz A, et al. Epidemiology and antibiotic resistance of prosthetic joint infections according to time of occurrence, a 10-year study. J Infect. 2022;85(5):492–8. 10.1016/j.jinf.2022.07.009. [DOI] [PubMed]
- 10.Høiby N, et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect. 2015;21(Suppl 1):S1-25. 10.1016/j.cmi.2014.10.024. [DOI] [PubMed]
- 11.Moran E, et al. Guiding empirical antibiotic therapy in orthopaedics: The microbiology of prosthetic joint infection managed by debridement, irrigation and prosthesis retention. J Infect. 2007;55(1):1–7. 10.1016/j.jinf.2007.01.007. [DOI] [PubMed]
- 12.Rupp M, et al. Is There a Difference in Microbiological Epidemiology and Effective Empiric Antimicrobial Therapy Comparing Fracture-Related Infection and Periprosthetic Joint Infection? A Retrospective Comparative Study. Antibiot (Basel). 2021;10(8):921. 10.3390/antibiotics10080921. [DOI] [PMC free article] [PubMed]
- 13.Depypere M, et al. Recommendations for Systemic Antimicrobial Therapy in Fracture-Related Infection: A Consensus From an International Expert Group. J Orthop Trauma. 2020;34(1):30–41. 10.1097/bot.0000000000001626. [DOI] [PMC free article] [PubMed]
- 14.Metsemakers WJ, et al. General treatment principles for fracture-related infection: recommendations from an international expert group. Arch Orthop Trauma Surg. 2020;140(8):1013–27. 10.1007/s00402-019-03287-4. [DOI] [PMC free article] [PubMed]
- 15.Metsemakers WJ, et al. Fracture-related infection: A consensus on definition from an international expert group. Inj. 2018;49(3):505–10. 10.1016/j.injury.2017.08.040. [DOI] [PubMed]
- 16.McNally M, et al. The EBJIS definition of periprosthetic joint infection. Bone Joint J. 2021;103-b(1):18–25. 10.1302/0301-620x.103b1.Bjj-2020-1381.R1. [DOI] [PMC free article] [PubMed]
- 17.Charlson ME, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. 10.1016/0021-9681(87)90171-8. [DOI] [PubMed]
- 18.Magiorakos AP, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–81. 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed]
- 19.Depypere M, et al. Pathogenesis and management of fracture-related infection. Clin Microbiol Infect. 2020;26(5):572–8. 10.1016/j.cmi.2019.08.006. [DOI] [PubMed]
- 20.Peng HM, et al. Microbiology analysis of periprothetic joint infection post total hip and knee arthroplasty of 9 centers in Beijing between 2014 and 2016. Zhonghua Wai Ke Za Zhi. 2019;57(8):596–600. 10.3760/cma.j.issn.0529-5815.2019.08.007. [DOI] [PubMed]
- 21.Ares O, et al. General assembly, prevention, host related local: proceedings of international consensus on orthopedic infections. J Arthroplasty. 2019;34(2s):S3-s12. 10.1016/j.arth.2018.09.049. [DOI] [PubMed]
- 22.Triffault-Fillit C, et al. Microbiologic epidemiology depending on time to occurrence of prosthetic joint infection: a prospective cohort study. Clin Microbiol Infect. 2019;25(3):353–8. 10.1016/j.cmi.2018.04.035. [DOI] [PubMed]
- 23.Ravi S, et al. Antibiotic resistance in early periprosthetic joint infection. ANZ J Surg. 2016;86(12):1014–8. 10.1111/ans.13720. [DOI] [PubMed]
- 24.Zeller V, et al. Analysis of postoperative and hematogenous prosthetic joint-infection microbiological patterns in a large cohort. J Infect. 2018;76(4):328–334. doi: 10.1016/j.jinf.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Charalambous LT, et al. Prosthetic knee infection with coagulase-negative staphylococcus: a harbinger of poor outcomes. J Arthroplasty. 2022;37(6s):S313–s320. doi: 10.1016/j.arth.2022.02.050. [DOI] [PubMed] [Google Scholar]
- 26.Benito N, et al. Time trends in the aetiology of prosthetic joint infections: a multicentre cohort study. Clin Microbiol Infect. 2016;22(8):732.e1–8. doi: 10.1016/j.cmi.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Azzam K, et al. Microbiological, clinical, and surgical features of fungal prosthetic joint infections: a multi-institutional experience. J Bone Joint Surg Am. 2009;91(Suppl 6):142–149. doi: 10.2106/jbjs.I.00574. [DOI] [PubMed] [Google Scholar]
- 28.Marmor S, et al. Multiplex antibody detection for noninvasive genus-level diagnosis of prosthetic joint infection. J Clin Microbiol. 2016;54(4):1065–1073. doi: 10.1128/jcm.02885-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cobo F, et al. Candida-induced prosthetic joint infection. A literature review including 72 cases and a case report. Infect Dis (Lond). 2017;49(2):81–94. doi: 10.1080/23744235.2016.1219456. [DOI] [PubMed] [Google Scholar]
- 30.Nett JE. Future directions for anti-biofilm therapeutics targeting Candida. Expert Rev Anti Infect Ther. 2014;12(3):375–382. doi: 10.1586/14787210.2014.885838. [DOI] [PubMed] [Google Scholar]
- 31.Pappas PG, et al. Clinical practice guideline for the management of candidiasis: 2016 Update by the infectious diseases society of America. Clin Infect Dis. 2016;62(4):e1–50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodríguez-Pardo D, et al. An update on surgical and antimicrobial therapy for acute periprosthetic joint infection: new challenges for the present and the future. Expert Rev Anti Infect Ther. 2015;13(2):249–265. doi: 10.1586/14787210.2015.999669. [DOI] [PubMed] [Google Scholar]
- 33.Wang B, et al. Epidemiology and microbiology of fracture-related infection: a multicenter study in Northeast China. J Orthop Surg Res. 2021;16(1):490. doi: 10.1186/s13018-021-02629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Depypere M, et al. The microbiological etiology of fracture-related infection. Front Cell Infect Microbiol. 2022;12:934485. doi: 10.3389/fcimb.2022.934485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baertl S, et al. What Is the Most Effective Empirical Antibiotic Treatment for Early, Delayed, and Late Fracture-Related Infections? Antibiotics (Basel). 2022;11(3):287. 10.3390/antibiotics11030287. [DOI] [PMC free article] [PubMed]
- 36.Kuehl R, et al. Time-dependent differences in management and microbiology of orthopaedic internal fixation-associated infections: an observational prospective study with 229 patients. Clin Microbiol Infect. 2019;25(1):76–81. doi: 10.1016/j.cmi.2018.03.040. [DOI] [PubMed] [Google Scholar]
- 37.Hao M, Peng AQ. Comparison of bacteria isolated from open fractures following debridement and subsequent infection. J Orthop Sci. 2021;26(2):243–246. doi: 10.1016/j.jos.2020.02.021. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, et al. Epidemiology and drug resistance of fracture-related infection of the long bones of the extremities: a retrospective study at the largest trauma center in Southwest China. Front Microbiol. 2022;13:923735. doi: 10.3389/fmicb.2022.923735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gallardo-Calero I, et al. Increased infection risk after hip hemiarthroplasty in institutionalized patients with proximal femur fracture. Injury. 2016;47(4):872–876. doi: 10.1016/j.injury.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 40.Cuchí E, et al. Relationship between skin and urine colonization and surgical site infection in the proximal femur fracture: a prospective study. Int Orthop. 2020;44(6):1031–1035. doi: 10.1007/s00264-020-04525-w. [DOI] [PubMed] [Google Scholar]
- 41.Yeramosu T, et al. Risk factors for infection and subsequent adverse clinical results in the setting of operatively treated pilon fractures. J Orthop Trauma. 2022 doi: 10.1097/bot.0000000000002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harding CM, Hennon SW, Feldman MF. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat Rev Microbiol. 2018;16(2):91–102. doi: 10.1038/nrmicro.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caricato A, et al. Risk factors and outcome of Acinetobacter baumanii infection in severe trauma patients. Intensive Care Med. 2009;35(11):1964–1969. doi: 10.1007/s00134-009-1582-5. [DOI] [PubMed] [Google Scholar]
- 44.Bryson DJ, et al. Antibiotic prophylaxis in orthopaedic surgery: difficult decisions in an era of evolving antibiotic resistance. Bone Joint J. 2016;98-b(8):1014–9. doi: 10.1302/0301-620x.98b8.37359. [DOI] [PubMed] [Google Scholar]
- 45.Kapadia BH, et al. Periprosthetic joint infection. Lancet. 2016;387(10016):386–394. doi: 10.1016/s0140-6736(14)61798-0. [DOI] [PubMed] [Google Scholar]
- 46.Zimmerli W, Sendi P. Orthopaedic biofilm infections. APMIS. 2017;125(4):353–364. doi: 10.1111/apm.12687. [DOI] [PubMed] [Google Scholar]
- 47.Murdoch DR, et al. Infection of orthopedic prostheses after Staphylococcus aureus bacteremia. Clin Infect Dis. 2001;32(4):647–649. doi: 10.1086/318704. [DOI] [PubMed] [Google Scholar]
- 48.Majumder MAA, et al. Antimicrobial Stewardship: Fighting Antimicrobial Resistance and Protecting Global Public Health. Infect Drug Resist. 2020;13:4713–4738. doi: 10.2147/idr.S290835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rupp M, Popp D, Alt V. Prevention of infection in open fractures: Where are the pendulums now? Injury. 2020;51(Suppl 2):S57–s63. doi: 10.1016/j.injury.2019.10.074. [DOI] [PubMed] [Google Scholar]
- 50.Dudareva M, et al. The microbiology of chronic osteomyelitis: Changes over ten years. J Infect. 2019;79(3):189–198. doi: 10.1016/j.jinf.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 51.Gramlich Y, et al. Salvage procedure for chronic periprosthetic knee infection: the application of DAIR results in better remission rates and infection-free survivorship when used with topical degradable calcium-based antibiotics. Knee Surg Sports Traumatol Arthrosc. 2020;28(9):2823–2834. doi: 10.1007/s00167-019-05627-8. [DOI] [PubMed] [Google Scholar]
- 52.Riesgo AM, et al. Vancomycin povidone-iodine protocol improves survivorship of periprosthetic joint infection treated with irrigation and debridement. J Arthroplasty. 2018;33(3):847–850. doi: 10.1016/j.arth.2017.10.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental table 1. Comparison of isolated microorganisms in early, delayed and late PJI.
Additional file 2: Supplemental table 2. Comparison of isolated microorganisms in early, delayed and late FRI.
Additional file 3: Supplemental table 3. Analysis of drug resistance rates and sensitivity rates of Gram-positive cocci in PJI.
Additional file 4: Supplemental table 4. Analysis of drug resistance rates and sensitivity rates of Gram-negative bacilli in PJI.
Additional file 5: Supplemental table 5. Analysis of drug resistance rates and sensitivity rates of Gram-positive cocci in FRI.
Additional file 6: Supplemental table 6. Analysis of drug resistance rates and sensitivity rates of Gram-negative bacilli in FRI.
Additional file 7: Supplemental Table 7. Isolated microorganisms in FRI after open fractures and closed fractures.
Data Availability Statement
All data used and analysed during the current study are available from the corresponding author on reasonable request.