Abstract
Background:
Out-of-pocket payment for prescription opioids is believed to be an indicator of abuse or diversion, but few studies describe its epidemiology. Prescription drug monitoring programs (PDMPs) collect controlled substance prescription fill data regardless of payment source and thus can be used to study this phenomenon.
Objective:
To estimate the frequency and characteristics of prescription fills for opioids that are likely paid out-of-pocket by individuals in the Oregon Medicaid program.
Research Design:
Cross-sectional analysis using Oregon Medicaid administrative claims and PDMP data (2012 to 2013).
Subjects:
Continuously enrolled nondually eligible Medicaid beneficiaries who could be linked to the PDMP with two opioid fills covered by Oregon Medicaid.
Measures:
Patient characteristics and fill characteristics for opioid fills that lacked a Medicaid pharmacy claim. Fill characteristics included opioid name, type, and association with indicators of high-risk opioid use.
Results:
A total of 33 592 Medicaid beneficiaries filled a total of 555 103 opioid prescriptions. Of these opioid fills, 74 953 (13.5%) could not be matched to a Medicaid claim. Hydromorphone (30%), fentanyl (18%), and methadone (15%) were the most likely to lack a matching claim. The 3 largest predictors for missing claims were opioid fills that overlapped with other opioids (adjusted odds ratio [aOR] 1.37; 95% confidence interval [CI], 1.34-1.4), long-acting opioids (aOR 1.52; 95% CI, 1.47-1.57), and fills at multiple pharmacies (aOR 1.45; 95% CI, 1.39-1.52).
Conclusions:
Prescription opioid fills that were likely paid out-of-pocket were common and associated with several known indicators of high-risk opioid use.
Keywords: drug abuse, drug utilization, Medicaid, pharmacoepidemiology, substance abuse
1 |. BACKGROUND
Prescription drug monitoring programs (PDMPs) are state-administered electronic databases of controlled medication fills that are used by clinicians and others to monitor for possible prescription drug abuse, diversion, and overdose risk. Implemented in 49 of 50 states, PDMPs have been enthusiastically embraced by federal and state health authorities. Emerging evidence suggests they are effective at reducing certain aspects of opioid prescribing and overdose deaths.1–3 Although a core function for PDMPs in most states is clinical monitoring, there is growing interest in using PDMP data for public health surveillance, epidemiologic, and health services research.4
One particular strength of PDMP data is its collection of all relevant prescription fills regardless of payer; thus, supplementing pharmacy claims with PDMP data has the potential to identify out-of-pocket cash payments. Individuals with insurance coverage for prescription drugs may choose to pay for medications completely out-of-pocket (as opposed to having the pharmacy submit a claim for full or partial reimbursement) for a variety of reasons, including paying a lower cost (eg, 4 dollar generic programs), circumventing pharmacy-based utilization controls, or minimizing administrative burden or oversight. Out-of-pocket payment for prescription opioids is believed to be associated with abuse and diversion, though this is poorly documented. There are few studies examining this behavior because most administrative data from payers or retail pharmacies do not capture out-of-pocket payments.5,6 Using data submitted directly from retail pharmacies, one study found that opioid pharmacy or prescriber shoppers were significantly more likely to pay out-of-pocket for opioids than those who were not opioid shoppers.6 In Washington state, one report found that over 2000 Medicaid beneficiaries had both a Medicaid pharmacy claim of and an out-of-pocket paid controlled substance prescription on the same day.7 Another recent study found that individuals pay out-of-pocket for opioids and benzodiazepines to avoid utilization management strategies used by state Medicaid programs.5
Coupling PDMP data to administrative claims provides an opportunity to better understand patient demographics, comorbidity, and health care utilization associated with out-of-pocket payment. The objective of this study was to estimate the frequency and characteristics of prescription fills for opioids that were likely paid out-of-pocket by individuals in the Oregon Medicaid program. In Oregon, the PDMP and the state’s Medicaid program are both housed in the Oregon Health Authority (OHA). We linked Oregon PDMP opioid fill data to Oregon’s Medicaid pharmacy claims data to quantify the frequency and characteristics of opioid prescription fills that lacked a pharmacy claim and likely were associated with out-of-pocket payment. We also examined whether these prescriptions were associated with indicators of abuse or diversion.
2 |. METHODS
2.1 |. Study overview
We identified a cohort of Oregon Medicaid beneficiaries with continuous, uninterrupted enrollment for a 2-year period from 2012 to 2013. By selecting a continuously enrolled cohort, we minimize the risk of missing Medicaid data. We linked Medicaid identifiers to the Oregon PDMP database containing Schedule II-IV controlled substance fills dispensed at outpatient pharmacies to Oregon residents using a probabilistic approach. Oregon’s PDMP does not collect payment source. We defined out-of-pocket payments as cases in which an opioid fill in the PDMP database could not be linked to a corresponding Medicaid pharmacy claim. Finally, we summarized patient and fill characteristics associated with these presumed out-of-pocket payments.
2.2 |. Data sources
We derived study data from 2 sources: Oregon’s PDMP and Medicaid administrative claims. Oregon’s PDMP became operational in 2011 and was fully implemented in 2012, with nearly all pharmacies reporting on a weekly basis. Oregon’s PDMP data include the following variables: patient name, date of birth, sex, address, prescriber name, pharmacy name, quantity dispensed, national drug code (NDC), and date dispensed. Oregon Medicaid administrative claims data include pharmacy claims, medical claims, demographics, and eligibility. Pharmacy claims data include variables such as patient identifier, NDC, quantity dispensed, days supply, and claim date. Claim date was the date the prescription claim was processed and not necessarily the date the prescription was dispensed to the patient.
2.3 |. Study sample
The first step was to create a linkage between individuals in the PDMP and Medicaid datasets. We identified a preliminary cohort of Medicaid beneficiaries with at least one pharmacy claim (of any drug) from January 2012 through December 2013. We then matched these individuals to a deduplicated database of individuals with at least one opioid fill in the PDMP database. Because there was no unique identifier common to both datasets (eg, social security number), we conducted probabilistic matching on last name, first name, date of birth, and zip code using LinkKing (v7.1). Blocking level was set to medium, suspicious dates of birth (eg, January 1, 2001) were manually reviewed, and zip codes were custom weighted, with matches being weighted positive and nonmatches being neutral. All other fields were weighted at their default levels. LinkKing creates ordinal “linkage certainty levels” where each level has a decreasing likelihood that the linked record pairs are a true match. We reviewed a random sample from each linkage certainty level. For those certainty levels less than 95% positive predictive value, we reviewed all potential matches within that certainty level.
The next step was to link the PDMP opioid fills to the Medicaid claims to identifying fills without matching claims. To reduce false positives (fills incorrectly categorized as not having a matching claim), we used an iterative matching algorithm intended to be as permissive as possible in matching fills to claims. We used a 4-step process of decreasing stringency. For the first step, we matched on NDC, quantity dispensed, and date (claim date in claim and dispense date in PDMP). Second, of the remaining unmatched fills and claims, we matched on NDC, quantity, and date dispensed within 7 days. Next, we collapsed and summed the quantity of any residual unmatched fills for the same NDC that were within 3 days to allow for the possibility that pharmacies sometime issue partial fills for non–Schedule II opioids (eg, hydrocodone before rescheduling). We assigned the earliest chronologic date to fills that were collapsed. We then matched these collapsed fills to unmatched claims by NDC, quantity, and date within 7 days. Finally, we matched remaining unmatched fills to claims if they could be matched on NDC, and date within 7 days, regardless of quantity. The remaining unmatched fills were considered potentially out-of-pocket paid fills.
After both matching processes, we applied the following patient inclusion criteria: at least one opioid fill that matched to a corresponding pharmacy claim in each year (a total of 2 years), continuous Medicaid eligibility without dual Medicare coverage, and no other insurance coverage (eg, workers’ compensation). We required one matching opioid fill in each study year to assure the cohort had active pharmacy benefits for the entire study period and ensure the patient linkage was accurate. We excluded beneficiaries who resided in a long-term care or community-based living facility because institutional pharmacies are exempt from PDMP reporting in Oregon.
Following our patient and prescription matching procedure, we summarized both patient and fill level characteristics. Patient characteristics included age, sex, race (white versus non-white), fill quantity, Medicaid enrollment type (disability versus other), and relevant diagnoses (spinal disorder, musculoskeletal pain, headache, cancer, and opioid, alcohol, and other drug-related use disorders) Comorbidities were identified using specific International Classification of Disease, 9th Revision Clinical Modification (ICD9-CM) (see Table S1 for definitions). We also summarized the following fill-level attributes: drug name, opioid type (long-acting versus short-acting opioids), and day of week dispensed. We excluded buprenorphine because it is used primarily for treating opioid use disorder. Methadone used for substance use disorder treatment was not captured by PDMP data.
Finally, we sought to evaluate the effect of unobserved out-of-pocket payments on indicators of high-risk opioid use, including when opioids overlap with other prescribed opioids or benzodiazepines, and multiple provider use.8 First, we compared differences in the measured frequency of these high-risk indicators using Medicaid data, PDMP data, and the combined Medicaid/PDMP linked dataset. The linked Medicaid/PDMP data included PDMP data that matched to a Medicaid claim as well as the residual Medicaid claims that were not matched to the PDMP. Next, we estimated the association between out-of-pocket payments and high-risk indicators. Because days’ supply was not included in the PDMP during the study period, we estimated overlapping fills using a proxy of prescriptions dispensed within 7 days of each other. We conducted sensitivity analyses based on a 4- and 14-day period as well. We defined multiple provider episodes as 4 or more prescriptions from different providers in a 90-day period.9 Estimating daily dosage was not possible because Oregon’s PDMP did not include day supply during our study period. We used multilevel hierarchical logistic regression to account for clustering of fills by patient to assess the statistical significance of these associations. For each model, the primary independent variable was a dichotomous indicator of whether the fill contributed to high-risk use (eg, was part of a multiple pharmacy episode) and the dependent variable was a dichotomous indicator of if fill was out-of-pocket or reimbursed by Medicaid. Adjusted estimates further account for person-level characteristics, specifically race (white versus non-white), age-quartile, and fill volume. Fill volume was defined as the number of opioid prescriptions over the 2-year period and was included because it could potentially confound the association between an out-of-pocket fill and high-risk indicators. Fill volume was standardized as (x−mean (x))/sd(x), where x is the total number of opioid fills. Statistical analyses were performed using SAS 9.2 and in R version 3.2.2 using the lme4 package.
3 |. RESULTS
3.1 |. Cohort summary
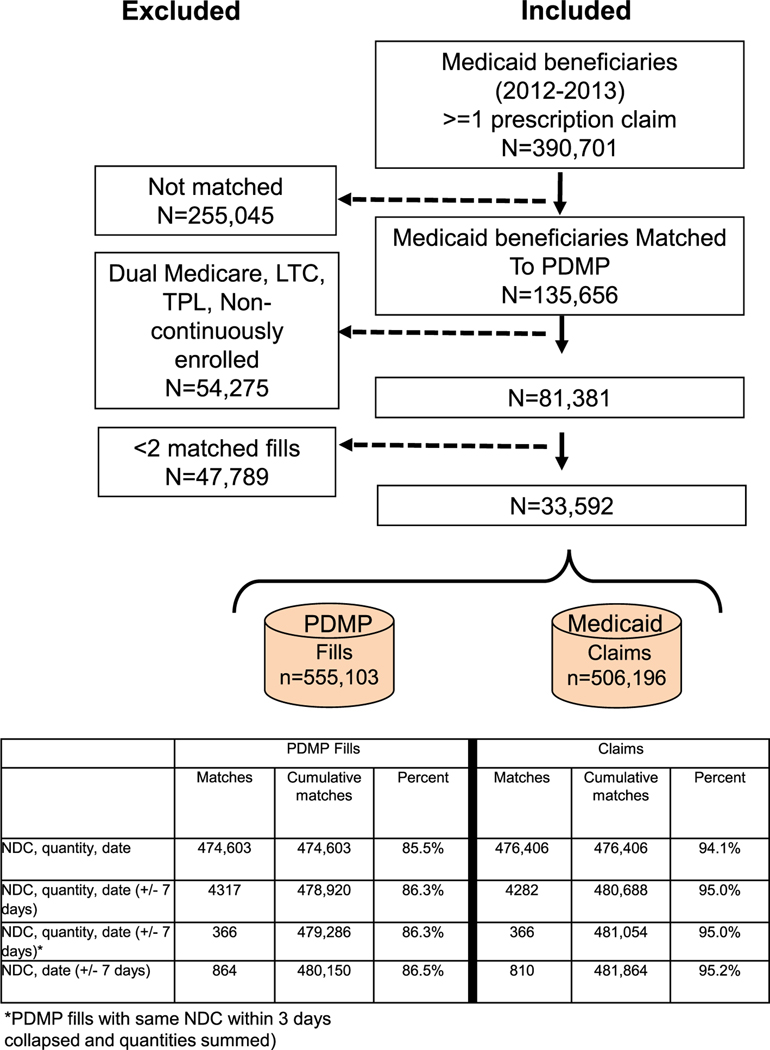
Figure 1 summarizes the results of the patient matching process and exclusions. We initially identified 390 701 unique Medicaid recipients with at least one outpatient pharmacy claim (any medication) from 2012 to 2013. Of this group, we matched 135 656 individuals with at least one opioid fill in the PDMP database. We excluded 54 275 individuals because they did not meet inclusion criteria and 47 789 individuals because they had fewer than 2 opioid fills that matched an outpatient pharmacy claim. The characteristics of the remaining 33 592 individuals in the study sample are summarized in Table 1. The majority of the sample was female and White, generally reflected the demography of Oregon’s Medicaid program. Most individuals were ages 30 to 49 (44%) with few younger than 20 or older than 64 years of age. Nearly one-third of the sample was eligible through disability criteria. A large majority of individuals had ICD9-CM diagnosis codes for either a spinal disorder (66%) or other musculoskeletal pain (75%). Nearly one-third (30%) had an ICD9-CM diagnosis code for a substance use disorder, with 10% having an opioid use disorder diagnosis.
FIGURE 1.
Summary of inclusion criteria. LTC, long-term care; NDC, national drug code; PDMP, prescription drug monitoring program; TPL, third party liability.
TABLE 1.
Demographic and diagnostic summary of study cohort;N = 33 592
| Characteristic Count (%) | N = 33 592 |
|---|---|
|
| |
| Female | 23 275 (69.3) |
| White | 27 585 (82.1) |
| Age | |
| <20 | 2216 (6.6) |
| 20–29 | 6379 (19.0) |
| 30–49 | 14 739 (43.9) |
| 50–64 | 10 117 (30.1) |
| >64 | 141 (0.4) |
| Opioid fills per patient (mean, sd) during study period | 16.9 (17.0) |
| Disability | 10 052 (29.9) |
| Diagnoses | |
| Spinal disorders | 22 193 (66.1) |
| Musculoskeletal pain | 25 091 (74.7) |
| Headache | 12 326 (36.7) |
| Cancer | 1857 (5.5) |
| Substance use disorder | 9983 (29.7) |
| Alcohol use disorder | 4108 (12.2) |
| Opioid use disorder | 3487 (10.4) |
| Other drug use disorder | 6449 (19.2) |
3.2 |. Matching PDMP fills to Medicaid claims
The study cohort generated 555 103 opioid fills and 506 196 opioid claims identified in the PDMP and Medicaid pharmacy claims data, respectively. Figure 1 summarizes results of matching procedure. Of the 555 103 PDMP fills, 474 603 (85.5%) were matched to a Medicaid claim with an exact match on NDC, date, and quantity. An additional 5547 (1.0%) PDMP fills were matched to a Medicaid claim after relaxing the matching criteria. Ultimately, we were unable to match 74 953 (13.5%) opioid fills in the PDMP to a Medicaid pharmacy claim. Of the 506 196 Medicaid claims, 24 332 (4.8%) could not be matched to a PDMP fill.
3.3 |. Characteristics of out-of-pocket opioid fills
As summarized in Table 2, the top 8 opioids in the PDMP dataset by fill volume represent over 98% of all opioids dispensed. The opioids with the highest proportion of PDMP fills lacking a Medicaid claim were hydromorphone (30%), fentanyl (18%), and methadone (17%).
TABLE 2.
Fills without corresponding Medicaid pharmacy claims by drug name
| Fills Without Medicaid Claim | Total Fills | Percent of Fills Without Medicaid Claim | |
|---|---|---|---|
|
| |||
| Hydrocodone/acetaminophen | 33 400 | 261 465 | 12.8% |
| Oxycodone | 13 974 | 112 794 | 12.4% |
| Oxycodone/acetaminophen | 9936 | 82 572 | 12.0% |
| Methadone | 5879 | 34 013 | 17.3% |
| Morphine sulfate | 4700 | 32 345 | 14.5% |
| Codeine/acetaminophen | 1110 | 10 018 | 11.1% |
| Hydromorphone | 2579 | 8558 | 30.1% |
| Fentanyl | 957 | 5207 | 18.4% |
| Other | 2418 | 8131 | 29.7% |
| Total | 74 953 | 555 103 | 13.5% |
In Table 3, we report the prevalence of high-risk opioid use using Medicaid data alone, PDMP data alone, and the combined dataset that includes PDMP data plus the residual Medicaid claims that were not matched to a PDMP fill (n = 24,332). At the prescription level, indicators of multiple provider were the most sensitive to using PDMP and PDMP/Medicaid linked data increasing 1.2% to 1.4% by including PDMP data. At the patient level, the prevalence of having any indicator increased by 1% to 2% by including PDMP data.
TABLE 3.
Comparison of high-risk opioid use patterns using Medicaid and prescription drug monitoring program data at patient and prescription level
| Prescription Level | Medicaid Data Count (%) N = 506 196 | PDMP Data Count (%) N = 555 103 | PDMP/Medicaid Linked Data Count (%) N = 579 435 |
|---|---|---|---|
|
| |||
| Opioid-opioid overlap | 184 967 (36.5%) | 204 507 (36.8%) | 214 025 (36.9%) |
| Opioid-benzodiazepine overlap | 64 823 (12.8%) | 74 478 (13.4%) | 77 756 (13.4%) |
| Multiple pharmacies | 28 408 (5.6%) | 38 864 (7%) | 40 486 (7%) |
| Multiple prescribers | 83 339 (16.5%) | 97 699 (17.6%) | 102 190 (17.6%) |
| Patient Level | Medicaid Data Count (%) N = 33 592 | PDMP Data Count (%) N = 33 592 | PDMP/Medicaid Linked Data Count (%) N = 33 592 |
|
| |||
| Opioid-opioid overlap | 18 246 (54.3%) | 18 248 (54.3%) | 18 938 (56.4%) |
| Opioid-benzodiazepine overlap | 8795 (26.2%) | 9353 (27.8%) | 9469 (28.2%) |
| Multiple pharmacies | 2047 (6.1%) | 2434 (7.2%) | 2593 (7.7%) |
| Multiple prescribers | 6625 (19.7%) | 7022 (20.9%) | 7418 (22.1%) |
Table 4 summarizes the association between PDMP fills lacking a Medicaid claim and fill and patient characteristics, including several indicators of high-risk opioid use. After adjusting for clustering and other patient characteristics, PDMP fills lacking pharmacy claims were significantly associated with opioid-opioid overlap, opioid-benzodiazepine overlap, multiple pharmacy and prescriber use, day of week (weekend versus otherwise), and opioid type (long versus short acting). The strength of associations was similar when we examined 4 and 14 days of opioid-opioid or opioid-benzodiazepine overlap (data not shown). The strongest association was multiple pharmacy use and opioid type. Fills that were part of a multiple pharmacy indicator were paid out-of-pocket 19.6% of the time, compared to 13.0% for fills not part of this indicator. In the multivariate model, opioids filled at multiple pharmacies had 45% higher odds, (adjusted odds ratio [aOR] 1.45; 95% confidence interval [CI], 1.39-1.52) of being paid out-of-pocket than opioids not part of this indicator. The odds of the patient paying out-of-pocket were 52% higher if the opioid was a long-acting opioid compared to a short-acting opioid (aOR 1.52; 95% CI, 1.47-1.57). Out-of-pocket payment for opioids was also significantly more common among individuals with diagnoses of spinal disorders, musculoskeletal pain, headache, and substance use disorders, but not cancer.
TABLE 4.
Proportion of fills without corresponding claims by fill and patient characteristics. Odds ratios are adjusted for clustering by individual, age, race, and number of fills
| Characteristic | Fills Without a Medicaid Claim 74 953 (A) | Total Fills 555 103 (B) | Percent of Fills Without Medicaid Claim (A/B) | Adjusted Odds Ratio (95% CI) |
|---|---|---|---|---|
|
| ||||
| Fill Characteristics | ||||
| Opioid-opioid overlap | ||||
| Yes | 32 043 | 204 507 | 15.7% | 1.37 |
| No | 42 910 | 350 596 | 12.2% | (1.34–1.4) |
| Opioid-benzodiazepine overlap | ||||
| Yes | 11 634 | 74 478 | 15.6% | 1.05 |
| No | 63 319 | 480 625 | 13.2% | (1.02–1.09) |
| Multiple pharmacies* | ||||
| Yes | 7627 | 38 864 | 19.6% | 1.45 |
| No | 67 326 | 516 239 | 13.0% | (1.39–1.52) |
| Multiple prescribers* | ||||
| Yes | 15 329 | 97 699 | 15.7% | 1.20 |
| No | 59 624 | 457 404 | 13.0% | (1.2–1.37) |
| Day of week | ||||
| Saturday or Sunday | 7623 | 51 768 | 14.7% | 1.18 |
| Monday to Friday | 67 330 | 503 335 | 13.4% | (1.14–1.22) |
| Opioid type | ||||
| Long-acting opioid | 11 846 | 72 934 | 16.2% | 1.52 |
| Short-acting opioid | 63 107 | 482 169 | 13.1% | (1.47–1.57) |
| Patient Characteristics | ||||
| Spinal disorders | ||||
| Yes | 60 851 | 434 236 | 14.0% | 1.31 |
| No | 14 102 | 120 867 | 11.7% | (1.24–1.39) |
| Musculoskeletal pain | ||||
| Yes | 62 969 | 462 886 | 13.6% | 1.11 |
| No | 11 984 | 92 217 | 13.0% | (1.04–1.18) |
| Headache | ||||
| Yes | 32 000 | 226 307 | 14.1% | 1.12 |
| No | 42 953 | 328 796 | 13.1% | (1.06–1.18) |
| Cancer | ||||
| Yes | 5705 | 41 327 | 13.8% | 0.99 |
| No | 69 248 | 513 776 | 13.5% | (0.9–1.1) |
| Alcohol use disorder | ||||
| Yes | 9548 | 66 791 | 14.3% | 1.15 |
| No | 65 405 | 488 312 | 13.4% | (1.06–1.23) |
| Opioid use disorder | ||||
| Yes | 13 137 | 84 866 | 15.5% | 1.15 |
| No | 61 816 | 470 237 | 13.1% | (1.06–1.24) |
| Other drug use disorder | ||||
| Yes | 16 977 | 120 810 | 14.1% | 1.07 |
| No | 57 976 | 434 293 | 13.3% | (1.01–1.14) |
Four or more fills/4 or more distinct providers in 90 days.
4 |. DISCUSSION
We found that over 13% of opioid prescriptions dispensed to Oregon Medicaid recipients could not be matched with a pharmacy claim, suggesting an out-of-pocket cash payment. Prescriptions for hydromorphone (30%), fentanyl (18%), and methadone (17%) had the highest likelihood of being paid for out-of-pocket. This is particularly alarming because fentanyl and methadone are among the highest risk of commonly used opioids. While it is not clear why the proportion of hydromorphone fills were paid out-of-pocket at a rate more than twice the overall average, misuse of hydromorphone has increased 438% nationally from 2004 to 2011, the greatest increase for any prescription opioid.10 Further, we found that several common indicators of abuse or diversion were significantly associated with probable out-of-pocket payment.
Our findings have at least 4 broad implications. First, there is a growing literature on the development and refinement of indicators of high-risk opioid use, misuse, or abuse and diversion. Studies document the frequency and harms associated with certain opioid misuse indicators such as doctor/pharmacy shopping or concurrent opioid use.8 Several organizations suggest that out-of-pocket payment is an indicator of misuse, but few studies have examined this because of inherent data limitations.11,12 One study using PDMP data from 5 states where source of payment is captured found that rates of out-of-pocket payment vary substantially, from 7.7% to 20.4%, although it was unclear what proportion of these individuals were paying out-of-pocket because they lacked coverage.13 Another study using retail pharmacy (rather than insurance company claims) fill data capturing payment source found that cash payment was significantly associated with opioid shopping behavior.6 Our findings are consistent with these reports. In addition to avoiding detection, individuals might opt for paying out-of-pocket to circumvent insurance program utilization controls such as copayments, early refill claim denials, or prior authorizations. During the study period, Oregon’s Medicaid program was administered through regional coordinated care organizations with distinct pharmacy benefit designs. Future research should be aimed at understanding how out-of-pocket payments affect opioid-directed utilization controls and vice versa.
Second, regardless whether the ultimate source of payment is out-of-pocket or another third-party payer, our findings indicate that state Medicaid programs likely lack a comprehensive picture of opioid utilization by their beneficiaries. This may affect the development of programs and policies aimed at reducing high-risk opioid prescriptions. A recent study of Medicaid recipients in North Carolina found that rates of opioid or benzodiazepine fills lacking a pharmacy claim increased from 16% to 55% following enrollment in the state’s lock-in program, which requires individuals to use the same prescriber and pharmacy for controlled substance prescriptions.5 This suggests that patients may circumvent utilization management tools aimed at reducing pharmacy shopping by paying cash. It is reasonable to assume a similar pattern may emerge when patients are faced with other opioid-related pharmacy benefit restrictions, such as prior authorization. In a more general sense, out-of-pocket payments likely distort analyses of interventions to combat opioid abuse when only pharmacy claims data are used.
Third, if out-of-pocket payment is an important indicator of potential opioid misuse, prescribers and pharmacists should consider adding payment source of previous prescriptions to checklists when reviewing patients’ PDMP data.
Finally, by recognizing that out-of-pocket payment could potentially make their utilization and pharmacy benefit management tools less effective, payers and state Medicaid programs can recommend their providers to check PDMP data for any high-risk and misuse indicators, so that these prescriptions can be avoided in the first place. The PDMPs should accurately collect source of payment to support its use by providers and payers. Currently, 45 of 49 state PDMPs collect source of payment.14
This study has several limitations. We restricted our study to include continuously enrolled Medicaid recipients with at least 2 covered opioid fills over a 2-year period. As such, our study sample is likely sicker and may not reflect a general Medicaid population with more dynamic enrollment, or a commercially insured population. Because there is no unique identifier common in both the Medicaid and PDMP data, we matched individuals in the Medicaid program to PDMP data using a probabilistic matching algorithm, and mismatches may have occurred. However, this seems unlikely because we also required individuals to have at least 2 opioid fills with matching claims. We may have failed to link all PDMP fills to existing claims for several reasons. Pharmacies may have partially dispensed certain (non–Schedule II fills) prescriptions over one or more days. The PDMP data include the date a prescription was “dispensed,” or released to the patient, while pharmacy claims data contain the service date, or the date that the prescription was processed by the pharmacy. After first matching on exact date matches, we allowed up to a 7-day difference between claim service date and PDMP dispense date to accommodate situations where the prescription was released to the patient more than a day after processing. We also cannot exclude data anomalies related to how the drug identity (NDC) was submitted in either data source. To accommodate these data anomalies, we applied an increasingly permissive algorithm to match PDMP opioid fills to Medicaid opioid claims, but false positives may remain. Although we used validated indicators of high-risk opioid use where possible, lack of day supply in the PDMP data source prevented us from computing overlap in the traditional way (ie, identifying overlapping episodes of therapy). Finally, we used nonmatching claims as a proxy measure of an out-of-pocket payments. Without PDMP payment source data, it is impossible to ascertain this with certainty. Replicating this study using data from a state with those data would validate our approach.
In summary, over 1 in 7 opioid fills by Medicaid recipients lacked an outpatient pharmacy claim, suggesting an out-of-pocket payment. This pattern was highest with several opioids with high abuse potential and other indicators of misuse or diversion. Future research should be directed at assessing the relationship between out-of-pocket payments and adverse patient outcomes such as overdose.8 State PDMPs would benefit from uniformly collecting these payment source data for programmatic and epidemiologic purposes. Payers, and specifically state Medicaid programs, should recognize that claims data provide an incomplete picture of opioid utilization. Sharing PDMP data within and across relevant state divisions would facilitate analyses with important public health implications.
Supplementary Material
KEY POINTS.
Out-of-pocket payment for prescription opioids is an understudied indicator of potential misuse, abuse, or diversion.
In this study of Medicaid beneficiaries, 13.5% of all prescription opioids identified in a state prescription drug monitoring program lacked a pharmacy reimbursement claim, indicating an out-of-pocket payment.
Hydromorphone, fentanyl, and methadone were the opioids most likely to be missing pharmacy claims.
Indicators of high-risk opioid use such as overlapping opioids, long-acting opioid use, and fills at multiple
ACKNOWLEDGEMENTS
This work is supported by a cooperative agreement with the Centers for Disease Control and Prevention in Atlanta, GA (1U011CE002500).
Funding information
Centers for Disease Control and Prevention in Atlanta, Grant/Award Number: 1U011CE002500
Footnotes
ETHICS STATEMENT
This study was approved by the Institutional Review Boards at the Oregon Health & Science University and Oregon Public Health Division. Data Use Agreements were executed with the Oregon Health Authority Department of Medical Assistance Programs and Public Health Division.
DISCLAIMER
The conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- 1.Patrick SW, Fry CE, Jones TF, Buntin MB. Implementation of prescription drug monitoring programs associated with reductions in opioid-related death rates. Health Aff. 2016;35(7):1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao Y, Pan Y, Taylor A, et al. Prescription drug monitoring programs are associated with sustained reductions in opioid prescribing by physicians. Health Aff. 2016;35(6):1045–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dowell D, Zhang K, Noonan RK, Hockenberry JM. Mandatory provider review and pain clinic laws reduce the amounts of opioids prescribed and overdose death rates. Health affairs (Project Hope). 2016;35(10): 1876–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Kane N, Hallvik SE, Marino M, et al. Preparing a prescription drug monitoring program data set for research purposes. Pharmacoepidemiol Drug Saf. 2016;25(9):993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts AW, Farley JF, Holmes GM, et al. Controlled substance lock-in programs: examining an unintended consequence of a prescription drug abuse policy. Health affairs (Project Hope). 2016;35(10): 1884–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cepeda MS, Fife D, Chow W, Mastrogiovanni G, Henderson SC. Opioid shopping behavior: how often, how soon, which drugs, and what payment method. J Clin Pharmacol. 2013;53(1):112–117. [DOI] [PubMed] [Google Scholar]
- 7.Using PDMPs to improve medical care: Washington State’s data sharing initiative with Medicaid and workers’ compensation. Prescription Drug Monitoring Program Center of Excellence at Brandeis University; 2013. [Google Scholar]
- 8.Park TW, Lin LA, Hosanagar A, Kogowski A, Paige K, Bohnert AS. Understanding risk factors for opioid overdose in clinical populations to inform treatment and policy. J Addict Med. 2016;10(6): 369–381. [DOI] [PubMed] [Google Scholar]
- 9.Yang Z, Wilsey B, Bohm M, et al. Defining risk of prescription opioid overdose: pharmacy shopping and overlapping prescriptions among long-term opioid users in Medicaid. The journal of pain: official journal of the American Pain Society. 2015;16(5):445–453. [DOI] [PubMed] [Google Scholar]
- 10.Atluri S, Sudarshan G, Manchikanti L. Assessment of the trends in medical use and misuse of opioid analgesics from 2004 to 2011. Pain Physician. 2014;17(2):E119–E128. [PubMed] [Google Scholar]
- 11.Stakeholders’ challenges and red flag warning signs related to prescribing and dispensing controlled substances national association of boards of pharmacy; March 2015 2015. [Google Scholar]
- 12.Pharmacists: on the front lines addressing prescription opioid abuse and overdose. 2016; http://www.cdc.gov/drugoverdose/pdf/pharmacists_brochure-a.pdf. Accessed November 4, 2016.
- 13.Paulozzi LJ, Strickler GK, Kreiner PW, Koris CM. Controlled substance prescribing patterns-prescription behavior surveillance system, eight states, 2013. Morbidity and mortality weekly report Surveillance summaries (Washington, DC: 2002). 2015;64(9):1–14. [DOI] [PubMed] [Google Scholar]
- 14.. Prescription drug monitoring program training adn technical assistance Center. 2016. http://www.pdmpassist.org/pdf/Payment_Method_Tracked.pdf. Accessed October 14, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.