Abstract
Objective:
Clinical interventions targeting co-occurring psychiatric disorders may represent a tangible target for improving retention in buprenorphine treatment for opioid use disorder. The aims of this study are to characterize receipt of antidepressants among patients receiving buprenorphine treatment and to examine the association between receiving antidepressants and retention in treatment.
Methods:
A retrospective cohort design was used. Using data from a large national commercially insured population, the cohort was selected as adults aged 18 to 64 years who initiated buprenorphine treatment in outpatient settings between January 1, 2016, and June 30, 2017. Receiving antidepressants was identified as prescription fills in the period between 6 months prior to buprenorphine initiation and during buprenorphine treatment. Buprenorphine discontinuation was defined as no buprenorphine prescription supply for at least 60 days following the end of the last buprenorphine prescription.
Results:
The cohort consisted of 11,619 individuals who initiated buprenorphine treatment and met our inclusion criteria. The cohort had a mean age of 36.3 years, 63% were male, and 55.7% received at least 1 antidepressant prescription at any time between 6 months prior to buprenorphine initiation and during treatment. Compared with those receiving no antidepressants at all, individuals starting antidepressants during buprenorphine treatment had an adjusted hazard ratio (HR) for treatment discontinuation of 0.72 (95% CI = 0.67–0.77), while receiving antidepressants only prior to buprenorphine initiation was associated with an increased risk of treatment discontinuation (HR = 1.40, 95% CI = 1.28–1.53).
Conclusions:
Findings suggest that receiving antidepressants during buprenorphine treatment is associated with improved retention. This highlights the critical importance of screening for and treating mental disorders concomitantly with treatment of opioid use disorder.
Driven by increases in overdoses involving synthetic opioids such as illicitly manufactured fentanyl, the opioid overdose epidemic in the US continues to worsen. Provisional data show more than 93,000 overdose deaths in the 12 months ending December 2020, a 29% increase from the 12 months ending December 2019.1 Similarly, syndromic surveillance data from emergency departments (EDs) in 29 states revealed that non-fatal opioid overdoses increased 9.7% between 2018 and 2019.2
Expanding access to medications for opioid use disorder (MOUD) treatment with buprenorphine in outpatient settings is a cornerstone of the US response to the opioid crisis.3,4 However, despite its well-documented effectiveness,5–8 buprenorphine treatment remains underutilized due to a number of patient and provider barriers.9–12 Further, the majority of patients prescribed buprenorphine are not retained in treatment for the recommended duration of minimum 6 months, with 50%–80% of patients discontinuing buprenorphine treatment within several weeks or a few months.13–16 Such discontinuation increases the risk of ED visits and opioid overdose, among other health and social problems associated with resumption of substance use.17–20
Several factors likely contribute to shorter patient retention in buprenorphine treatment, including stigma, attitudes of patients and providers about the role of MOUD, insurance-related barriers, and lack of provider training.21–23 More importantly, co-occurring substance use disorders (SUDs) and mental disorders such as depression have been identified as important clinical factors that undermine long-term retention in buprenorphine treatment and are linked to worse treatment outcomes.23–28 Clinical interventions targeting co-occurring psychiatric disorders may therefore represent a tangible strategy for improving retention in buprenorphine treatment.
Prior research has demonstrated the benefits of antidepressant treatment among patients receiving MOUD with co-occurring depression; however, much of this research focused on patients receiving methadone from opioid treatment programs and preceded the current epidemics of OUD and overdose.27,29 Few studies have used recent and large population level data to examine the prevalence of antidepressant receipt among patients receiving buprenorphine for OUD treatment and its impact on treatment retention.
To address this critical issue, we analyzed a large national commercial insurance claims database to examine and characterize antidepressant receipt among patients receiving buprenorphine for OUD treatment. In addition, we examined the association between antidepressant receipt and discontinuation from buprenorphine treatment while controlling for demographic characteristics, clinical factors, and use of other medications.
METHODS
Data Source
Data are from the IBM® MarketScan® Commercial Database. The database includes adjudicated and deidentified health insurance claims including inpatient, outpatient, prescriptions, and enrollment detail on approximately 27 million covered individuals each year from large employers and health plans across the US. Data from July 2015 to December 2018 were used for the analysis.
Design and Population
A retrospective cohort design was employed for this observational study. The study cohort consisted of commercially insured individuals aged 18–64 years who initiated buprenorphine treatment between January 2016 and June 2017. With data from July 2015 to December 2018, this period of initiation ensured a sufficient time period of baseline observation before initiation for every cohort member and ensured every cohort member could be followed up for as long as 18 months. Receiving buprenorphine was defined as buprenorphine fills that were identified from prescription claims by using National Drug Codes (NDCs). Buprenorphine products indicated for pain management (eg, Butrans, Belbuca) were excluded (Supplementary Table 1).
Individuals were included in the cohort if they (1) were continuously enrolled in insurance in the 6 months prior to and at least 6 months after the index buprenorphine prescription, (2) had both medical and prescription coverage during the enrolled period, (3) did not have a buprenorphine prescription during the 6 months prior to the index buprenorphine, and (4) had a buprenorphine treatment duration of at least 7 days in the follow-up period, in order to exclude individuals receiving buprenorphine possibly for short-term opioid withdrawal management.
Follow-up time started on the date of the index buprenorphine prescription, with maximum follow-up period of 18 months. Cohort members were censored if (1) they disenrolled before the event was observed or (2) their follow-up time reached 18 months before the event was observed. This study was reviewed by the Centers for Disease Control and Prevention (CDC) and determined to meet the definition of research as defined in 46.102(l) but did not involve human subjects as defined in 46.103(e)(1). The study was conducted consistent with applicable federal law and CDC policy.*
Outcome Measures and Daily Buprenorphine Dosage
The main outcomes were the event of discontinuation of buprenorphine treatment and treatment duration (time to discontinuation). We defined discontinuation as having > 60 follow-up days not covered by a buprenorphine supply following the end of the last fill of buprenorphine. Filling date and days’ supply were used to calculate treatment duration. The following rules were employed: if there was no overlap between prescriptions, days’ supply of all prescriptions and gaps between prescriptions were summed to calculate buprenorphine treatment duration; if there was a ≤ 7 day overlap between 2 prescriptions, we assumed that the second prescription started the day after the first prescription ended, ie, early refill; if the overlap was more than 7 days, the overlapping days were counted once. We created the following treatment duration categories: 7–30, 31–90, 91–180, 181–365, and > 365 days.15,18,30
To calculate daily buprenorphine dose, we first converted the dosage of all non-Suboxone buprenorphine to Suboxone dosage equivalent (conversion factors found in Supplementary Table 1).31–33 Daily dose was calculated as the mean daily dose of all buprenorphine prescriptions each person filled. Each person was grouped into one of the following dose categories: 2–8, 9–13, 14–16, 17–24, and > 24 mg.
Receipt of Antidepressants
Receipt of antidepressants among cohort members was defined as antidepressant prescription fills, which were identified from prescription claims using NDCs. We identified 4 categories of antidepressant prescriptions: selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, and other (Supplementary Table 2).
We defined receipt of antidepressants during buprenorphine treatment as a time-varying binary indicator that started being yes when first antidepressant was filled, regardless of the antidepressant type. In additional to the period during buprenorphine treatment, we captured any antidepressant fills during the 6 months prior to initiating buprenorphine and measured the total days’ supply of antidepressants received. To characterize antidepressant receipt among cohort members, they were categorized into 4 groups based on their status of antidepressant receipt prior to and during treatment: no antidepressant receipt at all, antidepressant receipt only in the 6 months prior to buprenorphine treatment, antidepressant receipt only during treatment, and antidepressant receipt both in the 6 months prior to and during treatment.
To measure the length of antidepressant use during buprenorphine treatment while accounting for survival time bias, we counted the number of treatment days that were covered by antidepressants so that the percent of total buprenorphine days with antidepressants could be calculated. We further subdivided covered treatment days by specific types of antidepressant.
Other Covariates
Gender and age (categorized as 18–34, 35–44, 45–54, and 55–64 years) when buprenorphine was initiated were obtained from the enrollment file. Three groups of co-occurring comorbidities among cohort members at baseline were identified with ICD-10-CM diagnosis codes from outpatient and/or inpatient medical claims in the 3-month period prior to buprenorphine treatment initiation: SUDs other than opioid (eg, alcohol use disorder, cannabis use disorder, cocaine use disorder), psychiatric disorders (eg, anxiety, bipolar disorder, major depression, schizophrenia), and chronic pain conditions (eg, back pain, neck pain, fibromyalgia, osteoarthritis) (Supplementary Table 3).
Receipt of major psychotropic medications by cohort members were identified from prescription claims across 4 medication classes: benzodiazepines, opioid analgesics, stimulants, and other psychotropic medications (Supplementary Table 4). Receipt of these psychotropic medications was defined and measured in similar ways as antidepressants, with the exception that they were not measured as time varying.
Statistical Analysis
Status of antidepressant receipt, percentage of buprenorphine treatment days covered by antidepressants, and all other covariates were compared between buprenorphine treatment duration categories to assess their relationships by using bivariate analyses. We used χ2 tests for dichotomous and categorical variables and Kruskal-Wallis test for continuous variables.
Kaplan-Meier curves accounting for time-varying antidepressant receipt during treatment were used to compare the probability of not being discontinued from buprenorphine treatment. Next, Cox proportional hazards models were used to estimate hazard ratios (HRs) for buprenorphine treatment discontinuation associated with time-varying status of antidepressant receipt during treatment. Thus, days of buprenorphine treatment before the start of antidepressants was counted as at risk, with the purpose of accounting for survival time bias. We controlled for the status of antidepressant receipt prior to buprenorphine treatment and included the interaction between it and receipt status during treatment. The model adjusted for all other covariates examined. Robust sandwich variance estimates were used to adjust standard errors, accounting for potential clustering of cohort members.
We conducted 3 sets of sensitivity analyses. First, we ran the Cox proportional hazard model restricted to individuals with current OUD diagnosis (ICD-10-CM codes F11.1X, F11.2X) during the 3 months prior to treatment. Second, we ran the Cox proportional hazard model by changing the definition of buprenorphine treatment discontinuation from 60 days to 30 days without supply. Third, we ran the Cox proportional hazard model restricted to individuals who did not receive antidepressants during buprenorphine treatment and those who received antidepressants for less than 2 weeks during buprenorphine treatment (short duration of antidepressant treatment is not effective in treating depression; thus, no impact on buprenorphine treatment retention). All analyses were conducted using Stata version 14.1 (StataCorp). Statistical significance was set at P < .05.
RESULTS
Sample
The cohort consisted of 11,619 individuals who initiated buprenorphine treatment between January 1, 2016, and June 30, 2017 (Table 1). Among cohort members, the mean age was 36.3 years; 63% were male. Mean and median buprenorphine treatment duration was 250.4 and 189 days, respectively. SUDs, psychiatric disorders, and chronic pain conditions at baseline were prevalent among 22.0%, 39.3%, and 38.2% of cohort members, respectively.
Table 1.
Baseline (3 Months Prior to Index Buprenorphine Prescription) Characteristics and Follow-up Buprenorphine Treatment Characteristics, by Buprenorphine Treatment Duration, Among a Cohort Who Initiated Buprenorphine Treatment Between January 2016 and June 2017
| Buprenorphine treatment duration | |||||||
|---|---|---|---|---|---|---|---|
| Overall | 7–30 Days | 31–90 Days | 91–180 Days | 181–365 Days | > 365 Days | P value | |
| Characteristic | (N = 11,619) | (N = 2,180) | (N = 1,731) | (N = 1,559) | (N = 2,478) | (N = 3,671) | |
| Age, mean, y | 36.3 | 35.3 | 33.9 | 35.4 | 36.4 | 38.3 | < .001 |
| Age groups, % | < .001 | ||||||
| 18–34 y | 50.0 | 54.9 | 59.0 | 53.9 | 49.7 | 41.4 | |
| 35–44 y | 22.8 | 17.6 | 17.6 | 21.1 | 24.4 | 28.1 | |
| 45–54 y | 16.2 | 14.7 | 14.3 | 15.2 | 15.9 | 18.5 | |
| 55–64 y | 11.0 | 12.8 | 9.1 | 9.8 | 10.0 | 12.0 | |
| Male, % | 63.0 | 61.3 | 62.2 | 62.9 | 62.6 | 64.6 | .120 |
| Baseline substance use disorder diagnosis, % | |||||||
| Alcohol use disorder | 7.5 | 10.3 | 8.4 | 7.2 | 6.6 | 6.2 | < .001 |
| Cannabis use disorder | 6.2 | 9.0 | 8.7 | 7.1 | 4.5 | 4.3 | < .001 |
| Cocaine use disorder | 3.5 | 5.2 | 5.2 | 4.0 | 2.4 | 2.3 | < .001 |
| Sedative and hypnotic related disorder | 6.0 | 7.7 | 7.9 | 6.0 | 5.0 | 4.5 | < .001 |
| Stimulant use disorder | 3.4 | 5.1 | 5.1 | 4.1 | 2.8 | 1.8 | < .001 |
| Other psychoactive substance use disorder | 11.5 | 14.0 | 14.2 | 11.6 | 9.8 | 9.8 | < 0001 |
| Any of the above substance use disorder diagnoses | 22.0 | 27.8 | 26.8 | 21.9 | 19.2 | 18.4 | < .001 |
| 2+ of the above substance use disorder diagnoses | 8.9 | 12.2 | 11.8 | 9.3 | 7.4 | 6.6 | < .001 |
| Baseline psychiatric disorder diagnosis, % | |||||||
| Anxiety | 25.1 | 26.0 | 26.8 | 24.8 | 24.6 | 24.2 | .222 |
| Bipolar disorder | 5.0 | 6.6 | 6.5 | 4.1 | 4.2 | 4.3 | < .001 |
| Major depression | 20.2 | 23.1 | 20.7 | 20.7 | 19.0 | 18.8 | .001 |
| Other mood disorder | 3.5 | 3.7 | 4.0 | 2.9 | 3.4 | 3.4 | .411 |
| ADHD | 6.5 | 6.4 | 6.8 | 6.2 | 6.9 | 6.2 | .744 |
| PTSD | 2.3 | 2.8 | 2.4 | 2.7 | 2.4 | 1.7 | .058 |
| Schizophrenia | 0.9 | 1.4 | 1.1 | 0.7 | 0.7 | 0.7 | .033 |
| Any of the above psychiatric diagnoses | 39.3 | 41.7 | 41.6 | 38.9 | 37.9 | 37.7 | .005 |
| 2+ of the above psychiatric diagnoses | 17.7 | 19.9 | 19.3 | 16.8 | 17.1 | 16.5 | .006 |
| Baseline pain condition diagnosis, % | |||||||
| Back pain | 26.8 | 29.5 | 25.7 | 26.0 | 25.6 | 26.7 | .020 |
| Neck pain | 10.8 | 12.5 | 10.4 | 10.5 | 10.0 | 10.5 | .055 |
| Migraine | 3.7 | 3.9 | 4.2 | 3.3 | 3.7 | 3.6 | .655 |
| Fibromyalgia | 2.2 | 3.0 | 2.1 | 1.7 | 2.3 | 1.9 | .052 |
| Osteoarthritis/joint cartilage | 6.3 | 6.6 | 5.2 | 5.6 | 5.4 | 7.4 | .003 |
| Inflammatory joint disorder | 14.2 | 15.9 | 13.6 | 13.8 | 13.5 | 14.0 | .129 |
| Periarticular/soft tissue disorders | 3.8 | 4.6 | 4.2 | 4.5 | 3.1 | 3.3 | .018 |
| Any of the above pain diagnoses | 38.2 | 41.0 | 37.0 | 37.1 | 36.4 | 38.8 | .015 |
| 2+ of the above pain diagnoses | 18.9 | 21.6 | 17.5 | 18.8 | 18.1 | 18.6 | .008 |
| Buprenorphine treatment characteristics | |||||||
| Days of buprenorphine treatment, mean (median) | 250.4 (189) | 19.4 (20) | 59.8 (58) | 134.4 (133) | 263.2 (257) | 518.2 (547) | < .001 |
| Daily dose in mg, mean (median) | 13.8 (15) | 12.6 (12) | 14.0 (15) | 14.2 (15) | 14.2 (15) | 14.1 (15) | < .001 |
| Daily dose categories, % | < .001 | ||||||
| 2–8 mg | 25.8 | 38.4 | 24.0 | 23.2 | 22.0 | 22.9 | |
| 9–13 mg | 15.2 | 11.7 | 17.3 | 15.8 | 15.2 | 15.8 | |
| 14–16 mg | 37.1 | 34.3 | 38.1 | 38.6 | 38.4 | 36.8 | |
| 17–24 mg | 19.6 | 13.9 | 17.3 | 19.4 | 22.5 | 22.4 | |
| > 24 mg | 2.3 | 1.7 | 3.3 | 3.0 | 2.0 | 2.1 | |
Abbreviations: ADHD = attention-deficit/hyperactivity disorder, PTSD = posttraumatic stress disorder.
Demographic and Baseline Clinical Characteristics by Treatment Duration Group
Age group distributions were significantly different across treatment duration groups (Table 1). Individuals with shorter treatment duration tended to be younger. For instance, 54.9% of individuals with buprenorphine duration of 7–30 days and 59.0% with a duration of 31–90 days were aged 18–34 years, whereas only 41.4% of individuals with treatment duration > 365 days were aged 18–34 years. Individuals with co-occurring cannabis use disorder and co-occurring cocaine use disorder were twice as likely to be in the shortest duration group compared to the longest duration group (9.0% vs 4.3%, 5.2% vs 2.3%, respectively). Unlike SUD, the prevalence of psychiatric and chronic pain conditions across different treatment durations was not always significantly different and varied by specific conditions.
Receipt of Antidepressants and Major Psychotropic Drugs
Overall, 55.7% of cohort members received antidepressants at any time between the 6 months prior to treatment and during treatment: 9.5% received antidepressants only prior to treatment, 17.4% received antidepressants only during buprenorphine treatment, and 28.8% received antidepressants in both periods (Table 2). Among those who received antidepressants during treatment, antidepressants covered an average of 31% of buprenorphine treatment days.
Table 2.
Characteristics of Received Antidepressants and Major Psychotropic Medications by Buprenorphine Treatment Duration Among a Cohort Who Initiated Buprenorphine Treatment Between January 2016 and June 2017
| Buprenorphine treatment duration | |||||||
|---|---|---|---|---|---|---|---|
| Overall | 7–30 Days | 31–90 Days | 91–180 Days | 181–365 Days | >365 Days | P value | |
| (N = 11,619) | (N = 2,180) | (N = 1,731) | (N = 1,559) | (N = 2,478) | (N = 3,671) | ||
| Antidepressants | |||||||
| Status of antidepressant use, % | |||||||
| No antidepressants during buprenorphine treatment | |||||||
| No antidepressants at all | 44.3 | 50.0 | 46.2 | 44.7 | 44.7 | 39.4 | < .001 |
| Antidepressants only in 6 mo prior to treatment | 9.5 | 20.2 | 11.9 | 8.6 | 6.1 | 4.7 | < .001 |
| Antidepressants during buprenorphine treatment | |||||||
| Antidepressants only during treatment | 17.4 | 11.2 | 13.2 | 16.9 | 18.2 | 22.9 | < .001 |
| Antidepressants both in 6 mo prior to and during treatment | 28.8 | 18.6 | 28.7 | 29.8 | 31.0 | 33.0 | < .001 |
| Days’ supply of antidepressant use prior to buprenorphine treatment, mean (median) | |||||||
| Among antidepressants only in 6 mo prior to treatment | 76.1 (60) | 90.7 (85) | 74.6 (60) | 66.0 (60) | 61.0 (53) | 62.8 (53) | < .001 |
| Among antidepressants both in 6 mo prior to and during treatment | 118.3 (131) | 118.3 (128) | 117.1 (132) | 119.0 (130) | 118.4 (129) | 119.3 (135) | .970 |
| Average percent of total buprenorphine treatment days covered by antidepressants, % | |||||||
| All types of antidepressants | 31.0 | 26.0 | 29.7 | 30.6 | 30.4 | 35.0 | < .001 |
| Antidepressant type | |||||||
| SSRI (eg, escitalopram, sertraline, fluoxetine) | 13.8 | 8.0 | 12.8 | 13.4 | 13.9 | 17.7 | < .001 |
| SNRI (eg, duloxetine, venlafaxine, desvenlafaxine) | 6.8 | 4.1 | 5.8 | 7.8 | 7.0 | 8.2 | < .001 |
| TCA (eg, amitriptyline, doxepin, nortriptyline) | 2.5 | 2.9 | 2.6 | 2.7 | 2.1 | 2.5 | .192 |
| Other (eg, trazodone, bupropion, mirtazapine) | 13.3 | 15.3 | 13.1 | 12.0 | 12.3 | 13.3 | < .001 |
| Major psychotropic medications | |||||||
| Benzodiazepines, % | |||||||
| Any benzodiazepine | 35.5 | 38.0 | 34.4 | 34.8 | 36.0 | 36.0 | .074 |
| Any benzodiazepine prior to treatment | 27.4 | 32.0 | 29.0 | 27.6 | 26.5 | 24.6 | < .001 |
| Any benzodiazepine during treatment | 25.2 | 22.5 | 21.7 | 25.0 | 27.8 | 28.2 | < .001 |
| Percent of total eligible buprenorphine treatment days with benzodiazepines | 16.0 | 21.6 | 15.5 | 15.3 | 16.2 | 13.2 | < .001 |
| Opioid analgesics, % | |||||||
| Any opioid analgesic | 46.5 | 45.1 | 44.3 | 42.8 | 45.4 | 51.9 | < .001 |
| Any opioid analgesic prior to treatment | 42.0 | 44.3 | 42.6 | 40.0 | 40.0 | 43.3 | .001 |
| Any opioid analgesic during treatment | 17.4 | 13.3 | 13.7 | 14.8 | 18.3 | 24.7 | < .001 |
| Percent of total eligible buprenorphine treatment days with opioid analgesics | 6.7 | 16.3 | 9.1 | 5.9 | 3.5 | 2.3 | < 0.001 |
| Stimulants, % | |||||||
| Any stimulant | 13.6 | 10.8 | 12.7 | 13.0 | 15.4 | 15.8 | < .001 |
| Any stimulant prior to treatment | 9.7 | 10.1 | 10.7 | 9.4 | 10.2 | 8.8 | .147 |
| Any stimulant during treatment | 10.8 | 6.0 | 8.4 | 10.6 | 13.6 | 14.1 | < .001 |
| Percent of total eligible buprenorphine treatment days with stimulants | 8.2 | 6.9 | 7.2 | 7.7 | 10.1 | 8.5 | < .001 |
| Other psychotropic medications, % | |||||||
| Any other psychotropic | 41.0 | 41.3 | 42.5 | 40.0 | 39.8 | 43.9 | .375 |
| Any other psychotropic prior to treatment | 28.1 | 31.2 | 31.9 | 28.4 | 26.0 | 25.8 | < .001 |
| Any other psychotropic during treatment | 32.4 | 27.6 | 31.2 | 30.9 | 33.8 | 38.7 | < .001 |
| Percent of total eligible buprenorphine treatment days with other psychotropics | 22.3 | 28.9 | 25.4 | 21.2 | 20.1 | 19.0 | < .001 |
Abbreviations: SNRI = serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor, TCA = tricyclic antidepressant.
Antidepressant receipt differed by treatment duration. The shortest duration group had the highest prevalence of receiving antidepressants only prior to treatment (20.2%), more than 4 times the prevalence of the longest duration group (4.7%). The inverse relationship was observed for the prevalence of receiving antidepressants during buprenorphine treatment, where prevalence of the longest duration group was twice that of the shortest group (55.9% vs 29.8%).
Receipt of major psychotropic medications was less common than antidepressants: 46.5% of cohort members received any opioid analgesics, followed by other psychotropic drugs (41.0%), benzodiazepines (35.5%), and stimulants (13.6%). Opioid analgesics were the most commonly received medications prior to treatment (42.0%), while other psychotropics were the most commonly received medications during treatment (32.4%).
Kaplan-Meier Curves and Cox Proportional Hazard Model
Kaplan-Meier curves in Figure 1 show the probability of not being discontinued from buprenorphine treatment by antidepressant receipt status. Individuals with no receipt of antidepressants during treatment had lower probability at any time. By contrast, those who received antidepressants during buprenorphine treatment had a higher probability of not being discontinued.
Figure 1.
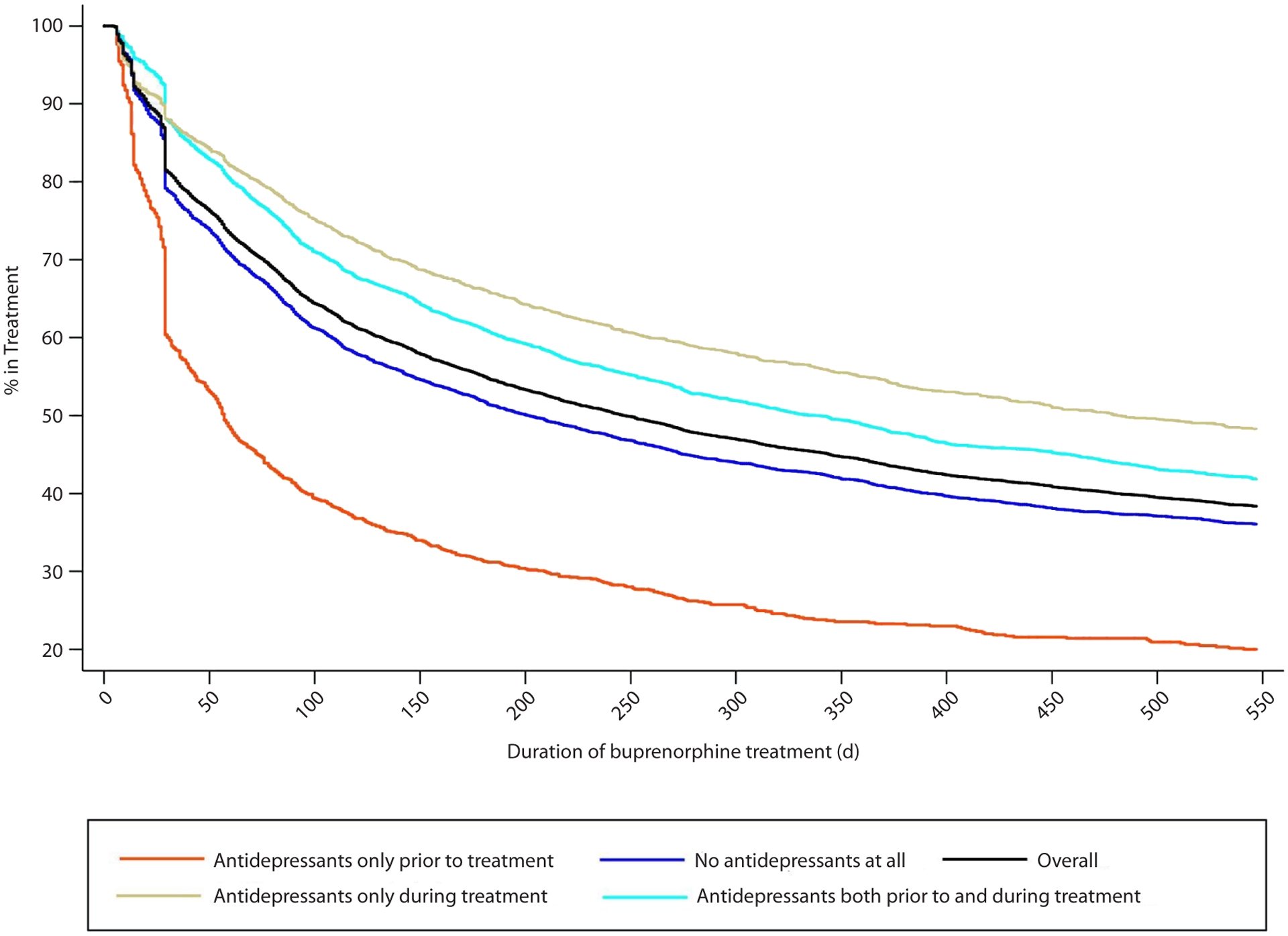
Kaplan-Meier Curves of Buprenorphine Treatment Duration by Status of Antidepressant Receipt
After adjusting for demographics, buprenorphine dosage, baseline clinical factors, and use of major psychotropics in a Cox proportional hazard model, receipt of antidepressants during buprenorphine treatment was associated with lower risk of treatment discontinuation compared with no receipt of antidepressants (Table 3, Figure 2). For instance, when compared with no receipt of antidepressants during buprenorphine treatment, individuals receiving antidepressants only during treatment had an HR of 0.72 (95% confidence interval [CI] = 0.67–0.77). Individuals receiving antidepressant both in the 6 months prior to and during treatment had the lowest HR of 0.51 [95% CI = 0.46–0.56]. Main results from the first 2 sets of sensitivity analyses were consistent with findings from the primary analysis. The association between receiving antidepressants and lower risk of buprenorphine treatment discontinuation was not found in the third sensitivity analysis, which indicated the robustness of our primary analysis (Supplementary Table 5).
Table 3.
Results of Cox Proportional Hazard Model for Buprenorphine Treatment Discontinuation
| Parameter | Level | Hazard ratio | z Value | P > |z| | 95% CI |
|---|---|---|---|---|---|
| Antidepressant status (ref = no antidepressants at all) | |||||
| Antidepressants in 6 mo prior to and during treatment | 0.505 | −14.48 | < .001 | 0.461–0.555 | |
| Age group (ref = 18–34 y) | |||||
| 55–64 y | 0.850 | −3.65 | < .001 | 0.779–0.928 | |
| Daily dose of buprenorphine (ref = 2–8 mg) |
|||||
| > 24 mg | 0.909 | −1.16 | .247 | 0.772–1.069 | |
| Gender (ref = female) | Male | 0.918 | −3.16 | .002 | 0.871–0.968 |
| Use of benzodiazepine (ref = no benzodiazepines at all) | |||||
| Benzodiazepines in 6 mo prior to and during treatment | 1.178 | 4.41 | < .001 | 1.095–1.267 | |
| Opioid analgesics in 6 mo prior to and during treatment | 0.935 | −1.55 | .120 | 0.858–1.018 | |
| Stimulants in 6 mo prior to and during treatment | 0.880 | −2.18 | .029 | 0.785–0.987 | |
| Use of other psychotropic prescriptions (OPP) (ref = no OPP at all) | |||||
| OPP in 6 mo prior to and during treatment | 1.001 | 0.03 | .977 | 0.930–1.078 | |
| Alcohol use disorder | Diagnosis present at baseline | 1.034 | 0.63 | .530 | 0.932–1.147 |
| Cannabis use disorder | Diagnosis present at baseline | 1.258 | 4.05 | < .001 | 1.125–1.406 |
| Cocaine use disorder | Diagnosis present at baseline | 1.250 | 3.09 | .002 | 1.085–1.439 |
| Sedative and hypnotic related disorder | Diagnosis present at baseline | 0.983 | −0.28 | .776 | 0.874–1.105 |
| Stimulant use disorder | Diagnosis present at baseline | 1.315 | 4.01 | < .001 | 1.150–1.504 |
| Stimulant use disorder | Diagnosis present at baseline | 1.315 | 4.01 | < .001 | 1.150–1.504 |
| Other psychoactive substance use disorder | Diagnosis present at baseline | 1.048 | 1.10 | .270 | 0.964–1.140 |
| Anxiety | Diagnosis present at baseline | 0.939 | −1.89 | .059 | 0.879–1.002 |
| Bipolar disorder | Diagnosis present at baseline | 1.116 | 1.86 | .063 | 0.994–1.253 |
| Major depression | Diagnosis present at baseline | 1.085 | 2.29 | .022 | 1.012–1.163 |
| Other mood disorder | Diagnosis present at baseline | 0.938 | −0.87 | .386 | 0.810–1.085 |
| ADHD | Diagnosis present at baseline | 1.091 | 1.41 | .159 | 0.966–1.231 |
| PTSD | Diagnosis present at baseline | 1.031 | 0.38 | .702 | 0.880–1.209 |
| Schizophrenia | Diagnosis present at baseline | 1.024 | 0.18 | .860 | 0.789–1.327 |
| Back pain | Diagnosis present at baseline | 1.040 | 1.16 | .247 | 0.973–1.111 |
| Neck pain | Diagnosis present at baseline | 1.066 | 1.45 | .146 | 0.978–1.161 |
| Migraine | Diagnosis present at baseline | 1.069 | 1.04 | .297 | 0.943–1.213 |
| Fibromyalgia | Diagnosis present at baseline | 1.176 | 1.95 | .051 | 0.999–1.383 |
| Osteoarthritis/joint cartilage | Diagnosis present at baseline | 0.986 | −0.25 | .800 | 0.886–1.098 |
| Inflammatory joint disorder | Diagnosis present at baseline | 1.000 | 0.01 | .991 | 0.923–1.085 |
| Periarticular/soft tissue disorders | Diagnosis present at baseline | 1.299 | 4.17 | < .001 | 1.149–1.469 |
Abbreviations: ADHD = attention-deficit/hyperactivity disorder, PTSD = posttraumatic stress disorder.
Figure 2.
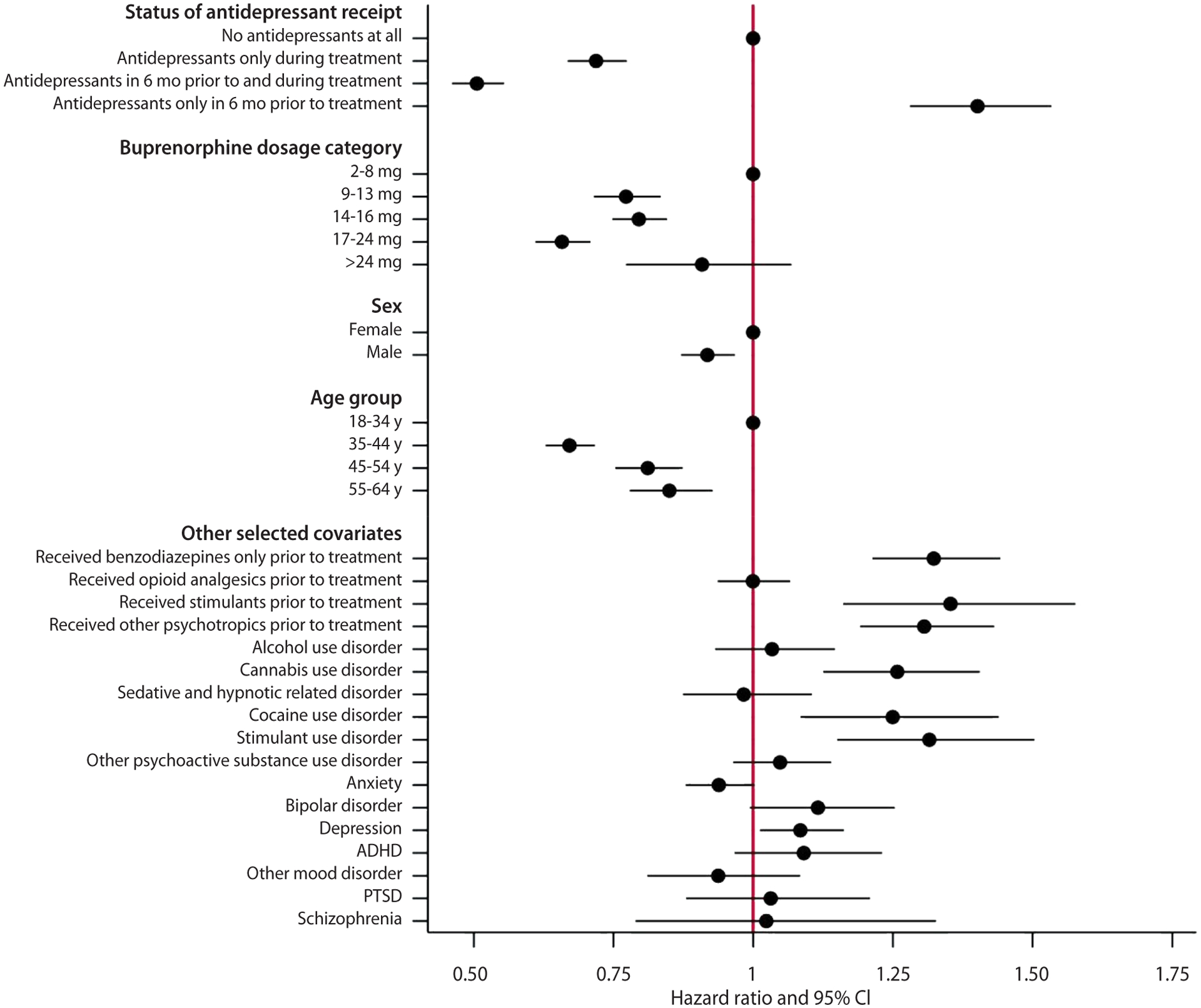
Hazard Ratios of Factors Associated With Discontinuing Buprenorphine Treatment
DISCUSSION
In this national sample of commercially insured individuals in the US, we found that receipt of antidepressants was common (55.7%) during any time between 6 months prior to buprenorphine initiation and during treatment in the overall cohort. SSRIs and other types (eg, trazodone, bupropion, mirtazapine) of antidepressants on average covered a higher percentage of buprenorphine treatment days among those who received antidepressants during treatment. Receipt of antidepressants during buprenorphine treatment was significantly associated with reduced risk of treatment discontinuation, even after accounting for demographics, buprenorphine dosage, receipt of other psychotropic medications, and co-occurring SUDs, psychiatric disorders, and pain conditions. In addition, the magnitude of this reduced risk varied by the status of whether receiving antidepressants prior to treatment initiation (49% reduction and 23% reduction, respectively). In contrast, no receipt of antidepressants during treatment was associated with increased risk of treatment discontinuation, particularly among those receiving antidepressants during 6 months prior to buprenorphine initiation, with an HR of 1.40 (95% CI = 1.28–1.53).
Our finding of an overall buprenorphine treatment retention rate of approximately 45% (Figure 1) at 1 year after initiation is consistent with retention rates previously observed among commercially insured populations and somewhat higher than those observed among Medicaid populations.23,34,35 Similar to prior research, we also found relatively high rates of co-occurring psychiatric disorders, SUDs, and pain conditions among individuals receiving buprenorphine.25,28,36 However, these rates could be underestimating the true prevalence due to the short 3-month look-back period for capturing them. Given the chronic nature of these co-occurring conditions, it was likely that patients’ diagnoses would be captured by claims data outside the 3-month window. Thus, our baseline co-occurring conditions should be interpreted more as current than as past.
Receipt of major psychotropic medications was common among cohort members during the study period, although not as common as antidepressants. Receipt of benzodiazepines, stimulants, and other psychotropic agents only prior to buprenorphine treatment was associated with an approximately 32%–35% increase in risk for treatment discontinuation. In contrast, we found no association between receipt of opioid analgesics prior to initiating buprenorphine and treatment discontinuation. Receipt of stimulant prescriptions during buprenorphine treatment or in both periods was associated with a significantly lower risk for discontinuation (HRs 0.52 and 0.88, respectively). However, these associations needed to be interpreted with caution. Future studies are needed to test if there was a potential therapeutic benefit from the combination of these two medications in the management of OUD, particularly for patients with co-occurring stimulant use disorders.
Although many co-occurring SUDs, psychiatric disorders, and pain conditions were common among cohort members, their association with risk of buprenorphine treatment discontinuation differed. First, our results showed that cannabis use disorder, cocaine use disorder, and stimulant use disorder at baseline were significantly associated with an increased risk of treatment discontinuation. These associations held in sensitivity analyses as well. The association could be due to unmanaged co-occurring cannabis or cocaine use disorder or due to certain zero tolerance policies toward other substance use by MOUD providers. Nevertheless, given the prevalence of polysubstance use and co-occurring SUDs among people with OUD,36 our findings highlight the importance of addressing co-occurring SUDs in people with OUD in order to improve treatment retention and outcomes. Second, we found no association between pain conditions and increased risk for treatment discontinuation except for the condition of periarticular and soft-tissue disorders, which could indicate that buprenorphine as a μ-opioid receptor partial agonist itself might provide analgesia for most people with chronic pain while receiving buprenorphine treatment. Third, unlike previous studies,37,38 we did not find that psychiatric diagnoses among individuals receiving buprenorphine were associated with improved retention. In fact, we found depression was associated with a small but statistically significant increased risk in treatment discontinuation while receipt of antidepressants was accounted for. The increased risk associated with a depression diagnosis at baseline might indicate the severity of depression and again highlight the importance of managing depression among people receiving buprenorphine treatment.
Limitations
This study is subject to several limitations. First, as with other studies using claims data, we assumed that medications were used as dispensed or received. Second, claims data lack information on several factors that might be associated with buprenorphine treatment retention, such as the severity, duration, and type (prescription vs illicit opioid) of OUD. We did conduct a sensitivity analysis by restricting the cohort to those who had OUD diagnosis at baseline, which did not change our main findings. Third, we did not adjust for the receipt of non-medication OUD treatments such as psychotherapy, which could be confounders for antidepressant receipt during buprenorphine treatment and improve treatment retention. Fourth, there might be other patient-level and provider-level confounding factors. For example, there might be unobserved patient characteristics that are associated with engagement for services and treatment as well as adherence to both receiving buprenorphine and antidepressants. In these MarketScan commercial claims data, we did not have information on who and what specialty prescribed buprenorphine and antidepressants, and whether they were prescribed by the same provider. Fifth, our data only contained the commercially insured population in the US aged < 65 years; thus, our results might not be generalizable to other insured or uninsured populations in the US, for instance, individuals publicly insured by Medicaid, which covers a higher proportion of patients with OUD and psychiatric disorders.39 Future studies should examine outcomes associated with receiving MOUD and antidepressants in these populations. Lastly, due to the observational nature of the study design and potential confounders discussed above, we cannot draw causal inferences on whether receipt of antidepressant during buprenorphine treatment is a direct cause of decreased risk in treatment discontinuation. Although baseline psychiatric disorders were common among cohort members, we did not know from these claims data the specific indications for which antidepressants were prescribed. Similarly, the reason for not receiving antidepressants during buprenorphine treatment among individuals who received antidepressants prior to treatment was not clear. Future research is needed to help understand the causal mechanism for these associations found by the current study.
CONCLUSION
In addition to shedding light on characteristics of antidepressant receipt among people receiving buprenorphine for OUD, our findings suggest that receiving antidepressants during buprenorphine treatment is associated with improved treatment retention, whether an individual starts antidepressants while receiving buprenorphine or continues with antidepressants during treatment. In contrast, discontinuing antidepressant use while starting buprenorphine treatment was found to be associated with a 40% increase in risk of buprenorphine discontinuation. Despite limitations, our main findings highlight the critical importance of screening for co-occurring OUD and depression, as using antidepressants concurrently with buprenorphine treatment for OUD is associated with improved MOUD treatment retention. Future research should also explore outcomes of screening and treating co-occurring OUD and other major psychiatric disorders and their influence on MOUD treatment retention.
Supplementary Material
Clinical Points.
Co-occurring psychiatric disorders are risk factors for early discontinuation from buprenorphine treatment for opioid use disorder (OUD).
This study found that using antidepressants concurrently with buprenorphine treatment for OUD is associated with improved treatment retention.
Clinicians should consider screening for depression and keeping patients on antidepressant treatment or initiating antidepressants during patients’ buprenorphine treatment for OUD.
Footnotes
Potential conflicts of interest: Dr Compton reports long-term holdings in General Electric Company, 3M Companies, and Pfizer, Incorporated, unrelated to the present work. The other authors report no competing interests.
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the National Institute on Drug Abuse, the National Institutes of Health, the Centers for Disease Control and Prevention, or the US Department of Health and Human Services.
Supplementary material: Available at PSYCHIATRIST.COM.
See, eg, 45 CFR part 46; 21 CFR part 56; 42 USC §241(d), 5 USC §552a, 44 USC §3501, et seq.
REFERENCES
- 1.Ahmad FB, Rossen LM, Sutton P. Provisional drug overdose death counts. Centers for Disease Control and Prevention. 2020. https://www.cdc.gov/nchs/nvss/vsrr/prhttps://www.cdc.gov/nchs/nvss/vsrr/provisional-drug-overdose.htm [Google Scholar]
- 2.Liu S, Scholl L, Hoots B, et al. Nonfatal drug and polydrug overdoses treated in emergency departments—29 States, 2018–2019. MMWR Morb Mortal Wkly Rep. 2020;69(34):1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strategy to Combat Opioid Abuse, Misuse, and Overdose-A Framework Based on the Five Point Strategy. US Department of Health and Human Services. 2018. Accessed May 15, 2020. https://www.hhs.gov/opioids/sites/default/files/2018-09/opioid-fivepoint-strategy-20180917-508compliant.pdf [Google Scholar]
- 4.National Academies of Sciences, Engineering, and Medicine. Medications for Opioid Use Disorder Save Lives. The National Academies Press; 2019. 10.17226/25310. [DOI] [PubMed] [Google Scholar]
- 5.Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2(2):CD002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krawczyk N, Mojtabai R, Stuart EA, et al. Opioid agonist treatment and fatal overdose risk in a state-wide US population receiving opioid use disorder services. Addiction. 2020;115(9):1683–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsui JI, Evans JL, Lum PJ, et al. Association of opioid agonist therapy with lower incidence of hepatitis C virus infection in young adult injection drug users. JAMA Intern Med. 2014;174(12):1974–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barocas JA, Gai MJ, Amuchi B, et al. Impact of medications for opioid use disorder among persons hospitalized for drug use-associated skin and soft tissue infections. Drug Alcohol Depend. 2020;215:108207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrilla CHA, Coulthard C, Larson EH. Barriers rural physicians face prescribing buprenorphine for opioid use disorder. Ann Fam Med. 2017;15(4):359–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beetham T, Saloner B, Wakeman SE, et al. Access to office-based buprenorphine treatment in areas with high rates of opioid-related mortality: an audit study. Ann Intern Med. 2019;171(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones CM, McCance-Katz EF. Characteristics and prescribing practices of clinicians recently waivered to prescribe buprenorphine for the treatment of opioid use disorder. Addiction. 2019;114(3):471–482. [DOI] [PubMed] [Google Scholar]
- 12.Beetham T, Saloner B, Gaye M, et al. Therapies offered at residential addiction treatment programs in the United States. JAMA. 2020;324(8):804–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ASAM National Practice Guideline for the Treatment of Opioid Use Disorder: 2020 Focused Update. American Society of Addiction Medicine. 2020. https://www.asam.org/Quality-Science/quality/2020-national-practice-guideline [Google Scholar]
- 14.Eastwood B, Strang J, Marsden J. Effectiveness of treatment for opioid use disorder: A national, five-year, prospective, observational study in England. Drug Alcohol Depend. 2017;176:139–147. [DOI] [PubMed] [Google Scholar]
- 15.Timko C, Schultz NR, Cucciare MA, et al. Retention in medication-assisted treatment for opiate dependence: A systematic review. J Addict Dis. 2016;35(1):22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volkow ND, Frieden TR, Hyde PS, et al. Medication-assisted therapies: tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063–2066. [DOI] [PubMed] [Google Scholar]
- 17.Samples H, Williams AR, Crystal S, et al. Impact of long-term buprenorphine treatment on adverse health care outcomes in Medicaid. Health Aff (Millwood). 2020;39(5):747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams AR, Samples H, Crystal S, et al. Acute care, prescription opioid use, and overdose following discontinuation of long-term buprenorphine treatment for opioid use disorder. Am J Psychiatry. 2020;177(2):117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parran TV, Adelman CA, Merkin B, et al. Long-term outcomes of office-based buprenorphine/naloxone maintenance therapy. Drug Alcohol Depend. 2010;106(1):56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daley DC. Family and social aspects of substance use disorders and treatment. Yao Wu Shi Pin Fen Xi. 2013;21(4):S73–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barry CL, McGinty EE, Pescosolido BA, et al. Stigma, discrimination, treatment effectiveness, and policy: public views about drug addiction and mental illness. Psychiatr Serv. 2014;65(10):1269–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin FR, Bisaga A, Sullivan MA, et al. A review of a national training initiative to increase provider use of MAT to address the opioid epidemic. Am J Addict. 2016;25(8):603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manhapra A, Agbese E, Leslie DL, et al. Three-year retention in buprenorphine treatment for opioid use disorder among privately insured adults. Psychiatr Serv. 2018;69(7):768–776. [DOI] [PubMed] [Google Scholar]
- 24.Goldner EM, Lusted A, Roerecke M, et al. Prevalence of Axis-1 psychiatric disorder and symptomatology among non-medical prescription opioid users in substance use treatment: systematic review and meta-analyses. Addict Behav. 2014;39(3):520–531. [DOI] [PubMed] [Google Scholar]
- 25.Han B, Compton WM, Blanco C, et al. Prescription opioid use, misuse, and use disorders in US adults: 2015 National Survey on Drug Use and Health. Ann Intern Med. 2017;167(5):293–301. [DOI] [PubMed] [Google Scholar]
- 26.Hasin D, Liu X, Nunes E, et al. Effects of major depression on remission and relapse of substance dependence. Arch Gen Psychiatry. 2002;59(4):375–380. [DOI] [PubMed] [Google Scholar]
- 27.Nunes EV, Sullivan MA, Levin FR. Treatment of depression in patients with opiate dependence. Biol Psychiatry. 2004;56(10):793–802. [DOI] [PubMed] [Google Scholar]
- 28.Volkow ND, Jones EB, Einstein EB, et al. Prevention and treatment of opioid misuse and addiction: a review. JAMA Psychiatry. 2019;76(2):208–216. [DOI] [PubMed] [Google Scholar]
- 29.Woody GE, O’Brien CP, McLellan AT, et al. The use of antidepressants with methadone in depressed maintenance patients. Ann N Y Acad Sci. 1982;398:120–127. [DOI] [PubMed] [Google Scholar]
- 30.Agbese E, Leslie DL, Manhapra A, et al. Early discontinuation of buprenorphine therapy for opioid use disorder among privately insured adults. Psychiatr Serv. 2020;71(8):779–788. [DOI] [PubMed] [Google Scholar]
- 31.Bunavail (buprenorphine and naloxone). Package insert. BioDelivery Sciences International, Inc. US Food and Drug Administration; website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/205637s020lbl.pdf#page=31. Revised October 2019. Accessed May 20, 2020. [Google Scholar]
- 32.Suboxone (buprenorphine and naloxone). Package insert. Indivior Inc. US Food and Drug Administration; website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022410s038lbl.pdf#page=32. Revised October 2019. Accessed May 20, 2020. [Google Scholar]
- 33.Zubsolv (buprenorphine and naloxone). Package insert. Orexo US, Inc. US Food and Drug Administration; website. Revised October 2019. Accessed May 20, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204242s017lbl.pdf#page=18. [Google Scholar]
- 34.Morgan JR, Schackman BR, Leff JA, et al. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. J Subst Abuse Treat. 2018;85:90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samples H, Williams AR, Olfson M, et al. Risk factors for discontinuation of buprenorphine treatment for opioid use disorders in a multi-state sample of Medicaid enrollees. J Subst Abuse Treat. 2018;95:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. 2019;197:78–82. [DOI] [PubMed] [Google Scholar]
- 37.Friesen EL, Kurdyak P. The impact of psychiatric comorbidity on treatment discontinuation among individuals receiving medications for opioid use disorder. Drug Alcohol Depend. 2020;216(1):108244. [DOI] [PubMed] [Google Scholar]
- 38.Griffin ML, Dodd DR, Potter JS, et al. Baseline characteristics and treatment outcomes in prescription opioid dependent patients with and without co-occurring psychiatric disorder. Am J Drug Alcohol Abuse. 2014;40(2):157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Substance Abuse and Mental Health Services Administration. Projections of National Expenditures for Treatment of Mental and Substance Use Disorders, 2010–2020. 2014. HHS; Publication No SMA-14–4883. Accessed July 2021. https://store.samhsa.g2020.ov/sites/default/files/d7/priv/sma14-4883.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


