Abstract
A literature review on new-onset autoimmune connective tissue diseases (ACTDs) following COVID-19 is lacking. We evaluated potential associations between COVID-19 and the development of new-onset ACTDs. The “population” was adults with disease terms for ACTDs, including systemic lupus erythematosus (SLE), Sjogren’s syndrome, systemic sclerosis (SSc), idiopathic inflammatory myositis (IIM), anti-synthetase syndrome, mixed CTD and undifferentiated CTD, and “intervention” as COVID-19 and related terms. Databases were searched for English-language articles published until September 2022. We identified 2236 articles with 28 ultimately included. Of the 28 included patients, 64.3% were female, with a mean age was 51.1 years. The USA reported the most cases (9/28). ACTD diagnoses comprised: 11 (39.3%) IIM (including four dermatomyositis); 7 (25%) SLE; four (14.3%) anti-synthetase syndrome; four (14.3%) SSc; two (7.1%) other ACTD (one lupus/MCTD overlap). Of eight, four (14.3%) patients (including that with lupus/MCTD) had lupus nephritis. The average time from COVID-19 to ACTD diagnosis was 23.7 days. A third of patients were admitted to critical care, one for treatment of haemophagocytic lymphohistiocytosis in SLE (14 sessions of plasmapheresis, rituximab and intravenous corticosteroids) and nine due to COVID-19. 80% of patients went into remission of ACTD following treatment, while three (10%) patients died—one due to macrophage activation syndrome with anti-synthetase syndrome and two from unreported causes. Our results suggest a potential association between COVID-19 and new-onset ACTDs, notably in young females, reflecting more comprehensive CTD epidemiology. The most common diagnosis in our cohort was IIM. The aetiology and mechanisms by which ACTDs emerge following COVID-19 remain unknown and require further research.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00296-023-05283-9.
Keywords: COVID-19, SARS-CoV-2, Pandemic, Rheumatic disease, Autoimmune connective tissue diseases
Introduction
The COVID-19 pandemic resulted in substantial mortality and morbidity [1]. Globally, an estimated 6.49 million people have died due to COVID-19 and its complications [2]. Although primarily a respiratory disease, SARS-CoV-2 infection has been linked to hyperinflammation in multiple organs due to cytokine storm and molecular mimicry [3, 4]. Several new autoimmune and autoinflammatory conditions have been reported among the SARS-CoV-2 survivors [5–7]. A systematic review (SR) by Saad et al. discovered that SARS-CoV-2 infection is associated with neurological, cardiological, and musculoskeletal inflammatory diseases [8].
Similarly, recent studies have linked SARS-CoV-2 infection to the onset of systemic autoimmune rheumatic diseases (SARD) following SARS-CoV-2 infection [9, 10]. An SR by Chaudhry et al. elucidated that eight patients developed new rheumatoid arthritis (RA) and several others had flare-ups of their existing RA after being infected with SARS-CoV-2 [11], aligning with another SR of literature on the vasculitides after COVID-19 infection [12]. Despite the emergence of new-onset autoimmune connective tissue diseases (ACTDs) following COVID-19 infection, an SR of the literature is lacking. Our objectives were twofold: (i) to investigate the prevalence, clinical outcomes, treatment, and prognosis of new-onset ACTDs after SARS-CoV-2 infection and (ii) to evaluate the potential association between COVID-19 infection and the development of new-onset ACTDs in adults.
Methods
This SR was conducted in accordance with the Cochrane Handbook and reported as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [13, 14].
The protocol was developed and registered in the PROSPERO database of SRs (CRD42022358750). The review question was: Is there an association between COVID-19 infection and the development of new-onset ACTDs in adults? We assessed the incidence of new cases of ACTDs developing after COVID-19 infection and their clinical characteristics, treatment, and outcomes.
Population
We included adults with ACTDs, including (but not limited to) systemic lupus erythematosus (SLE), Sjogren’s syndrome, systemic sclerosis (SSc), any idiopathic inflammatory myositis (IIM), anti-synthetase syndrome, mixed CTD and undifferentiated CTD (and related database specific indexing terms), with “intervention” as COVID-19 and related terms. All indexing terms and related keywords used are detailed within the supplementary materials.
We excluded patients developing new-onset ACTDs without prior SARS CoV-2 infection or patients without developing new-onset ACTD or flare of existing ACTDs.
Patients developing a systemic autoimmune rheumatic disease, not included in the above list, were excluded (such as inflammatory arthropathies and vasculitides).
Outcome
Outcomes were demographics, clinical characteristics and disease trajectory, treatment, and timing of developing new-onset ACTDs after SARS-CoV-2.
Intervention and comparator descriptors were not applied to this review.
Search strategy, databases and study selection
The search strategy is strategies are available in the online supplementary material. To ensure full comprehensive coverage, indexing terms (MeSH, applicable to Medline and Cochrane, and Emtree headings used on Embase) along with relevant keyword searching were incorporated. For terms for COVID-19, a dedicated search strategy developed by the National Institute for Clinical Excellence was used (Fig. 1). Medline, Embase, and Cochrane databases were searched from 2019 till September 2022, restricted to English-language articles only concerning adult populations. Eligible articles were: case reports and series (of any sample size), observational studies, qualitative studies and randomised controlled trials. Patients developing ACTDs without prior COVID-19 or reporting flares of existing ACTDs were excluded. Information was extracted on patient demographics, new ACTDs’ onset time, clinical characteristics, COVID-19 and ACTD treatment, and COVID-19 and ACTD outcomes.
Fig. 1.
Flow chart of the search strategy
Full-length articles were uploaded into EndNote V.X9 (Clarivate Analytics, Pennsylvania, USA), with duplicates removed (Fig. 2). Titles and abstracts were screened for eligibility, and articles meeting inclusion criteria were examined in further detail. For validation, 20% of the articles were screened. There were nil disagreements.
Fig. 2.
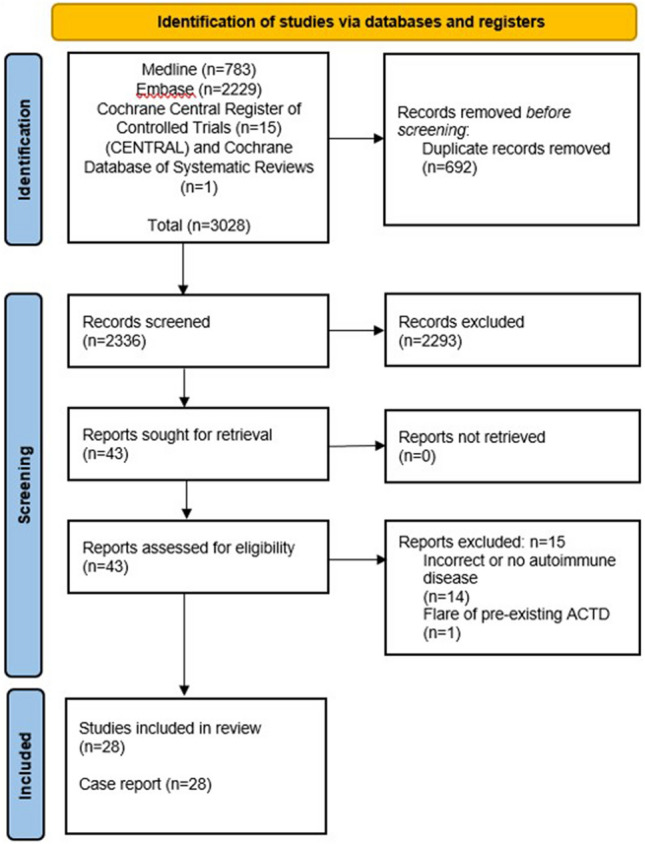
Flow diagram of stages of systematic literature review
All retrieved articles were either case reports or case series; therefore, no formal risk of bias assessment was possible.
Results
After deduplication, 2336 articles were identified. After screening the title and abstract, 2293 papers were excluded, with 43 proceeding to full-text screening. Ultimately, 28 articles (all single case reports) were included.
Article information and basic demographics are detailed in Table 1. Of the 28 included patients, 64.3% were female. The mean age was 51.1 years (range 20–89 years). Most case reports were from the USA (9/28), followed by Iran (4/28).
Table 1.
Summary of included case reports, with basic demographics, comorbidities and final ACTD diagnosis
| Title | Country | Gender | Mean age (years) | Comorbidities | ACTD diagnosis (as reported in article) |
|---|---|---|---|---|---|
| Zhang et al. 2022 [22] | USA | F | 58 | No | COVID‐19-associated myositis |
| Ramachandran et al. 2022 [30] | USA | M | 53 | Hypertension, CKD 3, cholecystectomy | SLE |
| Pereira et al. 2022 [40] | India | M | 57 | Unknown | Anti-synthetase syndrome |
| Okayasu et al. 2022 [23] | Japan | M | 89 | Hypertension, angina pectoris, dementia, clavicle fracture, previous lumbar vertebral compression fracture | Myositis and febrile neutropenia |
| Okada et al. 2022 [24] | Japan | F | 64 | Unknown | Dermatomyositis |
| Nunes et al. 2022 [38] | Portugal | F | 70 | Hypertension | Toxic epidermal necrolysis-like subacute cutaneous lupus |
| Kazzi et al. 2022 [31] | USA | M | 37 | No | SLE |
| Holzer et al. 2022 [19] | Germany | F | 20 | Unknown | Dermatomyositis |
| Giuggioli et al. 2022 [41] | Italy | F | 53 | Unknown | Raynaud's and systemic sclerosis |
| Chandra & Kahaleh 2022 [42] | USA | F | 48 | Anxiety, depression | Systemic Sclerosis |
| Bouchard Marmen et al. 2022 [43] | Canada | M | 62 | Unknown | Anti-synthetase syndrome |
| Blum et al. 2022 [44] | USA | M | 67 | None | Diffuse systemic sclerosis |
| Assar et al. 2022 [32] | Iran | F | 38 | Anxiety | SLE |
| Anderle et al. 2022 [45] | Austria | M | 20 | Unknown | Anti-MDA5 dermatomyositis |
| Amin et al. 2022 [27] | Pakistan | F | 52 | None | Polymyositis |
| Ali et al. 2022 [33] | Pakistan | F | 22 | Unknown | MCTD associated with a flare of lupus nephritis |
| Zamani et al. 2021 [34] | Iran | M | 39 | None | SLE |
| Slimani et al. 2021 [35] | Morocco | F | 23 | None | SLE and aPLS |
| Dadras et al. 2021 [25] | Iran | F | 58 | Diabetes mellitus, hypothyroidism, coronary artery | Dermatomyositis |
| Lokineni et al. 2021 [29] | USA | M | 51 | None | Necrotizing Myositis |
| Keshtkarjahromi et al. 2021 [46] | USA | F | 65 | Psoriasis, hypertension, hyperlipidaemia | MDA5-positive dermatomyositis complicated by MAS |
| Fineschi S 2021 [47] | Sweden | M | 47 | Unknown | Systemic Sclerosis |
| Borges et al. 2021 [20] | Brazil | F | 36 | Unknown | Dermatomyositis |
| Assar et al. 2021 [26] | Iran | F | 45 | OCD, hypothyroidism, migraine | Neutrophilic myositis |
| Ali et al. 2021 [36] | USA | F | 25 | Asthma, depression | SLE (complicated by HLH) |
| Aldaghlawi et al. 2021 [28] | USA | F | 69 | Stage IV chronic lymphocytic leukaemia | Myositis |
| Sacchi et al. 2020 [21] | Italy | F | 77 | Obesity, monoclonal gammopathy, diabetes mellitus, COPD, atrial fibrillation, CKD, cardiac failure | Myositis |
| Bonometti et al. 2020 [37] | Italy | F | 85 | None | SLE |
CKD chronic kidney disease, SLE systemic lupus erythematosus, MDA5 melanoma differentiation-associated protein 5, COPD chronic obstructive pulmonary disease
ACTD diagnoses comprised: 11 (39.3%) IIM (including 4 cases of dermatomyositis); 7 (25%) SLE; 4 (14.3%) anti-synthetase syndrome; 4 (14.3%) SSc; 2 (7.1%) other ACTD (one diagnosed with lupus/MCTD overlap). Of the eight patients diagnosed with SLE or lupus/MCTD, four (14.3%) were diagnosed with lupus nephritis. The average onset time from COVID-19 infection to ACTD diagnosis was 23.7 days.
The majority of cases (n = 16) were reported in 2022 and only one case of lupus nephritis was reported in 2020.
Investigations carried out varied markedly depending on geographic region (Table 2). Serum inflammatory markers (ESR and CRP) were gathered before the diagnosis of CTD in (9 ESR) and (14 CRP) cases with a mean of 70.2 mm/hr and 74.3 mg/L, respectively. One case had a normal ESR prior to diagnosis of ACTD and two patients had CRP levels reported within the normal range prior to ACTD diagnosis. Post-diagnosis of ACTD, there was reduction in both the ESR and CRP levels of those reported initially, with a mean of 53 mm/hr and 12.2 mg/L, respectively. One case had ESR within the normal range, and one had CRP within the normal range post-CTD diagnosis.
Table 2.
Summary of investigations leading to ACTD diagnosis
| Title | Time between onset of COVID-19 and autoimmune symptoms (days) | ESR pre-CTD-diagnosis (mm/hr) | CRP pre-CTD diagnosis (mg/L) | ESR post-CTD diagnosis and treatment (mm/hr) | CRP post-CTD diagnosis and treatment (mg/L) | CTD serology and basic biochemistry/haematology markers (where reported) | Imaging and findings (as reported) | Other investigations |
|---|---|---|---|---|---|---|---|---|
| Zhang et al. 2022 [22] | 21 | 94 | 110 | – | – | Positive anti-Ku, anti–SAE 1 IgG, anti–SS‐A | MRI: diffuse muscle oedema and enhancement, with region of myonecrosis | |
| Ramachandran et al. 2022 [30] | – | – | – | – | – |
Positive ANA 1:1280, speckled pattern; dsDNA 150 IU/ml Low C3 and C4 |
Nil described | Renal biopsy: focal segmental glomerulosclerosis, collapsing variant. Light microscopy: mild podocyte hyperplasia, increase in mesangial cellularity and matrix. Severe interstitial fibrosis and tubular atrophy involving 70–80% of the cortical parenchyma with focal dense inflammation. Electron microscopy: glomeruli with global sclerosis and intracapillary deposits. Stage IV lupus nephritis |
| Pereira et al. 2022 [40] | 56 | – | – | – | – |
Raised CPK (3736 IU/L) ANA and anti-smooth muscle antibodies were negative Positive anti-Jo-1 |
PET/CT MIP: abnormal increased FDG uptake in multiple muscle regions, more intense in the upper-limb muscles, suggesting possibility of inflammatory polymyositis (PM) and changes of interstitial lung disease with septal thickening and bronchiectatic changes in the right lower lobe | |
| Okayasu et al. 2022 [23] | 28 | – | 117 | – | – |
ANA < 40 C3 126, C4 31 KL-6 332 MPO-ANCA < 1.0; Positive anti-SSA/Ro < 1.0; Anti-SSB/La autoantibodies < 1.0; CCP Antibodies < 0.6; Cardiolipin antibodies < 4.0; IgG4 111 |
MRI (STIR) thighs: irregular high-intensity areas in both adductor muscle groups, suggesting necrotizing fasciitis. CT chest: no obvious interstitial pneumonia or lymphadenopathy suspicious for lymphoid species | |
| Okada et al. 2022 [24] | 28 | – | – | – | – |
Creatine kinase 1495 U/l D-dimer 6.1 µg/ml. Positive anti-NXP2 |
CT thorax, abdomen, pelvis: no malignancy nor interstitial lung disease MRI (STIR): intramuscular hyperintensity in proximal limbs |
|
| Nunes et al. 2022 [38] | 29 | 51 | 201 | – | – |
C3 0.66 g/L (0.83–1.93 g/L), C4 0.1 (0.15–0.57) Positive ANA 1:1,280, nuclear homogeneous pattern ENA antibodies SSA60 (Ro60)/SSB (La) positive |
Nil described | Urine analysis: 24 h proteinuria: 642 mg/24 h (50–80 mg/24 h) |
| Kazzi et al. 2022 [31] | 42 | – | – | – | – |
Positive ANA, positive double-stranded DNA antibody Hypocomplementemia, leukopenia |
CT thorax, abdomen, pelvis: subtle bilateral infiltrates may be secondary to atypical pneumonia. Mild thoracic, abdominal, and pelvic lymphadenopathy, non-specific. Mild mesenteric stranding may be secondary to mesenteric panniculitis, with possible pancreatitis | Proteinuria |
| Holzer et al. 2022 [19] | 14 | – | < 5 | – | – |
CK 19,647 Positive ANA: 1:640 Positive anti-NXP2 |
MRI muscle: Bilateral myositis of muscles of the pelvic hip girdle and thighs | |
| Giuggioli et al. 2022 [41] | 28 | – | 65 | – | – | Positive ANA, anticentromere pattern | Nailfold capillaroscopy: “early scleroderma pattern” | |
| Chandra and Kahaleh 2022 [42] | – | – | – | – | Positive ANA > 1:1280 | HRCT chest: interstitial lung disease, findings suggestive of non-specific interstitial pneumonia | ||
| Bouchard Marmen et al. 2022 [43] | 28 | – | 61 | – | – |
CK 7696, Positive ANA and anti-Jo-1 |
CT thorax: missed opacities with sub segmental consolidation MRI: oedema of the gluteal and thigh muscles consistent with myositis |
|
| Blum et al. 2022 [44] | 91 | – | – | – | – |
Positive ANA Positive anti-RNP and ani-SSA antibodies |
CT chest: ground-glass opacities possibility indicating interstitial lung disease | |
| Assar et al. 2022 [32] | 18 | 53 | – | 53 | – |
Positive ANA Positive anti-dsDNA Positive P-ANCA |
CT thorax and abdomen: pericardial and pleural effusion and enlarged liver and abdominal lymph nodes | |
| Anderle et al. 2022 [45] | – | – | 60 | – | – |
CK 246 U/L Positive ANA, fine speckled pattern 1:320 Anti-Ro-60 Ab at 23 U/mL (ULN ≤ 10 U/mL) Aanti-MDA-5 14 U/mL (ULN ≤ 10 U/mL) |
CT chest (week 2): patchy ill-defined consolidations and areas of ground-glass opacifications in the periphery of both lower lobes and subtle thickening of the bronchial walls and hepatic steatosis 3-Tesla, gadolinium contrast enhanced MRI: T2 fat saturated bilateral hyperintense signal alterations of bilateral proximal thigh muscles compatible with myositis |
|
| Amin et al. 2022 [27] | 112 | – | 40.5 | – | 12.2 | Nil described |
CT thorax, abdomen, pelvis: enlarged fatty liver and atrophic left kidney MRI shoulder and hip muscles: inflammatory changes in the muscles of the shoulder and pelvic girdle, chest, and anteromedial and lateral compartments of the thigh |
|
| Ali et al. 2022 [33] | – | 102 | 153 | – | – |
Positive ANA, anti-smith (Sm) and U1 small nuclear ribonucleoprotein (U1-RNP) Positive rheumatoid factor C3 and C4 within range |
Nil described | |
| Zamani et al. 2021 [34] | 56 | 74 | 34 | Normal | Normal |
Total complement activity (CH50), 45 (50–150); C3 133 mg/dL (90–180 mg/dL); C4 14 mg/dL (10–40 mg/dL) Anti-La/SSB, 160 U/ml (< 12 U/mL); anti-SSA/Ro, 200 U/mL (< 25 U/mL) Anti-CCP 48 IU/mL (< 20 IU/mL) Anti-dsDNA 70 IU/mL (< 35 IU/mL) Positive fluorescence ANA 1/160. Anticardiolipin, lupus anticoagulant, anti-beta-2 glycoprotein 1, C-ANCA, P-ANCA were negative |
CT chest: two ground-glass opacity nodules in the lower lobes of both lungs | Renal biopsy: mild mesangial hypercellularity (lupus nephritis class I) |
| Slimani et al. 2021 [35] | – | – | – | – | – |
Elevated PT, APTT Positive ANA, ds-DNA, anti-cardiolipin, beta-2-glycoprotein, lupus anticoagulant |
Nil described | |
| Dadras et al. 2021 [25] | – | 57 | – | – | – |
ANA, anti‐ds‐DNA, anti‐Smith antibody negative Myositis‐specific antibodies including anti‐Mi‐2, ‐Ku, ‐PM/Scl‐100, ‐Scl‐75, ‐SRP, ‐PL‐7, ‐PL‐12, ‐EJ, ‐OJ, ‐Jo‐1, and ‐Ro‐52 were negative |
CT abdomen and pelvis: normal CT chest: bilateral multifocal patchy consolidations with reverse halo view suggestive of the chronic phase of organising COVID‐19 pneumonia |
Three skin biopsies from different skin sites were taken with differential diagnoses of dermatomyositis and lupus erythematosus; the first was sent for examination under direct immune fluorescence, with findings in favour of lupus erythematosus. The second (from a Gottron papule) and third (from a vesicle on the extremities) biopsies were evaluated using hematoxylin‐eosin staining; findings indicated dermatomyositis‐lupus overlap features and were compatible with a collagen vascular disease |
| Lokineni et al. 2021 [29] | 84 | – | – | – | – | Nil described | Unknown | |
| Keshtkarjahromi et al. 2021 [46] | 56 | 20 | 67 | – | – |
Positive ANA, anti-MDA5, SSA-52 (Ro) Low C3 |
1st admission: Diagnostic imaging included MRI of right femur that demonstrated multiple scattered areas of proximal muscle oedema, which while non-specific, was felt to be consistent with an inflammatory myositis CT chest: mild bilateral patchy infiltrates 2nd admission: Repeat CT demonstrated a new, marked consolidative processes within the bilateral lower lobes in a peripheral distribution with pleural sparing |
Skin biopsy of the anterior chest was subsequently performed which demonstrated vacuolar interface dermatitis with an increase in dermal mucin |
| Fineschi S 2021 [47] | 21 | Normal | Normal | – | – |
Strongly positive ANA, nucleolar pattern Positive Anti-PM/Scl 75 and PM/Scl 100 Anti-Scl-70, anti-Jo-1, anti-RNA-polymerase III, and other autoantibodies tested negative |
HCRT: ground-glass opacities with predominantly peripheral and subpleural distribution such as in the early stages of interstitial lung disease | |
| Borges et al. 2021 [20] | 14 | – | – | – | – |
Positive fine speckled pattern ANA (1/640) Positive anti-Mi2, CPK 3518U/l |
Skin biopsy showed lamellar keratosis with foci of vascular changes in the epidermal layer and dilated vessels with a thickened wall and perivascular lymphocytic infiltrate | |
| Assar et al. 2021 [26] | 112 | 87 | – | – | – | Normal ANA, anti-dsDNA, antiphospholipid, anti-Ro, anti-La, ANCA, anti Jo1 antibodies | CT chest: peripheral and multi-lobar fibrotic areas in the lingula, right middle lobe and upper zones which were consistent with fibrotic changes due to previous COVID-19 infection | Electromyography and nerve conduction velocity studies (EMG/NCV) were compatible with inflammatory myopathy. There was no evidence of neuropathy and radiculopathy |
| Ali et al. 2021 [36] | 14 | – | Normal | – | – | Positive anti-dsDNA, anti-Smith, anti-RNP, anti-Ro, anti-La | Echocardiogram: large pericardial effusion | |
| Aldaghlawi et al. 2021 [28] | 21 | – | – | – | – |
CPK 2713 µ/L, lactate dehydrogenase 1348 µ/L, haptoglobin 196 mg/dL, haemoglobin 11.7 gm/dL, platelets 75 k/mm3, aspartate aminotransferase 96 µ/L, alanine aminotransferase 72 µ/L, creatinine 0.6 mg/dL, prothrombin 12.3 s, partial thromboplastin time 32.5 s, fibrinogen 599 mg/dL, IGG 333 mg/dL, immunoglobulin M 26 mg/dL, immunoglobulin A 83 mg/dL Peripheral blood smear revealed marked agglutination of red blood cells and a cold agglutinin with thermal amplitude of 30 °C was identified with complement C3B and C4 identified on red blood cell |
Unknown | Hepatitis B and C viral serologies were negative for acute infection |
| Sacchi et al. 2020 [21] | – | 59.4 | – | – | Positive ANA, cytoplasmic pattern (1:320) granular type, Anti-Ku and anti-MI 2b positivity | Unknown | ||
| Bonometti et al. 2020 [37] | – | – | – | – | – | Positive ANA with cytoplasmic (1: 160), homogeneous (1: 320) and granular (1: 320) pattern, Ku positivity and atypical ANCA | Unknown |
ESR erythrocyte sedimentation rate, CRP C-reactive protein, ANA anti-nuclear antibody, ANCA antineutrophil cytoplasmic antibodies, CT computed tomography, MRI magnetic resonance imaging, CTD Connective tissue diseases, DsDNA double-stranded deoxyribonucleic acid, CPK creatinine phosphokinase, COVID-19 Coronavirus disease 2019, CT computed tomography, RNP ribonucleoprotein
Regarding autoantibody levels (Table 2), anti-nuclear antibody (ANA) was the most commonly positive autoantibody in this cohort (n = 16), with a speckled pattern most commonly described. Where ANA was reported, two cases reported normal ANA titres and normal levels for the remaining autoantibody panel (including myositis-specific antigens). Details regarding all other autoantibodies are available in Table 2.
The most common imaging modality reported for our patients was computed tomography (CT) of the chest (n = 17) with the most common finding being “changes suggestive of interstitial lung disease (n = 13). Four cases did not find any pulmonary changes, out of which three cases had magnetic resonance (MRI) evidence suggestive of inflammatory myositis. Seven cases had MRI imaging of the muscles, which demonstrated muscle oedema suggestive of inflammatory myositis; one had electromyography to confirm the diagnosis.
The most commonly diagnosed CTD in our review was IIM, with 11 cases identified, four dermatomyositis. There was a wide age range (20–89 years), with female predominance (n = 9). CTD symptoms onset time also varied markedly, ranging from 14 to 112 days since COVID-19 diagnosis. Autoantibody serology also varied, with just three cases reporting positive ANA [19–21] and six reporting positivity for other autoantibodies, including NXP2, Mi2, Ku, and Ro [19–24]. In three cases with negative autoantibody serology, a diagnosis of myositis was made based on MRI muscle imaging, skin biopsy histology (consistent with dermatomyositis) and electromyography findings [23, 25–27]. Three cases did not report serology or imaging justification of diagnosis, with these diagnoses based on classic symptoms including “malaise, muscle weakness and skin lesions” and “severe intractable pain in bilateral lower extremities and subjective pelvic girdle weaknesses’’ associated with a high creatinine phosphokinase level [25, 28, 29].
COVID-19 treatment differed depending on the stage of pandemic and the country. Ten patients were admitted to critical care, one for ACTD treatment for SLE with haemophagocytic lymphohistiocytosis (HLH; 14 sessions of plasmapheresis, rituximab and intravenous corticosteroids) and nine for COVID-19. Five cases made explicit comments about the severity of COVID-19. However, no articles specified which grading system was used. There are several COVID-19 severity indices available, e.g. National Institute for Health, World Health Organisation, but none were mentioned in the texts. Nonetheless, three were classified as “mild”, one as “low severity” and one as “severe”.
Seventeen case reports provided details of treatment for COVID-19 (either the details of therapies given, or the fact that none were administered; Table 3). The following specific treatments were described for these patients: one case received a combination of tocilizumab, anticoagulation, hydroxychloroquine, and azithromycin; one received tapering corticosteroids and nintedanib for post-COVID-19 lung fibrosis; one received supplemental oxygen, dexamethasone, ipratropium bromide and enoxaparin; one received azithromycin, hydroxychloroquine; one received naproxen and Diphenhydramine syrup; one received hydroxychloroquine, cefazolin and azithromycin; one received “broad-spectrum antibiotics”, convalescent plasma and dexamethasone; one received remdesivir, corticosteroids, colchicine and plasmapheresis; one received levofloxacin and dexamethasone; one received oxygen, lopinavir/ritonavir, hydroxychloroquine, doxycycline, ceftriaxone and anticoagulant. Two cases of COVID-19 infection received no treatment.
Table 3.
Summary of treatment and outcomes for COVID-19 and ACTD
| Title | Severity of COVID-19 | Treatment of COVID-19 | ACTD diagnosis | Prior ACTD diagnosis | Treatment of CTD | ITU admission | CTD remission | Outcome |
|---|---|---|---|---|---|---|---|---|
| Zhang et al. 2022 [22] | Unknown | Tocilizumab, anticoagulation (drug name not specified), hydroxychloroquine, azithromycin | COVID‐19–associated myositis | No | IV methylprednisolone | No | Yes | Survived |
| Ramachandran et al. 2022 [30] | Unknown | Unknown | SLE | No | IV methylprednisolone 1 g/day for 3 days, then oral prednisolone 60 mg, with plasmapheresis (6 rounds), mycophenolate and hydroxychloroquine | No | Yes | Survived |
| Pereira et al. 2022 [40] | Unknown | Tapering corticosteroids and nintedanib for post-COVID lung fibrosis | Anti-synthetase syndrome | Unknown | Mycophenolate mofetil | No | Yes | Survived |
| Okayasu et al. 2022 [23] | Unknown | Unknown | Myositis and febrile neutropenia | No | Oral prednisolone 50 mg/day for 5 days | Unknown | Yes | Survived |
| Okada et al. 2022 [24] | Unknown | Unknown | Dermatomyositis | Unknown | 1 g IV methylprednisolone for 3 days, then oral prednisolone 60 mg/day | No | Yes | Survived |
| Nunes et al. 2022 [38] | Unknown | Supplemental oxygen, dexamethasone 6 mg/day, ipratropium bromide 40 µg 6-hourly, enoxaparin 40 mg/day, paracetamol 1 g as required | Toxic epidermal necrolysis-like subacute cutaneous lupus | No | Continuous surveillance and balneotherapy for 10 days. Subsequent hydroxychloroquine 400 mg/day and prednisolone 1 mg/kg/day (dose not specified) | Yes | Yes | Survived |
| Kazzi et al. 2022 [31] | Low | None | SLE | No | Antibiotics, corticosteroids and MMF 1500 mg twice daily with resolution. Subsequent hydroxychloroquine (dose unspecified) | No | Yes | Survived |
| Holzer et al. 2022 [19] | Unknown | Unknown | Dermatomyositis | Unknown | Corticosteroids, IVIG, MMF, ciclosporin A, tofacitinib, rituximab | No | Yes | Survived |
| Giuggioli et al. 2022 [41] | Unknown | Azithromycin for 5 days; hydroxychloroquine 400 mg twice a day for 1 day and then 200 mg every 12 h for 5 days | Raynaud's and systemic sclerosis | No | Nifedipine for Raynaud’s | No | Yes | Survived |
| Chandra & Kahaleh 2022 [42] | Unknown | Unknown | Systemic Sclerosis | No | MMF 1500 mg twice daily, amlodipine 5 mg daily, methotrexate 12.5 mg once weekly, prednisone 5 mg twice daily | No | No | Survived |
| Bouchard Marmen et al. 2022 [43] | Unknown | Unknown | Anti-synthetase syndrome | Unknown | Pulsed IV methylprednisolone, then oral prednisolone, cyclophosphamide, rituximab, IVIG | Yes | Yes | Survived |
| Blum et al. 2022 [44] | Unknown but complicated by CCF, AF and PE post-COVID infection | Unknown | Diffuse systemic sclerosis | No | MMF | Yes | No | Died |
| Assar et al. 2022 [32] | Mild | Naproxen 500 mg twice daily and diphenhydramine syrup four times a day orally on outpatient basis | SLE | No | Prednisolone 30 mg daily, hydroxychloroquine 200 mg daily and azathioprine 150 mg daily followed by MMF | No | Yes | Survived |
| Anderle et al. 2022 [45] | Unknown | Unknown | Anti-MDA5 dermatomyositis | Corticosteroid pulsed therapy (250 mg intravenous prednisolone), acyclovir and trimethoprim/sulfamethoxazole, cyclophosphamide and tacrolimus due to rapid disease progression. Colchicine due to the hyperinflammatory state. Caspofungin, piperacillin/tazobactam and doxycycline administered for infection prophylaxis | Yes | Yes | Survived (required ECMO and double lung transplant) | |
| Amin et al. 2022 [27] | Unknown | Unknown | Polymyositis | No | Oral prednisolone 60 mg/day, azathioprine 50 mg twice a day | No | Yes | Survived |
| Ali et al. 2022 [33] | Unknown | Unknown | MCTD associated with a flare of LN | Unknown | IV methylprednisolone 50 mg once daily throughout hospitalisation, in addition to oral hydroxychloroquine 200 mg once daily | No | Yes | Survived |
| Zamani et al. 2021 [34] | Mild | 400 mg hydroxychloroquine twice on the first day and 200 mg twice daily for a further 6 days | SLE | Prednisolone 30 mg daily and hydroxychloroquine, gabapentin, and vitamin B (300 mg daily) | No | Yes | Survived | |
| Slimani et al. 2021 [35] | Unknown | Unknown | SLE and aPLS | No | Nil described | Yes | No | Died |
| Dadras et al. 2021 [25] | Unknown | Cefazolin (2 g three times daily) and azithromycin (500 mg daily) | Dermatomyositis | Yes | Prednisolone 60 mg daily, methotrexate 15 mg weekly, hydroxychloroquine 400 mg daily | Unknown | Yes | Survived |
| Lokineni et al. 2021 [29] | Unknown | Broad-spectrum antibiotics (unspecified), convalescent plasma, dexamethasone | Necrotizing Myositis | No | Oral prednisone 60 mg daily, azathioprine 150 mg daily | No | Yes | Survived |
| Keshtkarjahromi et al. 2021 [46] | Unknown | Unknown | MDA5-positive dermatomyositis complicated by MAS | Yes |
1st admission: oral prednisone 60 mg daily, tapering regime. Discharged to rehabilitation centre with plans to continue steroid therapy with adjunctive trimethoprim-sulfamethoxazole for pneumocystis pneumonia prophylaxis 2nd admission: IV methylprednisolone 1 g/day for 3 days followed by 80 mg IV daily, IVIG 400 mg/kg/ day for 5 days |
Yes | No | Died |
| Fineschi S 2021 [47] | Mild | Unknown | Systemic Sclerosis | No | Calcium channel blocker, proton pump inhibitor, tear substitution | No | Unknown | Survived-awaiting further decision re immunosuppression |
| Borges et al. 2021 [20] | Unknown | Unknown | Dermatomyositis | Unknown | Pulsed IV methylprednisolone (unspecified) 5 days | No | Yes | Survived |
| Assar et al. 2021 [26] | Remdesivir, high doses of corticosteroids (unspecified), colchicine, plasmapheresis | Neutrophilic myositis | Yes | IVIG, 2 g/kg in four divided doses, prednisolone 1 mg/kg/day with gradual tapering (absolute doses unspecified) |
Yes, for COVID No for CTD |
Yes | Survived | |
| Ali et al. 2021 [36] | Unknown | None | SLE (complicated by HLH) | No | MMF 250 mg daily, hydroxychloroquine 400 mg daily. 14 sessions of plasmapheresis, 600 mg of rituximab twice, high-dose corticosteroids (dose unspecified) | Yes, for CTD | Not specified | Survived- requiring long-term rehabilitation |
| Aldaghlawi et al. 2021 [28] | Severe | Levofloxacin 750 mg daily, dexamethasone 6 mg daily. Discharged on supplemental oxygen 2 l on day 16 | Myositis | No | Oral prednisone 1 mg/kg daily for 4 weeks (dose unspecified); rituximab 375 mg/m2 weekly × 4 doses was initiated for cold agglutinin haemolytic anaemia; IVIG 1 g/kg daily × 2 doses on day 21 to address possible immune-related thrombocytopenia | No | Yes | Survived |
| Sacchi et al. 2020 [21] | Unknown | Oxygen supplementation then continued positive airway pressure therapy. Lopinavir/ritonavir, hydroxychloroquine, doxycycline, ceftriaxone, anticoagulation (unspecified) | Myositis | No | Corticosteroid 1 mg/kg (dose and type unspecified) |
Yes, for COVID No for CTD |
Yes | Survived |
| Bonometti et al. 2020 [37] | Swab negative, immunoglobulin positive | Unknown | SLE | No | Hydroxychloroquine and high-dose corticosteroids | No | Yes | Survived |
Regarding CTD treatment, of those described (27), different strengths of corticosteroids (methylprednisolone in 8 and oral prednisone in 15) were the most frequently prescribed (Table 3). This was followed by hydroxychloroquine (n = 9), mycophenolate mofetil (MMF) (n = 8), rituximab (n = 4), intravenous immunoglobulins (n = 3), azathioprine (n = 3), methotrexate (n = 2), cyclophosphamide (n = 2), plasmapheresis (n = 1), ciclosporin (n = 1), tofacitinib (n = 1), nifedipine (n = 1), tacrolimus (n = 1) and colchicine (n = 1). Antibiotic prophylaxis was administered in two cases, and vitamins B and D each in one case. The majority (80%) of patients experienced remission of ACTD following treatment. In comparison, three (10%) patients died—one from macrophage activation syndrome associated with anti-synthetase syndrome and two from unknown causes.
Discussion
This SR summarised the data on new-onset ACTDs following infection with SARS-CoV-2. Our findings from the 28 included cases suggest a potential association between COVID-19 infection and new-onset ACTDs, particularly in young females, reflective of wider CTD epidemiology. To our knowledge, this is the first SR to examine the association between COVID-19 and new-onset ACTDs, including the temporal relationship, diagnostic parameters and treatment.
Since March 2020, as the COVID-19 pandemic has progressed, so has our understanding of clinical sequelae arising following the infection. During the early stages of the pandemic, it was recognised that SARS-CoV-2 infection could cause a flare in SARD, including CTDs, which was well reported in the literature [15, 16]. ANA positivity was noted in 25% of hospitalised patients with acute COVID-19 infection, with a proportion of patients presenting with rheumatic manifestations, such as muscle weakness for myositis and rash and arthralgia for SLE, as in some of the cases described herein [16, 17]. An association was observed between severe COVID-19 and multisystem inflammatory syndromes and “cytokine storm”, similar to HLH and macrophage activation syndrome previously associated with ACTDs [17, 18]. Likewise, a temporal association between acute COVID-19 infection and the onset of ACTD became apparent in increasing number of cases.
The most common CTD following COVID-19 infection identified in our cohort was IIM, with four cases of dermatomyositis. Interestingly, IIM was diagnosed with negative autoantibody serology and solely on imaging or histology finding. In some cases, no investigative finding was reported, and a diagnosis was made based on symptoms [25, 28, 29]. It remains to be seen whether a subset of IIM is required within the nomenclature to account for such diagnoses arising post-COVID-19 infections, especially in the absence of typical serology.
Seven patients included in our review were diagnosed with SLE, and one with lupus/mixed connective tissue disease (MCTD) overlap [30–37]. Of these eight patients, four presented with lupus nephritis [30, 31, 33, 34]. Again, a wide age range was noted (22–85 years) with, 75% (6/8) of cases being female. One patient with SLE and antiphospholipid syndrome died following admission to the intensive therapy unit (ITU), although it was not specified which treatment she received for either COVID-19 or SLE [35]. Most patients required high-dose intravenous corticosteroids, followed by DMARDs, such as mycophenolate mofetil or hydroxychloroquine. One patient received plasmapheresis and rituximab after requiring ITU admission to treat ACTD and associated HLH [36]. In addition, Nunes et al. reported a case of toxic epidermal necrolysis-like lupus presentation following SARS-Cov-2 infection in a 70-year-old female with hypertension, who went into CTD remission following treatment with hydroxychloroquine and corticosteroids [38].
Our findings were consistent with those of Chaudry et al. [11], who conducted a similar literature review and discovered limited evidence of inflammatory arthritis developed following COVID-19 infection. However, this could be explained by the possible heterogeneity of the cases. On the other hand, an SR of case reports and case series by Wong et al. [12] found an association between COVID-19 and vasculitis. Moreover, COVID-19 has been linked to cytokine storm leading to an immune response to small vessel damage causing vasculitis and other immune-mediated inflammatory diseases [48]. Therefore, more research is needed to investigate the association between ACTDs and COVID-19.
The aetiology and mechanisms by which ACTDs emerge following COVID-19 infection remain unknown and require more robust epidemiological data. It is possible that patients had a mild asymptomatic disease in a genetically predisposed individual prior to COVID-19 infection, with SARS-CoV-2 triggering a flare due to the hyperinflammatory state [3]. Machado et al. recently proposed a new entity of “COVID-19-associated arthritis” in a similar review of inflammatory arthritis following COVID-19 infection. It may be that such nomenclature is required for those developing ACTD following SARS-COv-2 infection [39]. Further studies to elucidate the pathogenesis and aetiology of new-onset ACTD in these cases will aid the characterisation and understanding of these diseases.
Strengths and limitations
Our SR included a small number of cases due to the specific area of rheumatology it covered and the rarity of the ACTD subset we investigated. This might have resulted in biased results, and it is important not to infer causality solely from these cases. However, it is an important subset of ACTDs following SARS-CoV-2 infection, which are relatively unexplored. Our findings will pave the way for future research and better care for ACTD patients. This SR only included case reports that were limited in establishing a cause–effect relationship and, thus, were not generalisable. Therefore, extensive and longitudinal studies to determine causation are recommended to supplement the current literature.
In conclusion, we summarised 28 cases of new-onset ACTD in this SR, the most common presentations being IIM and SLE. However, cases of SSc and rarer diseases such as anti-synthetase syndrome were also identified. Further epidemiological studies of ACTD diagnosed post-COVID-19 infection will help us better understand this association and help identify those at risk of developing ACTD after contracting SARS-CoV-2 infection.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Conceptualization: KK, MD, AN; Methodology: KK, MD, HE, AN, Formal analysis and investigation: KK, MD; Writing—original draft preparation: KK, MD; Writing—review and editing: KK, MD, HE, AN; Supervision: AN. All co-authors take full responsibility for the integrity and accuracy of all aspects of the work.
Funding
Nil funding to declare.
Data availability
Data available upon request.
Declarations
Conflict of interest
The authors (KK, MD, HE, AN) declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Matta S, Chopra KK, Arora VK. Morbidity and mortality trends of Covid 19 in top 10 countries. Indian J Tuberc. 2020;67(4S):S167–S172. doi: 10.1016/j.ijtb.2020.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Coronavirus (COVID-19) dashboard | WHO Coronavirus (COVID-19) dashboard with vaccination data. https://covid19.who.int/?mapFilter=deaths.
- 3.Caso F, Costa L, Ruscitti P, Navarini L, Del Puente A, Giacomelli R, et al. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19(5):102524. doi: 10.1016/j.autrev.2020.102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Sawalha AH, Lu Q. COVID-19 and autoimmune diseases. Curr Opin Rheumatol. 2021;33(2):155–162. doi: 10.1097/BOR.0000000000000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yazdanpanah N, Rezaei N. Autoimmune complications of COVID-19. J Med Virol. 2022;94(1):54–62. doi: 10.1002/jmv.27292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dotan A, Muller S, Kanduc D, David P, Halpert G, Shoenfeld Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun Rev. 2021;20(4):102792. doi: 10.1016/j.autrev.2021.102792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galeotti C, Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol. 2020;16(8):413–414. doi: 10.1038/s41584-020-0448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saad MA, Alfishawy M, Nassar M, Mohamed M, Esene IN, Elbendary A. COVID-19 and autoimmune diseases: a systematic review of reported cases. Curr Rheumatol Rev. 2021;17(2):193–204. doi: 10.2174/1573397116666201029155856. [DOI] [PubMed] [Google Scholar]
- 9.Tang KT, Hsu BC, Chen DY. Autoimmune and rheumatic manifestations associated with COVID-19 in adults: an updated systematic review. Front Immunol. 2021;12:645013. doi: 10.3389/fimmu.2021.645013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nune A, Iyengar KP, Mulherin D, Ish P, Musat CA, Sapkota HR. Granulomatosis with polyangiitis and COVID-19 pneumonia. Indian J Rheumatol. 2022;17(2):210–212. doi: 10.4103/injr.injr_235_21. [DOI] [Google Scholar]
- 11.Chaudhry ZS, Nellessen N, Reis C, Sharip A. The development of inflammatory arthritis following SARS-CoV-2 infection: a systematic review of the literature. Fam Pract. 2022 doi: 10.1093/fampra/cmac029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong K, Farooq Alam Shah MU, Khurshid M, Ullah I, Tahir MJ, Yousaf Z. COVID-19 associated vasculitis: a systematic review of case reports and case series. Ann Med Surg. 2022;74:103249. doi: 10.1016/j.amsu.2022.103249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cochrane Handbook for Systematic Reviews of Interventions | Cochrane Training. https://training.cochrane.org/handbook/current.
- 14.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Iorio M, Cook CE, Vanni KMM, Patel NJ, D’Silva KM, Fu X, et al. DMARD disruption, rheumatic disease flare, and prolonged COVID-19 symptom duration after acute COVID-19 among patients with rheumatic disease: a prospective study. Semin Arthritis Rheum. 2022;55:152025. doi: 10.1016/j.semarthrit.2022.152025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed S, Zimba O, Gasparyan AY. COVID-19 and the clinical course of rheumatic manifestations. Clin Rheumatol. 2021;40(7):2611–2619. doi: 10.1007/s10067-021-05691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lerma LA, Chaudhary A, Bryan A, Morishima C, Wener MH, Fink SL. Prevalence of autoantibody responses in acute coronavirus disease 2019 (COVID-19) J Transl Autoimmun. 2020;3:100073. doi: 10.1016/j.jtauto.2020.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holzer MT, Krusche M, Ruffer N, Haberstock H, Stephan M, Huber TB, et al. New-onset dermatomyositis following SARS-CoV-2 infection and vaccination: a case-based review. Rheumatol Int. 2022;42(12):2267. doi: 10.1007/s00296-022-05176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borges NH, Godoy TM, Kahlow BS. Onset of dermatomyositis in close association with COVID-19-a first case reported. Rheumatology (Oxford) 2021;60(SI):SI96. doi: 10.1093/rheumatology/keab290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sacchi MC, Tamiazzo S, Lauritano EC, Bonometti R. Case report of COVID-19 in an elderly patient: could SARS-CoV2 trigger myositis? Eur Rev Med Pharmacol Sci. 2020;24(22):11960–11963. doi: 10.26355/eurrev_202011_23857. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Charmchi Z, Seidman R, Anziska Y, Velayudhan V, Perk J (2022) COVID-19 associated myositis with severe proximal and bulbar weakness (P9-9.006). Neurology 98(18 Supplement) [DOI] [PMC free article] [PubMed]
- 23.Okayasu T, Ohta R, Igarashi M, Kurita Y, Hayakawa M, Sano C. Coexistence of pancytopenia and myositis after developing COVID-19. Cureus. 2022 doi: 10.7759/cureus.26978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okada Y, Izumi R, Hosaka T, Watanabe S, Shijo T, Hatchome N, et al. Anti-NXP2 antibody-positive dermatomyositis developed after COVID-19 manifesting as type I interferonopathy. Rheumatology (Oxford) 2022;61(4):E90–E92. doi: 10.1093/rheumatology/keab872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shahidi Dadras M, Rakhshan A, Ahmadzadeh A, Hosseini SA, Diab R, Safari Giv T, et al. Dermatomyositis-lupus overlap syndrome complicated with cardiomyopathy after SARS-CoV-2 infection: a new potential trigger for musculoskeletal autoimmune disease development. Clin Case Rep. 2021;9(10):e04931. doi: 10.1002/ccr3.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Assar S, Pournazari M, Soufivand P, Mohamadzadeh D. Successful treatment of COVID-19 induced neutrophilic myositis with intravenous immunoglobulin and corticosteroids: a case report. Reumatismo. 2022;73(4):232–235. doi: 10.4081/reumatismo.2021.1437. [DOI] [PubMed] [Google Scholar]
- 27.Amin S, Rahim F, Noor M, Bangash A, Ghani F. Polymyositis: the comet tail after COVID-19. Cureus. 2022;14(6):e26453. doi: 10.7759/cureus.26453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aldaghlawi F, Shammah A, Kio E. SARS-CoV-2 infection complicated with cold agglutinin disease and myositis. Clin Case Rep. 2021;9(4):2196–2199. doi: 10.1002/ccr3.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lokineni S, Mortezavi M. Delayed-onset necrotizing myositis following COVID-19 infection. Eur J Case Rep Intern Med. 2021;8(4):002461. doi: 10.12890/2021_002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramachandran L, Dontaraju VS, Troyer J, Sahota J. New onset systemic lupus erythematosus after COVID-19 infection: a case report. AME Case Rep. 2022;6:14. doi: 10.21037/acr-21-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kazzi B, Fine D, Geetha D, Chung M, Monroy-Trujillo M, Timlin H. New-onset lupus nephritis associated with COVID-19 infection. Lupus. 2022;31(8):1007–1011. doi: 10.1177/09612033221098571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Assar S, Pournazari M, Soufivand P, Mohamadzadeh D. Systemic lupus erythematosus after coronavirus disease-2019 (COVID-19) infection: case-based review. Egypt Rheumatol. 2022;44(2):145–149. doi: 10.1016/j.ejr.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali S, Almas T, Zaidi U, Ahmed F, Shaikh S, Shaikh F, et al. A novel case of lupus nephritis and mixed connective tissue disorder in a COVID-19 patient. Ann Med Surg. 2022;78:103653. doi: 10.1016/j.amsu.2022.103653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zamani B, Moeini Taba SM, Shayestehpour M. Systemic lupus erythematosus manifestation following COVID-19: a case report. J Med Case Rep. 2021;15(1):1–4. doi: 10.1186/s13256-020-02582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slimani Y, Abbassi R, El Fatoiki FZ, Barrou L, Chiheb S. Systemic lupus erythematosus and varicella-like rash following COVID-19 in a previously healthy patient. J Med Virol. 2021;93(2):1184–1187. doi: 10.1002/jmv.26513. [DOI] [PubMed] [Google Scholar]
- 36.Ali R, Mehannek R, Patel A, Paige A, Reddy S, Guma M, et al. Systemic lupus erythematosus with hemophagocytic lymphohistiocytosis: is COVID-19 the inciting factor? Cureus. 2021;13(11):e19657. doi: 10.7759/cureus.19657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonometti R, Sacchi MC, Stobbione P, Lauritano EC, Tamiazzo S, Marchegiani A, et al. The first case of systemic lupus erythematosus (SLE) triggered by COVID-19 infection. Eur Rev Med Pharmacol Sci. 2020;24(18):9695–9697. doi: 10.26355/eurrev_202009_23060. [DOI] [PubMed] [Google Scholar]
- 38.Nunes AL, Simoes L, Figueiredo C, Carvalho R, Lima J, Santos RM. Toxic epidermal necrolysis-like lupus erythematous presentation following SARS-CoV-2 infection. J Med Cases. 2022;13(2):89–93. doi: 10.14740/jmc3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farisogullari B, Pinto AS, Machado PM. COVID-19-associated arthritis: an emerging new entity? RMD Open. 2022;8(2):e002026. doi: 10.1136/rmdopen-2021-002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pereira M, Shivdasani D, Roy D, Rungta R, Dang S, Singh N. Post-COVID-19 unusual inflammatory syndromes detected on 18F-FDG PET/CT scan. Clin Nucl Med. 2022;47(4):E363–E365. doi: 10.1097/RLU.0000000000004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giuggioli D, Spinella A, de Pinto M, Mascia MT, Salvarani C. From Raynaud phenomenon to systemic sclerosis in COVID-19: a case report. Adv Skin Wound Care. 2022;35(2):123–124. doi: 10.1097/01.ASW.0000795240.63966.53. [DOI] [PubMed] [Google Scholar]
- 42.Chandra A, Kahaleh B. Systemic sclerosis (SSc) after COVID-19: a case report. Cureus. 2022;14(3):e23179. doi: 10.7759/cureus.23179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bouchard Marmen M, Ellezam B, Fritzler MJ, Troyanov Y, Gould PV, Satoh M, et al. Anti-synthetase syndrome occurring after SARS-CoV-2 infection. Scand J Rheumatol. 2022;51(3):255–257. doi: 10.1080/03009742.2021.2024019. [DOI] [PubMed] [Google Scholar]
- 44.Blum FR, Sampath AJ, Gilbert AL, Foulke GT. Diffuse systemic sclerosis following COVID-19 infection. Scand J Rheumatol. 2022 doi: 10.1080/03009742.2022.2103935. [DOI] [PubMed] [Google Scholar]
- 45.Anderle K, Machold K, Kiener HP, Bormann D, Hoetzenecker K, Geleff S, et al. COVID-19 as a putative trigger of anti-MDA5-associated dermatomyositis with acute respiratory distress syndrome (ARDS) requiring lung transplantation, a case report. BMC Rheumatol. 2022 doi: 10.1186/s41927-022-00271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keshtkarjahromi M, Chhetri S, Balagani A, et al. Macrophage activation syndrome in MDA5 antibody-positive dermatomyositis and COVID-19 infection. BMC Rheumatol. 2021;5(1):59. doi: 10.1186/s41927-021-00225-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fineschi S. Case report: systemic sclerosis after Covid-19 infection. Front Immunol. 2021;12:2439. doi: 10.3389/fimmu.2021.686699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galeotti C, Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol. 2020;16:413–414. doi: 10.1038/s41584-020-0448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available upon request.