ABSTRACT
Many insects contain endosymbiotic bacteria within their bodies. In multiple endosymbiotic systems comprising two or more symbionts, each of the symbionts is generally localized in a different host cell or tissue. Bemisia tabaci (Sweet potato whitefly) possesses a unique endosymbiotic system where co-obligate symbionts are localized in the same bacteriocytes. Using fluorescence in situ hybridization, we found that endosymbionts in B. tabaci MEAM1 occupy distinct subcellular habitats, or niches, within a single bacteriocyte. Hamiltonella was located adjacent to the nucleus of the bacteriocyte, while Portiera was present in the cytoplasm surrounding Hamiltonella. Immunohistochemical analysis revealed that the endoplasmic reticulum separates the two symbionts. Habitat segregation was maintained for longer durations in female bacteriocytes. The same segregation was observed in three genetically distinct B. tabaci groups (MEAM1, MED Q1, and Asia II 6) and Trialeurodes vaporariorum, which shared a common ancestor with Bemisia over 80 million years ago, even though the coexisting symbionts and the size of bacteriocytes were different. These results suggest that the habitat segregation system existed in the common ancestor and was conserved in both lineages, despite different bacterial partners coexisting with Portiera. Our findings provide insights into the evolution and maintenance of complex endosymbiotic systems and highlight the importance of organelles for the construction of separate niches for endosymbionts.
IMPORTANCE Co-obligate endosymbionts in B. tabaci are exceptionally localized within the same bacteriocyte (a specialized cell for endosymbiosis), but the underlying mechanism for their coexistence remains largely unknown. This study provides evidence for niche segregation at the subcellular level between the two symbionts. We showed that the endoplasmic reticulum is a physical barrier separating the two species. Despite differences in co-obligate partners, this subcellular niche segregation was conserved across various whitefly species. The physical proximity of symbionts may enable the efficient biosynthesis of essential nutrients via shared metabolic pathways. The expression “Good fences make good neighbors” appears to be true for insect endosymbiotic systems.
KEYWORDS: insect symbiosis, symbiotic bacteria, whiteflies
INTRODUCTION
Numerous insects contain endosymbiotic bacteria within their bodies. Some endosymbionts are obligate, with crucial roles in host growth and reproduction, by providing essential nutrients, while others are facultative (1, 2). In multiple endosymbiotic systems comprising two or more symbionts, each of the symbionts is generally localized in a different host cell or tissue. This type of localization is observed in various insect species, such as aphids, spittlebugs, leafhoppers, scale insects, adelgids, psyllids, and cicadas (1, 3–13). The compartmentalization of multiple symbionts into different host cells is considered an important step in evolution, to reduce direct conflict between the multiple symbionts and control them within the same host (12, 14).
The sweet potato whitefly Bemisia tabaci (Hemiptera: Aleyrodidae) is a cryptic species complex comprising more than 44 genetic groups based on the mitochondria cytochrome oxidase I sequences (15). Among the genetic groups, Middle East Asia Minor 1 (MEAM1) and Mediterranean Q1 (MED Q1) are globally important pests, and both possess two phylogenetically distinct types of endosymbiotic bacterium, Candidatus Portiera aleyrodidarum and Candidatus Hamiltonella defense (hereafter called Portiera and Hamiltonella, respectively). They are co-obligate symbionts based on the virtually universal infection observed in natural populations (16–23), the decrease in host fitness caused by symbiont elimination (24, 25), and their genome contents for producing essential amino acids and cofactors (26–29), although mathematical metabolic models assuming limited conditions suggested that Hamiltonella shows a nutritionally parasitic state (30).
Interestingly, previous studies have reported that these symbionts in B. tabaci are co-localized in the same bacteriocytes (31–33), suggesting possible competition between the symbionts for limited space and resources. Nevertheless, Portiera and Hamiltonella populations are large and exhibit synchronous dynamics in MEAM1 (34). These data suggest that some mechanism exist to maintain the two essential symbionts in the same bacteriocytes. One mechanism for avoiding conflict between the symbionts would be niche segregation, although it has never been reported at the subcellular level. In this study, we demonstrated that habitats of the symbionts are segregated within a single bacteriocyte by the endoplasmic reticulum (ER). Our results also suggest that this segregation system operates not only in specific genetic groups of B. tabaci but also in other whitefly species.
RESULTS AND DISCUSSION
Temporal dynamics of co-obligate symbionts.
We used quantitative PCR to investigate the population dynamics of the two symbiotic bacteria Portiera and Hamiltonella in MEAM1. Both populations increased during nymphal growth, peaked in actively reproducing young adults, and declined in older whiteflies (Fig. S1). The primary role of these symbionts is to provide nutrients to the host (26–29). Consistent with that role, their population dynamics correspond to the necessity for the host growth and reproduction, as reported for other obligate symbionts of multiple insect hosts, including the pea aphid (35–37), cereal weevils (38), and a different MEAM1 strain (34). The populations of both symbionts in females were maintained at relatively higher levels for longer durations than those for the male populations (Fig. S1). These results suggest that the symbiont populations are differentially regulated depending on the host’s sex.
Portiera and Hamiltonella occupy the same bacteriocytes but segregate into different subcellular niches.
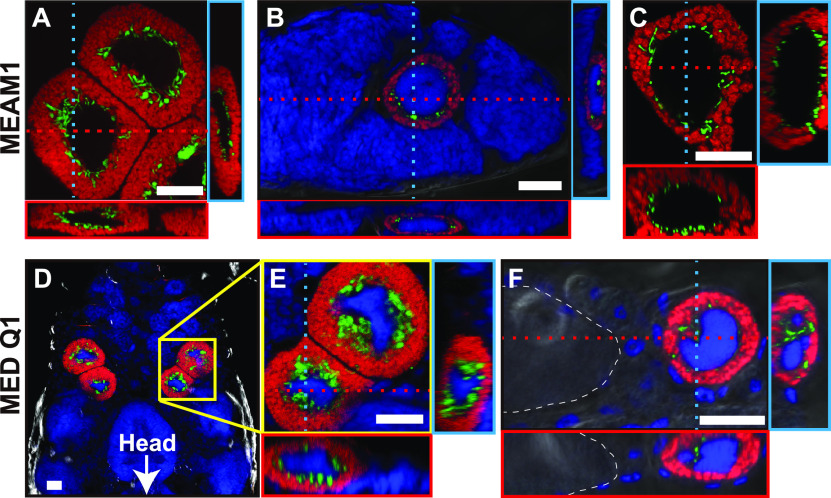
Previous studies using fluorescence in situ hybridization (FISH) analysis (39, 40) suggested that Hamiltonella is distributed around the nucleus of the bacteriocyte while Portiera is distributed in the cytoplasmic regions. However, the distribution pattern was not clear as the observations were made on a single slice of the bacteriocyte. Therefore, we acquired 3D spatial data depicting the distribution of the two symbionts in bacteriocytes using Z stack analysis. Confocal laser scanning microscopy of the teneral adults of MEAM1 revealed distinct niche for the two symbionts within the same bacteriocytes: Hamiltonella was located adjacent to the nucleus of the bacteriocyte, while Portiera was located in the cytoplasm, surrounding Hamiltonella (Fig. 1A; Movie S1). In contrast, Rickettsia, which generally infects MEAM1 as a facultative symbiont (16, 19–23, 41, 42), were scattered all over the body and rarely observed in the bacteriocytes (Fig. S2A and B).
FIG 1.
Localization of Portiera (red) and Hamiltonella (green) in bacteriocytes of Bemisia tabaci. (A to C) MEAM1: (A) bacteriocytes dissected from an adult female; (B) a 3-day-old egg; and (C) a bacteriocyte dissected from a fourth-instar nymph. (D to F) MED Q1 strain: (D) Bacteriocytes of the fourth-instar nymph; (E) enlarged image of the regions indicated by a yellow square in (D); and (F) a bacteriocyte just before entering the egg in a teneral adult female. In (A to C, E, and F), orthogonal views of Z-stack images are shown; red and blue dashed lines indicate corresponding points in the orthogonal planes. In (B, D, E, and F), host nuclear DNA is visualized in blue. In (F), white dashed lines indicate the outline of the egg. Bars, 20 μm.
In B. tabaci, an intact bacteriocyte-bearing symbiont is transferred to each egg in adult females (43) that persists through embryogenesis (44). Niche segregation between Portiera and Hamiltonella was observed in the bacteriocytes transferred into the eggs (Fig. 1B) of MEAM1 and was maintained from nymphs (Fig. 1C) to female and male young adults (Fig. S3A and B), during which the metabolic function of the symbionts becomes necessary for host growth and reproduction. At 15 days after adult eclosion, the observed habitat segregation was disrupted only in male bacteriocytes (Fig. S3C and D). This observation was consistent with the report that apoptosis and autophagy occurred in male bacteriocytes but not in females (33). Portiera and Hamiltonella titers remarkably declined only in males at this stage (Fig. S1); hence, it is conceivable that the long-term stable habitat in female bacteriocytes evolved to ensure the vertical transmission of both symbionts.
The endoplasmic reticulum segregates symbionts within bacteriocytes.
We performed electron microscopy to further understand the mechanisms underlying the subcellular niche segregation of symbionts in bacteriocytes. The results revealed the presence of rod-shaped bacteria next to the nucleus and unstructured hypertrophic bacteria on the outer cytoplasm (Fig. 2A). The shapes and locations of the two bacteria types were consistent with those of Hamiltonella and Portiera, as demonstrated by FISH (Fig. 1A to C). Interestingly, an ER-like multiple membrane structure was observed around Hamiltonella (Fig. 2B).
FIG 2.
Transmission electron micrographs (TEM) of a bacteriocyte in B. tabaci MEAM1. (A) Unstructured hypertrophic bacteria (Portiera) in the cytoplasm and rod-shaped bacteria (Hamiltonella) around the nucleus of the bacteriocyte. (B) Enlarged image of Hamiltonella. N, nucleus of a bacteriocyte; P, Portiera; H, Hamiltonella. Blue arrowhead, ER-like structures surrounding Hamiltonella. Bars, 2 μm.
The ER and Hamiltonella were simultaneously detected using a combination of immunohistochemistry and FISH at the young adult stage. Strong fluorescence signals for the ER marker were detected around the nuclei of bacteriocytes and surrounded the fluorescence signals for Hamiltonella (Fig. 3A and 4A). A three-dimensional analysis using composite Z-stack images revealed that the ER encompassed Hamiltonella (Fig. 3A). These results confirmed that the ER partitioned Hamiltonella and Portiera. At 15 days after adult eclosion, the ER and cell structure disruption was observed in males (Fig. S4C), possibly due to programmed cell death (33). In comparison, the ER was detected between the co-obligate symbionts in females (Fig. S4B). These results indicate that the ER-mediated subcellular niche segregation of the symbionts lasts longer in females. It suggests that the ER has an important role in maintenance of stable niche for each symbiont especially in female bacteriocytes which are needed to be transferred to the next generation (44).
FIG 3.
Localization of Hamiltonella and the ER in a bacteriocyte of a young adult female (1 to 5 days after eclosion) of MEAM1 (A) and MED Q1 (B). Orthogonal views of Z-stack images are shown. Red and blue dashed lines indicate corresponding points in the orthogonal planes. Hamiltonella and ER are shown in green and violet, respectively. Panels in the bottom right corner of each figure are DAPI-stained images, showing nuclei in the center of the bacteriocytes and Portiera and Hamiltonella around the nuclei. Yellow dashed lines indicate the outline of the bacteriocytes. Bars, 20 μm.
FIG 4.
Localization of Portiera (red) and Arsenophonus (green) in a putative young adult female B. tabaci Asia II 6. (A) Bacteriocytes in the abdomen. (B) developing egg within the female. Orthogonal views of Z-stack images are shown. Red and blue dashed lines indicate corresponding points in the orthogonal planes. Host nuclear DNA is visualized in blue. In (B), yellow dashed lines indicate the outline of the egg. Bars, 50 μm.
Subcellular niche segregation in other B. tabaci subgroups and more distantly related species.
The B. tabaci subgroup MED Q1 also possesses Portiera and Hamiltonella as co-obligate symbionts (16–21, 26). FISH analysis indicated that Portiera and Hamiltonella are segregated within individual bacteriocytes through earlier developmental stages in MED Q1 (Fig. 1D to F), as observed in MEAM1 (Fig. 1A to C). The facultative symbiont Cardinium in MED Q1 did not show specific localization in bacteriocytes (Fig. S2C and D). Combining FISH and immunohistochemistry, the ER membrane encompassed Hamiltonella and separated it from Portiera (Fig. 3B; Fig. S4D). Cytological staining of the dissected living bacteriocytes from young adults also demonstrated that Hamiltonella was sterically surrounded by the ER and was segregated from Portiera (Fig. S5).
Furthermore, we assayed for subcellular habitat segregation in a distantly related genetic group of B. tabaci, Asia II 6. Asia II group is frequently infected with Candidatus Arsenophonus sp. (hereafter called Arsenophonus) in addition to Portiera but not with Hamiltonella (19, 20, 45). Using FISH, we demonstrated that Arsenophonus is located adjacent to the nucleus of the bacteriocyte in both adults and developing eggs (Fig. 4). The localization pattern of Arsenophonus in Asia II 6 was identical to that of Hamiltonella in both MEAM1 (Fig. 1A to C) and MED Q1 (Fig. 1D to F). Moreover, Arsenophonus around the nucleus was surrounded by the ER (Fig. S4E), similar to that of Hamiltonella in MEAM1 and MED Q1 (Fig. S4A, B, and D).
Trialeurodes vaporariorum belongs to the same family as B. tabaci, Aleyrodinae (46). Arsenophonus has been detected at high frequencies in T. vaporariorum (22, 32, 47, 48) and is considered an essential symbiont, supplying or complementing the nutrients that are not produced by coexisting Portiera (49). FISH revealed that Portiera and Arsenophonus also exhibit subcellular habitat segregation in the same bacteriocytes of T. vaporariorum (Fig. 5A). Immunohistochemical staining with FISH indicated that the ER encloses Arsenophonus, separating it from Portiera (Fig. 5B; Fig. S4F). Bacteriocytes in T. vaporariorum (ca. 20 μm in diameter) are considerably smaller than those of B. tabaci (ca. 30 to 50 μm). Despite the morphological and phylogenetic differences, the same system in bacteriocytes is involved in symbiont niche partitioning. This suggests that the subcellular niche segregation of the symbionts evolved in the common ancestor and have been conserved to the present.
FIG 5.
Localization of Portiera (red), Arsenophonus (green), and the ER (violet) in bacteriocytes of Trialeurodes vaporariorum. (A) FISH image and (B) immunohistochemistry combined with FISH. Orthogonal views of Z-stack images are shown. Red and blue dashed lines indicate corresponding points in the orthogonal planes. In (B), panel in the bottom right corner is DAPI-stained images and yellow dashed lines indicate the outline of a bacteriocyte. Bars, 20 μm.
Biological implications, evolution, and possible mechanisms underlying subcellular symbiont segregation.
Previous ecological studies have shown that niche segregation reduces the strength of competition between species that exploit the same limiting resource (50–52). Niche segregation is found in various taxa, including endosymbiotic bacteria in insects, in which each endosymbiont generally localizes in different host cells or organs (1, 3–12). The compartmentalization of multiple symbionts into different host cells is considered an important step in the evolution to reduce direct conflict between the multiple symbionts and control them within the same host (14). The endosymbiotic system in B. tabaci is a rare exception because co-obligate symbionts occupy the same bacteriocytes (31, 32). In this study, we revealed that the endosymbionts in B. tabaci and T. vaporariorum are separated by the ER and show niche segregation at the subcellular level. This novel finding indicates that the niche segregation of endosymbionts can be established not only in different host cells but also within a single cell.
It is intriguing why the symbiont co-habitation has evolved exceptionally in the whiteflies. In certain whitefly species, including B. tabaci and T. vaporariorum, one or more entire bacteriocytes bearing symbionts are transferred to the developing egg (1, 43, 44, 53). Under the unique transmission manner, one of the symbiont partners could be lost by the failure of transmission if the two co-obligate symbiotic bacteria were separated into different cells. Hence, it is conceivable that the unique transmission machinery of the symbionts has driven the evolution of the symbiont colocalization in whiteflies. To clarify the evolutionary process of co-habitation and subcellular niche segregation of the symbionts, further studies are required on the vertical transmission and localization manners of the symbionts in various whitefly species.
In some co-obligate symbiotic systems in insects, each symbiont can complete the biosynthetic pathway of some essential nutrients, such as essential amino acids or vitamins, on its own or with host genes (13, 54–58). In other co-symbiotic systems, metabolic interdependence for some essential nutrients is present, wherein one of the symbionts possesses genes necessary for the first part of the biosynthetic pathway, and the other symbiont has genes necessary for the latter part of the pathway (10, 12, 13, 59, 60). In such a system, metabolic intermediates must diffuse or be translocated from one symbiont to another to produce essential nutrients in adjacent host cells. However, the co-obligate symbiotic system in B. tabaci has a more complex interdependence; enzymes for essential amino acids (e.g., lysine) are dispersed across both Portiera and Hamiltonella, and metabolic intermediates should be transported between the symbionts multiple times for production (26–28) (Fig. S6A). Although several metabolic duplications are present in the host genome (27, 28, 61), the physical proximity between Portiera and Hamiltonella in the same bacteriocyte, which is stably maintained during development (Fig. 1), may be adaptive for the efficient production of essential amino acids using intertwined biosynthetic pathways (Fig. S6C). It should be noted that mealybugs also have intertwined metabolic pathways in their co-obligate symbiotic systems; gamma-proteobacteria are located inside the betaproteobacterium Tremblaya, and metabolic intermediates are transported between them multiple times to produce nutrients (62–65). Accordingly, reducing the distance between symbionts could be a driving force for the evolution of complex interdependent biosynthetic pathways.
B. tabaci is a cryptic species complex with reportedly more than 44 genetic groups (15). In this study, we found the subcellular habitat segregation of two symbionts in three distinct genetic groups in B. tabaci, MEAM1, MED Q1, and Asia II 6 (Fig. 1 and 4; Fig. S4A to E). Moreover, the same localization pattern of symbionts was detected in T. vaporariorum (Fig. 5; Fig. S4F) belonging to the different genera. In Asia II 6 and T. vaporariorum, the subcellular niche occupied by Hamiltonella in MEAM1 and MED Q1 was occupied by Arsenophonus. Similar to Hamiltonella in B. tabaci, it has been suggested that Arsenophonus shares metabolic pathways for essential amino-acid synthesis with Portiera and the host T. vaporariorum (Fig. S6B) (49, 66). Arsenophonus might establish co-obligate symbiotic systems with Portiera in Asia II 6 as well as in T. vaporariorum. In many geographic regions, Hamiltonella and Arsenophonus are rarely found in the same individual of B. tabaci and T. vaporariorum (16–23, 67), suggesting that the two symbiotic bacteria are competing over functional and cytological niches. Moreover, it appears that the habitat segregation system was established in a common ancestor of species in Aleyrodinae and was conserved, while the bacterial partners coexisting with Portiera have been replaced (Fig. 6). To understand the generality and the evolutionary origin of the habitat segregation system, detailed analyses of other genetic groups in B. tabaci and diverse whitefly species are required.
FIG 6.
Phylogenetic relationships among whiteflies and their symbiotic system in bacteriocytes. Numbers at internal nodes indicate the divergence date (Mya, million years ago) estimated by Santos-Garcia et al. (66). The size of bacteriocytes is shown on the same scale.
As mentioned above, the bacterial partner coexisting with Portiera in bacteriocytes varied depending on the species and groups of host insects. This indicates that some molecular and cellular mechanisms underlying subcellular habitat segregation are mainly attributed to the host insects. It is also possible that the symbionts in whiteflies have some properties associated with the ER in bacteriocytes. It is noteworthy that human-pathogenic bacteria, such as Legionella pneumophila, Brucella abortus, and Simkania negevensis, exploit the host ER to generate a niche for replication (68–71). Recent studies have revealed that the organelles have an intimate association with endosymbiotic bacteria and play key roles in the maintenance and control of the endosymbiotic system in insects (72–76). This study provides novel insight into the interaction between symbionts and organelles. To clarify the molecular mechanisms underlying subcellular niche segregation, further studies are required from the perspectives of both the host and the symbionts. Super-resolution microscopy and cryo-electron microscopy will be useful in revealing details about the sites of interaction between the symbionts and ER and inferring molecular mechanisms.
MATERIALS AND METHODS
Materials.
Whitefly strains and their symbionts used in the study are listed in Table S1. B. tabaci was maintained on cabbage (Brassica oleracea) and Trialeurodes vaporariorum on cucumber (Cucumis sativus) at 25 ± 1°C and 40% to 60% relative humidity in a long-day regimen (16L: 8D). Adult B. tabaci Asia II 6 insects were collected from the field in May 2017 and immediately stored in 100% acetone until use (77). Additional detailed information is provided in Table S1 and Fig. S7.
DNA extraction and quantitative PCR analysis of symbionts.
Ten individuals of MEAM1 at each developmental stage (Fig. S7), from egg to adult, were collected. Eggs and nymphs were collected without distinction between males and females as the sex of whiteflies is indistinguishable at immature stages. In the adult stages, whiteflies from both sexes were separately collected. Samples were preserved in 100% acetone (77) until DNA extraction. The total genomic DNA of the insect individual and its symbionts was extracted using the NucleoSpin Tissue XS Kit (MACHEREY-NAGEL, Duren, Germany), according to the manufacturer’s instructions. From the eggs, the DNA was extracted from groups of 10 individuals. Portiera and Hamiltonella were quantified in terms of 16S rRNA gene copies using the Mx3005P system (Agilent Technologies, Santa Clara, CA, USA) with THUNDERBIRD SYBR qPCR Mix (Toyobo, Osaka, Japan), and specific primer sets (Table S2). PCR conditions were: 95°C for 3 min, followed by 40 cycles of 95°C for 30 s and 55°C for 30 s, and a final extension at 72°C for 30 s. The quantitative PCR analysis was conducted by using a standard curve method, as described previously (78).
Fluorescence in situ hybridization.
To examine the localization of the symbionts, the insect whole-body or dissected tissue specimens were fixed in Carnoy’s solution (EtOH: chloroform: glacial acetic acid, 6:3:1), bleached in 6% hydrogen peroxide in EtOH, and subjected to whole-mount FISH, as described (79). Fluorochrome-labeled oligonucleotide probes are listed in Table S2. Host-cell nuclei were counterstained with 4,6-diamino-2-phenylindole (DAPI). The specificity of in situ hybridization was confirmed using a no-probe control and an RNase digestion control (78).
Transmission electron microscopy.
Twenty teneral adults of MEAM1 (1 day old after eclosion) were dissected in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4), and the dissected bacteriocytes were prefixed with the fixative at 4°C overnight. Subsequently, the samples were postfixed with 2% osmium tetroxide in 0.1 M phosphate buffer (pH 7.4) at 4°C for 90 min before dehydration with a graded ethanol series. The dehydrated specimens were embedded in EPON 812 resin, processed into ultrathin sections (approximately 80 nm thick) using an ultramicrotome EM UC7 (Leica, Wetzlar, Germany), mounted on copper meshes, stained with uranyl acetate and lead citrate, and observed under a transmission electron microscope (80 kV; H-7600 Hitachi, Tokyo, Japan).
Simultaneous detection of symbionts and endoplasmic reticulum.
Ten to 20 mixed-sex individuals of 1 to 5 days or 15 days after adult eclosion were collected from the laboratory strains, B. tabaci MEAM1 and MED Q1 and T. vaporariorum (Table S1). For B. tabaci Asia II 6, acetone-preserved samples were used owing to the difficulty of obtaining fresh materials. After samples were washed with 70% EtOH, bacteriocytes were collected by dissection in phosphate-buffered saline (PBS). The bacteriocytes were transferred to 6.5-mm Transwell dishes with 8-μm pore polycarbonate membrane inserts (Corning, New York, NY, USA) and fixed in 4% paraformaldehyde (PFA in phosphate buffer) for 3 h at 25°C. After fixation, bacteriocytes were washed thrice in PBS with 0.3% TritonX-100 (PBSTx) for 30 min and then soaked thrice in hybridization solution (20 mM Tris-HCl pH 8.0, 0.9 M NaCl, 0.01% SDS and 30% formamide) for 5 min. Then, fluorochrome-labeled probes were hybridized overnight at 25°C. The oligonucleotide probes (Table S2) were used for Portiera detection. For Hamiltonella or Arsenophonus detection, Stellaris RNA FISH probe sets were used (Biosearch Technologies, Novato, CA, USA) (Table S2) as previously described (80). Host-cell nuclei were counterstained with DAPI during hybridization. Subsequently, the samples were refixed in 4% PFA for 7 h at 4°C as described (81) with minor modifications. Then, bacteriocytes were washed thrice in PBSTx and blocked with 1% gelatin in PBS for 30 min at 25°C. After blocking, bacteriocytes were incubated with the KDEL ER marker antibody (10C3) (Santa Cruz, Dallas, TX, USA; cat. no. sc-58774) diluted (1:50) in PBS containing 1% gelatin and 0.05% Tween 20 overnight at 4°C. Bacteriocytes were washed thrice in PBSTx and then incubated with Alexa Fluor Plus 555- or 647-conjugated goat anti-mouse IgG secondary antibody (Thermo Fisher Scientific, Waltham, MA, USA) at a dilution of 1:100 for 3 h at 25°C.
Laser confocal microscopy.
Samples were mounted with ProLong Diamond (Thermo Fisher Scientific). Images were obtained using a LSM5 Pascal or LSM710 microscope and analyzed using LSM5 Pascal Image and LSM ZEN2009 software (Carl Zeiss, Oberkochen, Germany).
Cytological staining.
Staining bacteriocytes was performed as described previously (82), with some modifications. Bacteriocytes were dissected from female B. tabaci MED Q1 (Table S1) within 3 days after adult eclosion in Buffer A (35 mM Tris–HCl, pH 7.6, containing 10 mM MgCl2, 25 mM KCl, and 250 mM sucrose) and stained with 4 μM SYTO16 (Thermo Fisher Scientific, Waltham, MA, USA) for nucleic acids (bacteria and host) and 4 μM ER-Tracker Red (Thermo Fisher Scientific) for ER in Buffer A for 2 h at 37°C on the SkyLight Glass Base Dish 3971-035-SK (IWAKI, Shizuoka, Japan). Images were obtained using a laser confocal microscope (LSM710) and analyzed using the LSM software ZEN2009 (Carl Zeiss, Oberkochen, Germany).
Statistics.
To evaluate differences in the symbiont titers between females and males at each adult stage (days 1, 15, and 30), the Mann-Whitney U test with Bonferroni’s correction was adopted. Analyses were conducted with R v.3.5.3 software (http://www.r-project.org).
Supplementary Material
ACKNOWLEDGMENTS
We thank Y. Horita, D. Hwang, K. Moronaga, N. Murakami, Y. Utsuno, and M. Watanabe for technical assistance; N. Haruyama, K. Kijima, T. Kitamura, J. Ohnishi, I. Ohta, and T. Uesato for providing insect samples; and S. Egoshi, K. Dodo, and M. Yoshida for useful comments. Part of this study was supported by JSPS KAKENHI Grant Number 18K05673 (to T.T.) and grants from the Project of the NARO Bio-oriented Technology Research Advancement Institution (Research program on the development of innovative technology). A.F. was supported by grants from the RIKEN Special Postdoctoral Researcher (SPDR) Program and Leading Initiative for Excellent Young Researchers (LEADER) Program.
A.F. and T.T. designed research; A.F. and Y.M. performed research; Y.K. contributed new analytic tools; A.F. and T.T. analyzed data and wrote the paper.
Footnotes
Supplemental material is available online only.
Contributor Information
Tsutomu Tsuchida, Email: tsuchida@sci.u-toyama.ac.jp.
John M. Chaston, Brigham Young University
REFERENCES
- 1.Buchner P. 1965. Endosymbiosis of animals with plant microorganisms. John Wiley and Sons, New York, NY. [Google Scholar]
- 2.Moran NA, McCutcheon JP, Nakabachi A. 2008. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet 42:165–190. doi: 10.1146/annurev.genet.41.110306.130119. [DOI] [PubMed] [Google Scholar]
- 3.Koga R, Bennett GM, Cryan JR, Moran NA. 2013. Evolutionary replacement of obligate symbionts in an ancient and diverse insect lineage. Environ Microbiol 15:2073–2081. doi: 10.1111/1462-2920.12121. [DOI] [PubMed] [Google Scholar]
- 4.Noda H, Watanabe K, Kawai S, Yukuhiro F, Miyoshi T, Tomizawa M, Koizumi Y, Nikoh N, Fukatsu T. 2012. Bacteriome-associated endosymbionts of the green rice leafhopper Nephotettix cincticeps (Hemiptera: Cicadellidae). Appl Entomol Zool 47:217–225. doi: 10.1007/s13355-012-0110-1. [DOI] [Google Scholar]
- 5.Tsuchida T, Koga R, Horikawa M, Tsunoda T, Maoka T, Matsumoto S, Simon JC, Fukatsu T. 2010. Symbiotic bacterium modifies aphid body color. Science 330:1102–1104. doi: 10.1126/science.1195463. [DOI] [PubMed] [Google Scholar]
- 6.Matsuura Y, Koga R, Nikoh N, Meng XY, Hanada S, Fukatsu T. 2009. Huge symbiotic organs in giant scale insects of the genus Drosicha (Coccoidea: Monophlebidae) harbor flavobacterial and enterobacterial endosymbionts. Zoolog Sci 26:448–456. doi: 10.2108/zsj.26.448. [DOI] [PubMed] [Google Scholar]
- 7.Nakabachi A, Ueoka R, Oshima K, Teta R, Mangoni A, Gurgui M, Oldham NJ, van Echten-Deckert G, Okamura K, Yamamoto K, Inoue H, Ohkuma M, Hongoh Y, Miyagishima SY, Hattori M, Piel J, Fukatsu T. 2013. Defensive bacteriome symbiont with a drastically reduced genome. Curr Biol 23:1478–1484. doi: 10.1016/j.cub.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 8.Manzano-Marin A, Szabo G, Simon JC, Horn M, Latorre A. 2017. Happens in the best of subfamilies: establishment and repeated replacements of co-obligate secondary endosymbionts within Lachninae aphids. Environ Microbiol 19:393–408. doi: 10.1111/1462-2920.13633. [DOI] [PubMed] [Google Scholar]
- 9.Matsuura Y, Moriyama M, Łukasik P, Vanderpool D, Tanahashi M, Meng X-Y, McCutcheon JP, Fukatsu T. 2018. Recurrent symbiont recruitment from fungal parasites in cicadas. Proc Natl Acad Sci USA 115:E5970–E5979. doi: 10.1073/pnas.1803245115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manzano-Marin A, Simon JC, Latorre A. 2016. Reinventing the wheel and making it round again: evolutionary convergence in Buchnera-Serratia symbiotic consortia between the distantly related lachninae aphids Tuberolachnus salignus and Cinara cedri. Genome Biol Evol 8:1440–1458. doi: 10.1093/gbe/evw085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toenshoff ER, Penz T, Narzt T, Collingro A, Schmitz-Esser S, Pfeiffer S, Klepal W, Wagner M, Weinmaier T, Rattei T, Horn M. 2012. Bacteriocyte-associated gammaproteobacterial symbionts of the Adelges nordmannianae/piceae complex (Hemiptera: Adelgidae). ISME J 6:384–396. doi: 10.1038/ismej.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monnin D, Jackson R, Kiers ET, Bunker M, Ellers J, Henry LM. 2020. Parallel evolution in the integration of a co-obligate aphid symbiosis. Curr Biol 30:1949–1957.e6. doi: 10.1016/j.cub.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Renoz F, Ambroise J, Bearzatto B, Fakhour S, Parisot N, Ribeiro Lopes M, Gala JL, Calevro F, Hance T. 2022. The di-symbiotic systems in the aphids Sipha maydis and Periphyllus lyropictus provide a contrasting picture of recent co-obligate nutritional endosymbiosis in aphids. Microorganisms 10:1360. doi: 10.3390/microorganisms10071360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chomicki G, Werner GDA, West SA, Kiers ET. 2020. Compartmentalization drives the evolution of symbiotic cooperation. Philos Trans R Soc Lond B Biol Sci 375:20190602. doi: 10.1098/rstb.2019.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanakala S, Ghanim M. 2019. Global genetic diversity and geographical distribution of Bemisia tabaci and its bacterial endosymbionts. PLoS One 14:e0213946. doi: 10.1371/journal.pone.0213946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gueguen G, Vavre F, Gnankine O, Peterschmitt M, Charif D, Chiel E, Gottlieb Y, Ghanim M, Zchori-Fein E, Fleury F. 2010. Endosymbiont metacommunities, mtDNA diversity and the evolution of the Bemisia tabaci (Hemiptera: Aleyrodidae) species complex. Mol Ecol 19:4365–4376. doi: 10.1111/j.1365-294X.2010.04775.x. [DOI] [PubMed] [Google Scholar]
- 17.Tsagkarakou A, Mouton L, Kristoffersen JB, Dokianakis E, Grispou M, Bourtzis K. 2012. Population genetic structure and secondary endosymbionts of Q Bemisia tabaci (Hemiptera: Aleyrodidae) from Greece. Bull Entomol Res 102:353–365. doi: 10.1017/S0007485311000757. [DOI] [PubMed] [Google Scholar]
- 18.GnankinÉ O, Mouton L, Henri H, Terraz G, HoundetÉ T, Martin T, Vavre F, Fleury F. 2013. Distribution of Bemisia tabaci (Homoptera: Aleyrodidae) biotypes and their associated symbiotic bacteria on host plants in West Africa. Insect Conservation and Diversity 6:411–421. doi: 10.1111/j.1752-4598.2012.00206.x. [DOI] [Google Scholar]
- 19.Bing XL, Ruan YM, Rao Q, Wang XW, Liu SS. 2013. Diversity of secondary endosymbionts among different putative species of the whitefly Bemisia tabaci. Insect Sci 20:194–206. doi: 10.1111/j.1744-7917.2012.01522.x. [DOI] [PubMed] [Google Scholar]
- 20.Fujiwara A, Maekawa K, Tsuchida T. 2015. Genetic groups and endosymbiotic microbiota of the Bemisia tabaci species complex in Japanese agricultural sites. J Appl Entomol 139:55–66. doi: 10.1111/jen.12171. [DOI] [Google Scholar]
- 21.Zchori-Fein E, Lahav T, Freilich S. 2014. Variations in the identity and complexity of endosymbiont combinations in whitefly hosts. Front Microbiol 5:310. doi: 10.3389/fmicb.2014.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marubayashi JM, Kliot A, Yuki VA, Rezende JA, Krause-Sakate R, Pavan MA, Ghanim M. 2014. Diversity and localization of bacterial endosymbionts from whitefly species collected in Brazil. PLoS One 9:e108363. doi: 10.1371/journal.pone.0108363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karut K, Karaca MM, Döker I, Kazak C. 2017. Analysis of species, subgroups, and endosymbionts of Bemisia tabaci (Hemiptera: Aleyrodidae) from southwestern cotton fields in Turkey. Environ Entomol 46:1035–1040. doi: 10.1093/ee/nvx093. [DOI] [PubMed] [Google Scholar]
- 24.Su Q, Oliver KM, Pan H, Jiao X, Liu B, Xie W, Wang S, Wu Q, Xu B, White JA, Zhou X, Zhang Y. 2013. Facultative symbiont Hamiltonella confers benefits to Bemisia tabaci (Hemiptera: Aleyrodidae), an invasive agricultural pest worldwide. Environ Entomol 42:1265–1271. doi: 10.1603/EN13182. [DOI] [PubMed] [Google Scholar]
- 25.Su Q, Xie W, Wang S, Wu Q, Liu B, Fang Y, Xu B, Zhang Y. 2014. The endosymbiont Hamiltonella increases the growth rate of its host Bemisia tabaci during periods of nutritional stress. PLoS One 9:e89002. doi: 10.1371/journal.pone.0089002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao Q, Rollat-Farnier PA, Zhu DT, Santos-Garcia D, Silva FJ, Moya A, Latorre A, Klein CC, Vavre F, Sagot MF, Liu SS, Mouton L, Wang XW. 2015. Genome reduction and potential metabolic complementation of the dual endosymbionts in the whitefly Bemisia tabaci. BMC Genomics 16:226. doi: 10.1186/s12864-015-1379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen W, Hasegawa DK, Kaur N, Kliot A, Pinheiro PV, Luan J, Stensmyr MC, Zheng Y, Liu W, Sun H, Xu Y, Luo Y, Kruse A, Yang X, Kontsedalov S, Lebedev G, Fisher TW, Nelson DR, Hunter WB, Brown JK, Jander G, Cilia M, Douglas AE, Ghanim M, Simmons AM, Wintermantel WM, Ling KS, Fei Z. 2016. The draft genome of whitefly Bemisia tabaci MEAM1, a global crop pest, provides novel insights into virus transmission, host adaptation, and insecticide resistance. BMC Biol 14:110. doi: 10.1186/s12915-016-0321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie W, Yang X, Chen C, Yang Z, Guo L, Wang D, Huang J, Zhang H, Wen Y, Zhao J, Wu Q, Wang S, Coates BS, Zhou X, Zhang Y. 2018. The invasive MED/Q Bemisia tabaci genome: a tale of gene loss and gene gain. BMC Genomics 19:68. doi: 10.1186/s12864-018-4448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang YB, Ren FR, Yao YL, Sun X, Walling LL, Li NN, Bai B, Bao XY, Xu XR, Luan JB. 2020. Intracellular symbionts drive sex ratio in the whitefly by facilitating fertilization and provisioning of B vitamins. ISME J 14:2923–2935. doi: 10.1038/s41396-020-0717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ankrah NYD, Luan J, Douglas AE. 2017. Cooperative metabolism in a three-partner insect-bacterial symbiosis revealed by metabolic modeling. J Bacteriol 199. doi: 10.1128/JB.00872-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gottlieb Y, Ghanim M, Gueguen G, Kontsedalov S, Vavre F, Fleury F, Zchori-Fein E. 2008. Inherited intracellular ecosystem: symbiotic bacteria share bacteriocytes in whiteflies. FASEB J 22:2591–2599. doi: 10.1096/fj.07-101162. [DOI] [PubMed] [Google Scholar]
- 32.Skaljac M, Zanic K, Ban SG, Kontsedalov S, Ghanim M. 2010. Co-infection and localization of secondary symbionts in two whitefly species. BMC Microbiol 10:142. doi: 10.1186/1471-2180-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li NN, Jiang S, Lu KY, Hong JS, Wang YB, Yan JY, Luan JB. 2022. Bacteriocyte development is sexually differentiated in Bemisia tabaci. Cell Rep 38:110455. doi: 10.1016/j.celrep.2022.110455. [DOI] [PubMed] [Google Scholar]
- 34.Su Q, Xie W, Wang S, Wu Q, Ghanim M, Zhang Y. 2014. Location of symbionts in the whitefly Bemisia tabaci affects their densities during host development and environmental stress. PLoS One 9:e91802. doi: 10.1371/journal.pone.0091802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koga R, Tsuchida T, Fukatsu T. 2003. Changing partners in an obligate symbiosis: a facultative endosymbiont can compensate for loss of the essential endosymbiont Buchnera in an aphid. Proc Biol Sci 270:2543–2550. doi: 10.1098/rspb.2003.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakurai M, Koga R, Tsuchida T, Meng XY, Fukatsu T. 2005. Rickettsia symbiont in the pea aphid Acyrthosiphon pisum: novel cellular tropism, effect on host fitness, and interaction with the essential symbiont Buchnera. Appl Environ Microbiol 71:4069–4075. doi: 10.1128/AEM.71.7.4069-4075.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simonet P, Duport G, Gaget K, Weiss-Gayet M, Colella S, Febvay G, Charles H, Vinuelas J, Heddi A, Calevro F. 2016. Direct flow cytometry measurements reveal a fine-tuning of symbiotic cell dynamics according to the host developmental needs in aphid symbiosis. Sci Rep 6:19967. doi: 10.1038/srep19967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vigneron A, Masson F, Vallier A, Balmand S, Rey M, Vincent-Monegat C, Aksoy E, Aubailly-Giraud E, Zaidman-Remy A, Heddi A. 2014. Insects recycle endosymbionts when the benefit is over. Curr Biol 24:2267–2273. doi: 10.1016/j.cub.2014.07.065. [DOI] [PubMed] [Google Scholar]
- 39.Luan JB, Shan HW, Isermann P, Huang JH, Lammerding J, Liu SS, Douglas AE. 2016. Cellular and molecular remodelling of a host cell for vertical transmission of bacterial symbionts. Proc R Soc B 283:20160580. doi: 10.1098/rspb.2016.0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ren FR, Sun X, Wang TY, Yao YL, Huang YZ, Zhang X, Luan JB. 2020. Biotin provisioning by horizontally transferred genes from bacteria confers animal fitness benefits. ISME J 14:2542–2553. doi: 10.1038/s41396-020-0704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gottlieb Y, Ghanim M, Chiel E, Gerling D, Portnoy V, Steinberg S, Tzuri G, Horowitz AR, Belausov E, Mozes-Daube N, Kontsedalov S, Gershon M, Gal S, Katzir N, Zchori-Fein E. 2006. Identification and localization of a Rickettsia sp. in Bemisia tabaci (Homoptera: Aleyrodidae). Appl Environ Microbiol 72:3646–3652. doi: 10.1128/AEM.72.5.3646-3652.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Himler AG, Adachi-Hagimori T, Bergen JE, Kozuch A, Kelly SE, Tabashnik BE, Chiel E, Duckworth VE, Dennehy TJ, Zchori-Fein E, Hunter MS. 2011. Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science 332:254–256. doi: 10.1126/science.1199410. [DOI] [PubMed] [Google Scholar]
- 43.Costa HS, Toscano NC, Henneberry TJ. 1996. Mycetocyte inclusion in the oocytes of Bemisia argentifolii (Homoptera: Aleyrodidae). Ann Entomol Soc Am 89:694–699. doi: 10.1093/aesa/89.5.694. [DOI] [Google Scholar]
- 44.Luan J, Sun X, Fei Z, Douglas AE. 2018. Maternal inheritance of a single somatic animal cell displayed by the bacteriocyte in the whitefly Bemisia tabaci. Curr Biol 28:459–465.e3. doi: 10.1016/j.cub.2017.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang XT, Cai L, Shen Y, Du YZ. 2018. Diversity and evolution of the endosymbionts of Bemisia tabaci in China. PeerJ 6:e5516. doi: 10.7717/peerj.5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mound LA, Halsey SH. 1978. Whitefly of the world: a systematic catalogue of the Aleyrodidae (Homoptera) with host plant and natural enemy data. John Wiley and Sons, Chichester, UK. [Google Scholar]
- 47.Kapantaidaki DE, Ovčarenko I, Fytrou N, Knott KE, Bourtzis K, Tsagkarakou A. 2015. Low levels of mitochondrial DNA and symbiont diversity in the worldwide agricultural pest, the greenhouse whitefly Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). J Hered 106:80–92. doi: 10.1093/jhered/esu061. [DOI] [PubMed] [Google Scholar]
- 48.Skaljac M, Zanic K, Hrncic S, Radonjic S, Perovic T, Ghanim M. 2013. Diversity and localization of bacterial symbionts in three whitefly species (Hemiptera: Aleyrodidae) from the east coast of the Adriatic Sea. Bull Entomol Res 103:48–59. doi: 10.1017/S0007485312000399. [DOI] [PubMed] [Google Scholar]
- 49.Santos-Garcia D, Juravel K, Freilich S, Zchori-Fein E, Latorre A, Moya A, Morin S, Silva FJ. 2018. To B or not to B: comparative genomics suggests Arsenophonus as a source of B vitamins in whiteflies. Front Microbiol 9:2254. doi: 10.3389/fmicb.2018.02254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hardin G. 1960. The competitive exclusion principle. Science 131:1292–1297. doi: 10.1126/science.131.3409.1292. [DOI] [PubMed] [Google Scholar]
- 51.Chesson P. 2000. Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst 31:343–366. doi: 10.1146/annurev.ecolsys.31.1.343. [DOI] [Google Scholar]
- 52.Levine JM, HilleRisLambers J. 2009. The importance of niches for the maintenance of species diversity. Nature 461:254–257. doi: 10.1038/nature08251. [DOI] [PubMed] [Google Scholar]
- 53.Xu XR, Li NN, Bao XY, Douglas AE, Luan JB. 2020. Patterns of host cell inheritance in the bacterial symbiosis of whiteflies. Insect Sci 27:938–946. doi: 10.1111/1744-7917.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCutcheon JP, Moran NA. 2007. Parallel genomic evolution and metabolic interdependence in an ancient symbiosis. Proc Natl Acad Sci USA 104:19392–19397. doi: 10.1073/pnas.0708855104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCutcheon JP, Moran NA. 2010. Functional convergence in reduced genomes of bacterial symbionts spanning 200 My of evolution. Genome Biol Evol 2:708–718. doi: 10.1093/gbe/evq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCutcheon JP, McDonald BR, Moran NA. 2009. Convergent evolution of metabolic roles in bacterial co-symbionts of insects. Proc Natl Acad Sci USA 106:15394–15399. doi: 10.1073/pnas.0906424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koga R, Moran NA. 2014. Swapping symbionts in spittlebugs: evolutionary replacement of a reduced genome symbiont. ISME J 8:1237–1246. doi: 10.1038/ismej.2013.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szabo G, Schulz F, Manzano-Marin A, Toenshoff ER, Horn M. 2022. Evolutionarily recent dual obligatory symbiosis among adelgids indicates a transition between fungus- and insect-associated lifestyles. ISME J 16:247–256. doi: 10.1038/s41396-021-01056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meseguer AS, Manzano-Marín A, Coeur d'Acier A, Clamens AL, Godefroid M, Jousselin E. 2017. Buchnera has changed flatmate but the repeated replacement of co-obligate symbionts is not associated with the ecological expansions of their aphid hosts. Mol Ecol 26:2363–2378. doi: 10.1111/mec.13910. [DOI] [PubMed] [Google Scholar]
- 60.Weglarz KM, Havill NP, Burke GR, von Dohlen CD. 2018. Partnering with a pest: genomes of hemlock woolly adelgid symbionts reveal atypical nutritional provisioning patterns in dual-obligate bacteria. Genome Biol Evol 10:1607–1621. doi: 10.1093/gbe/evy114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luan JB, Chen W, Hasegawa DK, Simmons AM, Wintermantel WM, Ling KS, Fei Z, Liu SS, Douglas AE. 2015. Metabolic coevolution in the bacterial symbiosis of whiteflies and related plant sap-feeding insects. Genome Biol Evol 7:2635–2647. doi: 10.1093/gbe/evv170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.von Dohlen CD, Kohler S, Alsop ST, McManus WR. 2001. Mealybug beta-proteobacterial endosymbionts contain gamma-proteobacterial symbionts. Nature 412:433–436. doi: 10.1038/35086563. [DOI] [PubMed] [Google Scholar]
- 63.Husnik F, Nikoh N, Koga R, Ross L, Duncan RP, Fujie M, Tanaka M, Satoh N, Bachtrog D, Wilson AC, von Dohlen CD, Fukatsu T, McCutcheon JP. 2013. Horizontal gene transfer from diverse bacteria to an insect genome enables a tripartite nested mealybug symbiosis. Cell 153:1567–1578. doi: 10.1016/j.cell.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 64.Husnik F, McCutcheon JP. 2016. Repeated replacement of an intrabacterial symbiont in the tripartite nested mealybug symbiosis. Proc Natl Acad Sci USA 113:E5416–E5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Szabó G, Schulz F, Toenshoff ER, Volland JM, Finkel OM, Belkin S, Horn M. 2017. Convergent patterns in the evolution of mealybug symbioses involving different intrabacterial symbionts. ISME J 11:715–726. doi: 10.1038/ismej.2016.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Santos-Garcia D, Vargas-Chavez C, Moya A, Latorre A, Silva FJ. 2015. Genome evolution in the primary endosymbiont of whiteflies sheds light on their divergence. Genome Biol Evol 7:873–888. doi: 10.1093/gbe/evv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mouton L, Henri H, Romba R, Belgaidi Z, Gnankiné O, Vavre F. 2022. Analyses of symbiotic bacterial communities in the plant pest Bemisia tabaci reveal high prevalence of Candidatus Hemipteriphilus asiaticus on the African continent. Peer Community J 2:e20. doi: 10.24072/pcjournal.103. [DOI] [Google Scholar]
- 68.Arasaki K, Toomre DK, Roy CR. 2012. The Legionella pneumophila effector DrrA is sufficient to stimulate SNARE-dependent membrane fusion. Cell Host Microbe 11:46–57. doi: 10.1016/j.chom.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Starr T, Child R, Wehrly TD, Hansen B, Hwang S, Lopez-Otin C, Virgin HW, Celli J. 2012. Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle. Cell Host Microbe 11:33–45. doi: 10.1016/j.chom.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mehlitz A, Karunakaran K, Herweg JA, Krohne G, van de Linde S, Rieck E, Sauer M, Rudel T. 2014. The chlamydial organism Simkania negevensis forms ER vacuole contact sites and inhibits ER-stress. Cell Microbiol 16:1224–1243. doi: 10.1111/cmi.12278. [DOI] [PubMed] [Google Scholar]
- 71.Escoll P, Mondino S, Rolando M, Buchrieser C. 2016. Targeting of host organelles by pathogenic bacteria: a sophisticated subversion strategy. Nat Rev Microbiol 14:5–19. doi: 10.1038/nrmicro.2015.1. [DOI] [PubMed] [Google Scholar]
- 72.Newton IL, Savytskyy O, Sheehan KB. 2015. Wolbachia utilize host actin for efficient maternal transmission in Drosophila melanogaster. PLoS Pathog 11:e1004798. doi: 10.1371/journal.ppat.1004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sheehan KB, Martin M, Lesser CF, Isberg RR, Newton IL. 2016. Identification and characterization of a candidate Wolbachia pipientis type iv effector that interacts with the actin cytoskeleton. mBio 7. doi: 10.1128/mBio.00622-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simonet P, Gaget K, Balmand S, Ribeiro Lopes M, Parisot N, Buhler K, Duport G, Vulsteke V, Febvay G, Heddi A, Charles H, Callaerts P, Calevro F. 2018. Bacteriocyte cell death in the pea aphid/Buchnera symbiotic system. Proc Natl Acad Sci USA 115:E1819–E1828. doi: 10.1073/pnas.1720237115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fattouh N, Cazevieille C, Landmann F. 2019. Wolbachia endosymbionts subvert the endoplasmic reticulum to acquire host membranes without triggering ER stress. PLoS Negl Trop Dis 13:e0007218. doi: 10.1371/journal.pntd.0007218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Strunov A, Schmidt K, Kapun M, Miller WJ. 2022. Restriction of Wolbachia bacteria in early embryogenesis of neotropical Drosophila species via endoplasmic reticulum-mediated autophagy. mBio 13:e0386321. doi: 10.1128/mbio.03863-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fukatsu T. 1999. Acetone preservation: a practical technique for molecular analysis. Mol Ecol 8:1935–1945. doi: 10.1046/j.1365-294x.1999.00795.x. [DOI] [PubMed] [Google Scholar]
- 78.Tsuchida T, Koga R, Fujiwara A, Fukatsu T. 2014. Phenotypic effect of “Candidatus Rickettsiella viridis,” a facultative symbiont of the pea aphid (Acyrthosiphon pisum), and its interaction with a coexisting symbiont. Appl Environ Microbiol 80:525–533. doi: 10.1128/AEM.03049-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koga R, Tsuchida T, Fukatsu T. 2009. Quenching autofluorescence of insect tissues for in situ detection of endosymbionts. Appl Entomol Zool 44:281–291. doi: 10.1303/aez.2009.281. [DOI] [Google Scholar]
- 80.De Clerck C, Fujiwara A, Joncour P, Leonard S, Felix ML, Francis F, Jijakli MH, Tsuchida T, Massart S. 2015. A metagenomic approach from aphid's hemolymph sheds light on the potential roles of co-existing endosymbionts. Microbiome 3:63. doi: 10.1186/s40168-015-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Larsen P, Nielsen JL, Otzen D, Nielsen PH. 2008. Amyloid-like adhesins produced by floc-forming and filamentous bacteria in activated sludge. Appl Environ Microbiol 74:1517–1526. doi: 10.1128/AEM.02274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nishikori K, Morioka K, Kubo T, Morioka M. 2009. Age- and morph-dependent activation of the lysosomal system and Buchnera degradation in aphid endosymbiosis. J Insect Physiol 55:351–357. doi: 10.1016/j.jinsphys.2009.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1. Download spectrum.04684-22-s0001.mov, MOV file, 0.9 MB (874.1KB, mov)
Movie S1 legend, Fig. S1 to S7, and Tables S1 and S2. Download spectrum.04684-22-s0002.pdf, PDF file, 6.5 MB (6.5MB, pdf)