ABSTRACT
Flagellins are the main constituents of the flagellar filaments that provide bacterial motility, chemotactic ability, and host immune elicitation ability. Although the functions of flagellins have been extensively studied in bacteria with a single flagellin-encoding gene, the function of multiple flagellin-encoding genes in a single bacterial species is largely unknown. Here, the model plant-growth-promoting bacterium Pseudomonas kilonensis F113 was used to decipher the divergent functions of duplicated flagellins. We demonstrate that the two flagellins (FliC-1 and FliC-2) in 12 Pseudomonas strains, including F113, are evolutionarily distinct. Only the fliC-1 gene but not the fliC-2 gene in strain F113 is responsible for flagellar biogenesis, motility, and plant immune elicitation. The transcriptional expression of fliC-2 was significantly lower than that of fliC-1 in medium and in planta, most likely due to variations in promoter activity. In silico prediction revealed that all fliC-2 genes in the 12 Pseudomonas strains have a poorly conserved promoter motif. Compared to the Flg22-2 epitope (relative to FliC-2), Flg22-1 (relative to FliC-1) induced stronger FLAGELLIN SENSING 2 (FLS2)-mediated microbe-associated molecular pattern-triggered immunity and significantly inhibited plant root growth. A change in the 19th amino acid in Flg22-2 reduced its binding affinity to the FLS2/brassinosteroid insensitive 1-associated kinase 1 complex. Also, Flg22-2 epitopes in the other 11 Pseudomonas strains were presumed to have low binding affinity due to the same change in the 19th amino acid. These findings suggest that Pseudomonas has evolved duplicate flagellins, with only FliC-1 contributing to motility and plant immune elicitation.
IMPORTANCE Flagellins have emerged as important microbial patterns. This work focuses on flagellin duplication in some plant-associated Pseudomonas. Our findings on the divergence of duplicated flagellins provide a conceptual framework for better understanding the functional determinant flagellin and its peptide in multiple-flagellin plant-growth-promoting rhizobacteria.
KEYWORDS: Pseudomonas, flagellin, Flg22, motility, plant immunity, flagella, plant-microbe interactions
INTRODUCTION
Pseudomonas, a diverse and ecologically significant genus, is widespread in the natural environment. In addition to the opportunistic Pseudomonas pathogens, some rhizospheric or soil-dwelling Pseudomonas species act as plant-growth-promoting rhizobacteria (PGPR) that play substantial roles in plant growth promotion and disease suppression (1). Flagellum-mediated movement allows PGPR to move to nutrient-rich habitats and avoid conditions adverse to their survival. The bacterial flagellum is a macromolecular machinery with a rotary basal body embedded in the cell membrane, a curved hook, and one or more extracellular helical filaments that are composed of up to 30,000 flagellin monomers (2). As such, flagellin is required for bacterial motility, a fundamental function required for host colonization by pathogens, commensals, and symbionts (3). It is generally believed that the flagellar filament is encoded by a single flagellin gene per genome. However, as more and more bacterial genomes are sequenced, supernumerary flagellar loci have been discovered to be relatively common features in a broad taxonomic spectrum of bacteria, such as the order Enterobacterales, in which five (flag-1 to flag-5) flagellar loci occur on the genomes of enterobacterial taxa (4). It was reported that multiple flagellin genes had been found in more than 45% of the annotated bacterial genomes encoding flagella (5, 6), for reasons that are mostly unknown. The number of flagellin genes in such species usually ranges from two to seven, with a minority possessing more copies (5, 6).
Flagellar assembly and motility have been well studied in single-flagellin bacteria, such as Escherichia coli, which utilizes a single flagellin at any phase to assemble the filaments and control movement. However, the role of different flagellins in multiflagellin bacteria remains largely unclear. A few studies have demonstrated that a high degree of functional redundancy occurs in some bacteria, including Salmonella enterica serovar Typhimurium (7), Sinorhizobium meliloti (8 to 10), Bdellovibrio bacteriovorus (11), Helicobacter pylori (12), and Vibrio spp. (13 to 15), in which the flagellar filaments are assembled from all or part of the flagellins encoded in their genome. Loss of certain flagellins in these bacteria may result in changes in filament assembly and motility, depending on the species.
Regardless of whether the flagellar filament is simple or complex, the structures of most bacterial flagellin subunits are highly conserved in terms of amino-acid composition and subunit organization of the flagellin monomers. In a wide range of bacteria, flagellins comprise a ubiquitous microbe-associated molecular pattern (MAMP) that triggers both innate and adaptive immune responses in eukaryotic hosts and manipulates host–bacterial interactions (16). Flagellin 22 (Flg22), a 22-amino-acid peptide located in the highly conserved N-terminal region of flagellin, is perceived by the pattern recognition receptor FLAGELLIN-SENSING2 (FLS2) to induce immune reactions in various plants, such as tomato (Solanum lycopersicum), potato (Solanum tuberosum), tobacco (Nicotiana tabacum and N. benthamiana), and the model plant Arabidopsis thaliana (17). A recent high-throughput analysis showed that the Flg22 peptides of γ- and β-proteobacteria trigger strong oxidative bursts, whereas peptides from other (ε-, δ-, and α-) proteobacteria trigger a weak response, depending on the sequence divergence of the Flg22 epitopes in each taxonomic class (18). A massive screening of 412 Flg22 variants in the flagellin gene fliC of P. aeruginosa indicated that up to 80% of the variants could not restore motility of the immotile P. aeruginosa fliC mutant (19). Further analysis of FLS2-Flg22 variant interactions showed that more than 70% of variants retained interactions with FLS2 (19). Flg22 sequences from β- and γ-proteobacteria, Bacillus, and Actinobacteria resemble the immunogenic P. aeruginosa Flg22 sequence, whereas Flg22 sequences from Rhizobiales and Caulobacterales are substantially more divergent (20). These findings suggest that single amino-acid changes in the Flg22 epitope could affect bacterial motility and host–bacterial interaction. However, little is known about the biological roles and immunogenic and motility functions of multiple flagellins and natural Flg22 variants in a single bacterium.
Pseudomonas exhibits parasitic, commensal, and mutual interactions with host cells. Most Pseudomonas species, including the model pathogenic species P. aeruginosa and P. syringae, have only one fliC gene. In contrast, some commensal PGPR Pseudomonas strains, such as P. kilonensis F113 (previously P. fluorescens) and 1855-344, P. brassicacearum LBUM300, P. fluorescens et76, and Pseudomonas sp. CBZ-4, harbor an extra copy of the fliC gene (21). In P. kilonensis strain F113, a model fluorescent pseudomonad used to study secondary metabolite production and plant–bacterial interactions, a fliC-1 mutant produced aflagellate bacterial cells, whereas a fliC-2 mutant had a similar motility phenotype as the wild type (21). Reverse transcription (RT)-PCR analysis revealed that the fliC-2 gene is not expressed in the wild-type strain F113, but is highly expressed in kinB and algU mutants, implying that the expression of different fliC copies may be influenced by environmental conditions (21). However, it is currently unknown whether the two fliC genes have redundancy in flagellar biogenesis and bacterial fitness or whether the two Flg22 epitopes differ in plant immune elicitation ability.
Here, phylogenetic analysis revealed that all FliC-1 and FliC-2 sequences from 12 Pseudomonas strains were clustered into two clades, and the evolutionary distances of the FliC-2 sequences were significantly shorter than those of the FliC-1. Knockout of fliC-1, but not fliC-2, impaired motility, flagellar biogenesis, plant immune elicitation, and root growth inhibition. We also demonstrated that when fliC-1 is expressed in medium and in planta, fliC-2 is sitting idle. Flg22-1 was more effective at eliciting plant immunity and inhibiting root growth than Flg22-2, mainly due to variation in the 19th amino-acid residue.
RESULTS
Characterization of the two flagellin genes in P. kilonensis F113.
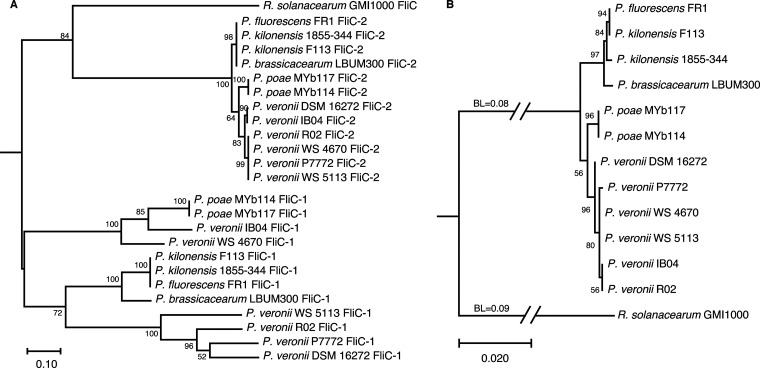
P. kilonensis strain F113 possesses two flagellin-encoding genes, fliC-1 (PSF113_1554) and fliC-2 (PSF113_0740), which are located in two separate flagellar clusters (21). The FliC-1 and FliC-2 proteins comprise 283 amino acids and 350 amino acids, respectively, which show 45.04% identity (Fig. S1). We performed a comprehensive BLAST analysis (Expect threshold was 0.05) to search for FliC-2 homologs in 967 genomes of the P. fluorescens group (taxid:136843) in NCBI, and 11 sequences were collected from P. veronii R02, P. veronii WS 4670, P. veronii WS 5113, P. veronii P7772, P. veronii IB04, P. veronii DSM 16272, P. fluorescens FR1, P. kilonensis 1855-344, P. brassicacearum LBUM300, P. poae MYb114, and P. poae MYb117. Phylogenetic analysis using MEGA X revealed that all FliC-1 and FliC-2 sequences clustered divergently into two clades (Fig. 1A), suggesting that these two kinds of flagellins (fliC-1 and fliC-2 in 12 Pseudomonas strains, respectively) might evolve independently. However, the evolutionary distances of the FliC-2 sequences were significantly shorter than those of the FliC-1 sequences. In addition, the six P. veronii strains in the FliC-1 clade were separated by a subgroup of P. kilonensis F113, FR1, 1855-344, and P. brassicacearum LBUM300. Conversely, P. veronii strains in the FliC-2 clade were clustered together. Although the taxonomic status of some collected strains remains to be determined, the FilC-2 phylogenetic tree seems to be more identical to the tree of the 16S rRNA genes (Fig. 1B), suggesting that FliC-2 is more appropriate than FliC-1 to illustrate the phylogenetic relationships among the collected 12 strains from the P. fluorescens complex (22).
FIG 1.
Phylogenetic analysis of flagellins of Pseudomonas strains. (A) Maximum-likelihood phylogeny based on protein sequence alignments of flagellins from 12 Pseudomonas strains and Ralstonia solanacearum GMI1000. The JTT matrix-based model with 500 bootstrap replicates was used. (B) Maximum-likelihood phylogeny based on 16S rRNA gene sequences from 12 Pseudomonas strains and Ralstonia solanacearum GMI1000. The Tamura–Nei model with 500 bootstrap replicates was used. 16S rRNA gene sequences and flagellin sequences from R. solanacearum GMI1000 were regarded as an outgroup. The numbers at the branches represent the confidence levels of the taxa clustered in the tree. The scale bar reflects evolutionary distance. BL, branch lengths.
A fliC-1 mutant is deficient in motility and flagellar biogenesis.
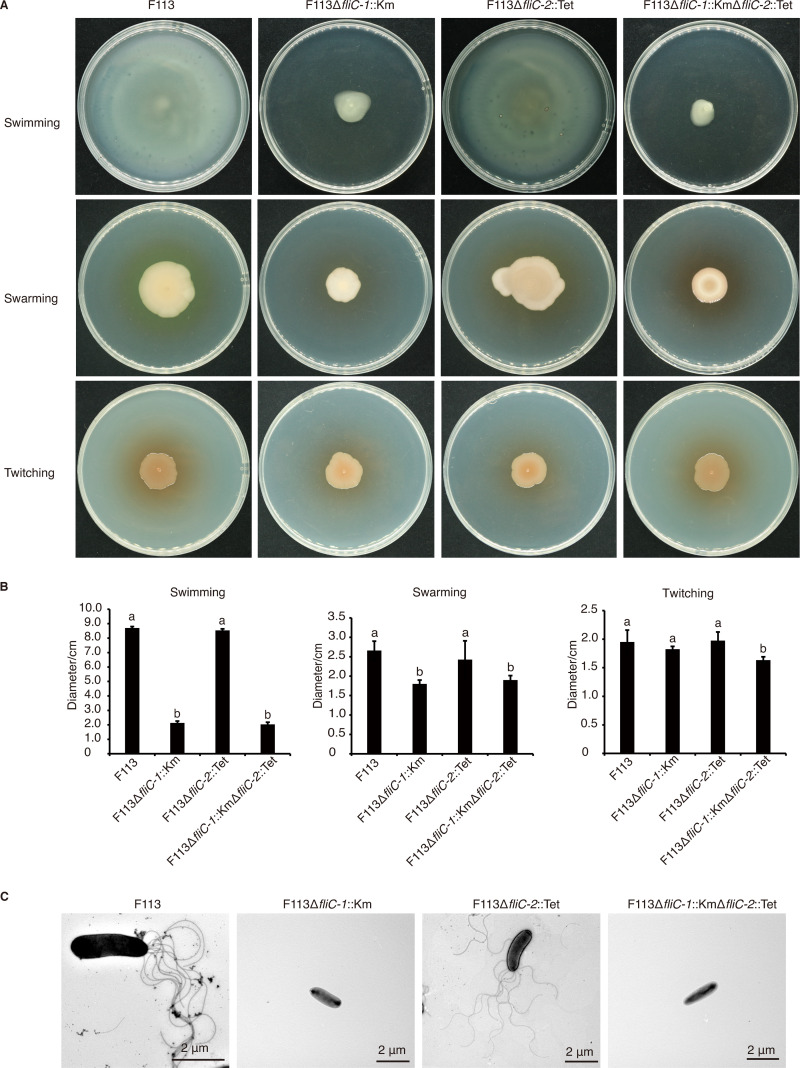
Bacteria deploy the flagellar apparatus as a power motor to change their motility patterns in order to occupy a more suitable habitat. To investigate which flagellin is required for motility and flagellar biogenesis, we generated mutants of the two flagellin genes. Strain F113 showed strong swimming and swarming activities on agar plates and formed expanding circular colonies after 24 h of incubation. The F113ΔfliC-1::Km mutant and F113ΔfliC-1::KmΔfliC-2::Tet double mutant were severely compromised in motility and formed significantly smaller colonies than the wild-type strain, whereas the fliC-2 mutation had no effect on swimming or swarming (Fig. 2A and B). Also, complemented with fliC-1 under the native promoter to F113ΔfliC-1::Km resulted in the recovery of swimming ability, whereas complemented with fliC-2 under the native promoter to F113ΔfliC-1::KmΔfliC-2::Tet did not (Fig. S2A and B). None of the single fliC mutants were affected in twitching motility (Fig. 2A and B). Morphological observation under a transmission electron microscope indicated that the wild-type strain F113 possessed one or more (up to 9) polar flagella (Fig. 2C and Fig. S2C). However, rather than the fliC-2 single mutant (F113ΔfliC-2::Tet), the fliC-1 single mutant (F113ΔfliC-1::Km) and the fliC-1 and fliC-2 double mutant (F113ΔfliC-1::KmΔfliC-2::Tet) were deficient in flagella production (Fig. 2C and Fig. S2C). These findings imply that fliC-1, rather than fliC-2, is essential for flagellar biogenesis, bacterial swimming, and swarming motility in strain F113 under laboratory conditions.
FIG 2.
Motility and flagellar examination of P. kilonensis F113 and its fliC mutants. (A) Swimming, swarming, and twitching motilities on KB swim plates (0.3%, 0.5%, and 1.0% agar, respectively). Photographs were taken 24 h (swimming), 48 h (swarming), or 96 h (twitching) after incubation. (B) The quantitative analysis of swimming, swarming, and twitching abilities of P. kilonensis F113 and the fliC mutants. Different letters indicate statistically significant differences between different treatments (one-way ANOVA, Tukey’s test; P < 0.05). (C) Transmission electron microscopy images of the flagella of P. kilonensis F113 and its derivatives. All of the experiments were repeated three times with similar results.
The fliC-1 mutant fails to stimulate plant immunity and affect root growth.
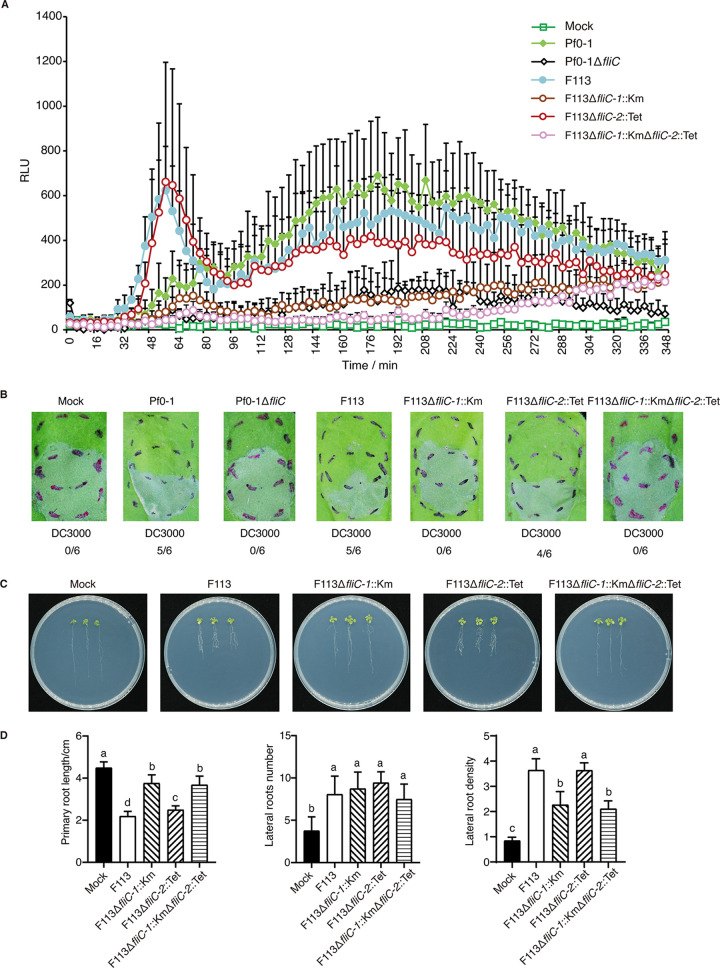
Flagellin derived from bacterial flagella is recognized as a typical MAMP perceived by the plant receptor FLS2 and stimulates plant innate immunity (17, 23). To assess whether the two fliC genes are involved in eliciting pattern-triggered immunity (PTI) responses, we first examined the ROS production induced in N. benthamiana by strain F113 and its fliC mutants. P. fluorescens Pf0-1 and its flagellin mutant Pf0-1ΔfliC were employed as positive and negative control, respectively (24). Strains F113 and F113ΔfliC-2::Tet stimulated similarly strong ROS bursts in tobacco leaves, as did the positive control P. fluorescens Pf0-1; however, no ROS bursts were observed after treatments with F113ΔfliC-1::Km, F113ΔfliC-1::KmΔfliC-2::Tet, and the negative control Pf0-1ΔfliC (Fig. 3A). Furthermore, challenge inoculation of strains F113 and F113ΔfliC-2::Tet compromised Pst DC3000-induced hypersensitive response (HR) in N. benthamiana, whereas the F113ΔfliC-1::Km and F113ΔfliC-1::KmΔfliC-2::Tet mutants failed to inhibit Pst DC3000-induced HR (Fig. 3B). These results were further confirmed by electrolyte leakage data (Fig. S3A), indicating that FliC-1, but not FliC-2, contributes to PTI activation.
FIG 3.
Contribution of the duplicated flagellins to plant immunity and root growth. (A) ROS induced by P. fluorescens Pf0-1, P. kilonensis F113, and the fliC mutants in N. benthamiana (RLU, relative light unit). (B) Challenge-inoculation HR assays for functional PTI were conducted by first infiltrating N. benthamiana leaves with 1 × 108 CFU/mL of the test Pseudomonas strains (upper circles). After 6 h, an overlapping inoculation of 5 × 106 CFU/mL of the HR-inducing strain Pst DC3000 (lower circles) was made. The fraction under each image indicates the number of times that the HR was inhibited compared to the number of test inoculations. (C) Effects of P. kilonensis F113 and its fliC mutants on shoot and root growth in A. thaliana accession Col-0. (D) Quantitative analysis of primary root length, number of lateral roots, and lateral root density. Different letters indicate statistically significant differences between different treatments (one-way ANOVA, Tukey’s test; P < 0.05). All experiments were repeated three times with similar results.
We next explored the effects of the mutants on Arabidopsis root growth. Compared to mock inoculation with ddH2O, strain F113 and all mutants significantly inhibited primary root growth. However, F113 and F113ΔfliC-2::Tet showed significantly stronger inhibitory abilities than F113ΔfliC-1::Km and F113ΔfliC-1::KmΔfliC-2::Tet (Fig. 3C and D). Further, strain F113 and all mutants significantly increased the number of lateral roots compared with the mock treatment, but there was no significant difference among the strains (Fig. 3C and D). It is noteworthy that lateral root density was significantly increased upon treatment with F113, whereas F113ΔfliC-1::Km, but not F113ΔfliC-2::Tet, strongly reduced lateral root density (Fig. 3C and D). Taken together, these data suggested that FliC-1 plays a major role in stimulating plant immunity and inhibiting root growth.
Transcriptional expression of fliC-1 is significantly higher than that of fliC-2 in medium and in planta.
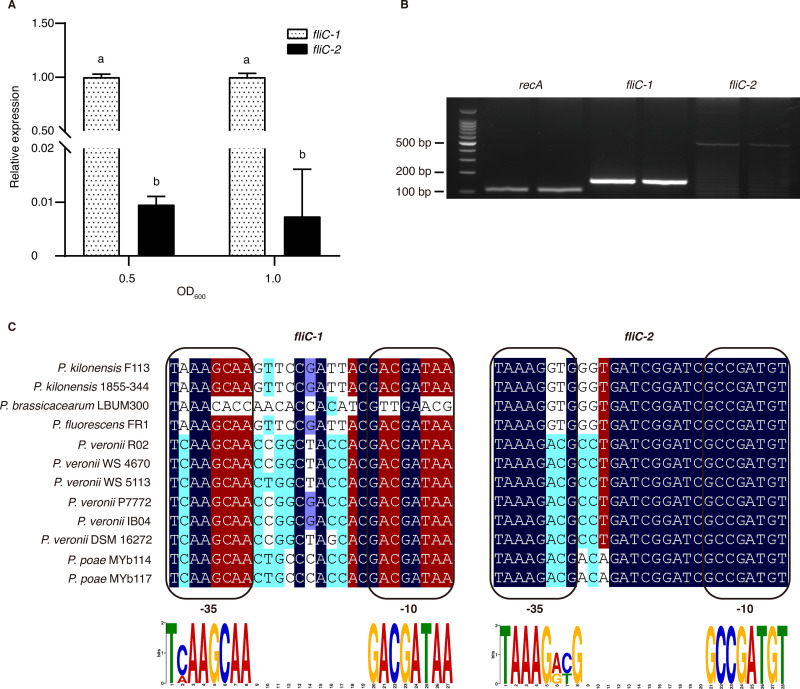
To investigate whether gene expression underlies the divergent functions of the two flagellin genes, we further examined the transcriptional expression of the two genes in medium and in planta. RT-qPCR results showed that fliC-1 was significantly more strongly expressed than fliC-2 in King’s medium B (KB) in both the logarithmic and stationary phases (Fig. 4A). Furthermore, RT-PCR assays demonstrated that fliC-2 expression was lower than that of the control recA, whereas fliC-1 expression was significantly higher than that of fliC-2 and recA (Fig. 4B; Fig. S3C). These results suggested that the two fliC genes show a significantly different transcriptional expression and may lead to divergent biological functions.
FIG 4.
fliC gene expression and promoter prediction. (A) RT-qPCR analysis of fliC-1 and fliC-2 expression in P. kilonensis F113 cultured in KB medium. (B) RT-PCR of fliC-1 and fliC-2 expression in P. kilonensis F113 infiltrated into N. benthamiana. (C) Alignment of the fliC-1 and fliC-2 promoter regions. The -10 and -35 regions for each promoter are indicated in boxes. Dark blue indicates 100% identity, red indicates ≥75% identity, cyan indicates ≥50% identity, and light blue indicates ≥33% identity. The height of each letter in the MEME LOGO represents the relative frequency of each base at different positions in the consensus sequence. Different letters indicate statistically significant differences between different treatments (one-way ANOVA, Tukey’s test; P < 0.05). All experiments were repeated three times with similar results.
The expression of the flagellar system is regulated by a complex hierarchy (25, 26). σ28 is a key master regulator that binds to a specific promoter region of the fliC genes (27, 28). To determine the promoters of the two fliC genes, we collected 13 well-studied fliC promoters from various bacteria (Table S5) and generated a fliC promoter diagram (Fig. S4A). Using this motif, we scanned the promoter regions of the two fliC genes of strain F113. The results demonstrated that fliC-1 has a conserved promoter (P = 1.88E-06), whereas the promoter of fliC-2 is more variable (P = 6.45E-06) (Fig. 4C; Table S6). We speculated that sequence polymorphism in the promoters may affect the expression levels of fliC-1 and fliC-2. Given the differential conservation of the two fliC promoters in strain F113, we asked whether the other 11 strains with two fliC genes would have the same characteristics. We performed promoter scanning and sequence alignments of all fliC-1 and fliC-2 genes (Fig. 4C). Notably, we observed that all fliC-1 promoters were more conserved than fliC-2 promoters, except in P. brassicacearum LBUM300, which has an untypical fliC-1 promoter (Fig. 4C). To test whether the promoter of fliC-1 could drive the expression of fliC-2, we developed a hybrid construct to quantify the transcription of fliC-2. Notably, the expression level of fliC-2 driven by the fliC-1 promoter in the double mutant F113ΔfliC-1::KmΔfliC-2::Tet was significantly increased (Fig. S4B). We then asked whether highly expressed fliC-2 could recover swimming motility or PTI elicitation in F113ΔfliC-1::KmΔfliC-2::Tet. However, swimming activity and ETI-cell death suppression were not observed after complementation with the fliC-2 gene driven by the fliC-1 promoter (Fig. S2A and D). Taken together, these findings imply that differential promoter activity results in divergent transcriptional expression of duplicated fliC genes in P. kilonensis F113. Although fliC-2 has low biological expression, artificially high expression of it cannot perform biological functions such as swimming motility and PTI elicitation.
Flg22-1 induces stronger plant immune responses than Flg22-2.
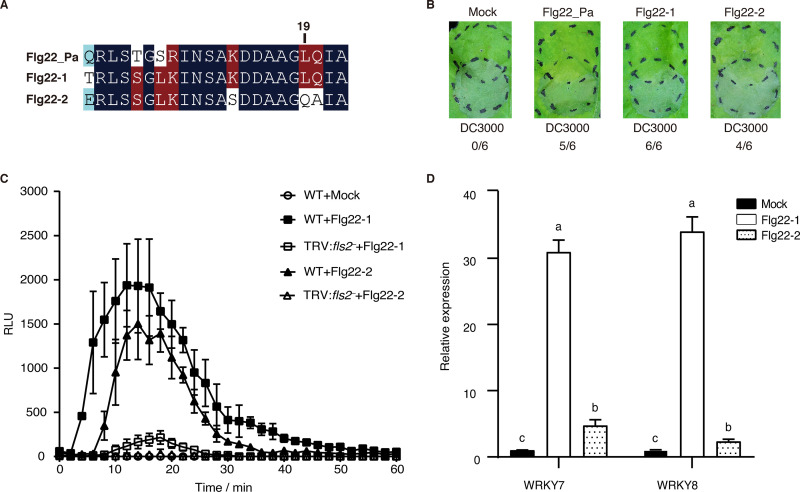
The Flg22 region in the bacterial flagellin N terminus carries the innate immune elicitation determinant that is recognized by FLS2 in many plant species (17). To examine the Flg22 peptides of the two flagellins in strain F113, we extracted the sequences of Flg22-1 and Flg22-2 and aligned them with the commercial Flg22_Pa (Fig. 5A). The results showed that Flg22-1 and Flg22-2 have 18 and 15 amino-acid residues identical to Flg22_Pa, respectively. Asp14, Asp15, Leu19, and Ile21 are key residues of Flg22_Pa for plant immunity elicitation (29). In strain F113, Flg22-1 has all four conserved amino acids, whereas Flg22-2 only has three; Leu19 is replaced by Gln. We synthesized Flg22-1 and Flg22-2 and examined their potential in stimulating plant immunity. Inoculation of Flg22-1 resulted in full inhibition of the HR triggered by Pst DC3000, whereas Flg22-2 exhibited moderate HR inhibition ability (Fig. 5B). ROS assays indicated that Flg22-1 induced stronger ROS production than Flg22-2 (Fig. 5C and Fig. S5). Notably, neither of the two peptides induced ROS production in fls2-silenced N. benthamiana (Fig. 5C and Fig. S5), suggesting that Flg22-1 and Flg22-2 are both sensed by FLS2. Further, both Flg22-1 and Flg22-2 induced callose deposition, but Flg22-1 induced more deposits than Flg22-2 (Fig. S6). Both Flg22-1 and Flg22-2 induced the expression of two PTI marker genes, WRKY7 and WRKY8, whereas Flg22-1 induced significantly higher levels of defense gene expression than Flg22-2 (Fig. 5D). Taken together, these results indicated that both Flg22-1 and Flg22-2 can induce plant immune responses, but Flg22-1 is more potent than Flg22-2.
FIG 5.
Plant immunity induced by Flg22-1 and Flg22-2. (A) Sequence alignment of Flg22_Pa from P. aeruginosa PAO1 and Flg22-1, and Flg22-2 from P. kilonensis F113. Dark blue indicates 100% identity, red indicates ≥50% identity, and light blue indicates ≥33% identity. (B) Challenge-inoculation HR assays for functional PTI were conducted by first infiltrating N. benthamiana leaves with 10 μM Flg22 peptides (upper circles). After 6 h, an overlapping inoculation of 5 × 106 CFU/mL of the HR-inducing strain Pst DC3000 (lower circles) was made. The fraction under each image indicates the number of times that the HR was inhibited to the number of test inoculations. (C) ROS induced by 0.1 μM Flg22 peptides in wild-type and fls2-silenced N. benthamiana plants. (D) PTI marker gene expression induced by 1 μM Flg22 peptides in N. benthamiana. Different letters indicate statistically significant differences between different treatments (one-way ANOVA, Tukey’s test; P < 0.05). All of the experiments were repeated three times with similar results.
Flg22-1 inhibits plant growth more robustly than Flg22-2.
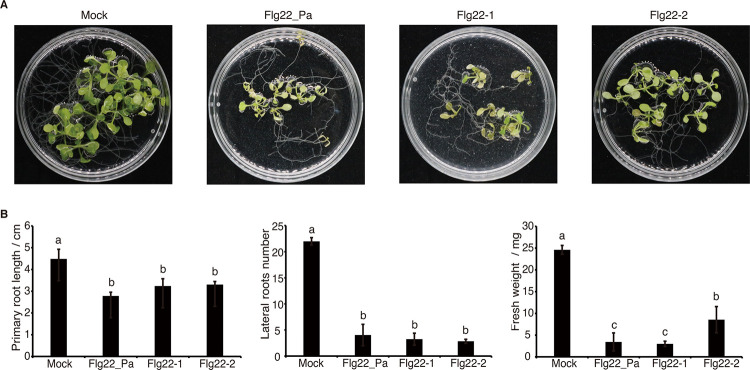
We next investigated whether the peptides Flg22-1 and Flg22-2 could inhibit plant growth. After 12 days, leaves from seedlings treated with Flg22_Pa and Flg22-1 developed significantly more chlorosis than those from plants treated with Flg22-2 (Fig. 6A and Fig. S7). Primary root lengths in the Flg22_Pa, Flg22-1, and Flg22-2 treatments were significantly shorter than those in the MS control, and lateral root numbers were reduced by approximately four times. Moreover, the fresh weights of whole plants in the Flg22-Pa, Flg22-1, and Flg22-2 treatments were reduced by approximately three to five times compared to those in the MS control, of which Flg22-2 showed weaker inhibition than Flg22_Pa and Flg22-1 (Fig. 6B).
FIG 6.
Effects of Flg22 peptides on plant growth. (A) Phenotypes of A. thaliana seedlings treated with Flg22_Pa, Flg22-1, and Flg22-2. (B) Quantitative analysis of primary root length, number of lateral roots formed, and fresh weight. Different letters indicate statistically significant differences between different treatments (one-way ANOVA, Tukey’s test; P < 0.05). All experiments were repeated three times with similar results.
The 19th residue of Flg22 is predicted to be critical for receptor interaction.
Given the differential ability of Flg22-1 and Flg22-2 to stimulate plant immunity and inhibit plant growth, we examined the key amino acids involved in receptor recognition. The binding affinity of Flg22 with FLS2 and BAK1 was predicted by in silico modeling using the MutaBind2 pipeline. Flg22-1 was predicted to bind the FLS2/BAK1 receptor complex with high affinity (ΔΔGbind of Flg22-1-FLS2 = −0.59 kcal/mol; Flg22-1-FLS2-BAK1 = −1.12 kcal/mol) (Table 1). In contrast, seven of the Flg22-2 residues differed from those of Flg22_Pa, and a significant reduction in binding was predicted for FLS2 (ΔΔGbind = 2.2 kcal/mol), whereas a moderate reduction was predicted for BAK1 (ΔΔGbind = 0.16 kcal/mol) (Table 1). To determine the critical residues, we performed single amino-acid replacements on Flg22_Pa based on the Flg22-2 sequence (Table 2). The predictions showed that K13S and Q20A in Flg22_Pa might have reduced the binding stability with FLS2 significantly. In contrast, L19Q in Flg22_Pa might have affected FLS2-Flg22-BAK1 complex formation significantly. It has been reported that Leu19 in Flg22 is the only contact site for binding to FLS2-BAK1 (20). Thus, mutation of the 19th amino acid in Flg22-2 might be the primary cause of reduced plant immunity and growth inhibition. Finally, we examined the identities of other Flg22-1 and Flg22-2 epitopes in the other 11 strains. All Flg22-2 sequences showed 100% identity to that of strain F113, whereas the Flg22-1 sequences were variable (Fig. S8). Remarkably, P. veronii strains showed significant variation; five contained Met rather than Leu at the 19th position. We assessed the binding affinities of the other Flg22-1 peptides with FLS2 and BAK1 using the MutaBind2 pipeline. Although some strains had a variable Flg22-1, none of them showed a significant change in binding affinity (Table S3, S4). These results suggested that Flg22-1 and Flg22-2 in all of the tested strains might have divergent effects on PTI stimulation.
TABLE 1.
Binding affinity between Flg22 and FLS2 or BAK1
| Peptide | Sequencea | Phase 1 (FLS2-Flg22) ΔΔGbind (kcal mol−1)b | Phase 2 (FLS2/Flg22-BAK1) ΔΔGbind (kcal mol−1) |
|---|---|---|---|
| Flg22-1 | TRLSSGLKIN SAKDDAAGLQIA | −0.59 | −1.12 |
| Flg22-2 | ERLSSGLKIN SASDDAAGQAIA | 2.2 | 0.16 |
| Flg22_Pa | QRLSTGSRIN SAKDDAAGLQIA | ||
Letters in bold indicate the mutated residues compared to Flg22_Pa.
ΔΔGbind (kcal mol−1): a positive value indicates a destabilizing mutation and a negative value a stabilizing mutation.
TABLE 2.
Binding affinity between Flg22 with a single mutation and FLS2 or BAK1
| Peptide | Mutated residue | Phase 1 (FLS2-Flg22) |
Phase 2 (FLS2/Flg22-BAK1) |
||
|---|---|---|---|---|---|
| ΔΔGbind (kcal mol−1) | Deleteriousa | ΔΔGbind (kcal mol−1) | Deleteriousa | ||
| Flg22-2 | Q1E | 1.38 | No | −0.44 | No |
| T5S | 0.72 | No | −0.65 | No | |
| S7L | −0.13 | No | −1.17 | No | |
| R8K | 1.37 | No | −0.36 | No | |
| K13S | 2.22 | Yes | –0.3 | No | |
| L19Q | 1.37 | No | 1.52 | Yes | |
| Q20A | 2.09 | Yes | −0.06 | No | |
Deleterious (yes/no): the MutaBind2 server uses ΔΔG ≥ 1.5 or ≤−1.5 kcal mol−1 to define whether or not a mutation is deleterious. ΔΔG ≥ 1.5 kcal mol−1 is deleterious.
DISCUSSION
Many bacterial species employ flagella to move to and colonize their preferred environmental niche. With the increase in genome sequences, multiple flagellin genes are increasingly being found in bacteria. However, the functions of multiple flagellins in bacteria remain largely elusive. Here, we demonstrated that the two flagellin proteins of P. kilonensis F113 are divergent evolutionarily, with FliC-1 functioning as the major determinant of motility and plant immunity stimulation. Notably, mutation of the 19th amino acid may have been a significant cause of reduced binding affinity with FLS2/BAK1 and plant immunity, as predicted by affinity prediction.
We found that FliC-1 and FliC-2 of P. kilonensis F113 show 45% identity, mostly in the two termini (Fig. S1). The middle region of FliC-1 of strain F113 is variable and 67 amino acids shorter than that of FliC-2 (Fig. S1). This was also observed for the 11 FliC-1 homologs from various Pseudomonas strains (data not shown). It has been reported that the conserved N- and C-terminal regions of flagellin proteins mediate filament assembly and harbor the hot spot recognized by the host immunity receptor, whereas the variable central region contains the surface-exposed D2-D3 domains (30 to 32). Although a previous study predicted that the second flagellar system of P. kilonensis F113 containing FliC-2 resulted from an insertion event based on a syntenic comparison of the genomes of strain F113 and P. brassicacearum NFM421 (21), the origin of FliC-2 is still unknown. Genome analysis of a broader range of related strains and a detailed phylogenetic description of relationships among Pseudomonas species are required to investigate the origin and selection rates of FliC-2 and to assess the extent of lateral gene transfer and recombination within these loci.
P. kilonensis F113 produces polar flagella, but only FliC-1 is required for flagellar assembly and mobility (Fig. 2) under laboratory conditions. These findings are consistent with those in Proteus mirabilis, in which flaA and flaB encode two flagellin proteins, but only FlaA is involved in filament assembly (33). However, a few studies showed that some bacteria deploy multiple flagellin proteins to build the flagellar complex (11, 33 to 35). B. bacteriovorus has six flagellin genes, fliC-1–6, all of which except fliC4, which expressed at a low level, synergistically encode the single polar flagellum (11). Notably, we discovered that P. kilonensis F113 produces 1 to 9 polar flagella, which differs from the single polar flagellum reported in a previous study (21). It has been suggested that polar flagella, as well as peritrichous flagella, are induced in solid medium or medium with a high viscosity (36, 37). Although we did not assess what determines the polar flagella number in P. kilonensis F113, further research into medium composition, culture conditions, and gene regulation would shed light on the mechanisms of flagella production.
We found that in P. kilonensis F113, fliC-1 is significantly more strongly expressed than fliC-2, which is largely dependent on the promoter activity. Notably, the fliC-1 promoter is more conserved than that of fliC-2 compared with the typical promoter regulated by the transcriptional regulator σ28. The same characteristics were found in the other Pseudomonas strains containing duplicated fliC genes. Interestingly, it has been reported that two flagellin genes in many Salmonella strains, fliC and fljB, encode phase 1 and phase 2 flagellins, respectively, which are not expressed simultaneously. The expression of the two loci is governed by a switch mechanism that is regulated by the invertible element hin, which appears to be unique to Salmonella (37). Given this, the second flagellin FljB is considered a genetic “spare tire” used in particular environmental circumstances that is used less often than FliC and is less critical to survival (38). Although it is unknown whether P. kilonensis F113 and its relatives are diphasic and capable of phase variation, the soil-dwelling and plant-colonizing properties of Pseudomonas imply that the second FliC could be used in unpredictable short-term emergencies, such as adapting to new niches, avoiding protozoan predation, and immunologic escape.
The FLS2/BAK1 complex is the direct receptor of Flg22 (19, 20, 29). The 19th amino acid, Leu, is the only residue that interacts with FLS2 and BAK1 and is located in the “message” area, which is involved in immune response activation (23, 29, 39). In all of the strains in this study, the 19th amino acid of Flg22-2 was Gln rather than Leu, resulting in reduced binding affinity with FLS2/BAK1. Some differences at the 19th amino acid of Flg22 have also been found in S. meliloti, A. tumefaciens, Candidatus Liberibacter solanacearum, and Candidatus Liberibacter asiaticus (40, 41), whose flagellins are nonimmunogenic, leading to immune evasion. However, a few studies have shown that replacements of Leu19 constrain motility and immunogenicity (19). For instance, lack of Leu19 in Flg22_Pa dramatically decreased plant immune activity (17, 23), and removal of Leu19 in Flg15-Δ3 even changed its biological activity into an antagonist (23). Interestingly, we found that some Pseudomonas strains had Met rather than Leu at the 19th residue of Flg22-1, which did not affect the binding affinity with FLS2/BAK1 (Table S3, S4). A recent study showed that Met at the 19th position of Flg225013 had no adverse effect on motility or FLS2 interaction (20). Previous and our findings combined fully explain the importance of Leu19 in Flg22, and this residue is functionally equivalent to Met, but not Gln. In conclusion, we hypothesize that the divergence of the two flagellins in P. kilonensis F113 and other strains with duplicated flagellins was driven by selection to evade plant immune detection. Our findings on the divergence of duplicated flagellins provide a conceptual framework for better understanding the functional determinant flagellin and its peptide in multiple-flagellin PGPRs.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table S1. Pseudomonas fluorescens Pf0-1, Pf0-1ΔfliC, P. syringae pv. tomato (Pst) DC3000, P. kilonensis F113, and the fliC mutants were grown in King’s medium B (KB) at 28°C (24, 42, 43). Escherichia coli (DH5α) was grown in Luria-Bertani (LB) broth at 37°C. To propagate plasmids and select transformants, antibiotics were added at the following final concentrations: 100 μg/mL ampicillin, 50 μg/mL kanamycin, and 20 μg/mL tetracycline, as required (44). pT18mob is a derivative of pT18mobsacB (24), in which the sacB gene fragment was removed by digestion with Aha III in this study.
Construction of fliC mutants and complemented strains.
To construct P. kilonensis F113ΔfliC-1::Km, a 509-bp fragment of the fliC-1 gene was amplified from P. kilonensis F113 using primers WHL1048 and WHL1049 and cloned into p2P24 (45). The final construct was transformed into P. kilonensis F113 by triparental mating. To construct P. kilonensis F113ΔfliC-2::Tet and P. kilonensis F113ΔfliC-1::KmΔfliC-2::Tet, a 634-bp fragment of the fliC-2 gene was amplified from P. kilonensis F113 using primers WHL1104 and WHL1105 and cloned into pT18mob. Triparental mating was used to introduce the vector into P. kilonensis F113 and the fliC-1 mutant. The primers used in this study are listed in Table S2. The complemented strains are created through triparental mating of pBBR1-MCS5-C1, pBBR1-MCS5-C2, and pBBR1-MCS5-C3.
Transmission electron microscopy and motility assays.
P. kilonensis F113 and derivatives were grown in KB medium for 24 h. Grids were stained, and photographs were taken as described previously (46). Swimming, swarming, and twitching motility were tested on KB media with 0.3%, 0.5% (plus 5 g/L glucose), and 1% agar according to a previous report (46). The plates were photographed after 24 h, and colony diameters were measured for quantitative analysis. All assays were repeated at least three times, with multiple replicates.
Reactive oxygen species (ROS) assay.
Bacterial suspensions at OD600 = 0.5 (5 × 108 CFU/mL) were infiltrated into N. benthamiana leaves. P. fluorescens Pf0-1 and Pf0-1ΔfliC were used as positive and negative controls, respectively. MgCl2 (10 mM) was used as a mock control. Six hours after infiltration, leaf disks (0.5 cm diameter) were punched out and soaked in 100 μL of 0.5 mM L-012 (Wako, Japan). The intensity of ROS production was determined by monitoring the chemiluminescence using a Tecan microplate reader (Tecan, Switzerland) (24).
For peptide-triggered ROS assays, N. benthamiana leaf discs were soaked in 100 μL of distilled water for 12 hours. Water was replaced with 100 μL of a solution containing 34 mg/mL of luminol, 10 μg/mL of horseradish peroxidase, and 0.1 μM Flg22. Luminescence was measured as described above (47). All assays were repeated at least three times, with multiple replicates.
Callose deposition assay.
Callose deposition in N. benthamiana was assayed as described previously (47), with slight adjustments. The leaves of N. benthamiana plants were infiltrated with 1 μM Flg22 peptide derived from P. aeruginosa (Flg22_Pa), Flg22-1, or Flg22-2. Sterile water was used as a mock control. Leaf disks (1 cm diameter) collected 6 h after infiltration were floated in 2 mL of 95% ethanol and incubated for decolorization at 37°C for 6 h until completely transparent (the decolorization time could be shortened to less than 4 h by refreshing the ethanol half-way the incubation). The decolorized leaves were washed twice with 70% ethanol and then thrice with sterile water. Aniline blue solution (1%, dissolved in 150 mM K2HPO4, adjusted to pH 9.5 with KOH) was added, and the leaf disks were placed in a dark environment for 1 h. Callose deposits were visualized using a confocal microscope (LSM 880, Zeiss). The assays were repeated at least three times, with multiple replicates.
Challenge-inoculation hypersensitive response (HR) assay.
Bacterial suspensions at OD600 = 0.1 (1 × 108 CFU/mL) or peptides at 10 μM were infiltrated into N. benthamiana leaves. After 6 h, the bacterial pathogen Pst DC3000 was inoculated into the prior infiltration area at OD600 = 0.005 (5 × 106 CFU/mL). After 2 days postinoculation (dpi), cell death was measured and photographs acquired. An ion leakage assay was conducted as previously reported (48). The assay was repeated at least three times, with multiple replicates.
Seedling growth inhibition assay.
Seeds of Arabidopsis thaliana Col-0 were surface-sterilized with 70% ethanol for 5 min and rinsed with sterile water thrice to remove the ethanol. The sterilized seeds were sown on 1× Murashige and Skoog (MS) plates supplemented with 0.5% sucrose and adjusted to pH 5.7 with KOH (49). Seedings were stratified at 4°C in the dark for 2 days and transferred to a growth chamber (21 to 23°C; 16 h light, 8 h dark; light intensity 100 μmol m−2 s−1) (50). P. kilonensis F113 and derivatives at 1 × 108 CFU/mL or peptides at 10 μM were dipped onto the root tips. Primary root length, number of lateral roots, and shoot fresh weight were measured at 14 dpi (51). Lateral root density was calculated by dividing the number of lateral roots on the primary root by root length as reported previously (52). The assay was repeated at least three times with multiple replicates.
Virus-induced gene silencing (VIGS) in N. benthamiana.
N. benthamiana plants were grown in a chamber with 16 h light/8 h dark, 60% humidity, and 24°C during the day and 22°C at night. For VIGS of FLS2 in N. benthamiana, Agrobacterium tumefaciens GV3101 carrying pTRV1 or pTRV2-FLS2 (53) was suspended in induction buffer (10 mM MES, 200 μM acetosyringone, pH 5.5) at OD600 = 0.5 and incubated at 28°C under shaking at 200 rpm for 3 h. pTRV2-PDS and pTRV2-EC1 were used as positive and negative controls, respectively (24). The cells were collected by centrifugation at room temperature, 3,000 × g for 2 min. Agrobacterium cells carrying pTRV1 and pTRV2 with FLS2, pTRV2-PDS, or pTRV2-EC1 were mixed at a 1:1 (vol/vol) ratio and resuspended in infiltration buffer (5 mM MES, pH 5.5). The mixtures were inoculated into all leaves of 2-week-old N. benthamiana plants (Fig. S3B). The efficiency of gene silencing was validated by quantitative RT-qPCR analysis. The experiment was repeated at least three times, with multiple replicates.
RT-qPCR and RT-PCR.
RT-qPCR assays were performed as described previously (54). Peptides at 1 μM were infiltrated into the leaves of N. benthamiana plants. After 3 h, leaf disks (1 cm diameter) were collected for cDNA preparation and RT-qPCR on an ABI QuantStudio6 Flex real-time PCR system (Applied Biosystems, USA). Gene expression levels were standardized to the level of the constitutively expressed NbEF1α, normalized to the expression in mock-treated plants, and calculated using the 2–ΔΔCt method.
For RT-PCR, total RNA was extracted using a Bacterial RNA kit (Omega). cDNA was synthesized using the Evo M-MLV RT Mix kit with gDNA Clean for qPCR (Accurate Biology). fliC-1 and fliC-2 mRNAs were amplified using the primers listed in Table S2. The recA gene was employed as a reference. All assays were repeated at least three times.
Phylogenetic analysis and binding affinity prediction.
The two flagellin-encoding genes were extracted from the genome of P. kilonensis. BLAST analysis was used to search for FliC homologs in the P. fluorescens group (taxid: 136843) in NCBI. MEGA X was used for multiple alignments, and the Tamura–Nei model and JTT matrix-based model were used to construct the phylogenetic tree, respectively (55 to 57). Estimates of the binding affinity of Flg22 peptides to FLS2 or BAK1 were calculated using the MutaBind2 server (58). The crystal structure of FLS2-Flg22-BAK1 (PDB: 4MN8) (29) was acquired from the Protein Data Bank (59) and uploaded to the MutaBind2 server. The residues of Flg22 were mutated to Flg22-1 and Flg22-2, respectively, and ΔΔGbind values were obtained (−1.5 ≤ ΔΔG ≤ 1.5 means not deleterious).
Statistical analysis.
All data were processed using software IBM SPSS Statistics 25.0 (SPSS, Chicago, IL, USA). The data were analyzed using one-way ANOVA, Tukey’s test. P <0.05 was considered statistically significant.
ACKNOWLEDGMENTS
This work was funded by the National Key R&D Program of China (2019YFD1002000), the Fundamental Research Funds for Central Public-Interest Scientific Institutions (Y2022PT12), the Science and Technology Programs of the Shandong Tobacco (KN273), and Zunyi Tobacco (2021XM03). H.-L.W. was supported by the Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences and the Beijing Innovation Consortium of Agriculture Research System (BAIC04-2022).
We declare no conflicts of interest.
H.-L.W. conceived the work and supervised the project. Y.L., J.W., and Y.-L.G. performed the experiments. H.-L.W. and Y.L. wrote the manuscript. J.W. and L.-Q.Z. revised the manuscript.
Footnotes
Supplemental material is available online only.
Contributor Information
Hai-Lei Wei, Email: weihailei@caas.cn.
Lindsey Price Burbank, USDA – San Joaquin Valley Agricultural Sciences Center.
REFERENCES
- 1.Vejan P, Abdullah R, Khadiran T, Ismail S, Nasrulhaq Boyce A. 2016. Role of plant growth promoting rhizobacteria in agricultural sustainability—a review. Molecules 21:573. doi: 10.3390/molecules21050573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minamino T, Namba K. 2004. Self-assembly and type III protein export of the bacterial flagellum. J Mol Microbiol Biotechnol 7:5–17. doi: 10.1159/000077865. [DOI] [PubMed] [Google Scholar]
- 3.Raina JB, Fernandez V, Lambert B, Stocker R, Seymour JR. 2019. The role of microbial motility and chemotaxis in symbiosis. Nat Rev Microbiol 17:284–294. doi: 10.1038/s41579-019-0182-9. [DOI] [PubMed] [Google Scholar]
- 4.De Maayer P, Pillay T, Coutinho TA. 2020. Flagella by numbers: comparative genomic analysis of the supernumerary flagellar systems among the Enterobacterales. BMC Genomics 21:670. doi: 10.1186/s12864-020-07085-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faulds-Pain A, Birchall C, Aldridge C, Smith WD, Grimaldi G, Nakamura S, Miyata T, Gray J, Li G, Tang JX, Namba K, Minamino T, Aldridge PD. 2011. Flagellin redundancy in Caulobacter crescentus and its implications for flagellar filament assembly. J Bacteriol 193:2695–2707. doi: 10.1128/JB.01172-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T, Yamanishi Y. 2008. KEGG for linking genomes to life and the environment. Nucleic Acids Res 36:D480–D484. doi: 10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McQuiston JR, Parrenas R, Ortiz-Rivera M, Gheesling L, Brenner F, Fields PI. 2004. Sequencing and comparative analysis of flagellin genes fliC, fljB, and flpA from Salmonella. J Clin Microbiol 42:1923–1932. doi: 10.1128/JCM.42.5.1923-1932.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scharf B, Schuster-Wolff-Bühring H, Rachel R, Schmitt R. 2001. Mutational analysis of the Rhizobium lupini H13-3 and Sinorhizobium meliloti flagellin genes: importance of flagellin A for flagellar filament structure and transcriptional regulation. J Bacteriol 183:5334–5342. doi: 10.1128/JB.183.18.5334-5342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pleier E, Schmitt R. 1989. Identification and sequence analysis of two related flagellin genes in Rhizobium meliloti. J Bacteriol 171:1467–1475. doi: 10.1128/jb.171.3.1467-1475.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sourjik V, Sterr W, Platzer J, Bos I, Haslbeck M, Schmitt R. 1998. Mapping of 41 chemotaxis, flagellar and motility genes to a single region of the Sinorhizobium meliloti chromosome. Gene 223:283–290. doi: 10.1016/s0378-1119(98)00160-7. [DOI] [PubMed] [Google Scholar]
- 11.Iida Y, Hobley L, Lambert C, Fenton AK, Sockett RE, Aizawa S. 2009. Roles of multiple flagellins in flagellar formation and flagellar growth post bdelloplast lysis in Bdellovibrio bacteriovorus. J Mol Biol 394:1011–1021. doi: 10.1016/j.jmb.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Josenhans C, Labigne A, Suerbaum S. 1995. Comparative ultrastructural and functional studies of Helicobacter pylori and Helicobacter mustelae flagellin mutants: both flagellin subunits, FlaA and FlaB, are necessary for full motility in Helicobacter species. J Bacteriol 177:3010–3020. doi: 10.1128/jb.177.11.3010-3020.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SE, Kim SY, Jeong BC, Kim YR, Bae SJ, Ahn OS, Lee JJ, Song HC, Kim JM, Choy HE, Chung SS, Kweon MN, Rhee JH. 2006. A bacterial flagellin, Vibrio vulnificus FlaB, has a strong mucosal adjuvant activity to induce protective immunity. Infect Immun 74:694–702. doi: 10.1128/IAI.74.1.694-702.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGee K, Hörstedt P, Milton DL. 1996. Identification and characterization of additional flagellin genes from Vibrio anguillarum. J Bacteriol 178:5188–5198. doi: 10.1128/jb.178.17.5188-5198.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung YC, Lee MA, Kim HS, Lee KH. 2021. Role of DegQ in differential stability of flagellin subunits in Vibrio vulnificus. NPJ Biofilms Microbiomes 7:32. doi: 10.1038/s41522-021-00206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stringlis IA, Pieterse CMJ. 2021. Evolutionary “hide and seek” between bacterial flagellin and the plant immune system. Cell Host Microbe 29:548–550. doi: 10.1016/j.chom.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Felix G, Duran JD, Volko S, Boller T. 1999. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18:265–276. doi: 10.1046/j.1365-313x.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- 18.Cheng JH, Bredow M, Monaghan J, DiCenzo GC. 2021. Proteobacteria contain diverse flg22 epitopes that elicit varying immune responses in Arabidopsis thaliana. Mol Plant Microbe Interact 34:504–510. doi: 10.1094/MPMI-11-20-0314-SC. [DOI] [PubMed] [Google Scholar]
- 19.Parys K, Colaianni NR, Lee HS, Hohmann U, Edelbacher N, Trgovcevic A, Blahovska Z, Lee D, Mechtler A, Muhari-Portik Z, Madalinski M, Schandry N, Rodríguez-Arévalo I, Becker C, Sonnleitner E, Korte A, Bläsi U, Geldner N, Hothorn M, Jones CD, Dangl JL, Belkhadir Y. 2021. Signatures of antagonistic pleiotropy in a bacterial flagellin epitope. Cell Host Microbe 29:620–634.e9. doi: 10.1016/j.chom.2021.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Colaianni NR, Parys K, Lee HS, Conway JM, Kim NH, Edelbacher N, Mucyn TS, Madalinski M, Law TF, Jones CD, Belkhadir Y, Dangl JL. 2021. A complex immune response to flagellin epitope variation in commensal communities. Cell Host Microbe 29:635–649.e9. doi: 10.1016/j.chom.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Barahona E, Navazo A, Garrido-Sanz D, Muriel C, Martinez-Granero F, Redondo-Nieto M, Martin M, Rivilla R. 2016. Pseudomonas fluorescens F113 can produce a second flagellar apparatus, which is important for plant root colonization. Front Microbiol 7:1471. doi: 10.3389/fmicb.2016.01471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hesse C, Schulz F, Bull CT, Shaffer BT, Yan Q, Shapiro N, Hassan KA, Varghese N, Elbourne LDH, Paulsen IT, Kyrpides N, Woyke T, Loper JE. 2018. Genome-based evolutionary history of Pseudomonas spp. Environ Microbiol 20:2142–2159. doi: 10.1111/1462-2920.14130. [DOI] [PubMed] [Google Scholar]
- 23.Meindl T, Boller T, Felix G. 2000. The bacterial elicitor flagellin activates its receptor in tomato cells according to the address-message concept. Plant Cell 12:1783–1794. doi: 10.2307/3871189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei HL, Chakravarthy S, Worley JN, Collmer A. 2013. Consequences of flagellin export through the type III secretion system of Pseudomonas syringae reveal a major difference in the innate immune systems of mammals and the model plant Nicotiana benthamiana. Cell Microbiol 15:601–618. doi: 10.1111/cmi.12059. [DOI] [PubMed] [Google Scholar]
- 25.Aldridge PD, Karlinsey JE, Aldridge C, Birchall C, Thompson D, Yagasaki J, Hughes KT. 2006. The flagellar-specific transcription factor, sigma28, is the Type III secretion chaperone for the flagellar-specific anti-sigma28 factor FlgM. Genes Dev 20:2315–2326. doi: 10.1101/gad.380406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saini S, Floess E, Aldridge C, Brown J, Aldridge PD, Rao CV. 2011. Continuous control of flagellar gene expression by the σ28-FlgM regulatory circuit in Salmonella enterica. Mol Microbiol 79:264–278. doi: 10.1111/j.1365-2958.2010.07444.x. [DOI] [PubMed] [Google Scholar]
- 27.Ohnishi K, Kutsukake K, Suzuki H, Iino T. 1990. Gene fliA encodes an alternative sigma factor specific for flagellar operons in Salmonella typhimurium. Mol Gen Genet 221:139–147. doi: 10.1007/BF00261713. [DOI] [PubMed] [Google Scholar]
- 28.Soutourina OA, Bertin PN. 2003. Regulation cascade of flagellar expression in Gram-negative bacteria. FEMS Microbiol Rev 27:505–523. doi: 10.1016/S0168-6445(03)00064-0. [DOI] [PubMed] [Google Scholar]
- 29.Sun Y, Li L, Macho AP, Han Z, Hu Z, Zipfel C, Zhou JM, Chai J. 2013. Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science 342:624–628. doi: 10.1126/science.1243825. [DOI] [PubMed] [Google Scholar]
- 30.Beatson SA, Minamino T, Pallen MJ. 2006. Variation in bacterial flagellins: from sequence to structure. Trends Microbiol 14:151–155. doi: 10.1016/j.tim.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Eckhard U, Bandukwala H, Mansfield MJ, Marino G, Cheng J, Wallace I, Holyoak T, Charles TC, Austin J, Overall CM, Doxey AC. 2017. Discovery of a proteolytic flagellin family in diverse bacterial phyla that assembles enzymatically active flagella. Nat Commun 8:521. doi: 10.1038/s41467-017-00599-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song WS, Jeon YJ, Namgung B, Hong M, Yoon SI. 2017. A conserved TLR5 binding and activation hot spot on flagellin. Sci Rep 7:40878. doi: 10.1038/srep40878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belas R. 1994. Expression of multiple flagellin-encoding genes of Proteus mirabilis. J Bacteriol 176:7169–7181. doi: 10.1128/jb.176.23.7169-7181.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belas R, Flaherty D. 1994. Sequence and genetic analysis of multiple flagellin-encoding genes from Proteus mirabilis. Gene 148:33–41. doi: 10.1016/0378-1119(94)90230-5. [DOI] [PubMed] [Google Scholar]
- 35.Blanco-Romero E, Redondo-Nieto M, Martínez-Granero F, Garrido-Sanz D, Ramos-González MI, Martín M, Rivilla R. 2018. Genome-wide analysis of the FleQ direct regulon in Pseudomonas fluorescens F113 and Pseudomonas putida KT2440. Sci Rep 8:13145. doi: 10.1038/s41598-018-31371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen RD, Baumann P. 1971. Structure and arrangement of flagella in species of the genus Beneckea and Photobacterium fischeri. J Bacteriol 107:295–302. doi: 10.1128/jb.107.1.295-302.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarter LL. 2001. Polar flagellar motility of the Vibrionaceae. Microbiol Mol Biol Rev 65:445–462. doi: 10.1128/MMBR.65.3.445-462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McQuiston JR, Fields PI, Tauxe RV, Logsdon JM, Jr.. 2008. Do Salmonella carry spare tyres? Trends Microbiol 16:142–148. doi: 10.1016/j.tim.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Belkhadir Y, Yang L, Hetzel J, Dangl JL, Chory J. 2014. The growth-defense pivot: crisis management in plants mediated by LRR-RK surface receptors. Trends Biochem Sci 39:447–456. doi: 10.1016/j.tibs.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gómez-Gómez L, Felix G, Boller T. 1999. A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. Plant J 18:277–284. doi: 10.1046/j.1365-313x.1999.00451.x. [DOI] [PubMed] [Google Scholar]
- 41.Hao G, Pitino M, Ding F, Lin H, Stover E, Duan Y. 2014. Induction of innate immune responses by flagellin from the intracellular bacterium, “Candidatus Liberibacter solanacearum.” BMC Plant Biol 14:211. doi: 10.1186/s12870-014-0211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buell CR, Joardar V, Lindeberg M, Selengut J, Paulsen IT, Gwinn ML, Dodson RJ, Deboy RT, Durkin AS, Kolonay JF, Madupu R, Daugherty S, Brinkac L, Beanan MJ, Haft DH, Nelson WC, Davidsen T, Zafar N, Zhou L, Liu J, Yuan Q, Khouri H, Fedorova N, Tran B, Russell D, Berry K, Utterback T, Van Aken SE, Feldblyum TV, D'Ascenzo M, Deng WL, Ramos AR, Alfano JR, Cartinhour S, Chatterjee AK, Delaney TP, Lazarowitz SG, Martin GB, Schneider DJ, Tang X, Bender CL, White O, Fraser CM, Collmer A. 2003. The complete genome sequence of the Arabidopsis and tomato pathogen Pseudomonas syringae pv. tomato DC3000. Proc Natl Acad Sci USA 100:10181–10186. doi: 10.1073/pnas.1731982100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scher FM, Baker R. 1982. Effect of Pseudomonas putida and a synthetic iron chelator on induction of soil suppressiveness to Fusarium wilt pathogens. Phytopathology 72:1567–1573. doi: 10.1094/Phyto-72-1567. [DOI] [Google Scholar]
- 44.Xia Z, Lei L, Zhang HY, Wei HL. 2018. Characterization of the ModABC molybdate transport system of Pseudomonas putida in nicotine degradation. Front Microbiol 9:3030. doi: 10.3389/fmicb.2018.03030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao H, Liu YP, Zhang LQ. 2019. In silico and genetic analyses of cyclic lipopeptide synthetic gene clusters in Pseudomonas sp. 11K1. Front Microbiol 10:544. doi: 10.3389/fmicb.2019.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Déziel E, Comeau Y, Villemur R. 2001. Initiation of biofilm formation by Pseudomonas aeruginosa 57RP correlates with emergence of hyperpiliated and highly adherent phenotypic variants deficient in swimming, swarming, and twitching motilities. J Bacteriol 183:1195–1204. doi: 10.1128/JB.183.4.1195-1204.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ngou BPM, Ahn HK, Ding P, Jones JDG. 2021. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 592:110–115. doi: 10.1038/s41586-021-03315-7. [DOI] [PubMed] [Google Scholar]
- 48.Serrano I, Gu Y, Qi D, Dubiella U, Innes RW. 2014. The Arabidopsis EDR1 protein kinase negatively regulates the ATL1 E3 ubiquitin ligase to suppress cell death. Plant Cell 26:4532–4546. doi: 10.1105/tpc.114.131540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Wees SC, Van Pelt JA, Bakker PA, Pieterse CM. 2013. Bioassays for assessing jasmonate-dependent defenses triggered by pathogens, herbivorous insects, or beneficial rhizobacteria. Methods Mol Biol 1011:35–49. doi: 10.1007/978-1-62703-414-2_4. [DOI] [PubMed] [Google Scholar]
- 50.Stringlis IA, Proietti S, Hickman R, Van Verk MC, Zamioudis C, Pieterse CMJ. 2018. Root transcriptional dynamics induced by beneficial rhizobacteria and microbial immune elicitors reveal signatures of adaptation to mutualists. Plant J 93:166–180. doi: 10.1111/tpj.13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JD, Felix G, Boller T. 2004. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428:764–767. doi: 10.1038/nature02485. [DOI] [PubMed] [Google Scholar]
- 52.Placido DF, Sandhu J, Sato SJ, Nersesian N, Quach T, Clemente TE, Staswick PE, Walia H. 2020. The LATERAL ROOT DENSITY gene regulates root growth during water stress in wheat. Plant Biotechnol J 18:1955–1968. doi: 10.1111/pbi.13355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chakravarthy S, Velásquez AC, Ekengren SK, Collmer A, Martin GB. 2010. Identification of Nicotiana benthamiana genes involved in pathogen-associated molecular pattern-triggered immunity. Mol Plant Microbe Interact 23:715–726. doi: 10.1094/MPMI-23-6-0715. [DOI] [PubMed] [Google Scholar]
- 54.Gu Y, Wang J, Xia Z, Wei HL. 2020. Characterization of a versatile plant growth-promoting rhizobacterium Pseudomonas mediterranea strain S58. Microorganisms 8:334. doi: 10.3390/microorganisms8030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tamura K, Nei M. 1993. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 57.Jones DT, Taylor WR, Thornton JM. 1992. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci 8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- 58.Zhang N, Chen Y, Lu H, Zhao F, Alvarez RV, Goncearenco A, Panchenko AR, Li M. 2020. MutaBind2: predicting the impacts of single and multiple mutations on protein-protein interactions. iScience 23:100939. doi: 10.1016/j.isci.2020.100939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. 2000. The protein data bank. Nucleic Acids Res 28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1-6. Download spectrum.03621-22-s0001.xlsx, XLSX file, 0.02 MB (24.3KB, xlsx)
Fig. S1 to S8. Download spectrum.03621-22-s0002.pdf, PDF file, 2.2 MB (2.2MB, pdf)