Abstract
Background
Few studies have examined influenza vaccine effectiveness (VE) among women during pregnancy in middle-income countries. We used data from a prospective cohort of women who were pregnant in Peru to estimate effectiveness of the 2018 Southern Hemisphere influenza vaccine.
Methods
Women at <28 weeks gestation were enrolled from 4 tertiary level hospitals in Lima, Peru at the start of the 2018 influenza season and followed until the end of their pregnancies. Participants had mid-turbinate nasal swabs collected and tested for influenza by reverse-transcription polymerase chain reaction (RT-PCR) with onset of ≥1 of myalgia, cough, runny nose or nasal congestion, sore throat, or difficulty breathing. Time-varying Cox proportional hazard regression models were used to estimate the risk of RT-PCR-confirmed influenza infection after adjusting for inverse probability treatment weight.
Results
We followed 1896 women for a median of 127 days (interquartile range [IQR], 86–174). Participants had a median age of 29 years (IQR, 24–34). Among the 1896 women, 49% were vaccinated with the 2018 influenza vaccine and 1039 (55%) developed influenza-like illness, 76 (7%) of whom had RT-PCR-confirmed influenza. Incidence rates of RT-PCR-confirmed influenza were 36.6 and 15.3 per 100 000 person-days among women who were unvaccinated and vaccinated, respectively. Adjusted influenza VE was 22% (95% confidence interval, −64.1% to 62.9%).
Conclusions
Participants vaccinated against influenza had more than 50% lower incidence of RT-PCR-confirmed influenza illness. Although the VE estimated through propensity weight-adjusted time-varying Cox regression did not reach statistical significance, our findings provide additional evidence about the value of maternal influenza vaccination in middle-income countries.
Keywords: influenza, maternal vaccination, Peru, pregnant, vaccine effectiveness
Incidence of RT-PCR-confirmed influenza illness was twice as high in participants who were unvaccinated compared to those vaccinated. In a time-varying Cox regression model with propensity score weight adjusted for, this translated to a VE of 22%.
Although influenza vaccines are recommended for women during pregnancy, little is known about their real-world effectiveness to guide immunization practice in low- and middle-income tropical countries. Individuals who are pregnant are at increased risk for influenza-associated hospitalization and may be at increased risk of late pregnancy loss and reduced birthweight of term babies [1–3]. Although there are studies showing that maternal vaccination during pregnancy provided protection to their infants [4, 5], there are still relatively few studies assessing the real-world effectiveness of influenza vaccines against influenza among women who are pregnant from low- and middle-income countries where most of the world's population lives [6].
After the 2009 influenza pandemic, the Peru Government introduced a policy in 2010 to offer influenza vaccination to women during pregnancy [7]. Vaccination is offered free of charge to individuals who are pregnant in the country. Despite free vaccination, there has been low uptake among these individuals [8]. Many studies have documented that healthcare provider (HCP) recommendations and HCP offers of influenza vaccination are strong motivators for women to receive influenza vaccine during pregnancy [9, 10]. Uncertainty about vaccine efficacy is one of the most commonly mentioned barriers to healthcare providers recommending influenza vaccine [11]. Providers are correct in asserting that there is a dearth of information about the real-world influenza vaccines effectiveness in low- and middle-income tropical countries in the Southern Hemisphere [6]. Information about influenza VE during pregnancy among women from middle-income countries might provide evidence to support HCP's recommendation of influenza vaccine to individuals who are pregnant and consequently increase influenza vaccine coverage.
Using data from a prospective cohort study of women that assessed the effect of influenza on pregnancy and perinatal outcomes [12, 13], we document influenza vaccination coverage and estimate VE among women who were pregnant during Peru's 2018 Southern Hemisphere influenza season [14]. In 2018, most influenza cases in Peru were caused by A(H1N1)pdm09 [15]. These viruses were antigenically similar to those in the 2018 World Health Organization (WHO)-recommended Southern Hemisphere influenza vaccine used in Peru (ie, A/Michigan/45/2015 (H1N1)pdm09-like virus, an A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus, and a B/Phuket/3073/2013-like virus [16].
METHODS
Study Design and Definitions
The Pregnancy and Influenza Multinational Epidemiologic (PRIME) study is a prospective, longitudinal cohort study of the incidence and impact of influenza virus infection among women during pregnancy in middle-income countries [12]. As part of the larger study, women aged ≥18 years, who were at <28 weeks gestation and had expected delivery dates ≥8 weeks after the start of the influenza season, were enrolled before or during Peru's Southern Hemisphere 2018 influenza season (ie, March 22, 2018) from prenatal clinics at 4 tertiary/referral level hospitals in Lima, Peru. To be eligible, individuals had to plan to remain in the study area for the study period, deliver at one of the study hospitals, and agree to be contacted twice a week during the study period for influenza surveillance purposes.
At enrollment, women completed a standardized interview to collect data on the following: sociodemographic characteristics; past medical and pregnancy history; and prenatal care for the current pregnancy. At the end of pregnancy, women were asked whether they received influenza vaccine during their pregnancy and if vaccinated, they were asked to provide the date of vaccination. Where available, medical records were used to verify influenza vaccination status of women who reported receiving influenza vaccination during the current influenza season. We verified vaccination status for 98% (910 of 929) of the women in the analytic sample who reported that they received the 2018 influenza vaccine. Throughout the follow-up period, participants were instructed to report any influenza-like symptoms (ie, new onset or sudden worsening of ≥1 of myalgia, cough, runny nose or nasal congestion, sore throat, or difficulty breathing) to study staff. In addition, participants were contacted twice weekly to ascertain whether they had influenza-like symptoms. Women with influenza-like symptoms had mid-turbinate nasal swabs collected by trained study staff. Local laboratories processed and tested the nasal swab specimens for influenza by real-time reverse-transcription polymerase chain reaction (RT-PCR) using Centers for Disease Control and Prevention (CDC)-approved protocols [13]. The real-time RT-PCR assays ascertained infection with influenza A or B viruses, and subtyping was performed to identify influenza A subtype (A/H1N1 and A/H3N2) and influenza B lineage (B/Yamagata and B/Victoria). The influenza testing and subtyping were completed using primers, probes, and reagents provided by US CDC. Laboratories that tested the specimens for PRIME had completed and passed either WHO- or US CDC-approved proficiency panels for influenza RT-PCR testing within 1 year before starting testing for the PRIME study.
In the present analysis, we included participants in the Peru PRIME cohort who contributed follow up during the 2018 influenza season, defined as March 22, 2018–December 31, 2018, based on surveillance data [12]. Thus, follow-up time began at the start of the 2018 influenza season (March 22, 2018) or at enrollment, whichever came later. Follow-up time ended on the last day of the influenza season (December 31, 2018) or at the end of pregnancy, whichever occurred first. Influenza vaccination status was defined based on participant self-report. Participants were classified as vaccinated ≥14 days after reported receipt of the 2018 influenza vaccine. We considered vaccination status to be indeterminate if a participant tested positive for influenza less than 14 days after receipt of the current influenza vaccine. All participants who did not receive the 2018 influenza vaccine throughout the follow-up period were considered unvaccinated. Participants who were followed for less than 14 days after receipt of the 2018 influenza vaccine were considered unvaccinated until the day they received the vaccine. For these participants, we censored the person-time after receipt of the 2018 influenza vaccine.
Statistical Analysis
Frequencies were calculated to describe the characteristics of participants by influenza vaccination status. We calculated incidence rate of RT-PCR-confirmed influenza illness overall and by vaccination status. Cox proportional hazard regression analysis with vaccination status as a time-varying covariate [17] and a timescale of days were used to estimate influenza VE against any type of RT-PCR-confirmed influenza illness and RT-PCR-confirmed influenza A (H1N1pdm09) illness separately. We censored person-time from the date of receiving the vaccine until 14 days after receipt of the influenza vaccine.
To control for any systematic differences between participants who got vaccinated and those who did not, we calculated the probability of vaccination based on potential confounding factors. We used the TWANG package in R to calculate propensity scores based on participants’ age in years, gestational age, number of prenatal visits, number of persons in household, health insurance status, gestational diabetes, gestational hypertension, highest educational level, body mass index before the current pregnancy, smoking status, alcohol consumption during the current pregnancy, household income level, whether participants worked outside the home, and any underlying chronic condition [13]. Underlying chronic conditions included human immunodeficiency virus, chronic respiratory conditions, chronic blood disorders, chronic endocrine disorders, chronic heart diseases, chronic neurological/neuromuscular disorders, and immunocompromised conditions (Table 1). An inverse probability treatment weight (IPTW) based on the propensity scores was calculated in SAS. The IPTW was adjusted in the Cox proportional hazard models.
Table 1.
Characteristics of Study Participants (n = 1896) PRI ME Study, 2018
| Variables | Total Sample | Unvaccinated | Vaccinateda | P Value |
|---|---|---|---|---|
| Number of prenatal visits (median IQR) | 8 (5–10) | 8 (5–10) | 9 (6–11) | .00 |
| Number of persons in household (median IQR) | 3 (2–4) | 3 (2–4) | 3 (2–4) | .61 |
| Gestational age in weeks at enrollment (median IQR) | 20.1 (14–25.9) | 20.4 (14–26) | 20 (14.3–25.6) | .73 |
| Prepregnancy BMI in kg/m2 (median IQR) | 24.7 (22.2–27.8) | 24.7 (22.2–27.7) | 24.7 (22.2–27.9) | .58 |
| Age (median IQR) | 29 (24–34) | 29 (24–34) | 28 (24–34) | .01 |
| Age Group, n (%) | .02 | |||
| 18 to 34 | 1466 (77.3) | 726 (75.1) | 740 (79.7) | |
| ≥35 | 430 (22.7) | 241 (24.8) | 189 (20.3) | |
| Education, n (%) | .99 | |||
| Up to secondary | 1008 (53.2) | 514 (53.2) | 494 (53.2) | |
| Postsecondary/University | 888 (46.8) | 453 (46.9) | 435 (46.8) | |
| Marital Status, n (%) | .90 | |||
| Married/living with partner | 1551 (81.8) | 790 (81.7) | 761 (81.9) | |
| Single/divorced/separated | 345 (18.2) | 177 (18.3) | 168 (18.1) | |
| Any underlying chronic condition, n (%) | 546 (28.8) | 284 (29.4) | 262 (28.2) | .57 |
| HIV | 9 (0.5) | 7 (0.7) | 2 (0.2) | .11 |
| Chronic respiratory condition | 82 (4.3) | 41 (4.2) | 41 (4.4) | .85 |
| Chronic blood condition | 48 (2.5) | 16 (1.7) | 32 (3.4) | .01 |
| Chronic endocrine condition | 141 (7.4) | 72 (7.5) | 69 (7.4) | .99 |
| Chronic heart condition | 49 (2.6) | 31 (3.2) | 18 (1.9) | .08 |
| Any other chronic conditionb | 325 (17.1) | 168 (17.4) | 157 (16.9) | .78 |
| Gestational Diabetes, n (%) | .43 | |||
| No | 1771 (93.4) | 899 (93.0) | 872 (93.9) | |
| Yes | 125 (6.6) | 68 (7.0) | 57 (6.1) | |
| Gestational Hypertension, n (%) | .41 | |||
| No | 1796 (94.7) | 912 (94.3) | 884 (95.2) | |
| Yes | 100 (5.3) | 55 (5.7) | 45 (4.8) | |
| Has Health Insurance, n (%) | .01 | |||
| No | 980 (51.9) | 472 (49.1) | 508 (54.9) | |
| Yes | 907 (48.1) | 490 (51.0) | 417 (45.1) | |
| Consumed Alcohol During Current Pregnancy, n (%) | .05 | |||
| No | 1248 (65.8) | 657 (67.9) | 591 (63.6) | |
| Yes | 648 (34.2) | 310 (32.1) | 338 (36.4) | |
| Smoked During Current Pregnancy, n (%) | .30 | |||
| No | 1800 (94.9) | 923 (95.5) | 877 (94.4) | |
| Yes | 96 (5.1) | 44 (4.5) | 52 (5.6) | |
| Monthly per Capita Incomec | .81 | |||
| Below poverty line | 1032 (54.4) | 529 (54.7) | 503 (54.1) | |
| Above poverty line | 864 (45.6) | 438 (45.3) | 426 (45.9) | |
| Total, n (%) | 1896 | 967 (51.0) | 929 (49.0) |
Abbreviation: BMI, body mass index; HIV, human immunodeficiency virus; IQR, interquartile range; PRIME, Pregnancy and Influenza Multinational Epidemiologic.
We classified participants as vaccinated ≥14 days after they had received the 2018 influenza vaccine.
Examples include chronic kidney conditions, cancer, chronic liver conditions, neurologic/neuromuscular disorders, and immunosuppressive disorders.
Poverty line is defined as monthly income <338 soles. P values were obtained from χ2 tests for categorical variables and analogous univariate analysis of variance for continuous variables. P values compare participants who received the influenza vaccine and those who did not receive the vaccine.
To aid in comparison with other vaccine effectiveness studies that treated vaccination status as a fixed variable, we conducted an additional Cox proportional hazard regression analysis with vaccination status as a fixed variable, that is, for those who were vaccinated, their follow-up period began from the day they enrolled if they qualified as vaccinated during enrollment by our definition or from the day they became vaccinated after enrollment; we did not account for time before being vaccinated.
As a sensitivity analysis to examine the effect of the timing of influenza vaccination among the study cohort, we conducted another time-varying Cox proportional hazard regression analysis with the start of the follow up set at when the first participants became vaccinated (April 27, 2018). In all analyses, a 2-tailed statistical significance level of 0.05 was set a priori. All analyses, except the propensity score calculation that was conducted in R (R version 4.1.2), were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Patient Consent Statement
The study was approved by the Naval Medical Research Unit 6 IRB (Protocol NAMRU6.2016.0015) in compliance with all applicable Federal regulations governing the protection of human subjects and by the Abt Associates Institutional Review Board (IRB). The IRB of the US Centers for Disease Control and Prevention relied on the review of the Abt Associates IRB. All participants provided written informed consent.
RESULTS
We enrolled 1967 women who were pregnant and excluded 63 (3%) because they withdrew from the study before the start of the influenza season or were missing data for the analysis. Eight participants tested positive within 14 days of receiving the vaccine and were excluded from the analysis because their vaccination status was indeterminate. Our final analytic sample was 1896 women aged 18 to 46 years (median, 29; interquartile range [IQR], 24–34); 6.6% had gestational diabetes, 5.3% had gestational hypertension, 34.2% consumed alcohol during the current pregnancy, 5.1% smoked cigarettes during the current pregnancy, 46.8% had postsecondary school education, and 28.8% had any underlying medical condition. Overall, 49.0% (n = 929) met our definition for vaccination (≥14 days after reported receipt of the 2018 influenza vaccine) (Table 1).
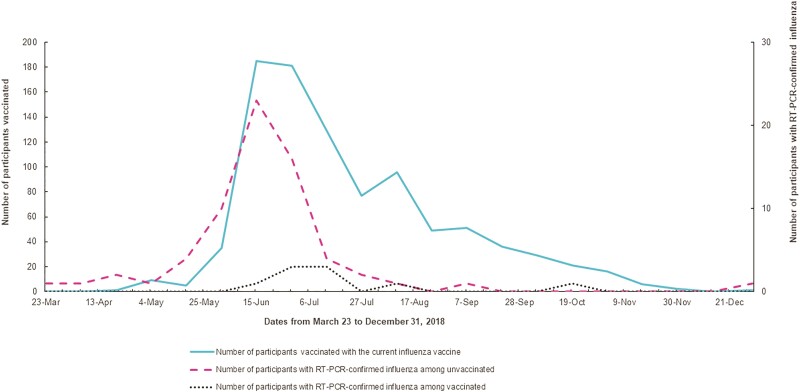
Of the 1896 participants, 1039 (54.8%) developed influenza-like illness, 76 (7%) of whom had RT-PCR-confirmed influenza during the follow up; 69 were caused by influenza A (66 A/H1N1, 3 A/H3N2) and 7 were caused by influenza B (5 B/Yamagata, 2 B/Victoria). The most reported symptoms among those with RT-PCR-confirmed influenza were runny nose (84.2%), sore throat (79.0%), cough (77.6%), myalgia (60.5%), and difficulty in breathing (18.4%) (Table 1). Approximately 17% of participants had been vaccinated with the current influenza vaccine during March 22 through June 22, 2018, (ie, the first 3 months of the season) when 76% (58 of 76) of the RT-PCR-confirmed influenza illnesses were identified (Figure 1).
Figure 1.
Timing of influenza vaccination and laboratory-confirmed influenza illness among pregnant women in Lima during 2018.
Note: 74% of reverse-transcription polymerase chain reaction (RT-PCR)-confirmed influenza illnesses in the cohort occurred between March 23 through June 22. During this period, only 17% of the participants (36% of those who were vaccicnated during the follow up) had been vaccinated with the current influenza vaccine.
Participants contributed 183 199 unvaccinated days (median, 91; IQR, 54–138) and 58 869 vaccinated days (median, 61; IQR, 34–93). The overall incidence rate of RT-PCR-confirmed influenza illness among study participants during the observation period was 31.4 per 100 000 person-days. The incidence rate among participants who were unvaccinated was 36.6 per 100 000 person-days, and among participants who were vaccinated the incidence rate was 15.3 per 100 000 person-days. Adjusting for IPTW, the VE against any type of influenza A and B in the cohort was 22% (95% confidence interval [CI], −64.1% to 62.9%). The VE against H1N1pdm09, the predominant influenza A subtyped that circulated in the country in 2018, was 15.9% (95% CI, −87.1% to 62.2%). In a sensitivity analysis with follow-up time starting on the date the first participant in our cohort became vaccinated, the adjusted VE was 20.1% (95% CI, −67.6% to 67.9%), which is similar to the VE we observed when follow up started on the first day of influenza season (Table 2). Conversely, when we treated vaccination status as a fixed variable, we observed a VE of 80.0% (95% CI, 59.7%–90.1%).
Table 2.
Estimated Vaccine Effectiveness Among Pregnant Women in Peru With Vaccination Status as a Time-Varying Covariate—PRIME Study, 2018
| Variable | Analysis With Follow up From Start of influenza Season Verified and Unverified Vaccination Status | Sensitivity Analysis With Follow up From When the First Participant Became Vaccinated | ||||
|---|---|---|---|---|---|---|
| Total | Unvaccinated | Vaccinateda | Total | Unvaccinated | Vaccinateda | |
| Person-days (Median, IQR) |
242 068 (127, 86–174) | 183 199 (91, 54–138) | 58 869 (61, 34–93) | 217 713 (112, 74–158) | 158 844 (76, 42–124) | 58 869 (61, 34–93) |
| Laboratory-confirmed influenza cases (n) | 76 | 67 | 9 | 72 | 63 | 9 |
| Incidence rate per 100 000 person-days | 31.4 | 36.6 | 15.3 | 33.1 | 39.7 | 15.3 |
| Adjusted VE* against all types of influenza (95% CI) | N/A | Ref | 22.0% (−64.1% to 62.9%) | N/A | Ref | 20.1% (−67.6% to 61.9%) |
| Influenza A/H1N1 cases | 66 | 59 | 7 | 65 | 58 | 7 |
| Adjusted VE* against only A/H1N1 (95% CI) | N/A | Ref | 15.9% (−87.1% to 62.2%) | N/A | Ref | 15.9% (−87.4% to 62.3%) |
Abbreviation: CI, confidence interval; IQR, interquartile range; N/A, not applicable; PRIME, Pregnancy and Influenza Multinational Epidemiologic; Ref, referent category; VE, vaccine effectiveness.
We classified participants as vaccinated 14 days after they had received the 2018 influenza vaccine.
*Adjusted for inverse probability treatment weight.
DISCUSSION
Summary of Key Findings
During 2018, the incidence of RT-PCR-confirmed influenza illnesses among participants who were unvaccinated in the Peru cohort was more than twice the incidence of those who were vaccinated, and the associated time-varying Cox regression VE point estimate was 22%. Although this Cox regression VE estimate was low and statistically not significant, the point estimate was similar in magnitude to VE reported elsewhere [4, 18]. For example, our estimate is similar to the US 2018 general population test-negative design VE of 29% (95% CI, 21%–35%) [19].
When we treated vaccination status as a fixed variable to aid comparison of our findings with other studies, we observed a VE of 80%, which was comparable to VE estimated through similar models among women who were pregnant in Greece during the 2018–2019 Northern Hemisphere influenza season (ie, 72%) [20]. Such findings are also similar to Australia's 2018 Southern Hemisphere VE of 68% in preventing general population primary care visit attributable to influenza illness and 58% against influenza-associated hospitalization [21]. The difference in point estimates is likely driven by differences in the underlying assumptions in the time-varying and fixed variable Cox regression models, which are especially apparent when sample size is small; additional studies to identify optimal approaches to analyze VE in longitudinal cohorts would be useful.
The benefits of influenza vaccines accrue as more individuals become vaccinated and are associated with illness prevention and attenuation [22], decreased presenteeism and absenteeism, and direct and indirect economic benefits [23–26]. Besides effectively preventing influenza illness and illness complications among women [4, 18], influenza vaccination during pregnancy has an added benefit of protecting against adverse birth outcomes, including preterm birth, low birthweight, and death. Using PRIME data from 3 middle-income countries, we previously reported that antenatal influenza infection was associated with late pregnancy loss and a reduction in mean birthweight [13]. Therefore, preventing antenatal influenza may improve birth outcomes. In addition, a meta-analysis of 2 randomized controlled trials (RCTs) from South Africa and Nepal suggests that maternal influenza vaccination was 34% effective in preventing laboratory-confirmed influenza in infants [4]. Pooled data from RCTs conducted in Mali, Nepal, and South Africa indicated that maternal vaccination was 42% and 35% efficacious against laboratory-confirmed influenza in women who were pregnant and infants aged up to 6 months, respectively [27]. Thus, the emerging evidence from low- and middle-income countries suggests the benefits of maternal influenza vaccination to women who are pregnant and their unborn children.
The influenza vaccine coverage among cohort participants was higher than previously recorded [8]. Despite this increased uptake, approximately half of our cohort did not receive influenza vaccines, which are offered free-of-charge in Peru prenatal clinics. Furthermore, a substantial proportion of individuals who were vaccinated did not receive the vaccine before their highest risk period. National data reported to the Pan American Health Organization (PAHO) showed that influenza test positivity in Peru peaked during the first 3 months of the 2018 influenza season [28]. In the present study, we observed that only one third of participants had been vaccinated during the first 3 months of the influenza season when more than three quarters of RT-PCR-confirmed influenza illnesses occurred (Figure 1). These findings suggest the potential value of a postintroduction evaluation of influenza vaccines to optimize coverage and timing of vaccination [1, 29].
Our results add to the limited evidence about the effectiveness of influenza vaccines in middle-income tropical countries in the Southern Hemisphere. Although the VE estimate from the time-varying covariate Cox regression analysis was not statistically significant, it was similar in magnitude to previously published VE studies [4, 18, 19] that do suggest that vaccination during pregnancy is an effective health intervention. The findings have significant implication for a continued promotion of free-of-charge vaccination among women who are pregnant in countries like Peru to prevent influenza illnesses and their complications among these individuals and unborn children. Our findings could also trigger a postintroduction evaluation to optimize coverage and the timing of vaccination such that more women who are pregnant are protected against influenza before the start of the season. Finally, the results from our study could be used as inputs in cost-effectiveness analysis to guide policy decision to expand and/or sustain maternal influenza vaccination investments and programs.
Strengths and Limitations of the Study
This study has many strengths. Twice a week, study staff actively asked cohort participants about influenza-like symptoms thus minimizing the probability of missing illness and misclassification of illness status. Furthermore, the final analysis accounted for the timing of observation of each participant to account for the changing influenza infection risk level during the season. All findings are presented according to STROBE guidelines for observational studies. Despite these strengths, the following are important limitations of the study. The relatively low incidence of RT-PCR-confirmed influenza illness in our study cohort might have contributed to the low and nonsignificant VE we observed through time-varying regression. Our findings may not be representative of women with pregnancy throughout Peru because participants were exclusively from the capital city. In addition, influenza vaccination may prevent severe outcomes of influenza infection; however, our analysis did not assess severity of disease among vaccinated versus unvaccinated due to small sample. Finally, because circulating influenza viruses and vaccine viruses may change over time, the VE reported in this study may not represent VE in other influenza seasons. Ongoing VE monitoring, for example through the PAHO multicountry Network for the Evaluation of Vaccine Effectiveness in Latin American and the Caribbean—influenza (REVELAC-i), are useful program evaluations for subregional countries using novel or locally sourced Southern Hemisphere influenza vaccine products that are understudied in the Northern Hemisphere [30].
CONCLUSIONS
Although approximately half of the women in our cohort were vaccinated with the 2018 influenza vaccine, only 1 in 3 of those individuals were vaccinated before the main epidemic. Those vaccinated had 50% lower incidence of subsequent influenza illness. The time-varying Cox regression VE was not statistically significant; however, the fixed variable VE estimate was high and statistically significant. Taken together, our findings reaffirm the Government of Peru's decision to invest in free-of-charge influenza vaccines to protect individuals who are pregnant from influenza illness. The findings could be used in risk communication messages to improve health literacy about the value of vaccination among providers and target groups such as individuals who are pregnant to increase and sustain influenza vaccination coverage. Preventing influenza during pregnancy is especially useful because it prevents illness and illness complications among mothers and their unborn babies [31].
Acknowledgments
We thank the women who participated in PRIME for their generosity. We also thank the field staff in participating hospitals and Michael Jhung for his critical review of study findings.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention or the US Navy.
Financial support. This study was funded by the US Centers for Disease Control and Prevention through the following funding mechanisms: Interagency Agreements 16FED1612328, 17FED1712076, and 18FED1812054IPD with the US Naval Medical Research Unit No. 6 and Contract Number HHSD2002013M53890B with Abt Associates.
Potential conflicts of interest. All authors: No reported conflicts of interest.
Contributor Information
Daniel Owusu, Influenza Division, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Fatimah S Dawood, Influenza Division, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Eduardo Azziz-Baumgartner, Influenza Division, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Yeny Tinoco, U.S. Naval Medical Research Unit No. 6, Bellavista, Peru.
Giselle Soto, U.S. Naval Medical Research Unit No. 6, Bellavista, Peru.
Oswaldo Gonzalez, Instituto Nacional Materno Perinatal, Lima, Peru.
Santiago Cabrera, Hospital Nacional Docente Madre Niño San Bartolomé, Lima, Peru.
Richard Florian, Hospital Nacional Arzobispo Loayza, Lima, Peru.
Edwin Llajaruna, Hospital Nacional Dos de Mayo, Lima, Peru.
Danielle Rentz Hunt, Abt Associates, Inc., Atlanta, Georgia, USA.
Meredith G Wesley, Influenza Division, Centers for Disease Control and Prevention, Atlanta, Georgia, USA; Abt Associates, Inc., Atlanta, Georgia, USA.
Tat Yau, Influenza Division, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Carmen S Arriola, Influenza Division, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
References
- 1. Buchy P, Badur S. Who and when to vaccinate against influenza. Int J Infect Dis 2020; 93:375–87. [DOI] [PubMed] [Google Scholar]
- 2. Bratton KN, Wardle MT, Orenstein WA, Omer SB. Maternal influenza immunization and birth outcomes of stillbirth and spontaneous abortion: a systematic review and meta-analysis. Clin Infect Dis 2015; 60:e11–9. [DOI] [PubMed] [Google Scholar]
- 3. Fiore AE, Shay DK, Broder K, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009; 58:1–52. [PubMed] [Google Scholar]
- 4. Jarvis JR, Dorey RB, Warricker FDM, Alwan NA, Jones CE. The effectiveness of influenza vaccination in pregnancy in relation to child health outcomes: systematic review and meta-analysis. Vaccine 2020; 38:1601–13. [DOI] [PubMed] [Google Scholar]
- 5. Nunes MC, Madhi SA. Influenza vaccination during pregnancy for prevention of influenza confirmed illness in the infants: a systematic review and meta-analysis. Hum Vaccin Immunother 2018; 14:758–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fell DB, Azziz-Baumgartner E, Baker MG, et al. Influenza epidemiology and immunization during pregnancy: final report of a World Health Organization working group. Vaccine 2017; 35:5738–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. PAHO . Immunization status in the Americas: 2010 summary. Available at: https://www.paho.org/en/documents/immunization-americas-2010-summary. Accessed 6 February 2023.
- 8. Reinders S, Romero C, Carcamo C, et al. A community-based survey on influenza and vaccination knowledge, perceptions and practices in Peru. Vaccine 2020; 38:1194–201. [DOI] [PubMed] [Google Scholar]
- 9. Arriola CS, Suntarattiwong P, Dawood FS, et al. What do pregnant women think about influenza disease and vaccination practices in selected countries. Hum Vaccin Immunother 2021; 17:2176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arriola CS, Vasconez N, Bresee J, Ropero AM. Knowledge, attitudes and practices about influenza vaccination among pregnant women and healthcare providers serving pregnant women in Managua, Nicaragua. Vaccine 2018; 36:3686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morales KF, Menning L, Lambach P. The faces of influenza vaccine recommendation: a literature review of the determinants and barriers to health providers’ recommendation of influenza vaccine in pregnancy. Vaccine 2020; 38:4805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dawood FS, Hunt D, Patel A, et al. The Pregnancy and Influenza Multinational Epidemiologic (PRIME) study: a prospective cohort study of the impact of influenza during pregnancy among women in middle-income countries. Reprod Health 2018; 15:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dawood FS, Kittikraisak W, Patel A, et al. Incidence of influenza during pregnancy and association with pregnancy and perinatal outcomes in three middle-income countries: a multisite prospective longitudinal cohort study. Lancet Infect Dis 2021; 21:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leite JA, Resende P, Araya JL, et al. Genetic evolution of influenza viruses among selected countries in Latin America 2017–2018. PLoS One 2020; 15:e0227962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. PAHO . Regional update, influenza. Epidemiological week 50–51—December 28, 2018. Available at: https://www.paho.org/en/node/62577. Accessed 7 June 2021.
- 16. WHO . Recommended composition of influenza virus vaccines for use in the 2018 southern hemisphere influenza season. Available at: https://www.who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2018-southern-hemisphere-influenza-season. Accessed 7 June 2021.
- 17. Thompson MG, Soto G, Peretz A, et al. Influenza vaccine effectiveness within prospective cohorts of healthcare personnel in Israel and Peru 2016–2019. Vaccine 2021; 39:6956–67. [DOI] [PubMed] [Google Scholar]
- 18. Steinhoff MC, Katz J, Englund JA, et al. Year-round influenza immunisation during pregnancy in Nepal: a phase 4, randomised, placebo-controlled trial. Lancet Infect Dis 2017; 17:981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Flannery B, Kondor RJG, Chung JR, et al. Spread of antigenically drifted influenza A(H3N2) viruses and vaccine effectiveness in the United States during the 2018–2019 season. J Infect Dis 2020; 221:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maltezou HC, Asimakopoulos G, Stavrou S, et al. Effectiveness of quadrivalent influenza vaccine in pregnant women and infants 2018–2019. Vaccine 2020; 38:4625–31. [DOI] [PubMed] [Google Scholar]
- 21. Australian Government Department of Health . Influenza Annual Epidemiology Report 2018, NSW. Available at: https://www.health.nsw.gov.au/Infectious/Influenza/Publications/2018/annual-2018-report.pdf. Accessed 6 February 2023.
- 22. Regan A, Arriola C, Couto P, et al. Severity of influenza illness associated with seasonal influenza vaccination among hospitalized patients in four South American countries 2013–2019: a surveillance-based cohort study. Lancet Infect Dis 2022; 23:222–32. doi: 10.1016/S1473-3099(22)00493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Biggerstaff M, Cohen C, Reed C, et al. A cost-effectiveness analysis of antenatal influenza vaccination among HIV-infected and HIV-uninfected pregnant women in South Africa. Vaccine 2019; 37:6874–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. D'Angiolella LS, Lafranconi A, Cortesi PA, Rota S, Cesana G, Mantovani LG. Costs and effectiveness of influenza vaccination: a systematic review. Ann Ist Super Sanita 2018; 54:49–57. [DOI] [PubMed] [Google Scholar]
- 25. Tinoco YO, Azziz-Baumgartner E, Rázuri H, et al. A population-based estimate of the economic burden of influenza in Peru 2009–2010. Influenza Other Respir Viruses 2016; 10:301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peasah SK, Azziz-Baumgartner E, Breese J, Meltzer MI, Widdowson MA. Influenza cost and cost-effectiveness studies globally–a review. Vaccine 2013; 31:5339–48. [DOI] [PubMed] [Google Scholar]
- 27. Omer SB, Clark DR, Madhi SA, et al. Efficacy, duration of protection, birth outcomes, and infant growth associated with influenza vaccination in pregnancy: a pooled analysis of three randomised controlled trials. Lancet Respir Med 2020; 8:597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pan American Health Organization . 2018 Weekly influenza report EW 50-51/2018. Available at: https://www3.paho.org/hq/index.php?option=com_docman&view=download&category_slug=see-influenza-reports-by-year-4302&alias=47387-regional-update-influenza-epidemiological-week-50-51-december-28-2018&Itemid=270&lang=en. Accessed 18 December 2022 .
- 29. WHO . Influenza Vaccine Post-Introduction Evaluation (IPIE). Available at: https://www.who.int/teams/immunization-vaccines-and-biologicals/diseases/seasonal-influenza/influenza-vaccine-post-introduction-evaluation-(ipie). Accessed 18 December 2022.
- 30. Sofia Arriola C, El Omeiri N, Azziz-Baumgartner E, et al. Influenza vaccine effectiveness against hospitalizations in children and older adults-data from South America 2013–2017. A test negative design. Vaccine X 2019; 3:100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Azziz-Baumgartner E, Grohskopf L, Patel M. Realizing the potential of maternal influenza vaccination. JAMA 2021; 325:2257–9. [DOI] [PMC free article] [PubMed] [Google Scholar]