Abstract
Summary
We examined serum IGF-1 in premenopausal IOP, finding relationships that were opposite to those expected: higher IGF-1 was associated with lower bone formation and higher body fat, and lower BMD response to teriparatide. These paradoxical relationships between serum IGF-1, bone, and fat may contribute to the mechanism of idiopathic osteoporosis in premenopausal women.
Introduction
Premenopausal women with idiopathic osteoporosis (IOP) have marked deficits in bone microarchitecture but variable bone remodeling. We previously reported that those with low tissue-level bone formation rate (BFR) are less responsive to teriparatide and have higher serum IGF-1, a hormone anabolic for osteoblasts and other tissues. The IGF-1 data were unexpected because IGF-1 is low in other forms of low turnover osteoporosis—leading us to hypothesize that IGF-1 relationships are paradoxical in IOP. This study aimed to determine whether IOP women with low BFR have higher IGF-1 and paradoxical IGF-1 relationships in skeletal and non-skeletal tissues, and whether IGF-1 and the related measures predict teriparatide response.
Methods
This research is an ancillary study to a 24 month clinical trial of teriparatide for IOP. Baseline assessments were related to trial outcomes: BMD, bone remodeling. Subjects: Premenopausal women with IOP(n = 34); bone remodeling status was defined by baseline cancellous BFR/BS on bone biopsy. Measures: Serum IGF-1 parameters, compartmental adiposity (DXA, CT, MRI), serum hormones, and cardiovascular-risk-markers related to fat distribution.
Results
As seen in other populations, lower BFR was associated with higher body fat and poorer teriparatide response. However, in contrast to observations in other populations, low BFR, higher body fat, and poorer teriparatide response were all related to higher IGF-1: IGF-1 Z-score was inversely related to BFR at all bone surfaces (r = −0.39 to −0.46; p < 0.05), directly related to central fat (p = 0.05) and leptin (p = 0.03). IGF-1 inversely related to 24 month hip BMD %change (r = −0.46; p = 0.01).
Conclusions
Paradoxical IGF-1 relationships suggest that abnormal or atypical regulation of bone and fat may contribute to osteoporosis mechanisms in premenopausal IOP.
Keywords: Body composition, Bone biopsy, Bone turnover markers, Marrow adiposity, Premenopausal osteoporosis, Teriparatide
Introduction
Idiopathic osteoporosis (IOP) in premenopausal women is a term used to describe osteoporosis that affects young, otherwise healthy individuals with intact gonadal function and no secondary cause of bone loss. Although IOP in premenopausal women is rare, it is of high clinical significance, not only because low trauma fractures cause considerable morbidity in these young women, but also because premenopausal fractures predict increased rates of postmenopausal fractures[1, 2]. Because women with IOP have no known medical condition or medication exposure associated with bone fragility, we hypothesized that they may have a primary or genetic etiology that has affected skeletal development, leading to early-onset osteoporosis that presents in young adulthood. However, our genetic studies utilizing lwhole exome sequencing [3] document very few known pathogenic mutations in cohorts with premenopausal IOP, suggesting that some have unknown genetic etiologies or other mechanisms of early-onset bone fragility.
We previously documented that premenopausal women with IOP have marked deficits in bone microarchitecture, including thinner cortices; fewer, thinner, more widely separated and heterogeneously distributed trabeculae; and reduced bone stiffness by finite element analysis [4, 5]. Bone remodeling rate assessed at the tissue level was quite variable, consistent with heterogeneous mechanisms and etiologies of osteoporosis in this cohort. Women with very low bone remodeling had the most profoundly compromised bone structure and microstructure [4], suggesting that defective osteoblast function could be leading to compromised bone formation. They also responded less well to the osteo-anabolic medication, teriparatide [6], providing further support for the notion that osteoblast function was deficient in some.
In the course of investigating potential mechanisms for these observations, we found higher serum insulin-like growth factor-1 (IGF-1) in women with low-turnover IOP compared to those with normal or high bone remodeling [4, 6]. This was unexpected because serum IGF-1 is generally considered to stimulate osteoblasts and IGF-1 is low in other forms of low turnover osteoporosis, including male IOP [7], obesity [8–10], and growth hormone deficiency [11]. This led us to hypothesize that premenopausal women with low-turnover IOP have IGF-1 resistance at the osteoblast level, and possibly also in non-skeletal tissues. IGF-1 resistance, whether due to abnormalities in the hormone, its binding proteins, or its receptors, should manifest clinical features similar to those of adult growth hormone deficiency of pituitary origin. Such features might include decreased BMD and lean body mass, increased visceral adiposity, insulin resistance, and elevated serum low-density-lipoprotein (LDL) cholesterol and cardiovascular risk markers such as C-reactive protein (CRP), interleukin-6 (IL-6), and homocysteine [11–14]. Because the growth hormone axis plays a role in regulation of adipocyte and osteoblast lineages within the bone marrow [15], IGF-1 resistance might also affect fat/bone relationships in the bone marrow space.
The goals of this study were to determine (1) whether premenopausal women with IOP and low bone formation have evidence of paradoxical IGF-1 relationships or IGF-1 resistance in skeletal and non-skeletal tissues, and (2) whether serum IGF-1 and skeletal and non-skeletal markers of IGF-1 resistance or paradoxical IGF-1 relationships predict their response to teriparatide. To address these questions, we leveraged a 24-month FDA-funded randomized clinical trial of teriparatide for premenopausal IOP [16] that enrolled 41 premenopausal IOP subjects. Study outcomes included changes in BMD by DXA, serum bone remodeling markers, and tissue-level bone remodeling assessed via quadruple labeled transiliac crest bone biopsy. In the majority, teriparatide was associated with large increases in spine and hip BMD, tissue level bone remodeling, and serum bone remodeling markers. However, the response to teriparatide was quite variable, with seven women (18%) manifesting virtually no increase in BMD [16]. This NIH-funded ancillary study to the clinical trial aimed to investigate whether IGF-1-related mechanisms are important in the etiology of premenopausal IOP and contribute to the variability in response to teriparatide in women with this disorder.
Methods
Study design and participants
The study design and inclusion/exclusion criteria have been previously described [16]. Subjects were recruited and participated at one of two tertiary osteoporosis centers: Columbia University Irving Medical Center, New York, NY and Creighton University, Omaha, NE. Institutional Review Boards at both institutions approved the parent and ancillary studies and all participants provided their written informed consent.
Eligible participants had to have history of regular menses, early follicular phase follicle-stimulating hormone levels less than 20 mIU/mL, no historical or biochemical secondary cause of osteoporosis, and no clinical evidence of known rare bone diseases (e.g., osteogenesis imperfecta, Ehlers-Danlos). All had radiographically documented adult, low-trauma fractures, and/or T-scores that were ≤ − 2.5 or Z-scores ≤ − 2.0 at the lumbar spine (LS), femoral neck (FN), or total hip (TH). Low trauma was defined as equivalent to a fall from a standing height or less; skull and digit fractures were excluded. At the request of the United States Food and Drug Administration (FDA), inclusion criteria varied by age. Participants aged 20–35 had at least one major osteoporotic fracture and T-score or Z-score ≤ − 1.5. Those over 35 required a history of fracture and/or T-score ≤ − 2.5 or Z-score ≤ − 2.0. A physician panel (ES, AC, RRR) reviewed radiographic reports and assessed level of trauma associated with the fractures. Women were excluded if they had delivered a baby within the past 12 months or weaned a child within the previous 6 months. Women with secondary causes of osteoporosis (estrogen deficiency, eating disorders, osteomalacia, celiac or gastrointestinal disease), renal insufficiency, abnormal liver function tests, hyperparathyroidism, urinary calcium above 300 mg/g creatinine, serum 25-hydroxyvitamin D (25-OHD) levels below 20 ng/mL, specific drug exposures (glucocorticoids, anticonvulsants, anticoagulants, methotrexate, depot progesterone, gonadotrophin releasing hormone agonists), and current therapy for osteoporosis (raloxifene, bisphosphonates, calcitonin, teriparatide, denosumab) were excluded. Effective contraception was required to participate in the treatment study.
The parent study was a 6M phase II randomized controlled trial followed by open extension, so that each participant was treated with 24 months of teriparatide in total. Participants and study personnel remained masked to study outcomes until participants completed 24 months of teriparatide. Detailed study design and randomization scheme have been previously described [16]. For the ancillary study, only data obtained at baseline and during the drug treatment period (not the placebo treatment period) were utilized.
Fasting morning serum/plasma was collected and stored at − 80 °C for batch analyses performed in the Biomarkers Core of the Irving Institute for Clinical and Translational Research. Serum N-terminal propeptides of procollagen type 1 (P1NP) by RIA, osteocalcin (OCN) by ELISA, and C-telopeptide (CTx) by ELISA were assessed as part of the parent study [16]. The following additional parameters were assessed on baseline serum/plasma samples: 25-OHD by LCMSMS (Agilent, Santa Clara, CA), whole PTH by RIA (Scantibodies Laboratory, Santee, CA), calcium and albumin by colorimetric assay (Cobas Integra 400 Plus, Roche Diagnostics, Indianapolis, IN), IGF-1 by chemiluminescent immunoassay (Immulite, Re-standardized 2017, Siemens Healthcare Diagnostics, Deerfield, IL), growth hormone (GH) and IGF binding proteins by chemiluminescent immunoassay (CLIA: Siemens Healthcare Diagnostics, Deerfield, IL), leptin by RIA (Linco Research, St Charles, MO), adiponectin by ELISA (Millipore, Billerica, MA), insulin and IL-6 by CLIA (Siemens Healthcare Diagnostics, Deerfield, IL), CRP, total cholesterol, HDL, triglycerides, LDL (calculated), homocysteine, and glucose utilizing the Cobas Integra 400 Plus (Roche Diagnostics, Indianapolis, IN). Twenty-four hour urine calcium/creatinine assessments were performed at a commercial laboratory (Quest Diagnostics, Secausus, NJ). IGF-1 varies with sex and age, thus IGF-1 Z scores were calculated using Siemens (restandardized) assay-specific age and sex-stratified norms. The molar ratio of IGF-1 to IGFBP3 was calculated [17]. Insulin resistance was determined by basal glucose and insulin by standard homeostatic model assessment (HOMAir) methods [18].
BMD was measured by DXA (Discovery, Hologic Inc., Walton, MA) at baseline and 6-month intervals at the LS, TH, FN, and distal radius (DR). Body composition was assessed by DXA at baseline. Short-term coefficient of variation is 0.7% (LS) and 1.4% (FN) at Columbia and 1.5% (LS and FN) at Creighton. Phantoms were circulated between the study sites. T- and Z-scores were generated using the manufacturer’s database.
The cross-sectional areas of visceral adipose tissue and subcutaneous adipose tissue in mm2 were calculated by the method of Zhao et al. [19], based on one axial image (10 mm thickness) obtained at the mid-L4 vertebra level [20], at the time that central QCT volumetric bone density studies were obtained for the parent study.
Bone marrow fat fraction at the spine and hip were quantified at baseline using proton (1H) magnetic resonance spectroscopy (MRS), an accurate and noninvasive method that separately assesses bone marrow lipid and water components within the region of interest [21]. MR images at mid L3 and femur (lower border of greater trochanter) were acquired on a research-dedicated 1.5 T whole-body Signa “LX” MR system (General Electric, Milwaukee, WI) at Columbia University Irving Medical Center. Spectra acquired from L3 and FN were exported for analysis (Precision 650 Workstation; Dell, Austin, Tex) using a time-domain fitting routine known as the variable projection, or VARPRO, method in the jMRUI software [22].
This study utilized tissue-level bone remodeling data from bone biopsy samples obtained as part of the parent study. Transiliac bone biopsy after tetracycline, which labels bone surfaces undergoing active formation, permits precise quantification of surface-based bone formation rate (BFR/BS). The parent study obtained bone biopsies after quadruple tetracycline labeling. With the quadruple labeling protocol, one set of double tetracycline labels (two 3-day courses separated by 12 days) was administered before treatment and a second set of double demeclocycline labels was administered after 2.5 months of teriparatide/placebo. A single biopsy was performed in the participants after the second set of labels to assess BFR/BS before and during drug treatment, as described [16]. Baseline BFR/BS data, utilized for subgroup designation, was obtained from the first (baseline, pretreatment) set of labels. Assessment of change in BFR/BS on teriparatide compared both sets of labels in each subject. For these change variables, only those biopsied on active teriparatide treatment (not placebo) were included in these analyses since we aimed to assess medication response. Biopsies were embedded, sectioned, and stained using established procedures [4, 16]. Histomorphometry was performed with a digitizing image-analysis system (OsteoMeasure, Version 4.00C, OsteoMetrics, Inc, Atlanta, GA). All variables were calculated according to the American Society for Bone and Mineral Research recommendations [23].
Statistical analyses
The study had three prespecified aims. In the first and second aims, we compared low and high tissue level BFR/BS subjects according to GH and IGF-1 (aim 1) and non-skeletal measures of IGF-1 action: fat distribution and cardiovascular risk markers (aim 2). The third aim assessed whether serum IGF-1 and non-skeletal measures of IGF-1 action predicted the effect of teriparatide on BMD change between baseline and 12 and 24 months, and on the change in bone formation rate between baseline and 3 months. Change in LS BMD at 12 months was the primary outcome.
The sample size was predetermined by the parent R01 FD003902. Low and high BFR groups were defined based on median baseline BFR/BS and compared by Student’s t tests. In post-hoc power analyses, assuming N of 17 per group, we had 80% power to detect a mean difference of 40 ng/mL for IGF-1 (assuming SD of 40 ng/mL) and a mean difference of 1.0 for IGF-1 Z score (assuming SD of 1), and 88% power to detect a mean difference of 4.0 kg/m2 for BMI (assuming SD of 3.6 kg/m2). Pearson correlations were used to test relationships between parameters: IGF-1, baseline bone remodeling, non-skeletal measures of IGF-1 action, and measures of teriparatide response. Analyses were repeated using Spearman correlations for all variables that were not normally distributed (Kolmogorov-Smirnov testing). An extreme outlier for CRP was omitted from analyses of correlations with CRP. P values < 0.05 were considered statistically significant. No adjustment for multiple comparisons was applied.
Role of the funding source
This study was supported by the National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases: R03 AR064016 (Cohen). The parent study was funded by the United States Food and Drug Administration (FDA) Orphan Products Clinical Trials Grants Program (R01 FD003902). The principal investigators, Dr. Cohen and Dr. Shane, designed and conducted the study. Eli Lilly, USA, supplied teriparatide and identical placebo but no other financial support, and had no role in study design, collection, analysis and interpretation of data, writing of the report, or decision to submit for publication. Investigators at Columbia University/Rutgers University performed all analyses. The MRS analysis was supported by the Image Analysis Core Laboratory (P30 DK26687). This study was also supported by K24 AR052665 (Shane) and K23 AR054127 (Cohen), the Simon-Strauss Foundation and the Thomas L. Kempner Jr. and Katheryn C. Patterson Foundation. Dr. Cohen had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
Forty-one women were enrolled in the FDA study [16], 32 based on fracture history and nine based on BMD criteria. Among the 32 with a history of fracture, 25 reported multiple fractures in adulthood; 15 had vertebral, nine had hip, two had pelvic, nine had rib, 14 had upper extremity, and 16 had lower extremity fractures. Forty women (97.5%) completed 12 months and 35 women (85.4%) completed 24 months of active teriparatide [16]. Table 1 shows characteristics of the 34 women (27 included based on fracture history and 7 based on BMD criteria) who were included in this analysis: those who completed at least 12 months of teriparatide treatment and had baseline laboratory data as well as a quadruple labeled transiliac bone biopsy. Assessments of abdominal adiposity by CT were available in 27 and marrow fat by MRS were available in 20 subjects.
Table 1.
Subject characteristics and comparisons between Low and High BFR subgroups
| All included subjects n = 34 | Low BFR n = 17 | High BFR n = 17 | p value (low vs. high) | ||
|---|---|---|---|---|---|
|
| |||||
| Age (years) | 38.0 ± 7.3 | 39.4 ± 7.4 | 36.6 ± 7.2 | 0.28 | |
| # of adult fractures | 3.5 ± 3.1 | 4.1 ± 3.4 | 3.0 ± 2.8 | 0.33 | |
| BMD Z-scores | Lumbar spine | − 2.08 ± 0.90 | − 2.05 ± 0.78 | − 2.11 ± 1.03 | 0.85 |
| Total hip | − 1.43 ± 0.68 | − 1.26 ± 0.64 | − 1.62 ± 0.68 | 0.13 | |
| Femoral neck | − 1.69 ± 0.77 | − 1.54 ± 0.68 | − 1.85 ± 0.86 | 0.26 | |
| Distal radius | − 0.05 ± 0.92 | 0.14 ± 0.69 | − 0.25 ± 1.08 | 0.22 | |
| BMD (g/cm2) | Lumbar spine | 0.794 ± 0.097 | 0.795 ± 0.085 | 0.793 ± 0.111 | 0.95 |
| Total hip | 0.746 ± 0.084 | 0.765 ± 0.080 | 0.727 ± 0.088 | 0.20 | |
| Femoral neck | 0.630 ± 0.092 | 0.643 ± 0.080 | 0.616 ± 0.103 | 0.40 | |
| Distal radius | 0.667 ± 0.052 | 0.677 ± 0.038 | 0.657 ± 0.062 | 0.26 | |
| TBS (L1-L4) | 1.294 ± 0.096 | 1.270 ± 0.104 | 1.318 ± 0.084 | 0.18 | |
| Bio-chemistries | Serum 25-OHD (ng/mL) | 42.3 ± 9.6 | 41.4 ± 9.5 | 43.2 ± 10.0 | 0.60 |
| Whole PTH (pg/mL; range 5–39 pg/ mL) | 19.2 ± 5.9 | 18.4 ± 4.4 | 20.0 ± 7.0 | 0.43 | |
| Serum calcium (albumin adjusted, mg/dL) | 8.9 ± 0.3 | 8.9 ± 0.3 | 8.8 ± 0.2 | 0.16 | |
| Urine calcium (mg/gCr) | 136 ± 62 | 125 ± 50 | 145 ± 71 | 0.38 | |
| Baseline Serum Bone Remodeling | CTX (ng/mL) | 0.332 ± 0.155 | 0.255 ± 0.096 | 0.409 ± 0.166 | 0.002 |
| Markers | OCN (ng/mL) | 15.0 ± 6.7 | 12.6 ± 6.3 | 17.5 ± 6.4 | 0.03 |
| P1NP (μg/mL) | 37.0 ± 13.1 | 32.3 ± 9.1 | 41.7 ± 15.0 | 0.03 | |
| Baseline bone biopsy dynamic histo-morphometry | Cancellous BFR/BS (mm2/mm/ year) * | 0.011 ± 0.009 | 0.005 ± 0.003 | 0.017 ± 0.009 | < 0.001 * |
| Endocortical BFR/BS (mm2/mm/year) | 0.021 ± 0.019 | 0.008 ± 0.008 | 0.032 ± 0.019 | < 0.001 | |
| Intracortical BFR/BS (mm2/mm/year) | 0.026 ± 0.023 | 0.019 ± 0.020 | 0.032 ± 0.024 | 0.12 | |
| Cancellous MS/BS (%) | 5.66 ± 4.57 | 2.66 ± 1.56 | 8.66 ± 4.63 | < 0.001 | |
| Endocortical MS/BS (%) | 10.28 ± 9.38 | 4.34 ± 4.10 | 16.23 ± 9.47 | < 0.001 | |
| Intracortical MS/BS (%) | 10.22 ± 9.18 | 6.81 ± 6.67 | 13.64 ± 10.21 | 0.03 | |
| Cancellous MAR (μm/day) | 0.510 ± 0.070 | 0.470 ± 0.068 | 0.547 ± 0.048 | 0.001 | |
| Endocortical MAR (μm/day) | 0.486 ± 0.103 | 0.418 ± 0.088 | 0.543 ± 0.078 | < 0.001 | |
| Intracortical MAR (μm/day) | 0.657 ± 0.132 | 0.677 ± 0.121 | 0.642 ± 0.142 | 0.48 | |
| BMD response to Teriparatide at 24 M | Lumbar spine | 12.7 ± 9.2 | 11.8 ± 8.5 | 13.8 ± 10.1 | 0.54 |
| (%change) | Total hip | 5.1 ± 4.0 | 3.8 ± 2.5 | 6.6 ± 4.9 | 0.06 |
| Femoral neck | 5.3 ± 5.8 | 4.6 ± 6.0 | 6.1 ± 5.8 | 0.52 | |
Parameter used to define groups
25-OHD 25 hydroxyvitamin D, BFR/BS bone formation rate/bone surface, BMD bone mineral density, DXA dual-energy x-ray absorptiometry, MS/BS mineralized surface over bone surface, MAR mineral apposition rate, CTX C-telopeptide, OCN osteocalcin, PINP N-terminal propeptide of procollagen type 1
Characteristics of women with Low and High BFR
Women with low and high tissue level cancellous BFR/BS based on median cancellous BFR/BS in the cohort (0.0086 mm2/mm/yr) are compared in Table 1. Mean (±SD) cancellous BFR/BS was 0.005 ± 0.003 mm2/mm/year in the Low BFR group and 0.017 ± 0.009 mm2/mm/year in the High BFR group. The groups did not differ by age, indices of calcium metabolism, or disease severity at presentation, including BMD and number of fractures. Highly effective contraception was required during this treatment study. Ten women used combination oral contraceptives (OCP) during the study. Among OCP users, 7 were in the Low BFR group, and 3 were in the High BFR group (chi-square p = 0.13).
As expected, serum remodeling markers (CTX, OCN, PINP) were significantly lower in the Low BFR group, as were tissue-level indices of bone remodeling: BFR/BS and mineral apposition rate at the cancellous and endocortical surfaces and mineralized surface over bone surface at all surfaces.
GH, IGF-1, and binding protein levels
Low BFR subjects had higher IGF-1 levels (p = 0.05) and significantly higher IGF-1 age-adjusted Z-scores (p = 0.005; Table 2, Fig. 1) than High BFR subjects. IGF-1 Z-scores were also inversely correlated (Table 3) with baseline BFR/BS at the cancellous, endocortical, and intracortical bone surfaces. In contrast, serum GH and binding protein levels did not differ between BFR groups and were not associated with BFR/BS at any surface.
Table 2.
Comparison of GH and IGF-1, and non-skeletal measures of IGF-1 action (fat distribution and cardiovascular risk markers) between subjects with low vs. high BFR on biopsy (mean ± SD)
| Variable | All subjects n = 34 | Low BFR N = 17 | High BFR N = 17 | p value | |
|---|---|---|---|---|---|
|
| |||||
| GH and IGF-1 | GH (ng/mL) | 2.24 ± 2.63 | 2.28 ± 3.11 | 2.19 ± 2.13 | 0.92 |
| IGF-1 (ng/mL) | 127.4 ± 40.4 | 140.8 ± 35.9 | 114.0 ± 41.2 | 0.05 | |
| IGF-1 Z score | − 0.35 ± 1.09 | 0.15 ± 1.06 | − 0.86 ± 0.88 | 0.005 | |
| IGF-BP1 (pg/mL) | 34,295 ± 24,962 | 29,232 ± 20,775 | 39,357 ± 28,268 | 0.24 | |
| IGF-BP2 (ng/mL) | 245.9 ± 86.8 | 224.3 ± 85.4 | 267.5 ± 85.3 | 0.15 | |
| IGF-BP3 (μg/mL) | 6.21 ± 1.26 | 6.42 ± 1.13 | 5.99 ± 1.38 | 0.33 | |
| IGF-1/IGF-BP3 Molar ratio | 0.078 ± 0.020 | 0.083 ± 0.017 | 0.073 ± 0.022 | 0.15 | |
| Body composition | Height (cm) | 162.6 ± 7.6 | 161.8 ± 8.1 | 163.5 ± 7.1 | 0.52 |
| Weight (kg) | 58.2 ± 10.2 | 61.3 ± 11.7 | 55.1 ± 7.7 | 0.07 | |
| BMI (kg/m2) | 22.1 ± 3.6 | 23.5 ± 4.1 | 20.6 ± 2.4 | 0.02 | |
| DXA body composition | |||||
| Whole body fat (% Fat) | 35.0 ± 6.8 | 36.4 ± 6.5 | 33.6 ± 7.0 | 0.23 | |
| Trunk fat (% Fat) | 30.2 ± 7.7 | 32.2 ± 7.0 | 28.2 ± 8.1 | 0.13 | |
| Compartmental fat: CT slice at L4 | |||||
| Subcutaneous fat (mm2) | 20,383 ± 9633 | 22,578 ± 10,133 | 17,639 ± 8595 | 0.19 | |
| Visceral fat (mm2) | 5218±2135 | 5437 ± 2135 | 4943±2196 | 0.56 | |
| Marrow fat fraction assessed by MRS (N = 20) | Spine (L3; %) | 43.4 ± 13.1 | 42.2 ± 11.6 | 45.0 ± 15.4 | 0.67 |
| Femoral neck (%) | 71.1 ± 13.9 | 68.4 ± 12.7 | 74.4 ± 15.2 | 0.35 | |
| Serum markers associated with fat distribution | Leptin (ng/mL) | 19.3 ± 11.7 | 21.8 ± 12.9 | 16.8 ± 10.0 | 0.21 |
| Adiponectin (ng/mL) | 10,141 ± 3657 | 9209 ± 3251 | 11,074 ± 3894 | 0.14 | |
| CRP (mg/L) | 3.59 ± 14.20 | 6.06 ± 20.0* | 1.12 ± 1.87 | 0.32 | |
| IL-6 (pg/mL) | 1.97 ± 3.08 | 2.79 ± 4.13 | 1.16 ± 1.04 | 0.13 | |
| Total cholesterol (mg/dL) | 186 ± 35 | 183 ± 42 | 189 ± 27 | 0.66 | |
| HDL (mg/dL) | 77 ± 19 | 73 ± 15 | 82 ± 21 | 0.16 | |
| Triglycerides (mg/dL) | 76 ± 30 | 85 ± 33 | 68 ± 25 | 0.10 | |
| LDL (calculated; mg/dL) | 95 ± 32 | 94 ± 37 | 96 ± 28 | 0.86 | |
| Homocysteine (umol/L) | 9.1 ± 4.7 | 9.4 ± 6.5 | 8.8 ± 2.1 | 0.71 | |
| HOMA-IR | 0.977 ± 0.733 | 1.027 ± 0.668 | 0.926 ± 0.802 | 0.70 | |
GH growth hormone, IGF-1 insulin-like growth factor 1, BP binding protein, BMI body mass index, DXA dual-energy x-ray absorptiometry, MRS magnetic resonance spectroscopy, CRP C-reactive protein, IL-6 interleukin-6, HDL high-density lipoprotein, LDL low-density lipoprotein, HOMA-IR homeostatic model assessment of insulin resistance.
One subject with very high CRP of 83.5 mg/L contributed to the substantial variability in the Low BFR group; results of the between groups comparison did not differ after removal of this subject. With outlier removed mean CRP 1.17 ± 1.56; mean in Low BFR group: 1.12 ± 1.20 mg/L
Fig. 1.
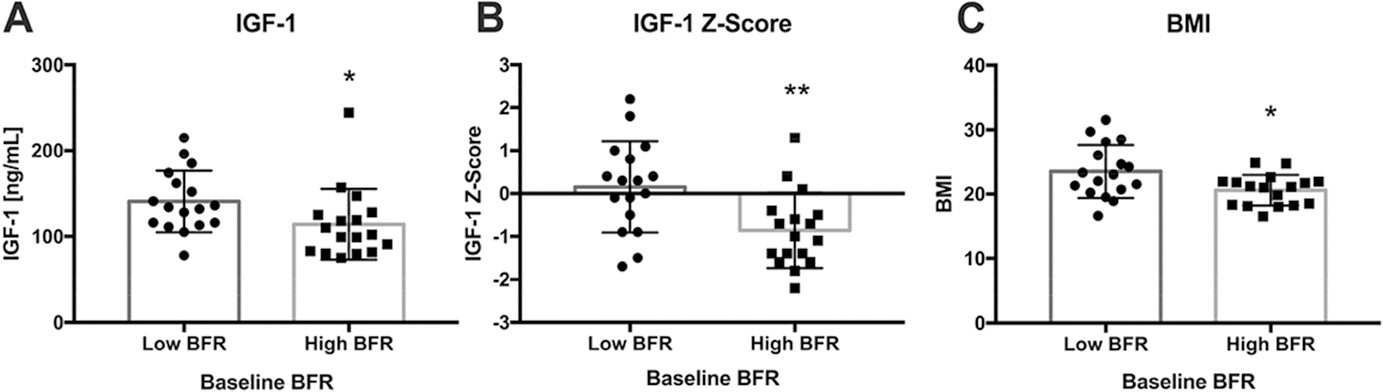
Comparisons between BFR subgroups in terms of A serum IGF-1, B serum IGF-1 Z-score, and C BMI. Subjects with Low BFR had higher IGF-1 (p = 0.05) and IGF-1 Z-scores, as well as higher BMI. *p ≤ 0.05; **p < 0.01. Box and hash marks denote mean±SD
Table 3.
Correlations (Pearson; r,p) with IGF-1, IGF-1 Z-score, and baseline BFR/BS
| IGF-1 | IGF-1 Z-score | BFR/BS |
||||
|---|---|---|---|---|---|---|
| Cancellous | Endocortical | Intracortical | ||||
|
| ||||||
| Age | − 0.28, 0.11 | 0.09, 0.60 | 0.01, 0.98 | 0.01, 0.96 | − 0.32, 0.08 | |
| Height | 0.12, 0.51 | 0.09, 0.60 | 0.03, 0.88 | 0.08, 0.65 | 0.01, 0.97 | |
| Weight | 0.30, 0.09 | 0.25, 0.15 | − 0.39. 0.02 | − 0.18, 0.33 | − 0.12, 0.52 | |
| BMI | 0.27, 0.12 | 0.24, 0.18 | − 0.45, 0.007 | − 0.26, 0.16 | − 0.15, 0.42 | |
| Baseline BMD by DXA | Lumbar spine | 0.11, 0.52 | 0.09, 0.61 | − 0.14, 0.42 | − 0.38, 0.04 | − 0.24, 0.18 |
| Total hip | 0.08, 0.65 | − 0.01, 0.96 | − 0.03, 0.88 | − 0.16, 0.39 | 0.02, 0.91 | |
| Femoral neck | 0.02, 0.90 | − 0.13, 0.48 | − 0.10, 0.58 | − 0.10, 0.62 | 0.27, 0.14 | |
| Distal radius | 0.03, 0.88 | 0.05, 0.79 | − 0.09, 0.61 | − 0.04, 0.82 | − 0.06, 0.75 | |
| DXA Body Composition | Whole body fat | 0.30, 0.09 | 0.32, 0.07 | − 0.30, 0.09 | − 0.10, 0.58 | 0.13, 0.50 |
| Trunk fat | 0.31, 0.08 | 0.34, 0.05 | − 0.38, 0.03 | − 0.19, 0.31 | − 0.001, 0.99 | |
| Compartmental Fat: | Subcutaneous fat | 0.31, 0.12 | 0.25, 0.21 | − 0.41, 0.03 | − 0.34, 0.10 | − 0.07, 0.75 |
| CT slice at L4 (n = 27) | Visceral fat | 0.18, 0.38 | 0.28, 0.16 | − 0.31, 0.12 | − 0.08, 0.70 | − 0.22, 0.30 |
| Marrow fat fraction assessed by MRS (n = 20) | L3 | 0.19, 0.45 | 0.16, 0.54 | − 0.09, 0.74 | 0.24, 0.34 | − 0.14, 0.61 |
| Femoral neck | 0.33, 0.15 | 0.26, 0.27 | − 0.22, 0.35 | − 0.01, 0.95 | − 0.12, 0.64 | |
| Serum markers associated with fat distribution | Leptin | 0.36, 0.04 | 0.38, 0.03 | − 0.30, 0.08 | − 0.14, 0.46 | − 0.07, 0.72 |
| Adiponectin | − 0.26, 0.13 | − 0.38, 0.03 | 0.19, 0.28 | 0.32, 0.08 | 0.22, 0.23 | |
| CRP * | 0.02, 0.92 | − 0.11, 0.55 | − 0.07, 0.69 | − 0.23, 0.22 | 0.19, 0.32 | |
| IL – 6 | 0.13, 0.45 | 0.24, 0.18 | − 0.20, 0.26 | − 0.16, 0.40 | − 0.07, 0.71 | |
| Total cholesterol | 0.17, 0.35 | 0.15, 0.38 | 0.12, 0.50 | 0.04, 0.84 | − 0.25, 0.16 | |
| HDL | − 0.19, 0.28 | − 0.23, 0.19 | 0.33, 0.06 | 0.22, 0.23 | − 0.03, 0.89 | |
| Triglycerides | 0.38, 0.03 | 0.34, 0.05 | − 0.22, 0.22 | − 0.22, 0.23 | − 0.30, 0.10 | |
| LDL (calculated) | 0.20, 0.27 | 0.23, 0.20 | − 0.02, 0.93 | − 0.05, 0.78 | − 0.22, 0.24 | |
| Homocysteine | 0.12, 0.52 | 0.10, 0.76 | 0.00, 0.99 | − 0.00, 099 | − 0.02, 0.93 | |
| HOMA-IR | 0.10, 0.56 | 0.01, 0.62 | − 0.12, 0.51 | − 0.01, 0.95 | 0.01, 0.97 | |
| Baseline serum bone re-modeling markers | CTX | − 0.24, 0.16 | − 0.32, 0.06 | 0.33, 0.06 | 0.47, 0.008 | 0.24, 0.18 |
| OCN | − 0.14, 0.44 | − 0.20, 0.27 | 0.34, 0.05 | 0.36, 0.049 | 0.10, 0.58 | |
| P1NP | − 0.27, 0.12 | − 0.20, 0.27 | 0.62, < 0.001 | 0.52, 0.003 | 0.23, 0.21 | |
| Baseline bone biopsy dynamic histo-morphometry: BFR/BS | Cancellous | − 0.39, 0.02 | − 0.46, 0.007 | N/A | N/A | N/A |
| Endocortical | − 0.33, 0.07 | − 0.41, 0.02 | N/A | N/A | N/A | |
| Intracortical | − 0.27, 0.14 | − 0.39, 0.03 | N/A | N/A | N/A | |
IGF-1 insulin-like growth factor 1, BMI body mass index, BFR/BS bone formation rate/bone surface, DXA dual-energy x-ray absorptiometry, MRS magnetic resonance spectroscopy, CRP C-reactive protein, IL-6 interleukin-6, HDL high-density lipoprotein, LDL low-density lipoprotein, HOMA-IR homeostatic model assessment of insulin resistance, CTX C-telopeptide, OCN osteocalcin, PINP N-terminal propeptide of procollagen type 1.
Outlier with CRP = 83.5 mg/L was omitted
Comparisons between the OCP users (n = 10) and nonusers (n = 24) showed no significant differences in IGF-1 or IGF-1 Z-score.
Fat distribution and cardiovascular risk markers
With regard to non-skeletal measures of IGF-1 action, such as adiposity, fat distribution, and cardiovascular risk markers, IGF-1 and IGF-1 Z score correlated directly with body and trunk fat by DXA, but results did not reach statistical significance (p = 0.05–0.09; Table 3). IGF-1 Z score correlated directly and significantly with serum leptin and inversely with adiponectin. IGF-1 correlated directly with serum triglyceride level; IGF-1 Z score tended to associate directly with triglyceride level (p = 0.05).
Subjects with Low BFR had higher BMI (Table 2, Fig. 1) and tended to weigh more. Compartmental fat assessed by DXA and CT slice at L4, and serum leptin and triglycerides, were higher in the Low BFR group, but the differences did not reach statistical significance. Other cardiovascular risk markers including CRP, IL-6, and lipid indices did not differ significantly between groups. Marrow fat assessments also did not differ between the groups.
In the group as a whole (Table 3), cancellous BFR/BS correlated inversely and statistically significantly with weight and BMI, DXA trunk fat, and subcutaneous fat by CT and tended to correlate inversely with serum leptin (p = 0.08).
Some variables (BMI, leptin, CRP, IL-6, homocysteine) were not normally distributed (Kolmogorov-Smirnov testing). Results in terms of median (IQR) and nonparametric analyses between groups are presented in a Supplemental Table and were not substantially different. For BMI, leptin, CRP, IL-6, and homocysteine, correlation analyses were repeated using Spearman correlations and were not substantially different. BMI remained significantly inversely related to cancellous BFR (r = −0.47, p = 0.005). Leptin remained significantly directly related to IGF-1 and IGF-1 Z score (r = 0.4, p = 0.02–0.03), and tended to be inversely related to cancellous BFR/BS (r = − 0.3, p = 0.09).
Response to teriparatide
The Low and High BFR/BS groups did not differ significantly with respect to effects of 12 months of teriparatide on percent change in BMD at any site (data not shown). At 24 months, BMD response at the total hip tended to be more favorable in the High BFR group (p = 0.06), but there were no statistically significant differences between the groups at any site. BMD response was quite variable, as previously described [16].
Across all subjects, the 12- and 24-month BMD responses to teriparatide directly correlated with baseline serum remodeling markers and tissue-level BFR/BS (Table 4, Fig. 2). Relationships were strongest between baseline cancellous BFR/BS and percent change in TH BMD at 24 months (r = 0.58; p < 0.01) and baseline endocortical BFR/BS and both 12- and 24-month percent change at the LS and TH (p = 0.44–55; p < 0.05). Excluding one outlier with a very high BFR/BS and large BMD change on teriparatide attenuated these relationships but those between endocortical BFR/BS and LS BMD change at 12 and 24 months remained statistically significant (both r = 0.4, p < 0.05).
Table 4.
Correlations (Pearson; r,p) with 12 (n = 34) and 24 month (n = 31) BMD response to teriparatide, and 3-month BFR/BS response to teriparatide on quadruple labeled bone biopsies (n = 21)
| Baseline Measures | BMD response to teriparatide (% change vs baseline) |
3-month BFR/BS response to teriparatide (absolute change, baseline vs 3-month on quadruple-labelled bone biopsy samples in teriparatide-treated subjects; n = 21) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 12 MLS BMD | 12 M TH BMD | 12 M FN BMD | 24 M LS BMD | 24 M TH BMD | 24 M FN BMD | Cancellous BFR | Endocortical BFR | Intracortical BFR | ||
|
| ||||||||||
| Age | −0.05,0.78 | 0.20, 0.27 | 0.22, 0.23 | 0.15, 0.41 | 0.30, 0.11 | 0.24, 0.22 | −0.21,0.36 | −0.17, 0.48 | 0.27, 0.27 | |
| Height | 0.23, 0.19 | 0.05, 0.80 | 0.11,0.57 | 0.20, 0.28 | 0.06, 0.77 | 0.29,0.13 | 0.00, 0.99 | 0.40, 0.08 | 0.07, 0.77 | |
| Weight | −0.11,0.53 | −0.22, 0.24 | −0.06, 0.75 | −0.19, 0.30 | −0.33, 0.08 | −0.14, 0.46 | −0.26, 0.25 | −0.35, 0.14 | 0.17,0.49 | |
| BMI | −0.28,0.12 | −0.26, 0.15 | −0.14, 0.45 | −0.35, 0.06 | −0.40,0.03 | −0.34, 0.07 | −0.28,0.21 | −0.62, 0.004 | 0.13,0.60 | |
| DXA body | Whole body | −0.12, 0.51 | −0.17, 0.35 | −0.13,0.47 | −0.19,0.31 | −0.17,0.37 | −0.31, 0.10 | −0.20, 0.39 | −0.54,0.01 | 0.17, 0.49 |
| composition (%Fat) | Trunk | −0.23,0.20 | −0.24, 0.19 | −0.18,0.35 | −0.29, 0.11 | −0.28,0.14 | −0.35, 0.06 | −0.10, 0.68 | −0.57,0.008 | 0.21, 0.40 |
| Compartmental | Subcutaneous | −0.48, 0.01 | −0.32, 0.11 | −0.16, 0.46 | −0.60, 0.002 | −0.45, 0.03 | −0.37, 0.08 | −0.16, 0.53 | −0.39, 0.11 | −0.04, 0.89 |
| fat: CT slice at L4 (n = 27) | Visceral | −0.15,0.47 | −0.17,0.40 | −0.03, 0.90 | −0.25, 0.24 | −0.23, 0.27 | −0.20, 0.36 | 0.10, 0.70 | −0.25, 0.31 | −0.01,0.96 |
| Marrow fat fraction assessed by MRS (N = 20) | L3 | 0.41, 0.10 | 0.01, 0.97 | 0.01, 0.98 | 0.48, 0.07 | 0.27, 0.33 | 0.21,0.44 | −0.00, 0.99 | 0.09, 0.77 | 0.06, 0.87 |
| Femoral neck | 0.11,0.66 | −0.25,0.30 | 0.02, 0.95 | 0.13,0.61 | −0.18, 0.50 | 0.01, 0.98 | −0.13,0.68 | −0.01,0.97 | −0.14, 0.69 | |
| IGF-1 | IGF-1 | −0.15, 0.39 | −0.18,0.32 | −0.08,0.66 | −0.16,0.39 | −0.46,0.01 | −0.34, 0.07 | −0.40, 0.08 | −0.19,0.42 | −0.09, 0.71 |
| IGF-1 Z score | −0.14, 0.44 | −0.07, 0.70 | 0.04,0.83 | −0.07,0.70 | −0.31, 0.09 | −0.22, 0.25 | −0.42, 0.06 | −0.25,0.29 | −0.03,0.91 | |
| Serum markers associated bution | Leptin | −0.18,0.33 | −0.30, 0.10 | −0.38, 0.04 | −0.24, 0.20 | −0.28, 0.14 | −0.48, 0.009 | −0.20, 0.38 | −0.38, 0.10 | −0.06, 0.79 |
| Adiponectin | 0.23, 0.20 | 0.12, 0.53 | 0.25, 0.18 | 0.10, 0.58 | 0.20, 0.29 | 0.36, 0.06 | 0.46,0.04 | 0.26, 0.27 | 0.05, 0.84 | |
| CRP* | −0.21,0.24 | −0.51,0.004 | −0.29,0.11 | −0.44, 0.01 | −0.54,0.003 | −0.35, 0.06 | 0.16, 0.49 | −0.15, 0.55 | −0.40, 0.10 | |
| IL-6 | −0.08,0.67 | −0.12, 0.52 | 0.12, 0.51 | −0.19,0.30 | −0.11,0.58 | −0.06,0.77 | −0.25, 0.27 | −0.18,0.44 | −0.18,0.46 | |
| Total cholesterol | −0.01,0.95 | 0.13, 0.49 | 0.07, 0.72 | −0.21,0.26 | −0.12, 0.53 | 0.07, 0.73 | 0.20, 0.39 | −0.09,0.70 | 0.18, 0.47 | |
| HDL | −0.13, 0.48 | 0.09, 0.62 | −0.10, 0.58 | −0.04, 0.85 | 0.16,0.41 | 0.02, 0.94 | 0.35, 0.12 | −0.14, 0.56 | 0.28, 0.24 | |
| Triglycerides | −0.09, 0.63 | −0.02, 0.91 | 0.08, 0.66 | −0.23,0.21 | −0.32, 0.09 | −0.19, 0.34 | −0.19,0.42 | −0.17, 0.47 | −0.13,0.60 | |
| LDL (calculated) | 0.04, 0.82 | 0.07, 0.71 | 0.07, 0.71 | −0.18,0.34 | −0.17, 0.37 | 0.08, 0.70 | 0.16, 0.50 | 0.02, 0.94 | 0.23, 0.37 | |
| Homocysteine | 0.16, 0.39 | 0.07, 0.70 | 0.15, 0.43 | 0.02, 0.90 | −0.01,0.95 | −0.01,0.94 | −0.04, 0.86 | −0.44, 0.05 | 0.05,0.83 | |
| HOMA-IR | −0.14, 0.45 | −0.06,0.73 | −0.24, 0.19 | −0.17, 0.36 | −0.16, 0.40 | −0.39, 0.04 | −0.34, 0.14 | −0.18, 0.45 | −0.03,0.90 | |
| Baseline serum bone re-modeling markers | CTX | 0.34, 0.05 | 0.20, 0.26 | −0.24, 0.20 | 0.40, 0.03 | 0.47, 0.009 | 0.23, 0.24 | 0.45,0.04 | 0.44, 0.06 | 0.15,0.53 |
| OCN | 0.27, 0.13 | 0.43, 0.01 | −0.13,0.48 | 0.23, 0.21 | 0.47, 0.01 | 0.04, 0.82 | −0.29, 0.20 | 0.01, 0.97 | 0.00, 0.99 | |
| P1NP | 0.39, 0.03 | 0.47, 0.007 | 0.03, 0.86 | 0.42, 0.02 | 0.72, <0.001 | 0.43,0.02 | 0.12, 0.61 | 0.33,0.16 | 0.13,0.61 | |
| Baseline bone biopsy dynamic histomorphometry: BFR/ BS | Cancellous | 0.34, 0.05 | 0.47, 0.007 | 0.03, 0.87 | 0.30, 0.10 | 0.58, 0.001 | 0.38,0.045 | 0.37, 0.10 | 0.40, 0.08 | 0.30, 0.22 |
| Endoc orticai | 0.54, 0.002 | 0.44, 0.02 | 0.06, 0.76 | 0.55, 0.002 | 0.55, 0.003 | 0.33, 0.10 | 0.27, 0.25 | 0.41, 0.08 | 0.48,0.04 | |
| Intrac orticai | 0.15,0.42 | 0.04, 0.85 | 0.16, 0.42 | 0.10, 0.63 | 0.36, 0.06 | 0.31,0.11 | 0.36, 0.13 | 0.43, 0.08 | 0.05,0.85 | |
BFR/BS bone formation rate/bone surface, BMD bone mineral density, DXA dual-energy x-ray absorptiometry, MRS magnetic resonance spectroscopy, CRP C-reactive protein, IL-6 interleukin-6, HDL high-density lipoprotein, LDL low-density lipoprotein, HOMA-IR homeostatic model assessment of insulin resistance, CTX C-telopeptide, OCN osteocalcin, PINP N-terminal propeptide of procollagen type 1
Outlier with CRP = 83.5 mg/L was omitted
Fig. 2.

Relationships between IGF-1, bone formation rate, and body fat and BMD response to 24 months of teriparatide treatment in terms of lumbar spine (LS), total hip (TH), and femoral neck (FN) percent change. Linear regression lines are shown. *p < 0.05; **p ≤ 0.01; ***p ≤ 0.001
IGF-1 was inversely related to 24-month percent change in TH BMD (r = − 0.46, p = 0.01) and tended to be inversely related to 24-month percent change in FN BMD (r = − 0.34, p = 0.07; Table 4, Fig. 2). Neither IGF-1 nor IGF-1 Z score were related to percent change in BMD at the LS (Table 4, Fig. 2). Both IGF-1 and IGF-1 Z score tended to associate inversely with the change in cancellous BFR based on quadruple-labeled bone biopsy sampling (p = 0.06–0.08).
Higher BMI, body fat, serum leptin, CRP, and HOMAIR were inversely related to BMD and/or tissue level bone remodeling response. Relationships were strongest between BMI, serum leptin, CRP, and subcutaneous fat and bone density response at the 24-month timepoint (Table 4, Fig. 2). BMI and body fat by DXA also related inversely to bone remodeling response at the endocortical surface (r = − 0.54 to − 0.62; p ≤ 0.01). For BMI, leptin, CRP, IL-6, and homocysteine, correlation analyses were repeated using Spearman correlations and were not meaningfully changed. BMI remained inversely related to BMD response at 24 months (at LS, r = − 0.38, p = 0.04, at TH r = − 0.32, trend p = 0.09). CRP remained inversely related to LS (r = − 0.45, p = 0.01) and TH (r = − 0.47, p = 0.01) BMD response at 24 months. Leptin remained inversely related to FN BMD response at 24 months (r = − 0.45, p = 0.01). Other cardiovascular risk markers associated with central adiposity did not significantly correlate with BMD or remodeling response. Marrow fat measures were not significantly related to BMD or bone remodeling response to teriparatide.
Discussion
This mechanistic ancillary study to a clinical trial of teriparatide for idiopathic premenopausal osteoporosis [16] aimed to determine whether premenopausal women with IOP and low bone formation have higher serum IGF-1 and evidence of paradoxical relationships with IGF-1 or IGF-1 resistance in both skeletal and non-skeletal tissues, and whether evidence of IGF-1 resistance in skeletal and/or non-skeletal tissues predicts their response to teriparatide. We found that those with low tissue-level bone formation rate at baseline had significantly higher IGF-1. Since the growth hormone-IGF-1 axis is generally osteoanabolic, this finding supports our hypothesis of paradoxical IGF-1 relationships at the skeletal level in IOP. IGF-1 was also directly correlated to some indices of body adiposity and related cardiovascular risk markers. Since obesity is associated with low IGF-1 [21], and adult growth hormone deficiency is associated with central adiposity and related cardiovascular risk markers [11–14], this provides some support for our hypothesis of “IGF-1 resistance”—or paradoxical relationships with IGF-1—in non-skeletal tissues. As hypothesized, we also found that both higher IGF-1 and higher body fat were associated with poorer response to the osteoanabolic drug, teriparatide, in this unusual group of young women with unexplained osteoporosis.
These findings are consistent with results of our previously reported pilot study of teriparatide in 21 premenopausal women with IOP. Women with no significant BMD response to teriparatide (nonresponders) had both lower biopsy-based baseline bone remodeling and higher serum IGF-1 levels [6]. BMI tended to be higher in non-responders than responders (24.5 ± 4.5 vs. 21.5 ± 2.8 kg/m2; p = 0.1) and BMI was inversely associated with the rise in serum bone turnover markers in response to teriparatide. Additionally, among a subset of these women, we utilized flow cytometry to explore the hypothesis that osteoblast and IGF-1 related mechanisms mediate differential response to teriparatide and documented increases in circulating osteoblast progenitors and IGF-1 receptor expression in response to teriparatide [24]. IGF-1 receptor expression in response to teriparatide predicted tissue-based bone formation rate and BMD response to teriparatide [24]. Less robust IGF-1 receptor expression response was associated with higher BMI. That we found similar relationships in two separate cohorts of premenopausal IOP women highlights the mechanistic importance of these paradoxical IGF-1 relationships.
Several possible mechanisms may explain how higher IGF-1 levels may be related to the phenotype documented here. It is possible that this is related to abnormal production of or association with IGF binding proteins, a proposed mechanism in some forms of short stature [25]. Several binding proteins assessed in this study did not differ between the groups. However, we acknowledge that the IGF binding protein system is quite complex, and such potential mechanisms require further investigation. It is also possible that higher IGF-1 levels could be explained by differences at the IGF-1 receptor level. Although the specific feedback loop leading to elevated IGF-1 in this setting requires additional study, elevated IGF-1 levels have been documented in several patients with genetic IGF-1 receptor defects [26, 27].
The relationship between higher body fat and lower bone remodeling, seen in this study, has been previously documented. In both premenopausal and postmenopausal women, visceral obesity or higher BMI have been associated with lower serum bone remodeling markers [8, 21, 28, 29]. In a bone biopsy study of healthy premenopausal women, we found significant inverse relationships between visceral adiposity and both bone formation rate and bone volume fraction [29]. The relationships we report here between baseline bone remodeling and response to teriparatide have been previously documented in several populations [30–32]. Additionally, inverse relationships between weight and teriparatide response have also been documented in postmenopausal women [31]. These IGF-1 data contrast with previous studies in which obesity is associated with lower IGF-1 [21, 33, 34] and lower bone remodeling [8, 21, 28, 29]. Moreover, both human and animal studies have shown that an intact GH/IGF-1 axis is required for PTH responsiveness [35, 36]. Thus, the relationships with IGF-1 are, in all respects, opposite to what would be expected. These paradoxical relationships lead us to hypothesize an IGF-1 resistant state in premenopausal IOP. As in other hormone-resistant conditions well-known in endocrinology, the clinical features of apparent hormone resistance also resemble those of hormone deficiency.
In this study, we measured marrow adiposity of L3 and the femoral neck by MRS. Within bone marrow, a common mesenchymal precursor differentiates into adipocytes or osteoblasts. In rodents, GH regulates the balance between bone formation and marrow adiposity [15]. In healthy obese premenopausal women (mean BMI 36.7 ± 4.2 kg/m2), vertebral bone marrow fat measured by MRS correlates positively with visceral adiposity and inversely with IGF-1, suggesting that the detrimental effect of visceral fat on bone health may be mediated in part by GH and/or IGF-1, important regulators of the fat/bone lineage [8]. Moreover, many studies have reported inverse relationships between marrow fat (assessed by bone biopsy or MRS) and bone mass [37–40]. Patients with osteoporosis have significantly higher adipocyte volume and lower bone volume [41] that is associated with decreased bone formation [42]. These data caused us to hypothesize that the inverse relationship between higher IGF-1 and lower bone formation rate was mediated by shifts in marrow lineage toward adipocytes rather than osteoblasts. However, we found no significant relationships between marrow fat measures and IGF-1, tissue-level bone remodeling, or response to teriparatide, and thus our data did not support this hypothesis. The MRI findings are, however, consistent with our prior biopsy-based marrow adiposity study conducted in a separate untreated premenopausal IOP cohort. Specifically, in the IOP cohort, we found higher adipocyte number (by 22%) and size (by 24%) on bone biopsy samples than controls (p < 0.0001 for all), even after adjusting for age, BMI, and bone volume [43]. We also found associations between marrow fat and bone formation rate or bone volume only in controls, and not in IOP subjects. Taken together, the findings from both studies suggest that the expected relationships between marrow fat and bone metabolism are disrupted in IOP subjects.
We measured compartmental body fat using both 2- and 3-dimensional methods (DXA, CT, MRS) and assessed serum markers (e.g., lipids, inflammation) that have been associated with adverse metabolic effects of visceral adiposity. Since our preliminary data suggested relationships between central adiposity and bone metabolism, we hypothesized that response to teriparatide would be inversely related to indices that reflect central (visceral) adiposity, namely adipokines and visceral fat assessed by CT and serum markers of dyslipidemia and inflammation. Results for leptin, adiponectin (a hormone known to inversely associate with visceral fat), BMI, and DXA-based 2-dimensional assessments of trunk fat were consistent with our hypothesis. However, when we directly measured compartmental fat by analyzing a CT slice at L4, we unexpectedly found that the inverse relationship to BMD response was more robust for subcutaneous fat than for visceral fat. This may have been due to the smaller CT sample size (n = 27) as well as the measurement technique involving assessments on one CT slice at L4, which may have limited our ability to detect expected relationships with visceral fat. Alternatively, drug pharmacokinetics could explain the relationships we observed between BMD response and subcutaneous fat. Body weight is known to affect the rate of teriparatide absorption and time to reach maximum concentration (Tmax) [44]. Subjects were performing daily abdominal subcutaneous injections of teriparatide during this study, and the amount of subcutaneous fat could affect drug pharmacokinetics and peak levels achieved. Thus, the inverse relationship between teriparatide responsiveness and subcutaneous fat could be due to differential pharmacokinetics rather than metabolic effects related to the central fat compartments.
This study explores the hypothesis of a mechanistic effect of higher IGF-1 in the Low BFR group. However, in examining IGF-1 Z score distribution, it might also be the case that those with High BFR are characterized by a lower IGF-1 state. One such low IGF-1 state is anorexia nervosa, which is associated with osteoporosis characterized by decreased bone formation, increased bone resorption, very low body fat indices, and higher marrow adiposity [45–47]. Although inclusion of women with anorexia nervosa could have affected the results, we included only women with regular menses and excluded women with eating disorders. Of two women in this study who had BMI < 18 kg/m2, one was in the Low BFR group and one was in the High BFR cohort. Thus, we believe that inclusion of constitutionally thin women did not affect our results.
This study has several limitations. The sample size was small because it was limited by the parent study. MRI, CT, and 3-month biopsy remodeling data on therapy were available in a subset, which may have affected our ability to detect significant relationships. Although compliance with teriparatide (assessed on returned vials and via dosing logs) was excellent (> 90%) and was not related to BMD response in the parent study [16], it remains possible that compliance could have affected these results. Premenopausal women with IOP in general and in this study are likely to have diverse etiologies of early-onset osteoporosis. Even within the Low BFR group, mechanisms related to IGF-1 or body composition may only be relevant to a subset. Further studies are needed to elucidate patient-specific mechanisms. We note that the relationships that we observed between variables were of modest strength and did not always consistently support the hypotheses tested. Although findings of this nature cannot prove the hypothesis of IGF-1 resistance, they do support atypical relationships with IGF-1 that should be further investigated. This small study of relationships cannot define mechanisms, but is instead intended to be hypothesis generating toward further studies to investigate mechanisms of idiopathic osteoporosis in premenopausal women.
In summary, in a group of premenopausal women with unexplained early-onset osteoporosis who were enrolled in an FDA-funded teriparatide treatment study, we found that lower baseline tissue level bone formation rate is associated with higher BMI and body fat and poorer response to the bone formation stimulating medication, teriparatide. These relationships have all been previously observed in other populations. However, in direct contrast to observations in other populations, low bone formation, higher body fat, and poorer response to teriparatide were all related to higher IGF-1, confirming our previously documented results in another group of premenopausal women with IOP. These observations suggest a paradoxical relationship between IGF-1 and bone (possibly a state of IGF-1 resistance) that may contribute to pathogenesis of osteoporosis in some women with IOP and low bone formation. Paradoxical IGF-1 relationships suggest that atypical regulation of bone and fat may contribute to mechanisms of osteoporosis in some women with IOP. This finding has implications for the much larger population of women with postmenopausal osteoporosis, in whom the very variable response to osteo-anabolic therapies for osteoporosis may also be related to body composition or IGF-1-related mechanisms. Further investigations will focus on a precision medicine approach to investigate such mechanisms at the individual cellular and genetic level.
Supplementary Material
Funding
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R03 AR064016), the United States Food and Drug Administration (FDA) Orphan Products Clinical Trials Grants Program (R01 FD003902), the National Institute of Diabetes and Digestive and Kidney Diseases (P30 DK26687), the Simon-Strauss Foundation, and the Thomas L. Kempner, Jr. and Katheryn C. Patterson Foundation. Eli Lilly, USA, supplied teriparatide and identical placebo.
Footnotes
Conflict of interest AC, ES, RRR, and JML receive research support from Amgen and Eli Lilly. DWD receives research support and consulting fees from Amgen, Eli Lilly, and Radius Health.
Code availability Not applicable.
Ethics approval Institutional Review Boards at both Columbia University, New York, NY and Creighton University, Omaha, NE approved the parent and ancillary studies.
Consent to participate All participants provided their written informed consent.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00198-021-06196-8.
Data availability
Not applicable.
References
- 1.Heshmati HM, Khosla S (1998) Idiopathic osteoporosis: a heterogeneous entity. Ann Med Interne (Paris) 149:77–81 [PubMed] [Google Scholar]
- 2.Hosmer WD, Genant HK, Browner WS (2002) Fractures before menopause: a red flag for physicians. Osteoporos Int 13:337–341 [DOI] [PubMed] [Google Scholar]
- 3.Cohen A, Hostyk J, Baugh EH, et al. (2021) Whole exome sequencing reveals potentially pathogenic variants in a small subset of premenopausal women with idiopathic osteoporosis. JBMR/JBMR Plus In Review: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen A, Dempster DW, Recker RR et al. (2011) Abnormal bone microarchitecture and evidence of osteoblast dysfunction in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab 96:3095–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen A, Liu XS, Stein EM, McMahon DJ, Rogers HF, Lemaster J, Recker RR, Lappe JM, Guo XE, Shane E (2009) Bone microarchitecture and stiffness in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab 94:4351–4360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen A, Stein EM, Recker RR et al. (2013) Teriparatide for idiopathic osteoporosis in premenopausal women: a pilot study. J Clin Endocrinol Metab 98:1971–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurland ES, Rosen CJ, Cosman F, McMahon D, Chan F, Shane E, Lindsay R, Dempster D, Bilezikian JP (1997) Insulin-like growth factor-I in men with idiopathic osteoporosis. J Clin Endocrinol Metab 82:2799–2805 [DOI] [PubMed] [Google Scholar]
- 8.Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, Harrington LM, Breggia A, Rosen CJ, Miller KK (2011) Determinants of bone mineral density in obese premenopausal women. Bone 48:748–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilsanz V, Chalfant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD (2009) Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab 94:3387–3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell M, Mendes N, Miller KK, Rosen CJ, Lee H, Klibanski A, Misra M (2010) Visceral fat is a negative predictor of bone density measures in obese adolescent girls. J Clin Endocrinol Metab 95:1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas JD, Monson JP (2009) Adult GH deficiency throughout lifetime. Eur J Endocrinol 161(Suppl 1):S97–S106 [DOI] [PubMed] [Google Scholar]
- 12.Abs R, Feldt-Rasmussen U, Mattsson AF, Monson JP, Bengtsson BA, Goth MI, Wilton P, Koltowska-Haggstrom M (2006) Determinants of cardiovascular risk in 2589 hypopituitary GH-deficient adults—a KIMS database analysis. Eur J Endocrinol 155:79–90 [DOI] [PubMed] [Google Scholar]
- 13.Carroll PV, Christ ER, Bengtsson BA et al. (1998) Growth hormone deficiency in adulthood and the effects of growth hormone replacement: a review. Growth Hormone Research Society Scientific Committee. J Clin Endocrinol Metab 83:382–395 [DOI] [PubMed] [Google Scholar]
- 14.Weaver JU, Monson JP, Noonan K, John WG, Edwards A, Evans KA, Cunningham J (1995) The effect of low dose recombinant human growth hormone replacement on regional fat distribution, insulin sensitivity, and cardiovascular risk factors in hypopituitary adults. J Clin Endocrinol Metab 80:153–159 [DOI] [PubMed] [Google Scholar]
- 15.Menagh PJ, Turner RT, Jump DB, Wong CP, Lowry MB, Yakar S, Rosen CJ, Iwaniec UT (2010) Growth hormone regulates the balance between bone formation and bone marrow adiposity. J Bone Miner Res 25:757–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen A, Shiau S, Nair N, et al. (2020) Effect of teriparatide on bone remodeling and density in premenopausal idiopathic osteoporosis: a phase II trial. J Clin Endocrinol Metab 105 (10):e3540–e3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedrich N, Wolthers OD, Arafat AM et al. (2014) Age- and sex-specific reference intervals across life span for insulin-like growth factor binding protein 3 (IGFBP-3) and the IGF-I to IGFBP-3 ratio measured by new automated chemiluminescence assays. J Clin Endocrinol Metab 99:1675–1686 [DOI] [PubMed] [Google Scholar]
- 18.Wallace TM, Levy JC, Matthews DR (2004) Use and abuse of HOMA modeling. Diabetes Care 27:1487–1495 [DOI] [PubMed] [Google Scholar]
- 19.Zhao B, Colville J, Kalaigian J, Curran S, Jiang L, Kijewski P, Schwartz LH (2006) Automated quantification of body fat distribution on volumetric computed tomography. J Comput Assist Tomogr 30:777–783 [DOI] [PubMed] [Google Scholar]
- 20.Bredella MA, Utz AL, Torriani M, Thomas B, Schoenfeld DA, Miller KK (2009) Anthropometry, CT, and DXA as predictors of GH deficiency in premenopausal women: ROC curve analysis. J Appl Physiol 106:418–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, Rosen CJ, Klibanski A, Miller KK (2011) Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring) 19:49–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen A, Shen W, Dempster DW et al. (2015) Marrow adiposity assessed on transiliac crest biopsy samples correlates with noninvasive measurement of marrow adiposity by proton magnetic resonance spectroscopy ((1)H-MRS) at the spine but not the femur. Osteoporos Int 26:2471–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28:2–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen A, Kousteni S, Bisikirska B et al. (2017) IGF-1 receptor expression on circulating osteoblast progenitor cells predicts tissue-based bone formation rate and response to teriparatide in premenopausal women with idiopathic osteoporosis. J Bone Miner Res 32:1267–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tollefsen SE, Heath-Monnig E, Cascieri MA, Bayne ML, Daughaday WH (1991) Endogenous insulin-like growth factor (IGF) binding proteins cause IGF-1 resistance in cultured fibroblasts from a patient with short stature. J Clin Invest 87:1241–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inagaki K, Tiulpakov A, Rubtsov P, Sverdlova P, Peterkova V, Yakar S, Terekhov S, LeRoith D (2007) A familial insulin-like growth factor-I receptor mutant leads to short stature: clinical and biochemical characterization. J Clin Endocrinol Metab 92:1542–1548 [DOI] [PubMed] [Google Scholar]
- 27.Walenkamp MJ, Losekoot M, Wit JM (2013) Molecular IGF-1 and IGF-1 receptor defects: from genetics to clinical management. Endocr Dev 24:128–137 [DOI] [PubMed] [Google Scholar]
- 28.Sowers MR, Zheng H, Greendale GA, Neer RM, Cauley JA, Ellis J, Johnson S, Finkelstein JS (2013) Changes in bone resorption across the menopause transition: effects of reproductive hormones, body size, and ethnicity. J Clin Endocrinol Metab 98:2854–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen A, Dempster DW, Recker RR et al. (2013) Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women: a transiliac bone biopsy study. J Clin Endocrinol Metab 98:2562–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen P, Satterwhite JH, Licata AA, Lewiecki EM, Sipos AA, Misurski DM, Wagman RB (2005) Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res 20:962–970 [DOI] [PubMed] [Google Scholar]
- 31.Heaney RP, Watson P (2011) Variability in the measured response of bone to teriparatide. Osteoporos Int 22:1703–1708 [DOI] [PubMed] [Google Scholar]
- 32.Miller PD, Delmas PD, Lindsay R et al. (2008) Early responsiveness of women with osteoporosis to teriparatide after therapy with alendronate or risedronate. J Clin Endocrinol Metab 93:3785–3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brick DJ, Gerweck AV, Meenaghan E, Lawson EA, Misra M, Fazeli P, Johnson W, Klibanski A, Miller KK (2010) Determinants of IGF1 and GH across the weight spectrum: from anorexia nervosa to obesity. Eur J Endocrinol 163:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pijl H, Langendonk JG, Burggraaf J, Frolich M, Cohen AF, Veldhuis JD, Meinders AE (2001) Altered neuroregulation of GH secretion in viscerally obese premenopausal women. J Clin Endocrinol Metab 86:5509–5515 [DOI] [PubMed] [Google Scholar]
- 35.Hock JM, Fonseca J (1990) Anabolic effect of human synthetic parathyroid hormone-(1–34) depends on growth hormone. Endocrinology 127:1804–1810 [DOI] [PubMed] [Google Scholar]
- 36.White HD, Ahmad AM, Durham BH, Peter R, Prabhakar VK, Corlett P, Vora JP, Fraser WD (2007) PTH circadian rhythm and PTH target-organ sensitivity is altered in patients with adult growth hormone deficiency with low BMD. J Bone Miner Res 22:1798–1807 [DOI] [PubMed] [Google Scholar]
- 37.Dunnill MS, Anderson JA, Whitehead R (1967) Quantitative histological studies on age changes in bone. J Pathol Bacteriol 94:275–291 [DOI] [PubMed] [Google Scholar]
- 38.Grey A (2009) Thiazolidinedione-induced skeletal fragility—mechanisms and implications. Diabetes Obes Metab 11:275–284 [DOI] [PubMed] [Google Scholar]
- 39.Meunier P, Aaron J, Edouard C, Vignon G (1971) Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res 80:147–154 [DOI] [PubMed] [Google Scholar]
- 40.Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB (2007) MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int 18:641–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M (2001) Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2:165–171 [DOI] [PubMed] [Google Scholar]
- 42.Verma S, Rajaratnam JH, Denton J, Hoyland JA, Byers RJ (2002) Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol 55:693–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen A, Dempster DW, Stein EM et al. (2012) Increased marrow adiposity in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab 97:2782–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Satterwhite J, Heathman M, Miller PD, Marin F, Glass EV, Dobnig H (2010) Pharmacokinetics of teriparatide (rhPTH[1–34]) and calcium pharmacodynamics in postmenopausal women with osteoporosis. Calcif Tissue Int 87:485–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, Ghomi RH, Rosen CJ, Klibanski A (2009) Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab 94:2129–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fazeli PK, Klibanski A (2014) Bone metabolism in anorexia nervosa. Curr Osteoporos Rep 12:82–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Misra M, Klibanski A (2014) Endocrine consequences of anorexia nervosa. Lancet Diabetes Endocrinol 2:581–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.


