ABSTRACT
Cystic fibrosis transmembrane conductance regulator (CFTR) modulators improve clinical outcomes with varied efficacies in patients with CF. However, the mutual effects of CFTR modulators and bacterial adaptation, together with antibiotic regimens, can influence clinical outcomes. We evaluated the effects of ivacaftor (IVA), lumacaftor (LUM), tezacaftor, elexacaftor, and a three-modulator combination of elexacaftor, tezacaftor, and ivacaftor (ETI), alone or combined with antibiotics, on sequential CF isolates. IVA and ETI showed direct antimicrobial activities against Staphylococcus aureus but not against Pseudomonas aeruginosa. Additive effects or synergies were observed between the CFTR modulators and antibiotics against both species, independently of adaptation to the CF lung. IVA and LUM were the most effective in potentiating antibiotic activity against S. aureus, while IVA and ETI enhanced mainly polymyxin activity against P. aeruginosa. Next, we evaluated the effect of P. aeruginosa pneumonia on the pharmacokinetics of IVA in mice. IVA and its metabolites in plasma, lung, and epithelial lining fluid were increased by P. aeruginosa infection. Thus, CFTR modulators can have direct antimicrobial properties and/or enhance antibiotic activity against initial and adapted S. aureus and P. aeruginosa isolates. Furthermore, bacterial infection impacts airway exposure to IVA, potentially affecting its efficacy. Our findings suggest optimizing host- and pathogen-directed therapies to improve efficacy for personalized treatment.
IMPORTANCE CFTR modulators have been developed to correct and/or enhance CFTR activity in patients with specific cystic fibrosis (CF) genotypes. However, it is of great importance to identify potential off-targets of these novel therapies to understand how they affect lung physiology in CF. Since bacterial infections are one of the hallmarks of CF lung disease, the effects (if any) of CFTR modulators on bacteria could impact their efficacy. This work highlights a mutual interaction between CFTR modulators and opportunistic bacterial infections; in particular, it shows that (i) CFTR modulators have an antibacterial activity per se and influence antibiotic efficacy, and (ii) bacterial airway infections affect levels of CFTR modulators in the airways. These findings may help optimize host- and pathogen-directed drug regimens to improve the efficacy of personalized treatment.
KEYWORDS: cystic fibrosis, CFTR modulator, Pseudomonas aeruginosa, Staphylococcus aureus, antibiotic, murine model, pharmacokinetics
INTRODUCTION
Cystic fibrosis (CF) is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene and affects mostly the lung, but also other organs. Respiratory failure in CF is caused by pulmonary infections and inflammation (1), with Pseudomonas aeruginosa and Staphylococcus aureus being recognized as the most prevalent pathogens. Their chronic persistence and pathogenicity are associated with their adaptation to CF airways (2).
Traditionally, treatments have focused on lessening infection and inflammation of CF disease, although the efficacy of antibiotic therapy is not optimal. Recently, new approaches to correct CFTR cellular misprocessing (correctors) and to restore its channel function (potentiators) have emerged (3). The CFTR potentiator ivacaftor (IVA) was introduced to target gating and other residual function deficits (4–6), while the CFTR correctors lumacaftor (LUM), tezacaftor (TEZ), and elexacaftor (ELX) were approved to correct the F508del-CFTR protein and were recently extended to correct other mutations causing processing and trafficking defects. Since the most common F508del-CFTR requires both correction and potentiation for clinical efficacy, two dual-agent modulators (LUM-IVA and TEZ-IVA) (7–11) and a triple-agent drug (ELX-TEZ-IVA, named ETI) (12) have been approved. Despite the clinical benefits of these treatments, marked variability in CF patient responses has been reported (4, 5, 7, 13). This supports the view that the response to CFTR-directed therapeutics is multifactorial and that CFTR-independent factors may contribute to treatment efficacy, but the mechanisms remain unknown.
There continues to be a substantial gap in our understanding of whether and how CFTR-directed therapies impact the microbiological profile in treated patients (14, 15). IVA treatment showed an initial reduction in the prevalence of bacterial isolation, but P. aeruginosa density rebounded after 1 year (16), and P. aeruginosa strains present before treatment were found to persist even after intensive antibiotic therapy (17). Recent studies based on airway microbial metagenomic sequencing showed that ETI combination therapy reduced the load of P. aeruginosa and S. aureus in both mild and severe CF patients (18). This microbiological shift is attributable to the ETI-mediated gain of CFTR activity and the possible effect on the airway microbiota that is converted into a community of commensals. Furthermore, CFTR modulators could also have a direct effect on major CF pathogens. In vitro studies have shown the anti-S. aureus activity of IVA and its synergy with anti-P. aeruginosa antibiotics (19–24). LUM and TEZ have also been shown to enhance the activity of polymyxin B (PMB) against some P. aeruginosa isolates (22), while the impacts of ELX and ETI remain largely unexplored. However, few data have been reported on the isolates tested in those studies or their origin, particularly on the colonization stage at which they were collected and whether or not they showed adaptation traits to the CF lung. Thus, it is still unclear whether the activities of CFTR modulators differ in CF strains associated with early and advanced stages of lung colonization.
To monitor possible interactions between CFTR modulators and CF pathogens, it is critical to know the drug concentration in the lung, particularly in the epithelial lining fluid (ELF), where bacteria are present (25). Most pharmacokinetic (PK) studies have evaluated the amount of CFTR modulators and their metabolites in the plasma of different hosts (26, 27). Recently, a study evaluated IVA concentration in plasma and nasal cells from CF patients under treatment (28). However, it remains unclear the amounts of CFTR modulators that reach the airway lumen and whether bacterial pneumonia can alter their PK profiles. Indeed, only a few data on human sputum have been reported (29). Considering that most CF patients are likely colonized by bacteria when CFTR modulator therapy is initiated (14), there is a clinical need to assess the impact of infection on drug metabolism to determine the correct dosage. It is recognized that the PK of many compounds can be altered by the presence of infection (30). Recently, it has been shown that TEZ, ELX, and IVA are metabolized by cytochrome P450 3A, whose enzymatic activity is influenced by environmental factors including infection and are sensitive to inhibition and induction (31, 32). So far, the PK of CFTR modulators was assessed in normal, healthy animals, with no additional assessments conducted in the infected models (26, 27), indicating a gap between clinical manifestations of CF disease and preclinical studies. Mouse models are not helpful for efficacy studies, since cross-species comparative studies have highlighted differences between human and mouse CFTR (33). However, they can facilitate PK analysis and provide much-needed knowledge on the interactions between CFTR modulators and CF pathogens while avoiding the variables of human studies.
Thus, we aimed to establish whether and how bacterial adaptation affects the antimicrobial activity of CFTR modulators, including ELX and ETI, and the impact of bacterial infection on PK. We designed the study to (i) determine the antimicrobial activity of CFTR modulators and their additive or synergistic effects with antibiotics against P. aeruginosa and S. aureus reference and clonal isolates collected from CF patients at early and advanced stages of lung colonization; and (ii) evaluate the concentration of IVA, as a model CFTR modulator, in plasma and airway samples and its changes during pneumonia in mice.
RESULTS
IVA and ETI showed the most potent antimicrobial activities against longitudinal S. aureus isolates, while P. aeruginosa isolates were not affected.
To evaluate the impact of bacterial adaptation to the CF lung on the antimicrobial activity of CFTR modulators, we measured minimum inhibitory concentrations (MICs) for clinical clonal isolates collected from CF patients at early and late stages of chronic colonization and previously characterized (see Tables S1 and S2 in the supplemental material) (34–37). We tested single CFTR modulators and the ELX-TEZ-IVA combination, at a ratio of 2:1:1.5, which is used for CF patients (38). IVA showed antimicrobial activity, with MICs ranging from 2 to 8 μg/mL for all the S. aureus isolates (Table 1). The MIC of ELX was 16 μg/mL for almost all S. aureus isolates, except for the J6 isolate (32 μg/mL). By contrast, the MICs of LUM and TEZ were >32 μg/mL, indicating little or no effect against any of the S. aureus isolates. The ELX-TEZ-IVA combination showed antimicrobial activity against all the S. aureus isolates, with MICs ranging from 4/2/3 to 8/4/6 μg/mL. By contrast, all CFTR modulators showed MICs of >32 μg/mL against P. aeruginosa isolates (Table S3). Our results demonstrated that adaptation does not change either the susceptibility of S. aureus strains to IVA and ETI or the resistance of P. aeruginosa to CFTR modulators.
TABLE 1.
MIC90 values for IVA, LUM, TEZ, ELX, and the triple combination ETI against S. aureus isolates collected from CF patients at different stages of colonization (early and late)a
| CFTR modulator | MIC90 (μg/mL) for S. aureus isolate (stage)b |
||||||
|---|---|---|---|---|---|---|---|
| Newman (ref) | A10 (early) | A12 (late) | J6 (early) | J9 (late) | F1 (early) | F5 (late) | |
| IVA | 4 | 2 | 4 | 8 | 8 | 8 | 8 |
| LUM | >32 | >32 | >32 | >32 | >32 | >32 | >32 |
| TEZ | >32 | >32 | >32 | >32 | >32 | >32 | >32 |
| ELX | 16 | 16 | 16 | 32 | 16 | 16 | 16 |
| ELX-TEZ-IVA | 4/2/3 | 4/2/3 | 4/2/3 | 8/4/6 | 8/4/6 | 8/4/6 | 4/2/3 |
S. aureus isolates were grown for 20 h in the presence of serial dilutions of IVA, LUM, TEZ, ELX, or the triple combination of ELX-TEZ-IVA. The concentrations tested ranged from 0.25 μg/mL to 32 μg/mL for IVA, LUM, TEZ, and ELX and from 0.5/0.25/0.375 μg/mL to 64/32/48 μg/mL for ELX-TEZ-IVA combination. The MIC90 was defined as the lowest compound concentration showing a reduction in the optical density at 620 nm of ≈90% in comparison to that for the bacteria grown with the vehicle after 20 h. Each experiment was performed at least two independent times (two technical replicates).
ref, reference strain; early, isolate collected at the early stage of chronic colonization; late, isolate collected after years of persistence, in the advanced stage of chronic colonization.
IVA and LUM potentiated the activities of all antibiotics against S. aureus.
Next, we determined the potential additive or synergistic effects of the CFTR modulators in combination with antibiotics against reference and clinical S. aureus isolates by checkerboard assays. In general, except for TEZ, interaction effects were observed on some isolates regardless of the stage of colonization (Table 2). The fractional inhibitory concentration (FIC) indexes of IVA showed broad-spectrum potentiation of linezolid (LZD) activity (6 out of 7 isolates), while a narrower spectrum was observed for amoxicillin (AMX), vancomycin (VAN), and teicoplanin (TEC). Synergistic effects were also observed between IVA and either VAN or TEC, but only on the Newman reference strain. By contrast, additive effects between either ELX or ETI and the tested antibiotics were more sporadic or completely absent, as in the case of LZD. LUM, which did not show direct antibiotic effects, behaved similarly to IVA in elevating the activities of the tested antibiotics and showed better synergistic effects with VAN or TEC against the Newman strain. These findings indicated that the CFTR modulators, mainly IVA and LUM, can enhance the performance of antibiotics used against S. aureus infections in CF patients. The potentiating effects appeared to be bacterial isolate dependent, but no correlation with the stage of chronic colonization was found.
TABLE 2.
FIC indexes for IVA, LUM, TEZ, ELX, and the triple combination ELX-TEZ-IVA in combination with common antibiotics against S. aureus isolates collected from CF patientsa
| CFTR modulator | Antibiotic | FIC index for S. aureus isolate (stage)b |
||||||
|---|---|---|---|---|---|---|---|---|
| Newman (ref) | A10 (early) | A12 (late) | J6 (early) | J9 (late) | F1 (early) | F5 (late) | ||
| IVA | AMX | 0.5 | 1 | 2 | 1 | 0.5 | 0.625 | 0.625 |
| VAN | 0.31 | 0.625 | 0.625 | 1 | 1 | 1 | 1 | |
| TEC | 0.31 | 1 | 1 | 1 | 0.75 | 1 | 0.75 | |
| LZD | 0.75 | 2 | 0.625 | 0.625 | 0.625 | 0.625 | 0.625 | |
| AZM | 0.531 | 2 | 2 | 2 | 2 | 2 | 2 | |
| LUM | AMX | 0.625 | 0.625 | 2 | 1 | 0.625 | 0.625 | 0.625 |
| VAN | 0.155 | 0.625 | 0.75 | 1 | 1 | 1 | 0.625 | |
| TEC | 0.185 | 0.625 | 0.625 | 2 | 2 | 0.625 | 0.75 | |
| LZD | 0.625 | 2 | 0.625 | 2 | 0.75 | 0.625 | 0.625 | |
| AZM | 0.75 | 1 | 1 | 2 | 2 | 1 | 2 | |
| TEZ | AMX | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| VAN | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| TEC | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| LZD | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| AZM | 2 | 2 | 2 | 2 | 1 | 2 | 2 | |
| ELX | AMX | 2 | 0.5 | 0.75 | 2 | 0.75 | 2 | 2 |
| VAN | 2 | 0.563 | 2 | 2 | 2 | 2 | 2 | |
| TEC | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| LZD | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| AZM | 0.75 | 1 | 1 | 0.75 | 0.75 | 0.5 | 2 | |
| ETI | AMX | 2 | 0.75 | 1 | 2 | 0.75 | 2 | 2 |
| VAN | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| TEC | 2 | 2 | 2 | 0.625 | 2 | 1 | 2 | |
| LZD | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| AZM | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
S. aureus isolates were grown for 20 h in the presence of serial dilutions of IVA, LUM, TEZ, ELX, or the triple combination ETI, along with the antibiotic AMX, VAN, TEC, LZD, or AZM. The concentrations of the CFTR modulators tested ranged from 0.5 to 32 μg/mL for IVA, LUM, and ELX and from 0.5/0.25/0.375 μg/mL to 32/16/24 μg/mL for the triple combination of ELX-TEZ-IVA (ETI), while antibiotics started from a concentration two-fold greater than the MIC90 for the isolate to 32 times below the MIC90 of the antibiotic alone. FIC indexes were calculated as (MIC90 of CFTR modulator in combination/MIC90 of CFTR alone) + (MIC90 of antibiotic in combination/MIC90 of antibiotic alone). Each experiment was performed at least two independent times (two technical replicates).
A FIC index of <0.5 indicated synergy (dark gray shading), a FIC index of ≥0.50 and <1.0 indicated an additive effect (light gray shading), and a FIC index of ≥1.0 and ≤4 indicated indifference (no shading). ref, reference strain; early, isolate collected at the early stage of chronic colonization; late, isolate collected after years of persistence, in the advanced stage of chronic colonization.
IVA and ETI potentiated the activities of only selected antibiotics against P. aeruginosa.
Despite the lack of intrinsic antibacterial activity of the CFTR modulators against P. aeruginosa (Table S3), we tested whether they enhanced the activity of antibiotics (Table 3). Strikingly, IVA was found to strongly enhance the activities of colistin (CST) and PMB against 95.45% of the isolates. Synergy, rather than additivity, was detected for most of the isolates. No effect was observed when IVA was combined with ciprofloxacin (CIP), meropenem (MER), or tobramycin (TOB). LUM, TEZ, and ELX showed only a few cases of additive or synergistic effects with selected antibiotics. For ETI, the FIC values indicated an additive effect on the activities of CST and PMB in several isolates, with synergy against the AA2 isolate reached with both antibiotics. In only two isolates did ETI potentiate the activity of CIP. No CFTR modulators affected azithromycin (AZM) activity on P. aeruginosa isolates. Notably, no negative interaction of the CFTR modulators with the antibiotics was observed. These findings indicated that IVA and ETI can enhance antibiotic activity, particularly that of CST and PMB, against P. aeruginosa in an isolate-dependent manner and independently of the stage of colonization.
TABLE 3.
FIC indexes for IVA, LUM, TEZ, ELX, and ETI in combination with common antibiotics against P. aeruginosa isolates collected from CF patientsa
| CFTR modulator | Antibiotic | FIC index for P. aeruginosa isolate (stage)b |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAO1 (ref) | AA2 (early) | AA43 (late) | AA44 (late) | MF1 (early) | MF51 (late) | KK1 (early) | KK2 (early) | KK71 (late) | KK72 (late) | RP73 (late) | ||
| IVA | CIP | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| MER | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| TOB | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| CST | 0.531 | 0.156 | 0.281 | 0.281 | 0.531 | 0.531 | 2 | 0.531 | 0.312 | 0.281 | 0.281 | |
| PMB | 0.562 | 0.281 | 0.281 | 0.281 | 0.281 | 0.281 | 0.375 | 0.516 | 0.312 | 0.281 | 0.281 | |
| AZM | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| LUM | CIP | 2 | 0.625 | 2 | 0.5 | 0.281 | 0.75 | 0.625 | 1 | 2 | 2 | 2 |
| MER | 2 | 2 | 2 | 0.5 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | |
| TOB | 1 | 2 | 2 | 0.5 | 2 | 2 | 1 | 0.75 | 1 | 1 | 0.75 | |
| CST | 1 | 0.625 | 2 | 2 | 2 | 2 | 2 | 2 | 0.5 | 0.375 | 2 | |
| PMB | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| AZM | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| TEZ | CIP | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| MER | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| TOB | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| CST | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0.75 | 2 | 2 | 2 | |
| PMB | 2 | 0.562 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| AZM | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| ELX | CIP | 2 | 2 | 2 | 2 | 0.687 | 2 | 2 | 2 | 2 | 2 | 2 |
| MER | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | |
| TOB | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| CST | 2 | 0.562 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| PMB | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| AZM | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| ETI | CIP | 2 | 2 | 2 | 2 | 0.438 | 1.25 | 2 | 0.594 | 1.25 | 2 | 2 |
| MER | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| TOB | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| CST | 0.523 | 0.273 | 0.523 | 0.547 | 0.547 | 2 | 2 | 2 | 0.594 | 0.547 | 2 | |
| PMB | 2 | 0.297 | 0.512 | 2 | 2 | 0.547 | 2 | 2 | 0.547 | 0.547 | 2 | |
| AZM | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
P. aeruginosa isolates were grown for 20 h in the presence of serial dilutions of IVA, LUM, TEZ, ELX, or the triple combination of ELX-TEZ-IVA (ETI) along with CIP, MER, CST, TOB, PMB, or AZM. The concentrations of the CFTR modulators tested ranged from 0.5 μg/mL to 32 μg/mL for IVA, LUM, TEZ, and ELX and from 0.5/0.25/0.375 μg/mL to 32/16/24 μg/mL for the triple combination ETI, while those of the antibiotics started from concentrations 2-fold greater than the MIC90 for the isolate to 32 times below the MIC90 of the antibiotic alone. FIC indexes are indicated and were calculated as (MIC90 of CFTR modulator in combination/MIC90 CFTR alone) + (MIC90 antibiotic in combination/MIC90 antibiotic alone). Each experiment was performed at least two independent times (two technical replicates).
A FIC index of <0.5 indicated synergy (dark gray shading), a FIC index of ≥0.50 and <1.0 indicated an additive effect (light gray shading), and a FIC index of ≥1.0 and ≤4 indicated indifference (no shading). ref, reference strain; early, isolate collected at the early stage of chronic colonization; late, isolate collected after years of persistence, in the advanced stage of chronic colonization.
IVA was differentially distributed in plasma, lung, and ELF, with concentrations affected by P. aeruginosa infection.
To establish the impact of bacterial infection on CFTR modulator PK, we exploited the mouse model of P. aeruginosa pneumonia (39) and evaluated IVA concentrations in murine plasma, lung, and ELF. A mouse model of acute infection established with P. aeruginosa reference strain PAO1 was used to mimic early treatment in patients with CF. We selected IVA as the model of a CFTR modulator, since it can be administered as a single compound to patients with CF and it showed the most striking antimicrobial activity in vitro. Mice were administered IVA at a dose approximately reflecting that used in humans, as detailed in Material and Methods. In addition, P. aeruginosa growth is not influenced by IVA and was therefore selected as a pathogenic model, to avoid potential confounding effects. Indeed, we did not observe any difference in the bacterial loads between the lungs of mice treated with IVA and those treated with the vehicle (Fig. S1). We first determined the protein binding of IVA and its metabolites M1 (pharmacologically active) and M6 (inactive) (40). The percentages of protein binding were very high in the plasma, with those of IVA, M1, and M6 reaching ≥99.5% (Table 4). Similar protein binding was observed in the lung for IVA (99.7%) and M1 (97.2%), while that of M6 was lower (80.3%).
TABLE 4.
Percentages of IVA and its metabolites M1 and M6 as protein-bound and free active compounds in murine plasma and lungsa
| Sample | Protein binding (%) |
||
|---|---|---|---|
| IVA | M1 | M6 | |
| Plasma | |||
| Bound fraction | 99.97 | 99.89 | 99.47 |
| Free active fraction | 0.03 | 0.11 | 0.53 |
| Lung | |||
| Bound fraction | 99.74 | 97.17 | 80.3 |
| Free active fraction | 0.26 | 2.83 | 19.7 |
Blood was collected from C57BL/6NCrlBR male mice (8 to 10 weeks of age) and processed to obtain plasma. Lungs were excised, homogenized, and centrifuged, and the supernatants were collected. Protein binding was measured by the dialysis method with rapid equilibrium dialysis inserts, with undiluted plasma or lung homogenate from 2-h samples derived from non-infected untreated mice. Samples were diluted 1:2 in phosphate-buffered saline (PBS) buffer spiked with a solution containing the three analytes against PBS buffer. Samples were analyzed with UPLC-MS/MS in positive multiple-reaction monitoring modes. Values are the means of three replicates.
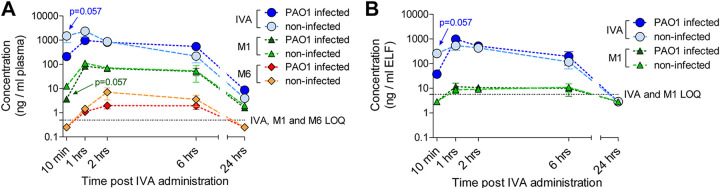
Next, the time-concentration curves of IVA and its metabolites showed that the parent drug accounted for the majority of the total drug, followed by M1 and then M6, in the plasma of both infected and non-infected mice (Fig. 1A). At early time points, IVA and M1 levels were lower in P. aeruginosa-infected mice than in non-infected mice (P = 0.057). However, at later time points, the IVA levels were slightly higher in infected mice than in non-infected mice. M6 levels ranged from low to undetectable in the plasma of all mice. Peak plasma levels of IVA, which were reached 1 h after treatment, were higher in non-infected compared to infected mice (Table 5), as were M1 levels (Table S4). Nonetheless, the area under the curve (AUC) for the period from 10 min (t10) to the last quantifiable concentration (AUClast) and the AUC to infinity (AUCinf) for IVA were higher in P. aeruginosa-infected than non-infected mice, indicating higher exposure in the presence of infection. This was confirmed by the higher last quantifiable concentration (Clast) at 24 h, half-life (t1/2), and mean residence time (MRT) in infected compared to non-infected mice. By contrast, M1 AUC, Clast, t1/2, and MRT values were similar in both groups.
FIG 1.
Ivacaftor, M1, and M6 concentrations in murine plasma and ELF. (A) C57BL/6NCrlBR male mice (8 to 10 weeks of age) were infected with 1 × 106 CFU of P. aeruginosa PAO1 by intratracheal administration. A non-infected control group was also tested in parallel. Thirty minutes after infection, the mice were treated with 3 mg/kg IVA in 10% PEG 400, 10% Tween 80, and 80% saline by intraperitoneal administration. Mice were sacrificed at 10 min and 1, 2, 6, and 24 h after IVA administration. Blood was collected and processed to obtain plasma. IVA, M1, and M6 concentrations in plasma were measured by HPLC-MS/MS. (B) BALF was collected and centrifuged, and the supernatant was used to quantify the IVA concentration. The volume of the ELF was determined by using the ratio of the urea concentration in BALF to that in plasma. The amounts of IVA and M1 were measured by HPLC-MS/MS. Data (derived from 3 to 4 mice) are represented as the mean values ± standard errors of the means (SEMs). Limits of quantification (LOQ) for IVA and its metabolites are indicated. A value corresponding to LOQ/2 was assigned to undetectable samples at specific time points (e.g., 10 min and/or 24 h). Statistical significance was calculated by the Mann-Whitney test comparing the infected and non-infected mouse groups at each time point.
TABLE 5.
Pharmacokinetic parameter estimates for IVA in plasma, ELF, and lungs from infected and non-infected micea
| Sample | Mouse group | Pharmacokinetic parametersb |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| tmax (h) | Cmax (ng/mL) | tlast (h) | Clast (h) | AUClast (h·ng/mL) | AUCinf (h·ng/mL) | t1/2 (h) | MRT (h) | ||
| Plasma | Non-infected | 1 | 2,345 | 24 | 4.0 | 7,482 | 7,498 | 2.9 | 2.9 |
| Infected | 1 | 968 | 24 | 8.6 | 9,111 | 9,152 | 3.3 | 4.7 | |
| ELF | Non-infected | 1 | 537 | 6 | 116.7 | 1,932 | 2,307 | 2.2 | 2.1 |
| Infected | 1 | 981 | 6 | 194.3 | 2,590 | 3,236 | 2.3 | 2.2 | |
| Lung | Non-infected | 1 | 249 | 6 | 68.2 | 946 | nd | nd | 2.2 |
| Infected | 1 | 364 | 24 | 4.4 | 4,010 | 4,033 | 3.7 | 5.2 | |
C57BL/6NCrlBR male mice (8 to 10 weeks of age) were infected with 1 × 106 CFU of P. aeruginosa PAO1 by intratracheal administration. A non-infected control group was also tested in parallel. Thirty minutes after infection, the mice were treated with 3 mg/kg IVA in 10% PEG 400, 10% Tween 80, and 80% saline by intraperitoneal administration. Mice were sacrificed at 10 min and 1, 2, 6, and 24 h after IVA administration. Blood was collected and processed to obtain plasma. BALF was collected and centrifuged, and the supernatant was used to quantify the IVA concentration. Lungs were excised, homogenized, and centrifuged, and the supernatants were used to quantify IVA concentrations. Plasma, lung homogenate, and BALF were added to a Phree phospholipid removal plate (Phenomenex) with acetonitrile and 0.1% formic acid in order to eliminate phospholipids, decreasing the matrix effect. Eluates were analyzed by UPLC-MS/MS with a linear gradient in multiple reaction monitoring positive mode. Calibration ranges were 0.5 to 750 ng/mL in plasma and BALF and 4 to 1,000 ng/g in the lung homogenate. Concentrations of IVA in ELF were determined using the ratio of the urea concentration in plasma to that in BALF: concentration in ELF = (drug concentration in BALF) × (urea in plasma)/(urea in BALF). The data are the geometric means of values from 3 to 4 mice.
tmax, time of maximum concentration; Cmax, maximum concentration; tlast, time of last quantifiable concentration; Clast, last quantifiable concentration; AUClast, AUC to the last quantifiable concentration level; AUCinf, AUC to infinity; t1/2, half-life; MRT, mean residence time; nd, not determined.
The IVA profile in the ELF followed that in the plasma samples up to 6 h, with higher levels in non-infected mice than in infected mice soon after treatment (P = 0.057) (Fig. 1B). P. aeruginosa infection resulted in higher IVA peak levels and AUClast, AUCinf, and Clast levels compared to levels in non-infected mice (Table 5). The partitioning to the ELF was estimated by comparing the AUC of the unbound compound (fAUC) in the plasma to that in the ELF. For the calculation of fAUC, a factor of 0.03% was used for the IVA parent drug in the plasma (Table 4). The ELF/plasma fAUC ratio of IVA was high, indicating that IVA penetrates ELF well (Table 6). M1 was only detected between 1 and 6 h post-administration in ELF of infected and non-infected mice (Fig. 1B). The ELF/plasma fAUC ratio was also high for M1, indicating high penetration in the ELF. M6 was not quantifiable for any conditions.
TABLE 6.
Penetration of IVA in ELF and lungsa
| Mouse group | PK value by sample type |
PK ratiosb |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Plasma |
Lung |
ELF |
Lung/plasma |
ELF/plasma |
||||||
| fCmax (ng/mL) | fAUC (h·ng/mL) | fCmax (ng/mL) | fAUC (h·ng/mL) | Cmax (ng/mL) | AUC (h·ng/mL) | C max | AUC | C max | AUC | |
| Non-infected | 0.70 | 2.20 | 0.65 | 2.44 | 537 | 1,910 | 0.93 | 1.11 | 767 | 868 |
| Infected | 0.29 | 2.72 | 0.94 | 10.62 | 981 | 2,587 | 3.24 | 3.90 | 3,383 | 951 |
The Cmax and AUC of plasma and lung homogenates were corrected for the free fraction according to the results of the protein binding experiment (0.03% for plasma and 0.26% for lung; see Table 4) to obtain the Cmax and AUC of unbound IVA (fCmax and fAUC). Data are the geometric means of values from 3 to 4 mice.
Lung/plasma values are the ratio of lung free maximum concentration or lung exposure to free maximum concentration divided by those in plasma, and ELF/plasma values are the ratio of ELF maximum concentration or ELF exposure to maximum concentration divided by plasma free maximum concentration or plasma exposure to free maximum concentration. fCmax and fAUC refer to unbound IVA in the plasma and lung.
The IVA profile in the lung homogenates showed similar levels in infected and non-infected mice up to 2 h, while the levels were higher in P. aeruginosa-infected mice after 6 h (Fig. S2). The lung/plasma fAUC ratio of IVA indicated that IVA penetrates the lung very well, particularly in infected mice (Table 6). M1 showed a profile in the lung similar to that in the ELF in both infected and non-infected mice (Fig. S2 and Tables S4 and S5). M6 was not quantifiable in either infected or non-infected mice at any time point.
These findings indicated that the IVA parent drug, accounting for the majority of the total drug, readily distributes to the airways and is higher during P. aeruginosa infection.
DISCUSSION
CFTR modulators correct the molecular defect underlying CF and disease manifestations. Since bacterial lung infections or colonization are one of the hallmarks of CF disease, the effect (if any) of CFTR modulators on bacteria could deeply affect the course of the disease. Here, we tested the impact of bacterial adaptation on the antimicrobial activity of CFTR modulators and that of bacterial infection on PK. We found that (i) IVA and the newly licensed ETI had the most potent intrinsic antimicrobial activity against longitudinal S. aureus isolates, while P. aeruginosa isolates were not affected; (ii) IVA and LUM potentiated the activities of all antibiotics against S. aureus, while IVA and ETI potentiated in particular that of polymyxins against P. aeruginosa; (iii) antimicrobial activity of CFTR modulators was independent of the stage of colonization for both bacterial species; and (iv) P. aeruginosa infection affected the distribution of IVA to plasma, lung, and ELF.
Our study, focused on previously characterized clinical clonal isolates collected from CF patients at early and late stages of chronic colonization, showed that IVA has a spectrum of relevant antibacterial activities that spans both early and late isolates of S. aureus. In addition, ETI is active against S. aureus, and its activity seems to be mainly driven by IVA, since the effective concentration of IVA in the triple combination was in the range of that as a single drug. In addition, we showed for the first time that ELX has broad antibacterial activity against S. aureus. Notably, the anti-S. aureus activity of CFTR modulators was independent of the stage of colonization. These activities deserve further investigation with additional biobanks, including those from CF patients under CFTR modulator treatment. Regarding CFTR modulator and antibiotic interactions, we expanded the notion that IVA can increase the activity of several classes of antibiotics against S. aureus. Furthermore, we showed that LUM, with no intrinsic antibacterial activity against S. aureus, can positively interact with several classes of antibiotics. The specific mechanism underlying the enhancement of antibiotic activities by IVA and LUM against S. aureus is unknown and should be investigated.
We observed a different scenario for P. aeruginosa clinical clonal isolates collected from CF patients at early and late stages of chronic colonization and previously characterized. IVA showed no antibacterial activity against any P. aeruginosa early or late isolates, suggesting that adaptation to the CF environment does not induce modification of bacterial structures or functions potentially causing resistance to IVA. This is in line with other reports showing that IVA is inactive against P. aeruginosa and other Gram-negative species (e.g., Klebsiella pneumoniae and Acinetobacter baumannii) (22). Gram-negative bacteria have a formidable outer membrane barrier, particularly against hydrophobic drugs such as CFTR modulators, and this can explain the absence of IVA activity. It was initially hypothesized that IVA inhibits DNA gyrase and topoisomerase IV, since it has a structural resemblance with quinolones. However, it has recently been demonstrated that its antibacterial activity is not due to a quinolone-like mode of action; rather, it inhibits P. aeruginosa cell envelope biogenesis, although this activity was shown only when combined with PMB (24). We also observed that IVA can specifically act in concert with polymyxins, such as CST and PMB, to enhance their antibacterial activities in almost all the isolates tested, while no additive or synergistic effects were observed for the other classes of antibiotics. Similar to IVA, also ETI potentiated the activities of CST and PMB in several isolates. Although the activity of ETI can be driven by IVA, some exceptions have been observed, suggesting potential antagonisms when IVA is combined in the triple combination in an isolate-dependent manner. To our knowledge, while synergies between IVA and antibiotics against S. aureus and P. aeruginosa have been previously described, no data have been provided for next-generation CFTR modulators, such as ELX and ETI. In addition, previous studies on IVA antibacterial activity were mainly performed on uncharacterized bacterial isolates. Our study aimed to clarify whether the stage of chronic colonization could affect the antibacterial activities of CFTR modulators. The finding that adaptation to the CF lung did not change the activity of these small molecules indicates their potential impact on P. aeruginosa and S. aureus during the entire course of colonization.
To further interpret these results, it is critical to know the amounts of CFTR modulators that reach the airway lumen and whether bacterial pneumonia can alter their concentrations. To investigate the PK profile and provide insight into the interactions between CFTR modulators and CF pathogens in the absence of confounding variables of human studies, we exploited a mouse model of P. aeruginosa pneumonia. We focused on the early stage of the disease, since IVA is now administered early in life and it is likely that also ETI will be approved for very early treatment. Our animal experiments were performed with PAO1 reference strain, given that no significant differences have been observed in the phenotypic characteristics and in vivo responses between reference and early P. aeruginosa clinical strains in previous testing (41). P. aeruginosa growth was not influenced by IVA and was therefore selected as a pathogenic model, avoiding potential confounding effects. Our results showed that the parent drug accounted for the majority of the total drug in plasma, lung, and ELF, similar to previous reports focused solely on murine plasma, but different from humans, who show higher concentrations of M1 in plasma (26). Interestingly, exposure of both plasma and airways to IVA was higher in mice with acute P. aeruginosa lung infection than in non-infected mice. In our study, the IVA Cmax in the plasma was in the range of a few micrograms per milliliter. However, the protein binding of IVA and its metabolites was extremely high in both the plasma and lung, indicating that the amount of free active compound was low, in agreement with previous studies (26). For instance, the Cmax of free active IVA in plasma was in the range of approximately 1 ng/mL in our model, similar to the peak free plasma concentration measured in CF patients treated with IVA (27).
When we focused on the airways, IVA showed good penetration into the ELF, reaching higher concentrations than in the plasma. The IVA Cmax was approximately 0.5 to 1 μg/mL in the ELF. Notably, we observed antimicrobial activity or additive or synergistic effects with CST at IVA concentrations ranging from 1 to 8 μg/mL, which were comparable to or moderately higher than those observed in the murine ELF. Schneider and colleagues measured an IVA concentration of 0.15 ± 0.05 μg/mL in the sputum of a CF patient at 2.5 h post-IVA administration (29), which would have excluded antimicrobial activities of CFTR modulators in CF patients. However, data from sputa of single patients cannot be considered conclusive, and IVA quantification in a cohort of patients is needed. In addition, IVA protein binding in airway secretions and how mucus impacts IVA availability should be evaluated, since they could extensively affect the activity. On the other hand, IVA is known to accumulate with repeated doses, particularly when it is taken with a fatty meal (42). Our work analyzed IVA concentrations in mice after administering a single dose, but higher levels would be expected during chronic treatment. In this regard, IVA levels following prolonged treatment in CF patients are unknown. Therefore, the antimicrobial activity of IVA in the airways of CF patients could be plausible and deserve further investigation. In addition, our findings support performing new studies on the potential benefits of pulmonary administration of CST in combination with IVA in treating P. aeruginosa lung infections (43).
Conclusions.
This work supports the interaction of CFTR modulators with opportunistic bacterial infection and antibiotics. Our results underline that CFTR modulators influence antibiotic efficacy through a direct or synergistic effect. This may orient the selection of specific antibiotics to treat infection in combination with CFTR modulators. Importantly, CFTR modulator efficacy is not influenced by specific bacterial phenotypes (early versus late adapted), indicating drug targeting at any stage of colonization. Since the bacterial strains used in this study were collected several years ago, they did not show some of the antibiotic resistances that have emerged in CF clinics in recent years. The challenge ahead is to validate our results with additional biobanks that include longitudinal strains isolated from CF patients under CFTR modulator therapies and with different patterns of antibiotic resistance.
So far, neither animal nor human studies have clarified the impact of infection on concentrations and biodistribution of CFTR modulators. Our results in a mouse model of P. aeruginosa pneumonia underline the importance of testing PK during infection, particularly in the airways. The challenge ahead is to improve these studies in mouse models by comparing initial and adapted bacterial isolates and including additional variables, such as polymicrobial infection and the concomitant use of other therapies, to further reflect the complexity of human diseases. Furthermore, these analyses should be extended to chronic infection mouse models with repeated administrations to determine the impacts both of drug accumulation and lung damage on biodistribution. This would help to optimize the drug regimens and improving their efficacy in the view of personalized treatments.
MATERIALS AND METHODS
Ethics statement.
The animal studies adhered to the Italian Ministry of Health guidelines for the use and care of experimental animals (IACUC 954). The experiments with CF P. aeruginosa and S. aureus isolates and storage of biological materials were approved by the Ethics Commissions of Hannover Medical School and University Hospital Münster (Germany).
Bacterial strains.
P. aeruginosa and S. aureus strains included reference PAO1 and Newman. CF clinical isolates recovered at early and late chronic colonization were RP73, AA2, AA43, AA44, MF1, MF51, KK1, KK2, KK71, and KK72 for P. aeruginosa and A10, A12, J6, J9, F1, and F5 (34–37) for S. aureus. Clinical strains were collected at Hannover Medical School and University Hospital Münster (Germany). Phenotype and antimicrobial resistance were previously characterized (see Tables S1 and S2 in the supplemental material) (34–37).
MIC measurements.
The MICs of CFTR modulators were determined using the broth microdilution susceptibility testing method as described in the supplemental material and elsewhere (34, 44).
Checkerboard assay.
The synergistic activities of the CFTR modulators combined with TOB, CIP, CST, PMB, MER, and AZM for P. aeruginosa and AMX, TEC, LZD, VAN, and AZM for S. aureus were determined by the checkerboard method in cation-adjusted Mueller-Hinton broth (45), as detailed in the supplemental material.
Mouse model.
C57BL/6NCrlBR male mice (8 to 10 weeks of age) were challenged with 1 × 106 colony forming units (CFU) of planktonic strain PAO1 by intratracheal injection. Mice were treated with 3 mg/kg IVA or vehicle (10% polyethylene glycol 400 [PEG 400], 10% Tween 80, and 80% saline) (20) by intraperitoneal injection half an hour after infection, according to the ARRIVE guidelines (46). IVA dose was calculated based on a single adult dose (150 mg) adjusted for mouse weight, assuming that an adult with CF weighs 50 kg (20). Blood, bronchoalveolar lavage fluid (BALF), and lung samples were recovered at different time points, as described elsewhere (39) for PK and as reported in the supplemental material.
Protein binding and concentration.
Protein binding was measured in mouse plasma and lung homogenate by rapid equilibrium dialysis. Samples were analyzed by ultraperformance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). Quantitation was performed via multiple reaction monitoring using the transitions reported in Table S6. Concentrations of IVA and its metabolites in ELF were determined by using the ratio of the urea concentration in BALF to that in plasma (47). Protein binding was expected to be negligible in ELF (47). The drug concentration in ELF was assumed to be equal to the unbound concentration. Additional details are provided in the supplemental material.
PK evaluation.
PK parameters were estimated using PK Solver Excel with a noncompartmental approach consistent with the intraperitoneal route of IVA administration. The AUC was calculated using the linear trapezoidal method for the period from t = 0 to the time of the last quantifiable concentration level (AUC0–last). Evaluation of the terminal elimination phase was not practical, as terminal phase concentration data were either not available (low doses) or sparse.
Statistics.
Statistical analyses were performed with Prism (GraphPad Software, Inc., San Diego, CA, USA) using a nonparametric two-tailed Mann-Whitney U test for compound concentrations and two-way analysis of variance with Bonferroni's multiple-comparison test for CFU. A P value of ≤0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank Marzia Giustra for her invaluable contribution to the in vitro assays and Burkhard Tümmler (Medizinische Hochschule Hannover, Germany) and Barbara C. Kahl (University Hospital Münster, Germany) for supplying, respectively, P. aeruginosa and S. aureus clinical isolates collected from CF patients. This study was supported by the Italian Cystic Fibrosis Research Foundation (FFC 15/2018 to C.C.), with contribution of the Delegazione FFC di Milano and Gruppo di Sostegno FFC di Morbegno, and to A.B. by the Cystic Fibrosis Foundation (BRAGON19G0). G.M. and F.C. have been Italian CF Research Foundation fellows (FFC 15/2018). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conceiving and Designing the Experiments, C.C. and A.B.; Performing Experiments, C.C., R.G., A.C., B.A.-F., G.M., F.C., C.B., and U.B.; Analyzing Data, C.C. and C.B.; Performing Statistical Analysis, C.C.; Interpretation of Experiment Results, C.C., R.G., A.C., B.A.-F., C.B., G.B., and A.B.; Preparing Figures, C.C.; Writing the Manuscript, C.C. and A.B.; Revising the Manuscript, C.C., G.B. and A.B.
Footnotes
Supplemental material is available online only.
Contributor Information
Cristina Cigana, Email: cigana.cristina@hsr.it.
Alessandra Bragonzi, Email: bragonzi.alessandra@hsr.it.
Joanna B. Goldberg, Emory University School of Medicine
REFERENCES
- 1.Gibson RL, Burns JL, Ramsey BW. 2003. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 168:918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 2.Camus L, Briaud P, Vandenesch F, Moreau K. 2021. How bacterial adaptation to cystic fibrosis environment shapes interactions between Pseudomonas aeruginosa and Staphylococcus aureus. Front Microbiol 12:617784. doi: 10.3389/fmicb.2021.617784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shteinberg M, Haq IJ, Polineni D, Davies JC. 2021. Cystic fibrosis. Lancet 397:2195–2211. doi: 10.1016/S0140-6736(20)32542-3. [DOI] [PubMed] [Google Scholar]
- 4.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevinek P, Griese M, McKone EF, Wainwright CE, Konstan MW, Moss R, Ratjen F, Sermet-Gaudelus I, Rowe SM, Dong Q, Rodriguez S, Yen K, Ordonez C, Elborn JS, VX08-770-102 Study Group . 2011. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durmowicz AG, Witzmann KA, Rosebraugh CJ, Chowdhury BA. 2013. Change in sweat chloride as a clinical end point in cystic fibrosis clinical trials: the ivacaftor experience. Chest 143:14–18. doi: 10.1378/chest.12-1430. [DOI] [PubMed] [Google Scholar]
- 6.Millar BC, McCaughan J, Rendall JC, Downey DG, Moore JE. 2018. Pseudomonas aeruginosa in cystic fibrosis patients with c.1652GA (G551D)-CFTR treated with ivacaftor: changes in microbiological parameters. J Clin Pharm Ther 43:92–100. doi: 10.1111/jcpt.12616. [DOI] [PubMed] [Google Scholar]
- 7.Wainwright CE, Elborn JS, Ramsey BW. 2015. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med 373:1783–1784. doi: 10.1056/NEJMc1510466. [DOI] [PubMed] [Google Scholar]
- 8.Konstan MW, McKone EF, Moss RB, Marigowda G, Tian S, Waltz D, Huang X, Lubarsky B, Rubin J, Millar SJ, Pasta DJ, Mayer-Hamblett N, Goss CH, Morgan W, Sawicki GS. 2017. Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): a phase 3, extension study. Lancet Respir Med 5:107–118. doi: 10.1016/S2213-2600(16)30427-1. [DOI] [PubMed] [Google Scholar]
- 9.Rowe SM, Daines C, Ringshausen FC, Kerem E, Wilson J, Tullis E, Nair N, Simard C, Han L, Ingenito EP, McKee C, Lekstrom-Himes J, Davies JC. 2017. Tezacaftor-Ivacaftor in Residual-Function Heterozygotes with Cystic Fibrosis. N Engl J Med 377:2024–2035. doi: 10.1056/NEJMoa1709847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor-Cousar JL, Munck A, McKone EF, van der Ent CK, Moeller A, Simard C, Wang LT, Ingenito EP, McKee C, Lu Y, Lekstrom-Himes J, Elborn JS. 2017. Tezacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med 377:2013–2023. doi: 10.1056/NEJMoa1709846. [DOI] [PubMed] [Google Scholar]
- 11.Donaldson SH, Pilewski JM, Griese M, Cooke J, Viswanathan L, Tullis E, Davies JC, Lekstrom-Himes JA, Wang LT, VX11-661-101 Study Group . 2018. Tezacaftor/ivacaftor in subjects with cystic fibrosis and F508del/F508del-CFTR or F508del/G551D-CFTR. Am J Respir Crit Care Med 197:214–224. doi: 10.1164/rccm.201704-0717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keating D, Marigowda G, Burr L, Daines C, Mall MA, McKone EF, Ramsey BW, Rowe SM, Sass LA, Tullis E, McKee CM, Moskowitz SM, Robertson S, Savage J, Simard C, Van Goor F, Waltz D, Xuan F, Young T, Taylor-Cousar JL, VX16-445-001 Study Group . 2018. VX-445-tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med 379:1612–1620. doi: 10.1056/NEJMoa1807120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, Ramsey BW, Taylor-Cousar JL, Tullis E, Vermeulen F, Marigowda G, McKee CM, Moskowitz SM, Nair N, Savage J, Simard C, Tian S, Waltz D, Xuan F, Rowe SM, Jain R, VX17-445-102 Study Group . 2019. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med 381:1809–1819. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi B, Dalpke AH, Boutin S. 2021. Changes in the cystic fibrosis airway microbiome in response to CFTR modulator therapy. Front Cell Infect Microbiol 11:548613. doi: 10.3389/fcimb.2021.548613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volkova N, Moy K, Evans J, Campbell D, Tian S, Simard C, Higgins M, Konstan MW, Sawicki GS, Elbert A, Charman SC, Marshall BC, Bilton D. 2020. Disease progression in patients with cystic fibrosis treated with ivacaftor: data from national US and UK registries. J Cyst Fibros 19:68–79. doi: 10.1016/j.jcf.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Hisert KB, Heltshe SL, Pope C, Jorth P, Wu X, Edwards RM, Radey M, Accurso FJ, Wolter DJ, Cooke G, Adam RJ, Carter S, Grogan B, Launspach JL, Donnelly SC, Gallagher CG, Bruce JE, Stoltz DA, Welsh MJ, Hoffman LR, McKone EF, Singh PK. 2017. Restoring cystic fibrosis transmembrane conductance regulator function reduces airway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am J Respir Crit Care Med 195:1617–1628. doi: 10.1164/rccm.201609-1954OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durfey SL, Pipavath S, Li A, Vo AT, Ratjen A, Carter S, Morgan SJ, Radey MC, Grogan B, Salipante SJ, Welsh MJ, Stoltz DA, Goss CH, McKone EF, Singh PK. 2021. Combining ivacaftor and intensive antibiotics achieves limited clearance of cystic fibrosis infections. mBio 12:e0314821. doi: 10.1128/mbio.03148-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pallenberg ST, Pust MM, Rosenboom I, Hansen G, Wiehlmann L, Dittrich AM, Tummler B. 2022. Impact of elexacaftor/tezacaftor/ivacaftor therapy on the cystic fibrosis airway microbial metagenome. Microbiol Spectr 10:e0145422. doi: 10.1128/spectrum.01454-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reznikov LR, Abou Alaiwa MH, Dohrn CL, Gansemer ND, Diekema DJ, Stoltz DA, Welsh MJ. 2014. Antibacterial properties of the CFTR potentiator ivacaftor. J Cyst Fibros 13:515–519. doi: 10.1016/j.jcf.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Payne JE, Dubois AV, Ingram RJ, Weldon S, Taggart CC, Elborn JS, Tunney MM. 2017. Activity of innate antimicrobial peptides and ivacaftor against clinical cystic fibrosis respiratory pathogens. Int J Antimicrob Agents 50:427–435. doi: 10.1016/j.ijantimicag.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Cho DY, Lim DJ, Mackey C, Skinner D, Zhang S, McCormick J, Woodworth BA. 2019. Ivacaftor, a cystic fibrosis transmembrane conductance regulator potentiator, enhances ciprofloxacin activity against Pseudomonas aeruginosa. Am J Rhinol Allergy 33:129–136. doi: 10.1177/1945892418815615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider EK, Azad MAK, Han M-L, Tony Zhou Q, Wang J, Huang JX, Cooper MA, Doi Y, Baker MA, Bergen PJ, Muller MT, Li J, Velkov T. 2016. An “unlikely” pair: the antimicrobial synergy of polymyxin B in combination with the cystic fibrosis transmembrane conductance regulator drugs KALYDECO and ORKAMBI. ACS Infect Dis 2:478–488. doi: 10.1021/acsinfecdis.6b00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thakare R, Singh AK, Das S, Vasudevan N, Jachak GR, Reddy DS, Dasgupta A, Chopra S. 2017. Repurposing ivacaftor for treatment of Staphylococcus aureus infections. Int J Antimicrob Agents 50:389–392. doi: 10.1016/j.ijantimicag.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Allobawi R, Ghelani DP, Schneider-Futschik EK. 2020. Metabolomic description of ivacaftor elevating polymyxin B mediated antibacterial activity in cystic fibrosis Pseudomonas aeruginosa. ACS Pharmacol Transl Sci 3:433–443. doi: 10.1021/acsptsci.0c00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodvold KA, George JM, Yoo L. 2011. Penetration of anti-infective agents into pulmonary epithelial lining fluid: focus on antibacterial agents. Clin Pharmacokinet 50:637–664. doi: 10.2165/11594090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Food and Drug Administration. 2012. Ivacaftor NDA 202188, clinical inspection summary, access data. FDA:203188Orif1s000. FDA, Silver Spring, MD. [Google Scholar]
- 27.Matthes E, Goepp J, Carlile GW, Luo Y, Dejgaard K, Billet A, Robert R, Thomas DY, Hanrahan JW. 2016. Low free drug concentration prevents inhibition of F508del CFTR functional expression by the potentiator VX-770 (ivacaftor). Br J Pharmacol 173:459–470. doi: 10.1111/bph.13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guimbellot JS, Ryan KJ, Anderson JD, Liu Z, Kersh L, Esther CR, Rowe SM, Acosta EP. 2020. Variable cellular ivacaftor concentrations in people with cystic fibrosis on modulator therapy. J Cyst Fibros 19:742–745. doi: 10.1016/j.jcf.2020.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider EK, Reyes-Ortega F, Wilson JW, Kotsimbos T, Keating D, Li J, Velkov T. 2016. Development of HPLC and LC-MS/MS methods for the analysis of ivacaftor, its major metabolites and lumacaftor in plasma and sputum of cystic fibrosis patients treated with ORKAMBI or KALYDECO. J Chromatogr B Analyt Technol Biomed Life Sci 1038:57–62. doi: 10.1016/j.jchromb.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez MN, Greene J, Kenna L, Kissell L, Kuhn M. 2020. The impact of infection and inflammation on drug metabolism, active transport, and systemic drug concentrations in veterinary species. Drug Metab Dispos 48:631–644. doi: 10.1124/dmd.120.090704. [DOI] [PubMed] [Google Scholar]
- 31.Choong E, Sauty A, Koutsokera A, Blanchon S, Andre P, Decosterd L. 2022. Therapeutic drug monitoring of ivacaftor, lumacaftor, tezacaftor, and elexacaftor in cystic fibrosis: where are we now? Pharmaceutics 14:1674. doi: 10.3390/pharmaceutics14081674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stavropoulou E, Pircalabioru GG, Bezirtzoglou E. 2018. The role of cytochromes P450 in infection. Front Immunol 9:89. doi: 10.3389/fimmu.2018.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bose SJ, Bijvelds MJC, Wang Y, Liu J, Cai Z, Bot AGM, de Jonge HR, Sheppard DN. 2019. Differential thermostability and response to cystic fibrosis transmembrane conductance regulator potentiators of human and mouse F508del-CFTR. Am J Physiol Lung Cell Mol Physiol 317:L71–L86. doi: 10.1152/ajplung.00034.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cigana C, Bernardini F, Facchini M, Alcala-Franco B, Riva C, De Fino I, Rossi A, Ranucci S, Misson P, Chevalier E, Brodmann M, Schmitt M, Wach A, Dale GE, Obrecht D, Bragonzi A. 2016. Efficacy of the novel antibiotic POL7001 in preclinical models of Pseudomonas aeruginosa pneumonia. Antimicrob Agents Chemother 60:4991–5000. doi: 10.1128/AAC.00390-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirschhausen N, Block D, Bianconi I, Bragonzi A, Birtel J, Lee JC, Dubbers A, Kuster P, Kahl J, Peters G, Kahl BC. 2013. Extended Staphylococcus aureus persistence in cystic fibrosis is associated with bacterial adaptation. Int J Med Microbiol 303:685–692. doi: 10.1016/j.ijmm.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Lore NI, Cigana C, De Fino I, Riva C, Juhas M, Schwager S, Eberl L, Bragonzi A. 2012. Cystic fibrosis-niche adaptation of Pseudomonas aeruginosa reduces virulence in multiple infection hosts. PLoS One 7:e35648. doi: 10.1371/journal.pone.0035648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bianconi I, Jeukens J, Freschi L, Alcala-Franco B, Facchini M, Boyle B, Molinaro A, Kukavica-Ibrulj I, Tummler B, Levesque RC, Bragonzi A. 2015. Comparative genomics and biological characterization of sequential Pseudomonas aeruginosa isolates from persistent airways infection. BMC Genomics 16:1105. doi: 10.1186/s12864-015-2276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoy SM. 2019. Elexacaftor/ivacaftor/tezacaftor: first approval. Drugs 79:2001–2007. doi: 10.1007/s40265-019-01233-7. [DOI] [PubMed] [Google Scholar]
- 39.Cigana C, Ranucci S, Rossi A, De Fino I, Melessike M, Bragonzi A. 2020. Antibiotic efficacy varies based on the infection model and treatment regimen for Pseudomonas aeruginosa. Eur Respir J 55:1802456. doi: 10.1183/13993003.02456-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Condren ME, Bradshaw MD. 2013. Ivacaftor: a novel gene-based therapeutic approach for cystic fibrosis. J Pediatr Pharmacol Ther 18:8–13. doi: 10.5863/1551-6776-18.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bragonzi A, Paroni M, Nonis A, Cramer N, Montanari S, Rejman J, Di Serio C, Doring G, Tummler B. 2009. Pseudomonas aeruginosa microevolution during cystic fibrosis lung infection establishes clones with adapted virulence. Am J Respir Crit Care Med 180:138–145. doi: 10.1164/rccm.200812-1943OC. [DOI] [PubMed] [Google Scholar]
- 42.Vertex Pharmaceuticals Incorporated. 2020. Kalydeco - Highlights of prescribing information. https://pi.vrtx.com/files/uspi_ivacaftor.pdf.
- 43.Chen J, Ahmed MU, Zhu C, Yu S, Pan W, Velkov T, Li J, Tony Zhou Q. 2021. In vitro evaluation of drug delivery behavior for inhalable amorphous nanoparticle formulations in a human lung epithelial cell model. Int J Pharm 596:120211. doi: 10.1016/j.ijpharm.2021.120211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.CLSI. 2014. Performance standards for antimicrobial susceptibility testing; 24th informational supplement. CLSI M100-S24. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 45.Sousa MGC, Xavier PD, Cantuaria APC, Porcino RA, Almeida JA, Franco OL, Rezende TMB. 2021. Host defense peptide IDR-1002 associated with ciprofloxacin as a new antimicrobial and immunomodulatory strategy for dental pulp revascularization therapy. Microb Pathog 152:104634. doi: 10.1016/j.micpath.2020.104634. [DOI] [PubMed] [Google Scholar]
- 46.Percie Du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Wurbel H. 2020. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol 18:e3000410. doi: 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Melchers MJ, Teague J, Warn P, Hansen J, Bernardini F, Wach A, Obrecht D, Dale GE, Mouton JW. 2019. Pharmacokinetics and pharmacodynamics of murepavadin in neutropenic mouse models. Antimicrob Agents Chemother 63. doi: 10.1128/AAC.01699-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download spectrum.04083-22-s0001.pdf, PDF file, 0.2 MB (256.3KB, pdf)