Case
A mother consults a pediatrician about problems she is having with her 6-year-old son. He is always fidgeting, squirming in his seat and moving constantly. He has difficulty waiting his turn when playing with other children and constantly interrupts his parents and teacher. She feels that these behaviours are caused by his high consumption of sugar: he loves candies and sugary soft drinks.
There are many popular beliefs about the effects of diet on mood and behaviour. No one doubts that food ingestion can influence mood and behaviour, but the mechanisms by which this happens are not fully understood. For example, who has not heard the statement “I'm feeling hypoglycemic”? What this statement probably means is that the person saying it feels somewhat tired and irritable and knows that she or he will feel better after eating. In fact, these “hypoglycemic” states are not usually accompanied by hypoglycemia1 and the cause of the initial state is unknown, as is the way in which food ingestion alleviates it. The acute behavioural effects of food can be striking. Perhaps the most dramatic effect is in the neonate. The transformation of an infant from crying and irritability (a state also not accompanied by hypoglycemia) to postprandial contentment and responsiveness that delights its parents can be dramatic.2 If a drug had a similar effect, it would be considered powerful. As with “hypoglycemia,” this behavioural effect is mediated by a change in brain function whose mechanism is not understood. Indeed, several different mechanisms probably operate, because the simple act of ingestion (for example, of water), the ingestion of nutrients and the ingestion of different macronutrients all have somewhat different effects on the state of neonates.2 Although the mechanisms that operate to influence various aspects of brain function after meal ingestion are mysterious, there is increasing understanding of how specific nutrients can influence both the brain and behaviour.
What is a food and what is a drug?
The behavioural effects of nutrients create problems for the legal regulation of foods and drugs. There is no ambiguity about the status of a snack eaten to relieve feelings of irritability or a cup of coffee taken to relieve tiredness, but problems start once nutrients are used in their purified form. The Canadian Food and Drugs Act3 defines food as “any article manufactured, sold or represented for use as food or drink for human beings” including chewing gum and “any ingredient that may be mixed with food for any purpose whatever.” The definition of a drug includes the statement that a drug is “any substance or mixture of substances manufactured, sold or represented for use in (a) the diagnosis, treatment, mitigation or prevention of a disease, disorder or abnormal physical state, or its symptoms, in human beings or animals, (b) restoring, correcting or modifying organic functions in human beings or animals.” These definitions rely more on what foods or drugs are sold for than what they are used for. This explains why a cup of coffee taken to increase arousal is a food, whereas a tablet of caffeine, containing the same amount of caffeine and taken for the same purpose, is a drug. In the United States, many natural compounds are treated as dietary supplements as long as the manufacturer makes no claims for their effects. Sales of these compounds depend on the fact that information about the effects of dietary supplements (whether true, unsubstantiated or false) is widely available in print and on the Web.
Tryptophan, serotonin, mood and aggression
The amino acid L-tryptophan is a dietary component that has been used in its purified form for many decades. Although at one time it was sold in Canada as a dietary supplement, it has been regulated as a drug since 1986. In the United States, it is a food supplement. Tryptophan is the precursor of the neurotransmitter serotonin. Its administration increases brain levels of tryptophan and may increase brain serotonin levels. Tryptophan seems to have an antidepressant effect in mild-to-moderate clinical depression, although it is probably not as effective as standard antidepressants in treating severe depression.4 Tryptophan decreases sleep latency in people with mild insomnia and has been used successfully to treat some types of pain. Both of these effects could result from increased brain serotonin. Although it is not as effective a hypnotic as the benzodiazepines, tryptophan preserves normal sleep architecture when administered in low doses (e.g., 1 g) and it does not cause dependence. Low brain serotonin may be associated with aggressive behaviour, and 2 studies suggest that tryptophan has some therapeutic effect in pathologically aggressive patients.5,6 Tryptophan may also be better than placebo in the treatment of premenstrual dysphoric disorder (premenstrual syndrome),7 which can be associated with moodiness and irritability.
The therapeutic action of tryptophan raises the question of the possible action of foods containing tryptophan. Will a bedtime glass of milk have a hypnotic effect because of its tryptophan content? The answer is no. Tryptophan is taken up into the brain by a transport system that it shares with all the other large neutral amino acids, and there is competition among them for entry into the brain. Because tryptophan is the least abundant amino acid in proteins, competition from the other large neutral amino acids will prevent any rise in brain tryptophan after ingestion of a meal containing protein.8
The dependence of brain serotonin on precursor availability has been demonstrated using the acute tryptophan depletion technique. Subjects ingest a mixture of amino acids lacking tryptophan. This induces protein synthesis, and circulating tryptophan levels fall as trytophan is incorporated into protein. Five hours after ingestion of the amino acid mixture, plasma tryptophan levels are only 10%–20% of control levels. This is accompanied by a marked decline in the synthesis of serotonin in the brain.9 Acute tryptophan depletion can produce a temporary lowering of mood in susceptible individuals, sometimes causing a full depressive symptom pattern in formerly depressed patients. The symptoms reverse as soon as tryptophan levels are restored. The tryptophan depletion technique can also elicit increased aggression in laboratory tests of aggression.10 These results suggest an involvement of low brain serotonin in the causes of depression and aggression. Although this use of dietary components in biological psychiatry research is artificial, and could not be duplicated by physiological changes in the diet, it illustrates clearly the potential of dietary components to cause dramatic changes in mood and behaviour.
S-adenosylmethionine, folic acid and mood
S-adenosylmethionine (SAMe) is another compound that is regulated as a dietary supplement in the United States but as a drug in Canada. It is one of the major methyl donors in animal metabolism and is a dietary constituent, although at very low levels. A number of placebo-controlled trials indicate that, when administered in pharmacological doses (up to 1 g/day), SAMe is an antidepressant and its use is relatively free of side effects.11 The metabolic precursor of SAMe is the essential amino acid L-methionine, and administration of methionine to rats raises levels of SAMe in the brain.12 This raises the possibility, still to be tested, that methionine could itself be an antidepressant. When given in very large doses (in the range of 10 g/day), methionine is known to exacerbate the symptoms of schizophrenia,13 and this may have prevented its consideration for use in more moderate doses to treat other conditions.
When SAMe donates its methyl group, the molecule is regenerated in a cycle that includes homocysteine and methionine. The methyl groups involved in this regeneration cycle come from serine via a separate demethylation– remethylation cycle that involves the B vitamin folic acid (Fig. 1). Folate deficiency can induce a decline in levels of SAMe in the brain and, moreover, this is associated with clinical depression in some patients.14 Depressed patients who are folate deficient recover faster when they receive folate supplements.15 Depression is commonly accompanied by anorexia, which can lead to folate deficiency, intensifying the depression and delaying recovery because of lowered levels of SAMe in the brain. Indeed, as many as 30% of psychiatric patients may be folate deficient.14 However, there is no reason to think that folate would enhance recovery in patients who are not folate deficient, because there is no evidence that pharmacological doses of folate will raise SAMe levels in the absence of folate deficiency.
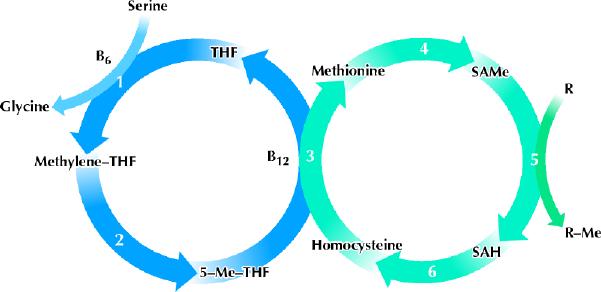
Fig. 1: In the left cycle, folate takes methyl groups from serine to form glycine and then uses them to methylate homocysteine, forming methionine in the right cycle. Methionine is the precursor of SAMe, a methyl donor that is involved in many methylation reactions. THF = tetrahydrofolate, Me = methyl group, SAMe = S-adenosylmethionine, SAH = S-adenosylhomocysteine, R = any molecule that can be methylated by SAMe. The enzymes involved in the metabolic pathways are (1) serine hydroxymethyltransferase (a pyridoxal phosphate–dependent enzyme), (2) methylenetetrahydrofolate reductase, (3) methionine synthase, also known as 5-methyltetrahydrofolate homocysteine methyltransferase (a cobalamin-dependent enzyme), (4) methionine adenosyltransferase, (5) a variety of SAMe-dependent methyltransferase enzymes, (6) adenosylhomocysteine hydrolase. Photo by: Lianne Friesen and Nicholas Woolridge
There is a connection between SAMe and serotonin, because folate deficiency can decrease serotonin synthesis in rats and humans, whereas SAMe can increase serotonin synthesis in both rats and humans.14 The mechanism of the link between SAMe and serotonin is unknown. SAMe is involved in over 35 methylation reactions,16 so the possibilities are many.
Carbohydrate, serotonin, acetylcholine, behaviour and memory
Although tryptophan and SAMe are among the most popular psychopharmacological drugs that are also dietary components, the dietary constituent that is most often associated with changes in mood and behaviour is undoubtedly carbohydrate. Carbohydrate may exert its mood-altering effects through a variety of mechanisms. One of these occurs before absorption and is seen only with the sugars. In human neonates, as in rats, a few drops of sweet solution placed on the anterior tongue have an analgesic effect that persists for several minutes beyond the perception of sweetness; this effect may be mediated via opioid receptors.17 Application of a sucrose solution to the tongue is used increasingly for the relief of procedural pain in human neonates.18
Most of the effects of carbohydrate on the brain are caused by mechanisms that operate after the carbohydrate has been absorbed in the gastrointestinal tract. Unlike the brief preabsorptive effects of sweet taste, these postabsorptive effects can last as long as the metabolic effects of carbohydrate persist. Popular beliefs about the effects of carbohydrate are very different from the information gleaned from controlled studies. Any discussion of the behavioural effects of food has to deal with the persistent myth that sugar causes hyperactivity in children. More than 20 short-term, placebo-controlled studies in a variety of settings failed to find any adverse effects of sugar ingestion, even in children identified by their parents as reacting adversely to sugar.19 Although these studies could be criticized as being too short in duration, a well-designed and extensive study lasting several weeks also found no adverse behavioural effects of sugar.20 This does not mean that an individual child will not respond with improved behaviour when sugar is removed from his or her diet. If the expectation of the parents and the child is that the child's behaviour will improve, and if the parents increase their involvement in the life of the child to the extent that they are aware of everything that the child ingests, an improvement in behaviour is quite possible. This may account for the many anecdotal reports of behavioural improvements accompanying the removal of sugars from the diet, and this may also explain the persistence of the idea that sugar causes hyperactivity despite convincing evidence to the contrary. Thus, although sugar does not cause hyperactivity, if the mother in the case described at the beginning of this article were to limit the sugar intake of her son, it might improve not only his nutritional intake but also his behaviour.
Although sucrose does not cause hyperactivity, controlled studies comparing different macronutrients have demonstrated effects on the brain of both simple and complex carbohydrates. The effects appear to be sedating rather than activating. Depending on the age and sex of the subject, carbohydrate can cause increased drowsiness or calmness.21 Drowsiness is also related to the amount of food energy ingested. A generous 4000-kJ (956-kcal) lunch will produce greater drowsiness than a more modest 1200-kJ (287-kcal) lunch, perhaps explaining the phenomenon known to ergonomists as the “post-lunch dip” in performance.22 A decline in human performance in the early afternoon can be demonstrated using a variety of measures, including as dramatic a one as falling asleep while at the wheel of a car.22
The sedating effect of carbohydrate has been attributed to stimulation of brain serotonin synthesis.23 According to this hypothesis, carbohydrate ingestion stimulates insulin release, and this in turn increases the branched-chain amino acid uptake into muscle. Because tryptophan competes with these amino acids for transport into the brain, their removal from the circulation enhances the uptake of tryptophan into the brain, increasing brain serotonin synthesis. Such a mechanism certainly operates in rats ingesting carbohydrate, but it probably does not account for changes in mood or behaviour related to the ingestion of real foods by humans. First, rats ingest a much higher proportion of their body weight as food than humans, and they have much greater fluctuations in plasma amino acid concentrations than humans do. Any increase in serotonin synthesis in the human brain after carbohydrate ingestion is too small to be detected by changes in the level of the serotonin metabolite 5-hydroxyindoleacetic acid in cerebrospinal fluid.24 Second, whereas ingestion of pure carbohydrate may tend to increase levels of serotonin in the brain, ingestion of carbohydrate with protein will counteract the effect of carbohydrate on serotonin. This is because, as discussed above, protein contains other large neutral amino acids that tend to inhibit the uptake of tryptophan into the brain. Amounts of protein as small as 4% in a carbohydrate meal can prevent the increase in levels of tryptophan in the brain.25 No real meals contain as little protein as this. Thus, the persistent myth that carbohydrate intake can improve mood and even decrease satiety, thereby helping weight loss because of its ability to increase serotonin, has no basis in fact.
Acetylcholine is another neurotransmitter associated with carbohydrate. In rats, glucose ingestion enhances the release of acetylcholine in the brain26 and also enhances memory. The memory-enhancing effect of glucose is also well established in humans,27 and this may in part account for the enhanced cognitive function of schoolchildren who eat breakfast.28 Glucose can also attenuate some of the effects of acute nicotine withdrawal, presumably by stimulating cholinergic pathways.29 Whether an enhancement of acetylcholine transmission is associated with other mental effects associated with carbohydrate ingestion is not known. Nor, indeed, is it known how glucose ingestion stimulates acetylcholine release.
Another nutrient-based strategy that has been used to enhance memory is raising the levels of the acetylcholine precursor choline, by ingestion of either choline or of lecithin (phosphatidylcholine).30 Small memory enhancements are only occasionally seen and usually at much higher doses of choline or lecithin than are present in over-the-counter formulations.
Fats and the brain
Fat is the macronutrient that best illustrates the expression “you are what you eat.” The lipid composition of the human brain in part reflects the dietary intake of different lipids.31 Rats fed diets containing different fats have different brain lipid contents and demonstrate differences in cognition and behaviour after only a few weeks on test diets.32 The mechanism that brings about these changes in brain function is not known, but it presumably involves changes in the properties of neuronal cell membranes and, therefore, changes in the receptors embedded in the membranes. Different human populations have widely different fat intakes, but to what extent any differences in behaviour and cognition that may occur in populations with widely differing diets regarding fat types and contents (such as the traditional Inuit or Hindu diets, or the current North American diet) are caused by different brain lipid content is entirely a matter for speculation.
Several studies have looked at the effects of supplements of omega-3 fatty acids. In a preliminary study, 9.6 g per day of omega-3 fatty acids helped to stabilize the condition of patients with bipolar affective disorder.33 A lower dose decreased aggression in students stressed by taking final examinations.34 Conflicting results have been obtained in 4 studies of patients with schizophrenia.35
Conclusion
Many of the popular beliefs about the effect of foods on mood and behaviour are incorrect. These include the postulated adverse effects of sugar on behaviour in children and the mood-elevating effect of carbohydrate because of its ability to increase brain serotonin. On the other hand, some beneficial effects of foods are perhaps not sufficiently appreciated. These include the analgesic effect of sweet taste in infants and the ability of food to enhance memory. Various dietary components such are tryptophan and SAMe are treated as drugs in Canada, but they are available over the counter in the United States where some Canadians buy their supplies of these drugs. Although dietary components used as drugs are relatively safe, as is the case with other drugs they can produce side effects and can interact with other drugs. Nonetheless, they may be perceived by those taking them as “natural” compounds.
Articles to date in this series .
Hoffer LJ. Clinical nutrition: 1. Protein–energy malnutrition in the inpatient. CMAJ 2001;165(10):1345-9.
Atkinson SA, Ward WE. Clinical nutrition: 2. The role of nutrition in the prevention and treatment of adult osteoporosis. CMAJ 2001;165(11):1511-4.
Footnotes
This series is supported, in part, by an unrestricted educational grant from the Danone Institute of Canada.
This article has been peer reviewed.
Competing interests: Dr. Young has received an honorarium for speaking from ICN Canada who market tryptophan.
Correspondence to: Dr. Simon Young, Department of Psychiatry, McGill University, 1033 Pine Ave. W, Montreal QC H3A 1A1; fax 514 398-4370; syoung@med.mcgill.ca
References
- 1.Palardy J, Havrankova J, Lepage R, Matte R, Belanger R, D'Amour P, et al. Blood glucose measurements during symptomatic episodes in patients with suspected postprandial hypoglycemia. N Engl J Med 1989;321(21):1421-5. [DOI] [PubMed]
- 2.Oberlander TF, Barr RG, Young SN, Brian JA. Short-term effects of feed composition on sleeping and crying in newborns. Pediatrics 1992;90:733-40. [PubMed]
- 3.Food and Drugs Act, R.S.C. 1985, c.F-27, s.2. Available: http://lois.justice.gc.ca/en/F-27/52692.html (updated 2001 Apr 30) (accessed 2001 Dec 10).
- 4.Young SN. The clinical psychopharmacology of tryptophan. In: Wurtman RJ, Wurtman JJ, editors. Food constituents affecting normal and abnormal behaviors. vol 7 of Nutrition and the brain series. New York: Raven Press; 1986. p. 49-88.
- 5.Morand C, Young SN, Ervin FR. Clinical response of aggressive schizophrenics to oral tryptophan. Biol Psychiatry 1983;18:575-8. [PubMed]
- 6.Volavka J, Crowner M, Brizer D, Convit A, van Praag HM, Suckow RF. Tryptophan treatment of aggressive psychiatric inpatients. Biol Psychiatry 1990;28:728-32. [DOI] [PubMed]
- 7.Steinberg S, Annable L, Young SN, Liyanage N. A placebo-controlled clinical trial of L-tryptophan in premenstrual dysphoria. Biol Psychiatry 1999; 45: 313-20. [DOI] [PubMed]
- 8.Wurtman RJ, Hefti F, Melamed E. Precursor control of neurotransmitter synthesis. Pharmacol Rev 1981;32:315-35. [PubMed]
- 9.Nishizawa S, Benkelfat C, Young SN, Leyton M, Mzengeza S, de Montigny C, et al. Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci U S A 1997;94(10):5308-13. [DOI] [PMC free article] [PubMed]
- 10.Moore P, Landolt HP, Seifritz E, Clark C, Bhatti T, Kelsoe J, et al. Clinical and physiological consequences of rapid tryptophan depletion. Neuropsychopharmacology 2000;23(6):601-22. [DOI] [PubMed]
- 11.Bressa GM. S-Adenosyl-L-methionine (SAMe) as antidepressant: meta-analysis of clinical studies. Acta Neurol Scand 1994;89:7-14. [DOI] [PubMed]
- 12.Rubin RA, Ordonez LA, Wurtman RJ. Physiological dependence of brain methionine and S-adenosylmethionine concentrations on serum amino acid pattern. J Neurochem 1974;23:227-31. [DOI] [PubMed]
- 13.Cohen SM, Nichols A, Wyatt R, Pollin W. The administration of methionine to chronic schizophrenic patients: a review of ten studies. Biol Psychiatry 1974; 8: 209-25. [PubMed]
- 14.Young SN, Ghadirian AM. Folic acid and psychopathology. Prog Neuropsychopharmacol Biol Psychiatry 1989;13:841-63. [DOI] [PubMed]
- 15.Godfrey PS, Toone BK, Carney MW, Flynn TG, Bottiglieri T, Laundy M, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet 1990;336(8712):392-5. [DOI] [PubMed]
- 16.Bottiglieri T, Hyland K. S-Adenosylmethionine levels in psychiatric and neurological disorders: a review. Acta Neurol Scand 1994;89:19-26. [DOI] [PubMed]
- 17.Barr RG, Young SN. A two phase model of the soothing taste response: implications for a taste probe of temperament and emotion regulation. In: Lewis M, Ramsay D, editors. Soothing and stress. Mahwah (NJ): Lawrence Erlbaum Associates; 1999. p. 109-37.
- 18.Stevens B, Taddio A, Ohlsson A, Einarson T. The efficacy of sucrose for relieving procedural pain in neonates: a systematic review and meta-analysis. Acta Paediatr 1997;86:837-42. [DOI] [PubMed]
- 19.Wolraich ML, Wilson DB, White JW. The effect of sugar on behavior or cognition in children: a meta-analysis. JAMA 1995;274:1617-21. [DOI] [PubMed]
- 20.Wolraich ML, Lindgren SD, Stumbo PJ, Stegink LD, Appelbaum MI, Kiritsy MC. Effects of diets high in sucrose or aspartame on the behavior and cognitive performance of children. N Engl J Med 1994;330:301-7. [DOI] [PubMed]
- 21.Spring BJ, Lieberman HR, Swope G, Garfield GS. Effects of carbohydrates on mood and behavior. Nutr Rev 1986;44:51-60. [DOI] [PubMed]
- 22.Craig A. Acute effects of meals on perceptual and cognitive efficiency. Nutr Rev 1986;44(Suppl):163-71. [DOI] [PubMed]
- 23.Wurtman RJ, Wurtman JJ. Brain serotonin, carbohydrate-craving, obesity and depression. Obes Res 1995;3:S477-80. [DOI] [PubMed]
- 24.Teff KL, Young SN, Marchand L, Botez MI. Acute effect of protein and carbohydrate breakfasts on human cerebrospinal fluid monoamine precursor and metabolite levels. J Neurochem 1989;52:235-41. [DOI] [PubMed]
- 25.Teff KL, Young SN, Blundell JE. The effect of protein or carbohydrate breakfasts on subsequent plasma amino acid levels, satiety and nutrient selection in normal males. Pharmacol Biochem Behav 1989;34:829-37. [DOI] [PubMed]
- 26.Ragozzino ME, Unick KE, Gold PE. Hippocampal acetylcholine release during memory testing in rats: augmentation by glucose. Proc Natl Acad Sci U S A 1996;93:4693-8. [DOI] [PMC free article] [PubMed]
- 27.Gold PE. Role of glucose in regulating the brain and cognition. Am J Clin Nutr 1995;61:S987-95. [DOI] [PubMed]
- 28.Pollitt E, Cueto S, Jacoby ER. Fasting and cognition in well- and undernourished schoolchildren: a review of three experimental studies. Am J Clin Nutr 1998;67(4):779S-84S. [DOI] [PubMed]
- 29.Helmers KF, Young SN. The effect of sucrose on acute tobacco withdrawal in women. Psychopharmacology 1998;139:217-21. [DOI] [PubMed]
- 30.Bartus RT, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982;217:408-14. [DOI] [PubMed]
- 31.Farquarson J, Cockburn F, Patrick WA, Jamieson EC, Logan RW. Infant cerebral cortex phospholipid fatty-acid composition and diet. Lancet 1992; 340:810-3. [DOI] [PubMed]
- 32.Greenwood CE, McGee CD, Dyer JR. Influence of dietary fat on brain membrane phospholipid fatty acid composition and neuronal function in mature rats. Nutrition 1989;5:278-81. [PubMed]
- 33.Stoll AL, Severus WE, Freeman MP, Rueter S, Zboyan HA, Diamond E, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry 1999;56(5):407-12. [DOI] [PubMed]
- 34.Hamazaki T, Sawazaki S, Itomura M, Asaoka E, Nagao Y, Nishimura N, et al. The effect of docosahexaenoic acid on aggression in young adults. A placebo-controlled double-blind study. J Clin Invest 1996;97(4):1129-33. [DOI] [PMC free article] [PubMed]
- 35.Maidment ID. Are fish oils an effective therapy in mental illness: an analysis of the data. Acta Psychiatr Scand 2000;102:3-11. [DOI] [PubMed]


