Abstract
Pancreatic adenocarcinoma is one of the leading causes of cancer-related deaths, and its therapy remains a challenge. Our proposed therapeutic approach is based on the intratumoral injections of mannan-BAM, toll-like receptor ligands, and anti-CD40 antibody (thus termed MBTA therapy), and has shown promising results in the elimination of subcutaneous murine melanoma, pheochromocytoma, colon carcinoma, and smaller pancreatic adenocarcinoma (Panc02). Here, we tested the short- and long-term effects of MBTA therapy in established subcutaneous Panc02 tumors two times larger than in previous study and bilateral Panc02 models as well as the roles of CD4+ and CD8+ T lymphocytes in this therapy. The MBTA therapy resulted in eradication of 67% of Panc02 tumors with the development of long-term memory as evidenced by the rejection of Panc02 cells after subcutaneous and intracranial transplantations. The initial Panc02 tumor elimination is not dependent on the presence of CD4+ T lymphocytes, although these cells seem to be important in long-term survival and resistance against tumor retransplantation. The resistance was revealed to be antigen-specific due to its inability to reject B16-F10 melanoma cells. In the bilateral Panc02 model, MBTA therapy manifested a lower therapeutic response. Despite numerous combinations of MBTA therapy with other therapeutic approaches, our results show that only simultaneous application of MBTA therapy into both tumors has potential for the treatment of the bilateral Panc02 model.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-02920-9.
Keywords: Pancreatic adenocarcinoma, TLR ligands, Mannan, Cancer immunotherapy, Metastases, Checkpoint inhibitors
Introduction
Pancreatic cancer is one of the leading causes of cancer-related deaths worldwide [1]. Patients with this type of cancer show a very limited response to currently available treatment approaches with only a 7% 5-year survival rate [2]. Its poor response and aggressivity can be explained by low antigenicity and its unique desmoplastic tumor stroma [3]. Low antigenicity results in the inability of T cells to recognize malignant cells, whereas the unique desmoplastic stroma can act as a physical barrier and block the penetration of anti-cancer drugs. Therefore, the blockade of the two most studied checkpoint inhibitors, programmed death-1 (PD-1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), in pancreatic cancer failed in phase I and II of clinical trials, respectively [4, 5]. Lower effectiveness of these checkpoint inhibitors and their combinations has also been observed in other cancer patients, except those with non-small cell lung cancer, melanoma, renal cell carcinoma, etc. [6, 7]. We thus speculate certain immunotherapy limitations in the narrow effect of the checkpoint inhibitors on the immune system. Checkpoint inhibitors are particularly involved in the last steps of an immune response, which represents the feedback of control mechanisms. This suggests that effective cancer immunotherapy has to be more complex and has to affect the immune system particularly during the initial activation of innate immunity and subsequent activation of adaptive immunity.
Previously, we studied cancer immunotherapy in murine models directed on the activation of the natural mechanisms of immune response and thereby involving both arms of the immune response, i.e., innate and adaptive immunity [8]. This novel approach combines toll-like receptor (TLR) ligands and a phagocytosis-activating ligand and exhibits promising results in the murine melanoma B16-F10 model. Here, TLR ligands support the infiltration of immune cells into a tumor and activation of immune cells [9–11]. The combination of resiquimod (R-848), polyinosinic–polycytidylic acid (poly(I:C)), and lipoteichoic acid (LTA) was assessed as the most potent. Mannan, a phagocytosis-activating ligand, artificially binds to tumor cell surfaces via a biocompatible anchor for cell membrane (BAM) and labels them for the infiltrated immune cells [9–12]. For the pancreatic adenocarcinoma (Panc02), pheochromocytoma (MTT), and colon carcinoma (CT26) mouse model, the combined application of mannan-BAM, R-848, poly(I:C), and LTA together with anti-CD40 antibody (called MBTA therapy) improved the survival of experimental mice [11–13].
The aim of this present study was to evaluate the effect of MBTA therapy in subcutaneous Panc02 tumors with a higher burden to more mimic the real situation of patients with pancreatic adenocarcinoma often during initial diagnosis. Specifically, mice with established pancreatic adenocarcinoma larger than so far studied and mice with two tumors, i.e., a bilateral Panc02 model, which enables to better study a systemic response to therapies, were used. Our results strongly indicate that MBTA therapy is effective for the treatment of established Panc02 tumors. Additionally, tumor rechallenge experiments revealed antigen-specific memory and rejection of Panc02 cells after subcutaneous and intracranial reinjections. CD4+ T lymphocytes had a minimal role during initial tumor reduction, but they were important in the survival of treated mice. In the bilateral Panc02 model, MBTA therapy only led to a decrease in the progression of both tumors. Therefore, to improve its applicability, we focused on its combination with checkpoint inhibitors, modification of tumor desmoplasia, chemoablation, or radiotherapy (RT) of the parallel tumor. Only simultaneous application of MBTA therapy into both tumors achieved effective therapeutic response in this challenging tumor model.
Materials and methods
Materials
Tissue culture media, media supplements, mannan from Saccharomyces cerevisiae, lipoteichoic acid (LTA) from Bacillus subtilis, polyinosinic–polycytidylic acid, sodium salt (poly (I:C)), and hyaluronidase (Type I-S) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Resiquimod (R-848) was obtained from Tocris Bioscience (Bristol, UK). Biocompatible anchor for cell membrane (BAM, Mw 4000) was purchased from NOF EUROPE (Grobbendonk, Belgium). Monoclonal anti-CD40 antibody (rat IgG2a, clone FGK4.5/FGK45), anti-CTLA-4 antibody (hamster IgG, clone (9H10), and anti-PD-L1 antibody (rat IgG2b, clone 10F.9G2) were purchased from BioXCell (West Lebanon, NH, USA). Heat-killed Listeria monocytogenes (HKLM) was purchased from InvivoGen (Toulouse, France).
Cell lines and mice
The murine pancreatic adenocarcinoma cell line Panc02 was obtained from Prof. Lars Ivo Partecke (Greifswald, Germany). Cells were maintained in Dulbecco’s modified eagle media (DMEM) supplemented with 10% heat-inactivated fetal bovine serum and antibiotics (PAA, Pasching, Austria). Murine melanoma B16-F10 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in RPMI 1640 media supplemented with 10% heat-inactivated fetal bovine serum and antibiotics (PAA, Pasching, Australia). Both cell lines were cultured at 37 °C in humidified air with 5% carbon dioxide.
SPF C57BL/6 mice were purchased from Charles River Laboratories (Sulzfeld, Germany). B6.129S2-Cd4tm1Mak/J mice (CD4−/− mice) and B6.129S2-Cd8atm1Mak/J mice (CD8−/− mice) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). All mice weighing between 18 g and 20 g were housed in specific pathogen-free barrier facilities with free access to sterile food and water; the photoperiod was 12/12.
Synthesis of mannan-BAM
Mannan-BAM was synthesized as previously described [10].
Tumor transplantation
Subcutaneous transplantation
Mice were subcutaneously injected with 4 × 105 Panc02 cells in 0.1 ml of DMEM or 4 × 105 B16-F10 cells in 0.1 ml of RPMI 1640 without additives into the previously shaved lower dorsal site (right or both right and left).
Intracranial transplantation
Mice were intraperitoneally injected with a mixture of ketamine (Narkamon, Bioveta, Czech Republic, 100 mg/kg) and xylazine (Rometar, Bioveta, Czech Republic, 5 mg/kg). Subsequently, the mice were intracranially injected with 1 × 105 Panc02 cells in 0.03 ml of DMEM without additives.
Treatment and its evaluation
MBTA therapy
50 µl of the therapeutic mixture consisting of 0.5 mg R-848 (HCl form), 0.5 mg poly(I:C), 0.5 mg LTA, and 0.4 mg anti-CD40 per ml of 0.2 mM mannan-BAM in PBS was injected intratumorally on the following days: 0, 1, 2, 8, 9, 10, 16, 17, 18, 24, 25, and 26.
MBT therapy
Anti-CD40 was not included.
Anti-CTLA-4, HKLM, and EtOH therapy
Anti-CTLA-4 antibody (1 mg/ml of PBS), HKLM (109/ml of PBS), and EtOH (ethanol absolute) were injected intratumorally (50 µl/mouse) on the following days: 20, 27, and 34.
Hyaluronidase and hyaluronidase + anti-CD40 antibody therapy
Hyaluronidase (40,000 U/ml of PBS), anti-CD40 antibody (0.8 mg/ml of PBS), or their combination was injected intratumorally (50 µl/mouse) on following days: 1, 7, 14, 21, and 28.
Radiotherapy (RT)
RT was applied using a TrueBeam linear electron accelerator system (Varian Medical System, Palo Alto, California, USA). For anesthesia, the mice were intraperitoneally injected with a mixture of ketamine (100 mg/kg) and xylazine (5 mg/kg). Subsequently, the mice received a single fraction of 12-Gray on days 0, 14, and 28 (focused on relevant tumors only).
Anti-PD-L1 therapy
Anti-PD-L1 antibody (0.4 mg/ml of PBS) was injected intratumorally (50 μl/mouse) on the following days: 15, 18, 21, 24, 29, and 32.
All mice were housed individually. Tumor size was measured every other day using a caliper. The formula V = (π/6) AB2, where A is the largest dimension of the tumor and B is the smallest dimension, was used to calculate tumor volume [14]. The reduction in tumor growth (%) as compared with control was calculated on day 14 using the following equation:
Statistical analysis
For analyses involving multiple groups and times, the area under the curve (AUC) was calculated, and statistical analysis was performed on AUC values using one-way ANOVA with Tukey’s post hoc test. Kaplan–Meier survival curves were compared using a log-rank test. Data were analyzed using STATISTICA 12 (StatSoft, Inc., Tulsa, OK, USA). Error bars indicate the standard error of the mean (SEM).
Results
Eradication of established Panc02 tumors using MBTA therapy
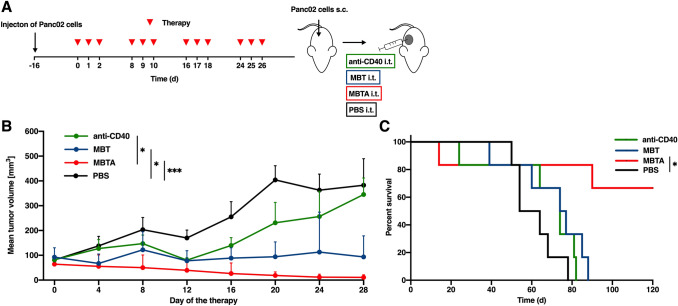
In our previous study [11], MBTA therapy eradicated 80% of smaller Panc02 tumors with an average tumor volume of 44.1 ± 9.9 mm3 (at the beginning of the therapy, i.e., 12 days after Panc02 cells transplantation) and volume range of 19.5–78.9 mm3. Here, we applied MBTA therapy to established Panc02 tumors larger than in the previous study, averaging 79.7 ± 30.7 mm3 (at the beginning of the therapy, i.e., 16 days after Panc02 cells transplantation) and ranging from 32.8 to 136.5 mm3 in tumor volume. Despite the degree of tumor size, MBTA therapy achieved 67% complete eradication (Fig. 1). The experiment was repeated four times with different tumor sizes. It was confirmed that the complete elimination is dependent on initial size of the tumor (Fig. S1, Supplementary materials). We also confirmed the crucial role of anti-CD40 antibody in MBTA therapy for the effective treatment of Panc02 tumors. Although MBT therapy (without anti-CD40 antibody) only led to partial elimination of tumor growth, mice survival was comparable to that of the control group.
Fig. 1.
MBTA therapy in established Panc02 tumors. a C57BL/6 mice were subcutaneously injected with Panc02 cells in the right flank. After 16 days, mice were randomized into 4 groups (n = 6/group): the group treated with anti-CD40 antibody; the group treated with MBT therapy; the group treated with MBTA therapy; the group treated with PBS. Therapy was given intratumorally on days 0, 1, 2, 8, 9, 10, 16, 17, 18, 24, 25, and 26. The tumor volume was measured with a caliper. b The tumor volume growth is presented as a growth curve (*p < 0.05, ***p < 0.005). c The survival analysis is presented as a Kaplan–Meier curve (*p < 0.05)
Additionally, MBTA-treated mice with complete tumor elimination (4 out of 6) were rechallenged on day 120 (day 0 as the start of therapy) with 1 × 106 Panc02 cells per mouse and revealed complete resistance against Panc02 retransplantation. The rechallenged mice initially developed small tumors in the first days after the transplantation, but subsequently, all tumors were eradicated (data not shown).
Underlying mechanisms of MBTA therapy in Panc02 tumors
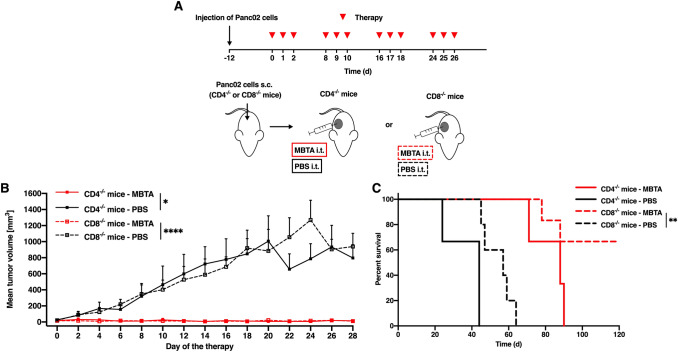
In the first experiment, we used mice lacking CD4+ T lymphocytes (CD4−/− mice) or CD8+ T lymphocytes (CD8−/− mice). MBTA therapy used in these immunodeficient mice revealed a reduction in tumor growth in both groups (Fig. 2). In CD4−/− mice, growth reduction was only temporary, and tumor regrowth followed 45 days after the start of the therapy. Meanwhile, in CD8−/− mice, eradication of tumors was observed in 4 out of 6 mice. Subsequently, 3 out of these 4 cured mice were resistant to subcutaneous retransplantation of Panc02 cells (4 × 105 cells/mouse) performed on day 120 (day 0 as the start of the therapy) (data not shown). Our findings suggest a strong initial effect of innate immunity and the important role of adaptive immunity (mainly CD4+ T lymphocytes) in complete eradication of tumor and resistance to its recurrence.
Fig. 2.
MBTA therapy of Panc02 tumors and the role of CD4+ T lymphocytes. a The CD4−/− and CD8−/− mice were subcutaneously injected with Panc02 cells in the right flank. After 12 days, both CD4−/− and CD8−/− mice were randomized into 4 groups (i) the CD4−/− mice treated with MBTA therapy (n = 3); (ii) CD4−/− mice treated with PBS (n = 3) (iii) the CD8−/− mice treated with MBTA therapy (n = 6); (iv) the CD8−/− mice treated with PBS (n = 5). Therapy was given intratumorally on days 0, 1, 2, 8, 9, 10, 16, 17, 18, 24, 25, and 26. The tumor volume was measured with a caliper. b The tumor volume growth is presented as a growth curve (*p < 0.05, ****p < 0.001). c The survival analysis is presented as a Kaplan–Meier curve (**p < 0.01)
Antigen specificity of immune memory induced by MBTA therapy
Mice-bearing Panc02 tumors were treated using MBTA therapy as described in Fig. 1. MBTA-treated mice with complete tumor elimination (4 out of 6 mice) were rechallenged with 4 × 105 Panc02 cells per mouse on day 120. All the mice were fully resistant and did not manifest subsequent tumor growth (data not shown). The rechallenge experiment was repeated on day 164 with 4 × 105 B16-F10 melanoma cells per mouse and resulted in rapid tumor growth (Fig. S2, Supplementary materials). Subsequent euthanization of these mice on day 203 followed due to the tumor growth. These results confirmed that the memory induced by MBTA therapy may be antigen specific.
Immune memory protects mice against intracranial Panc02 cells retransplantation
For this experiment, mice-bearing Panc02 tumors were also treated by MBTA therapy as described in Fig. 1 and 6 mice with complete tumor elimination (data not shown) were retransplanted with 1 × 105 Panc02 cells per mouse intracranially on day 120. For the control group, 8 healthy mice of the same age were used. Interestingly, MBTA-treated mice showing complete tumor elimination were fully resistant to Panc02 intracranial transplantation. In contrast, all mice in the control group died 11–14 days after the Panc02 intracranial transplantation (Fig. S3, Supplementary materials). Dissection of these mice revealed intracranial Panc02 tumors averaging 17.1 ± 15.5 mm3 in volume with a range of 2.1 – 32.8 mm3.
MBTA therapy of metastatic Panc02: the bilateral tumor model
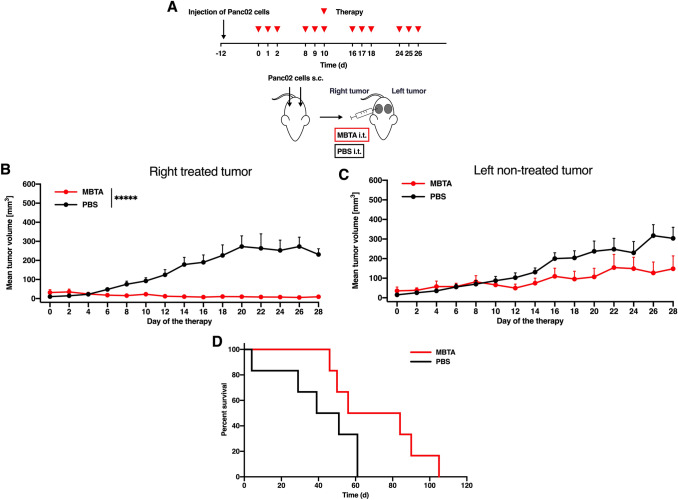
Metastatic disease is the biggest challenge of cancer therapy. As previously described [11], MBTA therapy can effectively suppress micrometastases in murine melanoma. However, for clinical purposes, more robust therapy is necessary to effectively eliminate advanced metastatic disease often caused by high-burden tumors. Therefore, we used the bilateral Panc02 model in our study. MBTA therapy was injected into the right tumor, and the left parallel (non-treated) tumor was observed for changes in its size (Fig. 3). The growth of the right MBTA-treated tumors was significantly reduced compared to that of the control (Fig. 3b), and tumor growth reduction was also observed in the left parallel (non-treated) tumors. The growth of the left parallel (non-treated) tumors was twice slower compared to that of the control (Fig. 3c). Interestingly, we observed that the presence of the left parallel (non-treated) tumor negatively affected the efficacy of MBTA therapy in the right tumor. In one tumor model, the reduction in tumor size caused by MBTA therapy led to the complete elimination of tumors in the majority of the treated mice [11, 12]. In contrast, in the bilateral Panc02 tumor model, the initial reduction in size of right MBTA-treated tumors relapsed, and the Panc02 tumors grew in half of the experimental animals.
Fig. 3.
MBTA therapy of the bilateral Panc02 model. a C57BL/6 mice were subcutaneously injected with Panc02 cells in both right and left flanks. After 12 days, mice were randomized into 2 groups (n = 6/group): (i) the group treated with MBTA therapy; (ii) the group treated with PBS. Therapy was given intratumorally into the right tumor on days 0, 1, 2, 8, 9, 10, 16, 17, 18, 24, 25, and 26. The tumor volume of both tumors was measured with a caliper. b The tumor volume growth of the right MBTA-treated and (c) the left parallel (non-treated) tumors is presented as a growth curve (*****p < 0.0005). d The survival analysis is presented as a Kaplan–Meier curve
Combination of MBTA therapy with other therapeutic approaches
Our results from the bilateral Panc02 tumor model suggested that MBTA therapy of only one tumor (the right MBTA-treated tumor) is not sufficient to eliminate the left parallel (non-treated) tumor. To boost the therapeutic effect of our proposed MBTA therapy, we simultaneously applied other therapeutic approaches on the left parallel tumor.
We first tested the intratumoral applications of anti-CTLA-4 antibody, heat-killed Listeria monocytogenes, and EtOH into the left parallel tumor with simultaneous application of MBTA therapy into the right tumor. Interestingly, we did not observe any augmentation on the therapeutic effect (Fig. S4, Supplementary materials).
Next, we focused on the manipulation of the Panc02 microenvironment, specifically on the reduction in extensive desmoplasia represented by the abundant presence of collagens, fibronectin, and hyaluronan in the intracellular space of Panc02 tumors. Desmoplasia limits the penetration of immune cells into the tumor, thus potentially suppressing immunotherapeutic outcomes [15]. The right tumors were treated by MBTA therapy, whereas the left parallel tumors were injected with anti-CD40 and hyaluronidase to decrease the density of protein fraction and to degrade hyaluronan, respectively [16, 17]. Interestingly, the effects of anti-CD40 antibody, hyaluronidase, and their combination were negligible (Fig. S5, Supplementary materials).
Simultaneous MBTA therapy and its combination with radiotherapy in the bilateral Panc02 model
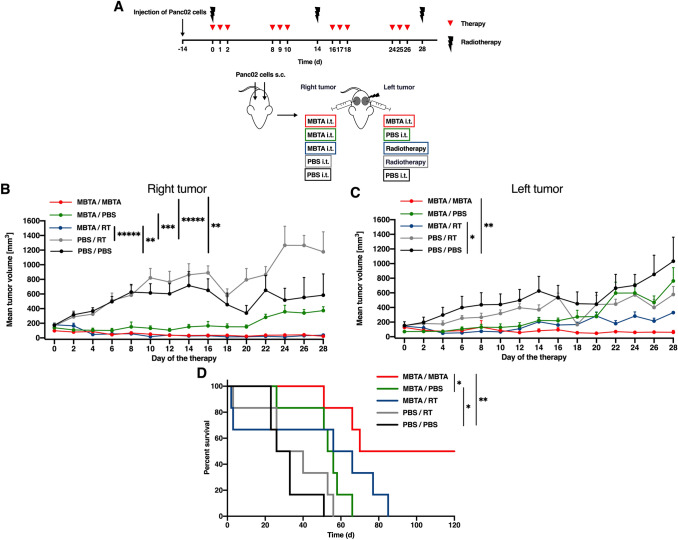
Lastly, we tested the simultaneous application of MBTA therapy into both right and left tumors and combined MBTA therapy of the right tumor with RT of the left parallel tumor in the bilateral Panc02 model. Panc02 tumors were utilized with the right tumors averaging 150.5 ± 62.9 mm3 with a range of 72.3–247.0 mm3, whereas the left parallel tumors averaged 129.9 ± 76.5 mm3 and ranged from 55.1 to 398.8 mm3.
Simultaneous application of MBTA therapy resulted in 96% reduction in right tumor volume and 87% reduction in left tumor volume as compared to that of the control (Fig. 4b, c). The reduction in tumor volume was accompanied by a significant prolongation of survival (Fig. 4d). Meanwhile, combined MBTA therapy with RT exhibited a therapeutic effect, as manifested by 67% growth reduction of left parallel tumors compared to that of the control group (Fig. 4c) with slight prolongation of survival (Fig. 4d). Because RT results in elevated expression of PD-L1 expression in tumors [18], we additionally tested the intratumoral application of anti-PD-L1 antibodies into the left parallel irradiated tumors. However, no additional therapeutic effects were observed (data not shown).
Fig. 4.
Simultaneous MBTA therapy in the right and left parallel Panc02 tumors and irradiation of the left parallel tumor. a C57BL/6 mice were subcutaneously injected with Panc02 cells in both right and left flanks. After 12 days, mice were randomized into 5 groups (n = 6/group): the group treated with MBTA therapy (right and left tumor); the group treated with MBTA therapy (right tumor) and PBS (left tumor); the group treated with MBTA therapy (right tumor) and radiotherapy (RT) (left tumor); the group treated with PBS (right tumor) and RT (left tumor); the group treated with PBS (both tumors). Therapy was given intratumorally into the right or left parallel tumor on days 0, 1, 2, 8, 9, 10, 16, 17, 18, 24, 25, and 26. The left parallel tumors were irradiated on days 0, 14, and 28. The tumor volume of both tumors was measured with a caliper. b The tumor volume growth of the right MBTA-treated and (c) the left parallel-treated tumors is presented as a growth curve (*p < 0.05, **p < 0.01, ***p < 0.005, *****p < 0.0005). d The survival analysis is presented as a Kaplan–Meier curve (*p < 0.05, **p < 0.01)
Moreover, we observed that the presence of the left parallel (non-treated) tumors negatively affected the efficacy of MBTA therapy in the right tumors (Fig. 4b, c, green curves), which corresponds with the aforementioned observation discussed in “MBTA therapy of metastatic Panc02: the bilateral tumor model.”
Discussion
We showed that MBTA therapy had a significant therapeutic effect not only in smaller but also in larger murine Panc02 tumors, as evidenced by the significant tumor growth reduction and tumor eradication in 67% of the treated animals and subsequent resistance against tumor rechallenge. Focusing on the underlying immune mechanisms involved in MBTA therapy, we observed that the presence of CD4+ T lymphocytes was not critical for significant tumor growth reduction. However, CD4+ T lymphocytes seem to be essential for delayed tumor response, long survival, and resistance against tumor rechallenge. When MBTA therapy was applied to subcutaneous murine bilateral Panc02 model, we observed a partial therapeutic effect on the left parallel (non-treated) tumor. To boost its therapeutic effect, we combined MBTA therapy with several different therapeutic approaches, such as intratumoral applications of anti-CTLA-4 antibody, heat-killed Listeria monocytogenes (HKLM), and EtOH (chemoablation), targeting the Panc02 microenvironment (reduction in extensive desmoplasia), simultaneous application of MBTA therapy into the distant tumor, and its combination with RT. Among these, only simultaneous application of MBTA therapy on the primary and distant tumors showed the most promising therapeutic outcomes in murine bilateral Panc02 model.
Tumor size is an important factor for successful cancer treatment. Although small tumors show better response to various therapeutic options, high-burden tumors remain a major challenge [19]. In a previous study, we successfully applied MBTA therapy in smaller subcutaneous Panc02 tumors (± 44.1 mm3). In the present study, we investigated its therapeutic effect on larger established Panc02 tumors (± 79.7 mm3) because they more closely mimic the real situation in patients during initial diagnosis and present a great challenge in cancer therapy. Interestingly, we achieved 67% complete eradication of Panc02 tumors using MBTA therapy, which was slightly less than the 80% complete eradication of smaller Panc02 from our previous study [11]. We also verified the importance of the presence of anti-CD40 antibodies in MBTA therapy, ensuring long-lasting survival of treated mice and tumor resistance during retransplantation. This is consistent with previously published Panc02 and murine pheochromocytoma data where the addition of anti-CD40 to MBT therapy also resulted in an increase in the overall survival of treated mice. Interestingly, the addition of anti-CD40 does not have any significant effect on reduction of tumor growth [11, 12].
We then focused on the underlying mechanisms of MBTA therapy in the Panc02 tumor model. Surprisingly, MBTA therapy fully suppressed tumor growth in the absence of either CD4+ T lymphocytes (CD4−/− mice) or CD8+ T lymphocytes (CD8−/− mice). However, the presence of CD4+ T lymphocytes seems to be important for long-lasting survival of mice and for resistance against tumor retransplantation. Resistance against retransplantation (75%) in CD8−/− mice could be interpreted based on the CD4+ T lymphocyte-driven macrophage activity [20–22]. However, a further study focused on the role of CD4+ in immune memory after MBTA therapy must be performed.
Subsequently, we also studied the antigen specificity involved in the mechanism of immune memory induced by MBTA therapy. MBTA therapy has shown 100% resistance against retransplantation of Panc02 tumor cells [11]. However, it remains unknown if this resistance is antigen specific. Therefore, mice with MBTA-therapy-eradicated Panc02 tumors were rechallenged with Panc02 tumor cells and B16-F10 murine melanoma cells. Interestingly, all mice manifested resistance against Panc02, but resistance against B16-F10 was not observed, suggesting that the immunological memory induced by MBTA therapy is antigen specific for each type of tumor.
To further investigate the immune memory induced by MBTA therapy, we retransplanted Panc02 cells into the immune-privileged site—the central nervous system (intracranial tumor cell retransplantation). All mice previously treated by MBTA therapy showed resistance as compared to the control group, in which all animals died 11–14 days after Panc02 injection. These data are consistent with MBTA therapy of CT26, where all mice were protected against subcutaneous and intracranial retransplantation with the same tumor cells [13]. This revealed the ability of the immune memory induced by MBTA therapy to cross over the blood–brain barrier, confirming its potential applicability in metastatic brain tumors.
The eradication of metastases is an important aspect of successful cancer treatment. Previously, we observed that MBTA therapy of the primary tumor is associated with growth reduction or elimination of small metastases [11, 12]. However, in larger distant Panc02 metastases, particularly in the bilateral Panc02 model, only limited anti-tumor effect was observed, and we, therefore, focused on the enhancement of this distant therapeutic effect. In the first set of experiments, we tested the application of anti-CTLA-4 antibody, heat-killed Listeria monocytogenes (HKLM), and EtOH (chemoablation) into the left tumor with simultaneous application of MBTA therapy into the right tumor. Anti-CTLA-4 antibody was expected to enhance the immune attack through depletion of Tregs [23]. HKLM was supposed to stimulate acute inflammation in the tumor [24] and change the pro-tumor environment into an anti-tumor one. Chemoablation is known to lead to cell membrane lysis, protein denaturation, vascular occlusion, and eventually tumor cell death [25]. Despite their proven individual anti-tumorigenic effects, no therapeutic improvement was detected when combined with MBTA therapy.
In the second set of experiments, we focused on the manipulation of Panc02 environment, specifically on the reduction in desmoplasia. Anti-CD40 antibody and hyaluronidase were applied into the left tumor with the simultaneous application of MBTA therapy into the right tumor. Anti-CD40 antibody was used to decrease the density of protein fraction (collagen I, fibronectin) [17], whereas hyaluronidase was used to degrade hyaluronan [16]. Neither of these substances nor their combination increased the therapeutic effect of MBTA therapy on distant tumors. As previously mentioned, advanced tumors possess protection against immune attack on many different levels; therefore, targeting just one of these levels is insufficient.
To improve the MBTA therapy’s outcome on distant tumors, we also tested its simultaneous application into the primary tumor (right tumor) and distant metastases (left tumor), resulting in 87% left tumor growth reduction and complete eradication of both tumors in 50% of the mice. These results suggest that the targeted application of MBTA therapy into primary tumors with simultaneous application into metastases can result in successful treatment of metastatic disease. The combination of MBTA therapy and RT showed promising results in tumor growth reduction but did not show a significant difference in survival of treated mice.
Panc02 tumors with higher tumor burden remain a challenge for modern cancer treatment. Even though the proposed MBTA therapy showed promising results in established murine Panc02 tumors and bilateral Panc02 tumors, we are still far away from completely curing these challenging tumors. Nevertheless, our study showed that only simultaneous application of MBTA therapy into the primary tumor and distant metastases improved the outcomes of large established and metastatic Panc02 tumors. As we discussed previously [8], this combination of TLR ligands, mannan-BAM, and anti-CD40 antibody has the potential to result ineffective treatments for patients with inoperable tumors or as a neoadjuvant therapy before surgery. Moreover, several TLR ligands and anti-CD40 antibodies are already in clinical trials or FDA-approved which can significantly speed up the potential application of MBTA therapy in the clinic [26, 27].
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the staff of Department of Radiology, the Nemocnice Ceske Budejovice Hospital, for the possibility to use linear electron accelerator system and their help and L. I. Partecke for the generous gift of Panc02 cells.
Abbreviation
- CTLA-4
Cytotoxic T-lymphocyte-associated antigen 4
- HKLM
Heat-killed Listeria monocytogenes
- MBT therapy
Mannan-BAM + TLR ligands
- MBTA therapy
Mannan-BAM + TLR ligands + anti-CD40 antibody
- RT
Radiotherapy
- TLR
Toll-like receptor
Funding
This study was funded by the Research Support Foundation (Vaduz, Fürstentum Liechtenstein) and the Intramural Research Program of the National Institutes of Health, Eunice Kennedy Shriver National Institutes of Child Health and Human Development.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standards
All experimental procedures involving mice were performed in accordance with the laws of the Czech Republic. The experimental Project was approved by the Ministry of Education, Youth, and Sports of the Czech Republic (Protocol No. 12098/2016-2).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ondrej Uher and Veronika Caisova have contributed equally to this work.
References
- 1.Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol. 2019;10(1):10–27. doi: 10.14740/wjon1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH, Neoptolemos JP. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. doi: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 3.Neesse A, Bauer CA, Ohlund D, Lauth M, Buchholz M, Michl P, Tuveson DA, Gress TM. Stromal biology and therapy in pancreatic cancer: ready for clinical translation? Gut. 2019;68(1):159–171. doi: 10.1136/gutjnl-2018-316451. [DOI] [PubMed] [Google Scholar]
- 4.Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, Rosenberg SA. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33(8):828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50(12):165. doi: 10.1038/s12276-018-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39. doi: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Uher O, Caisova V, Hansen P, Kopecky J, Chmelar J, Zhuang Z, Zenka J, Pacak K. Coley’s immunotherapy revived: innate immunity as a link in priming cancer cells for an attack by adaptive immunity. Semin Oncol. 2019 doi: 10.1053/j.seminoncol.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janotova T, Jalovecka M, Auerova M, Svecova I, Bruzlova P, Maierova V, Kumzakova Z, Cunatova S, Vlckova Z, Caisova V, Rozsypalova P, Lukacova K, Vacova N, Wachtlova M, Salat J, Lieskovska J, Kopecky J, Zenka J. The use of anchored agonists of phagocytic receptors for cancer immunotherapy: B16-F10 murine melanoma model. PLoS ONE. 2014;9(1):e85222. doi: 10.1371/journal.pone.0085222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waldmannova E, Caisova V, Faberova J, Svackova P, Kovarova M, Svackova D, Kumzakova Z, Jackova A, Vacova N, Nedbalova P, Horka M, Kopecky J, Zenka J. The use of Zymosan A and bacteria anchored to tumor cells for effective cancer immunotherapy: B16-F10 murine melanoma model. Int Immunopharmacol. 2016;39:295–306. doi: 10.1016/j.intimp.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Caisova V, Uher O, Nedbalova P, Jochmanova I, Kvardova K, Masakova K, Krejcova G, Padoukova L, Chmelar J, Kopecky J, Zenka J. Effective cancer immunotherapy based on combination of TLR agonists with stimulation of phagocytosis. Int Immunopharmacol. 2018;59:86–96. doi: 10.1016/j.intimp.2018.03.038. [DOI] [PubMed] [Google Scholar]
- 12.Caisova V, Li L, Gupta G, Jochmanova I, Jha A, Uher O, Huynh TT, Miettinen M, Pang Y, Abunimer L, Niu G, Chen X, Ghayee HK, Taieb D, Zhuang Z, Zenka J, Pacak K. The significant reduction or complete eradication of subcutaneous and metastatic lesions in a pheochromocytoma mouse model after immunotherapy using mannan-BAM, TLR ligands, and anti-CD40. Cancers (Basel) 2019 doi: 10.3390/cancers11050654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medina R, Wang HR, Caisova V, Cui J, Indig IH, Uher O, Ye J, Nwankwo A, Sanchez V, Wu TX, Nduom E, Heiss J, Gilbert MR, Terabe M, Ho W, Zenka J, Pacak K, Zhuang ZP. Induction of immune response against metastatic tumors via vaccination of mannan-BAM, TLR ligands, and anti-CD40 antibody (MBTA) Adv Ther Germany. 2020 doi: 10.1002/adtp.202000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Piao YF, Jiang Z, Chen L, Sun HB. Silencing of signal transducer and activator of transcription 3 expression by RNA interference suppresses growth of human hepatocellular carcinoma in tumor-bearing nude mice. World J Gastroenterol. 2009;15(21):2602–2608. doi: 10.3748/wjg.15.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunk PR, Bauer TW, Slingluff CL, Rahma OE. From bench to bedside a comprehensive review of pancreatic cancer immunotherapy. J Immunother Cancer. 2016;4:14. doi: 10.1186/s40425-016-0119-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kultti A, Li X, Jiang P, Thompson CB, Frost GI, Shepard HM. Therapeutic targeting of hyaluronan in the tumor stroma. Cancers (Basel) 2012;4(3):873–903. doi: 10.3390/cancers4030873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long KB, Gladney WL, Tooker GM, Graham K, Fraietta JA, Beatty GL. IFNgamma and CCL2 cooperate to redirect tumor-infiltrating monocytes to degrade fibrosis and enhance chemotherapy efficacy in pancreatic carcinoma. Cancer Discov. 2016;6(4):400–413. doi: 10.1158/2159-8290.CD-15-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azad A, Yin Lim S, D’Costa Z, Jones K, Diana A, Sansom OJ, Kruger P, Liu S, McKenna WG, Dushek O, Muschel RJ, Fokas E. PD-L1 blockade enhances response of pancreatic ductal adenocarcinoma to radiotherapy. EMBO Mol Med. 2017;9(2):167–180. doi: 10.15252/emmm.201606674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackaman C, Bundell CS, Kinnear BF, Smith AM, Filion P, van Hagen D, Robinson BW, Nelson DJ. IL-2 intratumoral immunotherapy enhances CD8 + T cells that mediate destruction of tumor cells and tumor-associated vasculature: a novel mechanism for IL-2. J Immunol. 2003;171(10):5051–5063. doi: 10.4049/jimmunol.171.10.5051. [DOI] [PubMed] [Google Scholar]
- 20.Haabeth OA, Tveita AA, Fauskanger M, Schjesvold F, Lorvik KB, Hofgaard PO, Omholt H, Munthe LA, Dembic Z, Corthay A, Bogen B. How do CD4(+) T cells detect and eliminate tumor cells that either lack or express MHC class II molecules? Front Immunol. 2014;5:174. doi: 10.3389/fimmu.2014.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bogen B, Fauskanger M, Haabeth OA, Tveita A. CD4(+) T cells indirectly kill tumor cells via induction of cytotoxic macrophages in mouse models. Cancer Immunol Immunother. 2019 doi: 10.1007/s00262-019-02374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisel D, Das K, Dickes E, Konig R, Osen W, Eichmuller SB. Cognate interaction with CD4(+) T cells instructs tumor-associated macrophages to acquire M1-like phenotype. Front Immunol. 2019;10:219. doi: 10.3389/fimmu.2019.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang F, Du X, Liu M, Zheng P, Liu Y. Anti-CTLA-4 antibodies in cancer immunotherapy: selective depletion of intratumoral regulatory T cells or checkpoint blockade? Cell Biosci. 2018;8:30. doi: 10.1186/s13578-018-0229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park DW, Kim JS, Chin BR, Baek SH. Resveratrol inhibits inflammation induced by heat-killed Listeria monocytogenes. J Med Food. 2012;15(9):788–794. doi: 10.1089/jmf.2012.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathers BW, Harvey HA, Dye CE, Dougherty-Hamod B, Moyer MT. Endoscopic ultrasound-guided ethanol ablation of a large metastatic carcinoid tumor: success with a note of caution. Endosc Int Open. 2014;2(4):E256–E258. doi: 10.1055/s-0034-1377612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anwar MA, Shah M, Kim J, Choi S. Recent clinical trends in Toll-like receptor targeting therapeutics. Med Res Rev. 2019;39(3):1053–1090. doi: 10.1002/med.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vonderheide RH. CD40 agonist antibodies in cancer immunotherapy. Annu Rev Med. 2020;71:47–58. doi: 10.1146/annurev-med-062518-045435-045435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.