Case 1
A 60-year-old man is discharged from hospital after his second bout of renal colic in 2 years. Both times, he passed a stone without any need for surgical intervention. There is no other relevant history. The stones were analyzed biochemically and found to be composed of pure calcium oxalate. Imaging studies do not suggest the presence of residual calculi in the renal tract. The patient is, understandably, reluctant to pass any more kidney stones. The results of relevant laboratory investigations are as follows: serum biochemistry normal, urine volume 1.75 L over 24 hours, urinary calcium excretion 2.9 mmol/day, urinary phosphate excretion 24 mmol/day, urinary uric acid excretion 5.5 mmol/day, urinary oxalate excretion 270 μmol/day and urinary citrate excretion 0.6 mmol/day. The patient asks you what the probable cause is of his recurrent nephrolithiasis. What medication could be prescribed to help him and what side effects could he anticipate? What other measures could the patient take to reduce his risk of stone recurrence?
Case 2
A 55-year-old woman with recurrent uric acid stones has begun treatment with potassium citrate, 60 mEq/day, in divided doses and allopurinol, 300 mg/day. She finds the potassium citrate unpalatable but, on your advice, agrees to continue the medication. She comes to your office 10 days after starting the medications describing general malaise, weakness, mild dysphagia and nausea. She has a generalized rash and some oral ulcers. What are the side effects of the prescribed medication? What is your diagnosis? What measures would you recommend?
Kidney stones are rarely, if ever, fatal. The main impact of nephrolithiasis is felt by young, otherwise healthy adults in the form of acute renal colic, causing symptoms of pain, nausea, vomiting and hematuria. The estimated cost of this condition in the United States for 1993 was US$1.83 billion.1 The lifetime risk of passing a kidney stone is about 8%–10% among North American males, and the peak age of incidence is 30 years. The rate of kidney stone formation in women is about half that in men, with 2 peaks, the first among women aged 35 years and the second among those aged 55 years.2 Among patients who have passed one kidney stone, the lifetime recurrence rate is 60%–80%.2 There is significant geographic and seasonal variation in rates of stone formation. The reasons for this variation are not entirely clear, but they may relate to climate and the mineral content of drinking water. The frequency of kidney stones (notably calcium oxalate stones) has increased with improved standards of living.2
This review will not concentrate on the acute management of nephrolithiasis but deals with the investigation and management of recurrent nephrolithiasis.
Pathophysiology
Kidney stone formation is the end result of a physicochemical process that involves nucleation of crystals from a supersaturated solution. The common constituents of kidney stones are listed in Table 1. The factors that influence crystal generation are urine volume, concentration of stone constituents (a function of urine volume), the presence of a nidus and the balance among various physicochemical factors that inhibit or promote stone formation.
Table 1
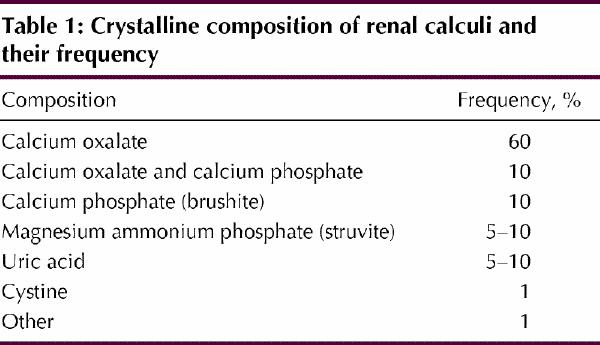
Most people's urine is supersaturated with the common components of renal stones, including calcium phosphate, calcium oxalate and, frequently, uric acid. Supersaturation of the urine constitutes a driving force within the solution favouring crystal nucleation and growth.
A great deal of attention has been focused recently on the interactions between crystals that are being formed and the cell surfaces in the renal tubules.3,4 The most common constituent of kidney stones, calcium oxalate monohydrate, binds electrostatically to anionic sites on cell surfaces. Thereafter, the crystals may be internalized, or they may remain on the cell surface, which allows further binding and propagation of the crystals. Soluble anions, such as citrate, may inhibit this process, as may urinary glycoproteins; these compounds thus act as inhibitors of the early phase of stone formation.
There is a fine balance in urine among substances that readily form crystals, such as calcium, oxalate and uric acid; promoters of crystallization, including pH, stasis and low volume; and inhibitors of this process, such as high urine volume and flow, citrate (which forms a complex with calcium to prevent its crystallization with oxalate) and urinary glycoproteins. The following section outlines how various factors affect the formation of stones.
Urine volume
One of the most important inhibitors of stone formation is urine volume. A high volume of urine helps to reduce the relative supersaturation of the crystal components. In addition, high volume implies high urine flow rates, which will tend to wash out any crystals that have formed. The value of increasing urine volume was demonstrated in a randomized clinical trial lasting 5 years.5 In that study, stones recurred in only 12 (12%) of the 99 patients who maintained a urine volume of about 2.6 L/day over 5 years, whereas stones recurred in 27 (27%) of the 100 patients in the control group, whose urine volume was about 1.2 L/day (p = 0.008). In addition, the time to recurrence was longer in the experimental group than in the control group (38.7 [standard deviation 13.2] months v. 25.1 [standard deviation 16.4] months; p = 0.016). The relative risk reduction ratio for subjects with higher fluid intake (and hence greater urine volume) was about 0.45. Another study6 has shown that most fluids consumed (with the possible exception of grapefruit juice) are associated with a positive effect, including tea, which contains high levels of oxalate, a factor that on its own can usually promote nephrolithiasis
Urinary oxalate
Oxalate is the end product of several metabolic pathways, including those involving serine, glycine, hydroxyproline and ascorbate, and is excreted renally. Only about 10%–20% of urinary oxalate is derived from dietary sources. Foods rich in oxalate include spinach, cranberries, chocolate and tea.
There is much less oxalate than calcium in urine. Therefore, changes in oxalate concentration have a greater impact than changes in calcium concentration on the relative supersaturation of calcium oxalate crystals. Indeed, the rate of change in supersaturation is 23 times greater for increases in oxalate than for comparable increases in calcium.7
Severe hyperoxaluria, classified as types I and II, is a feature of rare, inherited hepatic enzyme defects. These conditions typically present with multiple stones in childhood and may cause renal failure, although presentation in adulthood is also possible. In patients with malabsorption syndrome because of small-bowel resection or Crohn's disease, rapid transit of unbound oxalate through the gastrointestinal tract allows increased colonic absorption of oxalate and a syndrome of enteric hyperoxaluria.
Urinary calcium
Hypercalciuria is present in about half of patients with recurrent kidney stones. The most common reason appears to be enhanced gastrointestinal absorption of calcium, so-called absorptive hypercalciuria. The mechanism of the hyperabsorption is not known, but it appears to be inherited in an autosomal dominant fashion. It has been postulated that hyperresponsiveness to vitamin D in the jejunal mucosa is responsible (for a review of this phenomenon, see Asplin and colleagues2). Absorptive hypercalciuria is very common, but most people with this anomaly are asymptomatic and do not experience stone formation. A moderate calcium intake (1 g/day) may in fact be reasonable for patients, because a low-calcium diet may actually permit greater oxalate absorption from the bowel and thus lead to hyperoxaluria.
In about 5% of individuals with recurrent stone formation, primary hyperparathyroidism is the cause of hypercalciuria, so-called resorptive hypercalciuria. A renal calcium leak with resulting secondary hyperparathyroidism is seen in a further 2% of those with recurrent stone formation.
Uric acid
Uric acid is the end product of purine metabolism. It can be derived from dietary sources such as meat and fish or through endogenous production during cell turnover.
Pure uric acid stones are relatively rare, but they tend to recur in anyone who does have this type of stone. They form primarily in acid urine (pH < 5.5) and are unlikely to form if urine pH is above 6.5.
A number of inborn errors of metabolism are associated with excessive synthesis of uric acid.8 Hyperuricosuria is also important in the pathogenesis of calcium-containing stones, because uric acid crystals can act as a nidus for calcium oxalate precipitation.
Citrate
Citrate is derived from both endogenous (tricarboxylic acid cycle) and exogenous sources (citrus fruits such as oranges and grapefruit), however, the bulk of urinary citrate is the result of renal tubular cell excretion. In the presence of intracellular acidosis and hypokalemia, renal tubular citrate excretion is decreased. Hypocitraturia is a common, correctable cause of recurrent stone formation (pure calcium phosphate [brushite] stones). Hypocitraturia is also a frequent isolated finding in individuals with recurrent calcium stones.9 Urinary citrate forms a complex with calcium and inhibits early and late stages of crystal formation and propagation. Low levels of urinary citrate are seen in patients with systemic acidosis (including renal tubular acidosis), chronic diarrhea and hypokalemia, or it may occur idiopathically. Diets rich in animal protein lead to increased secretion of acid by the kidney and reduction of urinary citrate.
Stasis and infection
Crystal precipitation is more likely in stagnant urine. Calculi frequently form in areas of the pelvicalyceal system that do not drain adequately. Infection of the urine also predisposes to stone formation. Urea-splitting organisms such as Proteus, Klebsiella, Serratia and Mycoplasma create alkaline urine and thus enhance the production of magnesium ammonium phosphate (struvite) stones. These stones present a special therapeutic challenge, because they are themselves infected.
Urinary glycoproteins
A number of urinary glycoproteins have been identified as inhibitors of the process of calcium oxalate stone formation at various stages of development in vitro (reviewed in Khan10). These include Tamm-Horsfall protein, bikunin, nephrocalcin and a urinary form of prothrombin fragment 1. The physiologic role of these agents is the subject of intense investigation, as it is hoped that better understanding of their mode of action may lead to new therapeutic strategies.
Investigations
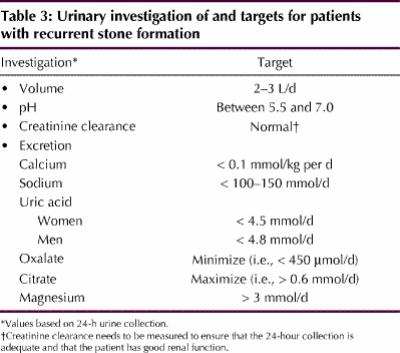
Some controversy exists about the extent of investigation required after the passage of a single stone. Because the rate of recurrence is high, some experts favour an exhaustive evaluation for anyone who has passed a stone. Basic investigations for a patient who has passed a kidney stone are listed in Table 2. However, a comprehensive urinary evaluation (Table 3) or referral to a “stone specialist” is required for patients in whom multiple stones are detected clinically or radiographically (including those with nephrocalcinosis), patients with cystine or uric acid stones detected by biochemically testing the stones provided by the patient, those with anatomic abnormalities of the renal tract and those with a strong family history of nephrolithiasis.
Table 2
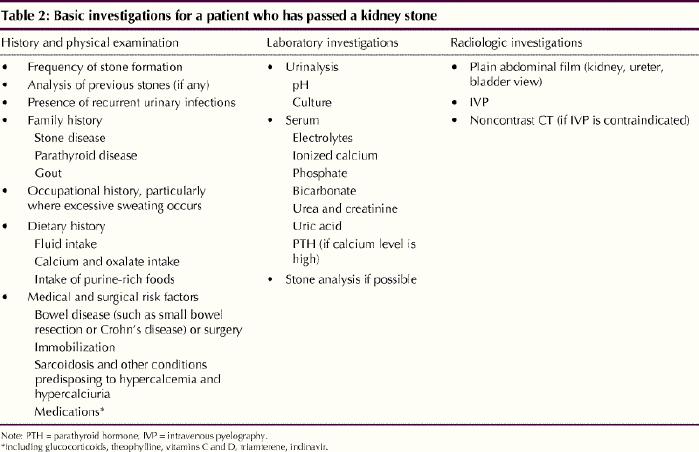
Table 3
A number of imaging techniques, including plain abdominal radiography, ultrasonography, intravenous pyelography (IVP), helical CT scanning and MRI scanning, can be used to demonstrate stones in the renal tract. Although IVP has been considered the criterion standard for many years, helical CT scanning has significant advantages in the setting of acute renal colic. Where available, helical CT scans can be performed rapidly, without the use of intravenous contrast, and can identify other causes of abdominal pain masquerading as renal colic. The emergency evaluation of kidney stones has been the subject of a recent review.11 For radio-opaque stones, annual radiography can be used to follow the growth of existing stones and to identify the appearance of new stones to help determine the efficacy of therapy.
Management of nephrolithiasis
Medical management
Medical interventions for kidney stones can be classified as general or specific. General measures, which are advisable for all patients, include increasing fluid intake to produce a urine volume of 2–3 L/day. Data on the benefits of dietary interventions are less conclusive,6,12 and well-conducted, prospective studies are lacking. Good advice is to reduce the intake of animal protein and sodium, because reduction in the renal excretion of sodium will also reduce renal calcium excretion and thus render the urine less lithogenic. As described earlier, patients should moderate their calcium intake to 1 g/day.
These general measures should be instituted in all patients with recurrent stone formation to take advantage of the well-recognized phenomenon of the “stone clinic effect,” which, it has been suggested, can reduce the 5-year stone recurrence rate by 60%.13 Specific interventions depend on stone type and identifiable factors in the urine that may increase lithogenicity, as outlined here.
Calcium oxalate stones
A recently published meta-analysis of randomized trials on preventing the recurrence of calcium oxalate stones suggested that the greatest benefit from pharmacologic intervention is associated with thiazide diuretics.14 Five trials involving thiazide diuretics and a single trial with the closely related drug indapamide suggested a risk reduction for developing new stones of 21.3% (95% confidence interval –29.2% to –13.4%) in the patients receiving medication. The combination of hydrochlorothiazide, 25–50 mg, with amiloride, 5 mg, to prevent potassium wasting was appropriate. Benefits were seen only after 3 years of treatment, so therapy should not be abandoned if stones recur early. Less benefit was conferred by citrate-containing alkalinizing compounds and allopurinol. However, oral potassium citrate, 30–80 mEq daily, is indicated for patients in whom hypocitraturia has been detected.
A randomized, placebo-controlled study showed a benefit of allopurinol, 100 mg 3 times daily, in people with hyperuricosuria and formation of calcium oxalate stones.15 In addition to a requirement for both hyperuricosuria and normocalciuria, patients who were selected for the study had formed 2 or more calculi in the 5 years before the study and at least one calculus in the previous 2 years. The rate of new stone events (defined as a composite of stone passage and the appearance of a new stone, or enlargement of an existing stone on abdominal radiography) was much higher among the 31 patients receiving placebo than among the 29 receiving allopurinol (0.26 v. 0.12 per patient year, p < 0.02).
There is no strong evidence to support the use of measures to reduce urinary oxalate, such as oral calcium supplementation and administration of cholestyramine.
Uric acid stones
Uric acid is soluble in alkaline urine. Thus, management of uric acid stones generally involves alkalinization of the urine. The target range for urine pH should be 6.0–6.5 for therapy, because formation of another type of stone, calcium phosphate, may be induced by urinary pH above 7. Urine can be alkalinized with either oral potassium citrate or sodium bicarbonate. The former has the advantage of avoiding a sodium load, which may lead to hypercalciuria, and has been shown to reduce significantly the recurrence of uric acid stones when given at doses of 30–80 mEq/day,16 but it should be used cautiously in combination with potassium-sparing diuretics because of concerns about hyperkalemia.
Struvite stones
Struvite stones form in infected urine and are frequently large enough to fill the entire pelvicalyceal system; in this situation, they are known as staghorn calculi. In rare cases, staghorn calculi are composed purely of cystine or calcium oxalate monohydrate. Because of their size and the presence of infection, a combined surgical and medical approach, including increased fluid intake and the use of antibiotics, is required. The American Urological Association has published clinical practice guidelines for the management of staghorn calculi.17 It recommends extracorporeal shock wave lithotripsy (ESWL) combined with percutaneous stone removal. Removal of all stone fragments and antibiotic therapy to sterilize the urine are required to prevent recurrence.
Cystine stones
Despite the rarity of cystine stones (1%–2% in adults, 6%–8% in children), they deserve special mention. Cystinuria is an inborn error of metabolism characterized by defective tubular and intestinal transport of cystine, ornithine, lysine and arginine. Although large amounts of all of these amino acids appear in the urine, only the cystine precipitates at physiologic pH. A high fluid intake throughout each 24-hour period decreases the rate of recurrence. Sodium restriction (to 80–100 mmol/day) significantly reduces cystine excretion.18 Other therapies for cystine stones include drugs such as penicillamine, tiopronin and captopril.19 These medications increase the solubility of cystine by cleaving the disulfide bond of the amino acid.
Surgical management
There have been major advances in the surgical management of renal and ureteric calculi over the past 15 years. Renal calculi that are increasing in size or causing symptoms or obstruction should be dealt with by one of the techniques that result in complete clearance of the stone and any fragments.
First described by Chaussy in 1982,20 ESWL is now the primary surgical modality for most renal calculi, although the clearance rate for all stone fragments is not as high as originally reported. Plain radiography indicates stone-free status in approximately 34% of patients, but nephrotomography indicates this degree of success in only 17%.21 The success of ESWL varies with the composition, location and size of the stone, the type of generator used and the “targetability” of the stone. Close follow-up to ensure passage and removal of all stone fragments is required. The rate of recurrence after ESWL varies according to stone type but is as high as 20% in patients with infected stones. If stone fragments are retained, regrowth rates are about 33% at 3.6 years.21
For most ESWL procedures conducted with modern equipment, anesthesia is not required, and patients are treated in the outpatient setting with light sedation. During the treatment, the patient receives about 2000 shocks from an ellipsoid generator; this lasts about 30 minutes depending on the size of the stone and the number of shocks that have to be delivered. This is associated with some discomfort, hematuria and the passage of multiple small stone fragments.
A number of other minimally invasive ureteroscopic and percutaneous surgical procedures are available. The combination of these with ESWL for large and staghorn calculi has given rise to the term “sandwich therapy.”22 This combined therapy entails percutaneous fragmentation and removal of as much stone as possible, followed by ESWL to those areas that could not be reached initially. Fewer percutaneous passes are required, and the risk of bleeding and infection is lessened. Second-look percutaneous nephrostomy passing through previously created tracts can be performed to remove stone fragments that are not passed after ESWL. Follow-up surveillance and antibiotic therapy, where appropriate, form an integral part of this therapy.
Newer techniques involving retrograde approaches to ureteral and renal calculi use miniature flexible and semi-rigid ureteroscopes, which permit access to the entire ureter and collecting system. There are few stones that cannot be managed by minimally invasive techniques. Open stone removal is necessary only for patients with anatomic anomalies and those for whom the newer modalities have failed.
Cases revisited
The patient described in case 1 probably has calcium oxalate nephrolithiasis because it is the most common form of nephrolithiasis, although there is nothing in the urine analysis to help confirm the diagnosis. Stone analysis would be very helpful, emphasizing the need to retain kidney stones whenever possible. Individuals who form kidney stones have low urine volume, thus an increase in fluids taken orally to produce a urine output of 2.5 L/day is recommended. Urinary citrate excretion is borderline, and dietary changes including reduction in animal protein intake and sodium intake are advisable. It would be reasonable to prescribe potassium citrate, 30–80 mEq/day.
The patient described in case 2 has probably developed Stevens–Johnson syndrome as a complication of the allopurinol. The medication should be discontinued immediately and the patient referred to an internist for urgent admission to hospital. Treatment is mainly supportive, with attention to concomitant organ dysfunction (liver, kidneys), nutrition and, possibly, the use of corticosteroids. This condition may progress to toxic epidermal necrolysis. Such severe reactions to allopurinol are very rare, but it is important to recognize them.
Comment
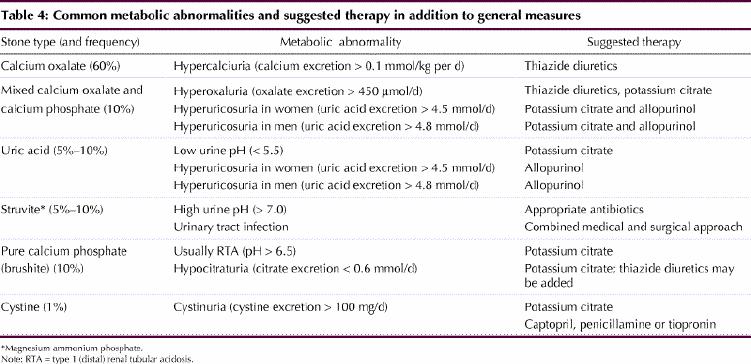
Renal stone disease remains common. A logical medical and surgical approach will reduce both the acute and recurrent morbidity associated with this condition. A summary of metabolic abnormalities associated with stone formation and suggested therapies is presented in Table 4.
Table 4
Key points .
Risk factors for recurrent stones
· Low urine volume
· Elevated urinary oxalate excretion
· Elevated urinary calcium excretion
· Elevated urinary uric acid excretion
· Low urinary level of inhibitors of stone formation, particularly citrate
· Low or high urine pH
· Urine stasis and infection
Considerations for referral for specialist evaluation
· Young age (children)
· Strong family history of kidney stones
· Radiographic identification of multiple stones, anatomic abnormalities or nephrocalcinosis
· Cystine or uric acid stones revealed by laboratory analysis
· Presence of infected stones
· Requirement for surgical intervention (including extracorporeal shock wave lithotripsy)
General measures to prevent recurrence of stones
· Increase fluid intake to produce 2–3 L of urine daily
· Decrease dietary oxalate (spinach, rhubarb, chocolate, nuts, cranberries)
· Decrease intake of animal protein
· Restrict sodium (< 100 mmol/d [2–3 g/d]) rather than calcium (should be 1 g/d)
Indications for surgical removal of stones
· Nonprogression of ureteric stones
· Persistent obstruction
· Persistent symptoms
· Presence of infection
Footnotes
Competing interests: None declared.
Correspondence to: Dr. A. Ross Morton, Renal Unit, Kingston General Hospital, 76 Stuart St., Kingston ON K7L 2V7; fax 613 548-0686; mortona2@kgh.kari.net
References
- 1.Clark JY, Thompson IM, Optenberg SA. Economic impact of urolithiasis in the United States. J Urol 1995;154:2020-4. [PubMed]
- 2.Asplin JR, Favus MJ, Coe FL. Nephrolithiasis. In: Brenner BM, editor. Brenner and Rector's the kidney. 5th ed. Philadelphia: WB Saunders; 1996. p. 1893–935.
- 3.Lieske JC, Deganello S, Toback FG. Cell-crystal interactions and kidney stone formation. Nephron 1999;81(Suppl 1):8-17. [DOI] [PubMed]
- 4.Wu W, Gerard DE, Nancollas GH. Nucleation at surfaces: the importance of interfacial energy. J Am Soc Nephrol 1999;10:S355-8. [PubMed]
- 5.Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol 1996;155:839-43. [PubMed]
- 6.Curham GC, Willett WC, Speizer FE, Stampfer MJ. Beverage use and risk for kidney stones in women. Ann Intern Med 1998;128:534-40. [DOI] [PubMed]
- 7.Rodgers A. Aspects of calcium oxalate crystallization: theory, in vitro studies, and in vivo implementation. J Am Soc Nephrol 1999;10:S351-4. [PubMed]
- 8.Low RK, Stoller ML. Uric acid-related nephrolithiasis. Urol Clin North Am 1997;24:135-48. [DOI] [PubMed]
- 9.Rudman D, Kutner MH, Redd SC II, Waters WC IV, Gerron GG, Bleier J. Hypocitraturia in calcium stone formers. J Clin Endocrinol Metab 1982; 55(6):1052-7. [DOI] [PubMed]
- 10.Khan SR, editor. Proceedings of the 2nd Finlayson Symposium on Urolithiasis. J Am Soc Nephrol 1999;10(Suppl 14):S232-466.
- 11.Manthey DE, Teichman J. Nephrolithiasis. Emerg Med Clin North Am 2001;19(3):633-54. [DOI] [PubMed]
- 12.Parivar F, Low RK, Stoller ML. The influence of diet on urinary stone disease. J Urol 1996;155:432-40. [PubMed]
- 13.Hoskins DH, Erickson SB, Vandenberg CJ. The stone clinic effect in patients with idiopathic calcium urolithiasis. J Urol 1983;130:1115-8. [DOI] [PubMed]
- 14.Pearle MS, Roehrborn CG, Pak CYC. Meta-analysis of randomized trials for medical prevention of calcium oxalate nephrolithiasis. J Endourol 1999; 13:679-85. [DOI] [PubMed]
- 15.Ettinger B, Tang A, Citron JT, Livermore B, Williams T. Randomized trial of allopurinol in the prevention of calcium oxalate calculi. N Engl J Med 1986; 315:1386-9. [DOI] [PubMed]
- 16.Pak CYC, Sakhaee K, Fuller C. Successful management of uric acid nephrolithiasis with potassium citrate. Kidney Int 1986;30:422-8. [DOI] [PubMed]
- 17.Segura JW, Preminger GM, Assimos DG, Dretler SP, Kahn RI, Lingeman JE, et al. Nephrolithiasis Clinical Guidelines Panel summary report on the management of staghorn calculi. J Urol 1994;151:1648-51. [DOI] [PubMed]
- 18.Norman RW, Manette WA. Dietary restriction of sodium as a means of reducing urinary cystine. J Urol 1990;143:1193-5. [DOI] [PubMed]
- 19.Cohen TD, Streem SB, Hall P. Clinical effect of captopril on the formation and growth of cystine calculi. J Urol 1995;154:164-6. [PubMed]
- 20.Chaussy C. Extracorporeal shock wave lithotripsy: new aspects in the treatment of kidney stone disease. Munich: Karger; 1982.
- 21.Lahme S, Wilbert DM, Schneider M, Bichler KH. Fate of clinically insignificant residual fragment (CIRF) after ESWL. In: Rodgers AL, Hibbert BE, Hess B, Kahn SR, Preminger GM, editors. Urolithiasis 2000. Proceedings of the 9th International Symposium on Urolithiasis; 2000 Feb 13-17; Cape Town (South Africa). Rondebosch (South Africa): University of Cape Town Publishers; 2000. p. 748-9.
- 22.Streem SB. Sandwich therapy. Urol Clin North Am 1997;24:213-23. [DOI] [PubMed]


