Abstract
Background
The role and impact of RSV in the adult population is not well understood and comparative data of RSV infection, influenza A/B and SARS-CoV-2 in the elderly hospitalized for respiratory infections is limited.
Methods
In a retrospective, monocentric study we analyzed data of adult patients with respiratory infections tested positive by PCR for RSV, Influenza A/B and SARS-CoV-2 over a four-year period from 2017 to 2020. Symptoms on admission, laboratory results, and risk factors were assessed, and the clinical course and outcomes were studied.
Results
A total of 1541 patients hospitalized with respiratory disease and PCR positive for one of the 4 viruses were enrolled in the study. RSV was the second most prevalent virus before the COVID-19 pandemic and RSV patients represent the oldest group in this study with an average age of 75 years. Neither clinical nor laboratory characteristics differ clearly between RSV, Influenza A / B and SARS-CoV-2 infections. Up to 85% of patients had risk factors, with COPD and kidney disease found particularly frequently in RSV infections. Hospital stay was 12.66 days for RSV patients and thus significantly longer than for influenza A / B (10.88 and 8.86, respectively, p < 0.001), but shorter than for SARS-CoV-2 (17.87 days, p < 0.001). The risk for ICU admission and the rate of mechanical ventilation were also higher for RSV than for influenza A (OR 1.69 (p = 0.020) and 1.59 (p = 0.050)) and influenza B: (1.98 (p = 0.018) and 2.33 (p < 0.001)), but lower than for SARS-CoV-2 (0.65 (p < 0.001) and 0.59 (p = 0.035)). The risk of hospital mortality for RSV was increased compared with influenza A (1.55 (p = 0.050)) and influenza B (1.42 (p = 0.262)), but lower compared to SARs-CoV-2 (0.37 (p < 0.001).
Conclusion
RSV infections in elderly are frequent and more severe than those with influenza A/B. While the impact of SARS-CoV-2 most likely decreased in the elderly population due to vaccination, RSV can be expected to continue to be problematic for elderly patients, especially those with comorbidities and thus, more awareness on the disastrous impact of RSV in this age group is urgently needed.
Keywords: Severe respiratory infections, RSV, Influenza A/B, SARS-CoCV-2, Elderly
1. Background
Respiratory syncytial virus (RSV) is the most common cause for hospitalization in children during the winter months [1]. There is evidence that RSV can also cause severe infections in adults [2]. Especially elderly people who are already susceptible to severe respiratory infections are at increased risk of requiring emergency treatment or hospitalization in the course of RSV infections [3,4]. No vaccine preventing RSV infection is yet available and in the absence of an antiviral therapy for RSV, treatment can only be symptomatic and supportive. Estimating the true incidence of RSV in the adult population has historically been associated with difficulties in diagnosing clinical infections correctly [5], technical difficulties in testing [6] and low public awareness of RSV in adults.
For the post-pandemic winter season of 2022/2023, with widely dropped intervention measures against SARS-CoV-2 infections which were effective also against other respiratory infections) [7], an early and intense wave of influenza is widely expected, given the lack of influenza during the COVID-19 pandemic and the decline in population immunity [8,9]. The situation for RSV infections is similar [10] but the awareness for the impact of RSV on morbidity and mortality in adults is lacking.
There are data comparing characteristics and outcomes in hospitalized adult patients with severe respiratory infections caused by RSV and influenza A/B [11], but comparisons of RSV with influenza as well as SARS-CoV-2 infections have been limited. As we have begun to systematically collect such data since 2017, we have been able to examine patient characteristics, clinical presentation, risk factors, and outcomes such as length of hospital stay, complications, and need for critical care in adults with RSV infection compared to influenza A/B and SARS-CoV-2
2. Material and methods
2.1. Study setting and data collection
Data was collected retrospectively during four consecutive seasons (2017 – 2020) from a maximum care hospital with 905 beds. All adult patients with a positive PCR test for RSV, Influenza A / B and SARS-CoV- 2 and influenza-like symptoms or an expanded symptom complex (fatigue, enteritis) at admission between 2017 and 2020 were included in the data analyses. All patients with SARS-CoV-2 infections were recorded in 2020, when no variants of concern (VOC) circulated in Germany. The indication for testing was made by the attending physician based on a work case definition of the official German Healthcare Services (Robert Koch Institute (RKI)) or on clinical experience with older patients published elsewhere [12], [13], [14]. Factors known to influence the morbidity and mortality of respiratory infections (age, sex, heart insufficiency / coronary heart disease, chronic obstructive lung disease (COPD), end stage renal disease, diabetes and cancer) were collected from patient-records based on ICD10. However, data on influenza vaccination status were not available.
Clinical symptoms of respiratory infection (cough, headache, body temperature) and expanded symptom complex in case of suspicion of a masked course (weakness, enteritis) were recorded at admission and collected from patient-records. Blood tests were performed on admission and parameters for inflammation (C-reactive protein, leukocytes) and disease severity (lactate dehydrogenase, glucose) were collected from the laboratory information system.
2.2. Testing for respiratory viruses
Nasal (RSV), nasopharyngeal (RSV / Influenza A / B, SARS-CoV-2) or oropharyngeal (SARS) swabs were taken when testing was indicated. Reverse transcription polymerase chain reactions (RT-PCR) for RSV, Influenza A and influence B were performed with the commercial triplex assay Xpert™ Xpress FLU / RSV (2017 – 11/2020) on the GeneExpert™ platform (Cepheid Inc., USA). For testing of SARS-CoV-2 by RT-PCR, first the LightMix™ Modular SARS and WUHAN CoV-E-gene test on a LightCycler™ 480 (Roche, Germany) combined with a RNA extraction step (Nucleospin RNA Virus, Machery Nagel, Germany) was introduced for routine testing in 02/2020 (Roche Diagnostic, Germany) followed by the Xpert™ Xpress CoV-2 test (Cepheid) in 04/2020. In 12/2020, the quadruplex Xpert™ Xpress CoV-2 / Flu / RSV (Cepheid) was introduced for routine testing of the four viruses. Particular with regard to SARS-CoV-2 testing, the distinct RT-PCR-tests have comparable specificities and sensitivities as published elsewhere.
With regard to triplex or quadruplex testing for viruses, only test results that were requested by the physician were reported into LIS and statistically analyzed (test results that were not requested by the physiscian were only kept in the daily data memory of the devices and then automatically deleted).
2.3. Outcomes
To characterize the clinical course of patients with RSV compared to the other virus infections, the duration of hospital stay was calculated. Furthermore, the rate of complications like pneumonia (definition: infiltrate of the lung in an x-ray image), the rate of intensive care stay, frequency of mechanical ventilation (invasive or non-invasive), the rate of bacterial superinfections (documentation within the patient file by the attending physiscian and antibiotic use) and the hospital mortality were of interest.
2.4. Statistics
Analysis of possible differences between groups were performed with SPSS, version 21. Comparisons based on Fishers exact test including calculation of odds ratios with associated confidence intervals were performed using R software, version 4.2.0.
2.5. Ethics
The present study was reviewed and approved by the Ethics Committee of the University Hospital Regensburg (no. 22–2973–104).
3. Results
3.1. Infections and hospitalizations
In the present study, a total of 1537 patients with respiratory symptoms / or extended symptom complex and positive PCR test results for RSV (n = 318) / Influenza A (591) / B (289),and SARS CoV-2 (342) were evaluated from 2017 to 2020. RSV and Influenza A / B in-patients were collected during four seasons (each year between January to April) while for SARS CoV-2, all in-patients with and symptoms during 2020 were included. Possible patients with co-infections have been included. Although we do not have information on such patients, we assume that such patients are very rare and did not have a significant impact on the statistical analysis.
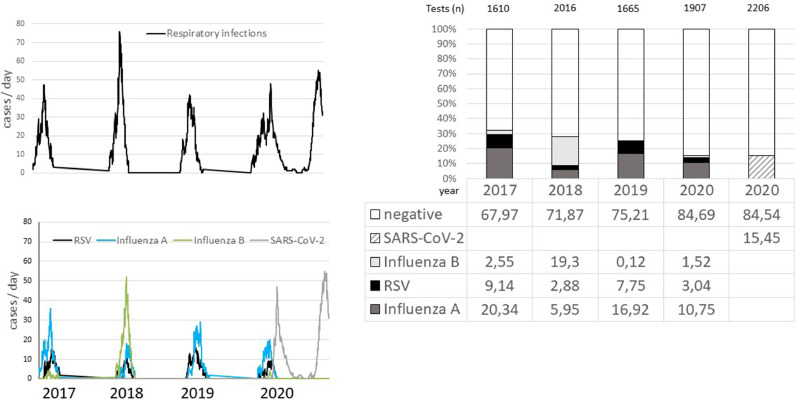
Hospitalization of patients with RSV, Influenza A / B and SARS-CoV-2 was recorded from 2017 to 2020 (Fig. 1 A and B). During the 2020 winter season, infections with RSV, influenza A and SARS occurred with overlap or in quick succession, respectively. In general, peaks for RSV were found between calendar week (CW) 7 and 9, for Influenza A between CW 6 and 9, for Influenza B between CW 8 and 9 and for SARS CoV-2 at CW 15 (first wave) and CW 49 (second wave). The cumulative detection rate of RSV or influenza A/B decreased from 32.12% in 2017 to 15.31% in 2020. Positivity rates of RSV ranged from 2.9% to 9.1% per season, being more frequent than influenza B in 3 of 4 seasons (Fig. 1C). Overall, the evaluation of PCR test results shows a high proportion of negative test results (between 64.31% and 84.69%). Cumulative positivity rates for both SARS-CoV-2 waves were 15.45%. It should be mentioned that the positivity rates based on panel testing may be lower than if only mono-tests had been performed.
Fig. 1.
A. Time course relating to severe respiratory infections during the season 2017 – 2020 (cumulative hospitalized patients per day): the highest activity was found in 2018 with up to 78 patients / day. B. Time course of infections related to RSV, influenza A, influenza B and SARS-CoV-2. The highest activity was found during the second wave of the corona pandemic with up to 55 patients / day and during the season 2018 with a high influenza activity. The data show that the infection waves with RSV and Influenza usually overlap: peaks for RSV were found between week 7 and 9 / for Influenza A between 6 and 9 / for Influenza B beween 8 and 9. C. The stack bars show the distribution of PCR detection rates for RSV (black bar) compared to the other virus detections (Influenza A; deep gray bar; Influenza B: gray bar; SARS-CoV-2: hatched bar). The total number of tests / season is given above the respective year bar in the graph.
3.2. Patient characteristics, clinical symptoms and laboratory parameters
Overall, patients with RSV infections appear to be the oldest patient group. With an average of 75.1 years, they were significantly older than SARS patients with 70.8 years when hospitalized (Table 1 ). Coughing was most prevalent in Patients with RSV and least prevalent in SARS, while headaches were most frequently found in Influenza B and relatively rare in RSV patients. Fatigue is common in all patients but most prominent in Influenza B and SARS patients, while enteritis was most common with Influenza A and B. Overall, no characteristic pattern of symptoms was found for any of these infections. Fever is not common in patients with any of these viral infections. Concerning laboratory parameters, LDH was lower in RSV infections compared to SARS CoV-2, and leukocytes were increased compared to Influenza B /SARS-CoV-2. Again, there was no typical pattern of laboratory parameters discriminating these infections in the elderly.
Table 1.
Demographic, clinical and laboratory parameters of RSV compared to Influenza A, Influenza B and SARS-CoV-2.
| Parameter / virus (n) | RSV (318) | Influenza A (591) | Influenza B (289) | SARS-Cov-2 (342) | p |
| Demographic data | |||||
| Age years | 75.1 (14.3)& | 72.9 (15.3)&& | 75.7 (13.7)&& | 70.8 (15.9)&&& | < 0.001 |
| Gender f/m | 135/183 | 261/330 | 135 / 154 | 168 / 174 | 0.301 |
| Clinical symptoms | |||||
| Cough% | 71& | 68 | 66 | 51&& | < 0.001 |
| Headache% | 7*,** | 13 | 18 | 14 | < 0.001 |
| Weakness% | 67#,## | 67###,#### | 83 | 78 | < 0.001 |
| Enteritis | 10$ | 21 | 20 | 17 | < 0.001 |
| Laboratory parameters | |||||
| Body temperture °C | 37.31 (0.97)& | 37.54 (1.03)&&,&&& | 37.27 (0.86) | 37.31 (0.93) | < 0.001 |
| Leukcocytes n/ul | 10.1 (7.6)*,** | 8.4 (5.8) | 7.4 (6.7) | 8.5 (11.7) | < 0.001 |
| C-reactive protein mg/dL | 62.6 (77.1)# | 65.9 (81.0) | 48.8 (68.8)## | 83.4 (83.1)### | < 0.001 |
| Lactate dehyrogenase IU/mL | 265 (114)% | 309 (474) | 273 (176) | 409 (632)%% | < 0.001 |
| Glucose mg/dL | 145 (64)$ | 137 (57) | 135 (69) | 129 (53) | 0.03 |
(): standard deviation.
Demographic data:.
& RSV vs. SARS: p = 0.01 (&&Influenza B vs. Influenza A: p = 0.048 / &&&SARS—CoV-2 vs. Influenza A / B: p = 0.001, respectively).
Clinical Symptoms:.
&RSV vs. SARS-CoV-2: p < 0.001 (&&SARS-CoV-2 vs. Influenza A / B: p < 0.001, respectively).
*RSV vs. Influenza B: 0.04; **RSV vs. SARS-CoV-2 < 0.001.
#RSV vs. Influenza B: p < 0.001, ##RSV vs. SARS-CoV-2: p = 0.005 (###Influemza A vs. Influenza B: p < 0.001; ####Influenza A vs. SARS-CoV-2: p = 0.001).
$RSV vs. Influenza A: < 0.001.
Laboratory parameters.
&RSV vs. Influenza A: p = 0.014 (&&Influenza A vs. Influenza B: p = 0.014 / &&&Influenza A vs. SARS-CoV-2: p = 0.010.
*RSV vs. Influenza A: p = 0.015 / ⁎⁎RSV vs. Influenza B: p < 0.001.
#RSV vs. SARS-CoV-2: p < 0.01 (##Influenza B vs. Influenza A: p = 0.016 / ###SARS-CoV-2 vs. Influenza A / B: p < 0.01, respectively).
%RSV vs. SARS-CoV-2: p < 0.01 (%%SARS vs. Influenza A / B: p < 0.01, respectively).
§RSV vs. SARS-CoV-2: p = 0.020.
3.3. Co-morbidities
The majority of the patients included in the study had at least one of the five risk factors for a severe course of respiratory disease 69.2% for SARS to 85.2% in RSV patients (Table 2). In RSV patients, COPD was significantly more prevalent compared to Influenza B and SARS CoV-2. Furthermore, RSV patients had more often kidney diseases compared to Influenza A and SARS.
3.4. Disease severity and outcomes
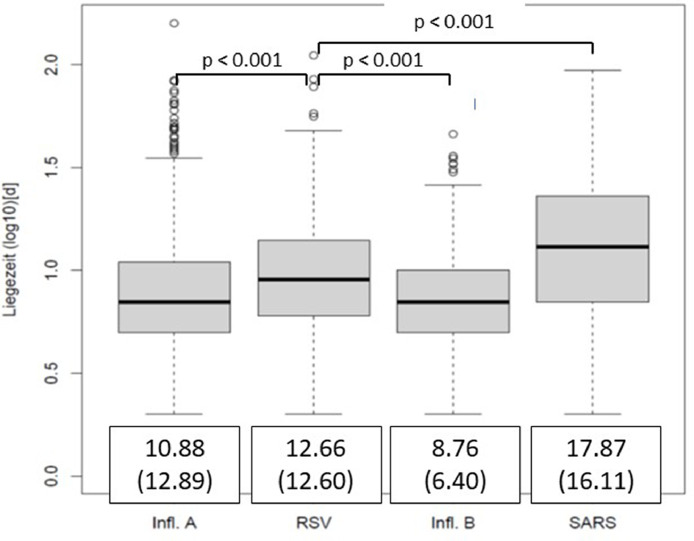
On average, patients with RSV stayed significantly longer in the hospital than patients with Influenza A or Influenza B, but shorter than SARS CoV-2 patients (Fig. 2 ). The risk to develop pneumonia, acquire bacterial superinfection, go on mechanical ventilation and for a fatal outcome was higher with RSV than in influence A/B but lower than with SARS-CoV-2 infections (ORs 95% CI are given in Fig. 3A ). The same trends were observed for the risk to be transferred to ICU, for which RSV patients had the second highest risk after SARS-CoV-2 patients (Fig. 3B).
Fig. 2.
Comparison of hospital stay of RSV patients to Influenza A / B and SARS-CoV-2 (box plots, days (log)). Patients with RSV infections stay longer at hospital compared to patients with Influenza A / B, and shorter compared to SARS-CoV-2 (mean days (standard deviations) of hospital stay are given framed below the box plot).
Fig. 3.
A.The figure shows the proportion of patients with viral infections who had a severe clinical course characterized by having pneumonia, mechanical ventilation, bacterial superinfections and a fatal outcome (mortality). OR (95%CI, p) were calculated for RSV compared to other viruses (table). B.The figure shows the proportion of patients with viral infections who had to be transferred to intensive care units: Patients with RSV infections had a significantly higher risk (OR (95%CI, p) of intensive care than patients with Influenza A / B, but a lower risk than patients with SARS-CoV-2.
4. Discussion
Our data show that RSV is a highly prevalent and severe infection in the elderly. Second only to the original strain of SARS-CoV-2, it leads to significantly more complications, a more severe course with more admissions to ICU, a longer stay in the hospital and even more fatalities than influenza A/B. RSV, Influenza A / B and SARS-CoV-2 could not be distinguished from each other on the basis of clinical appearance and routine blood analyses performed; ultimately, a PCR test must be performed for differentiation.
RSV, Influenza A and Influenza B epidemics have annual and overlapping peaks in winter months, which was also observed for the first waves of SARS-CoV-2 in Germany [15]. In our study, RSV was detected in up to 10% of these patients before the occurrence of SARS-CoV-2, which is comparable to results from other countries [16], [17], [18]. Despite using a multiplex PCR, RSV, Influenza A / B and SARS-CoV-2 were only detected in about 1/3 of tests. Detection rates of respiratory pathogens causing serious infections could be increased to 70% at best by extending the PCR panel to additional viruses and bacteria [19]. The patients with influenza B infection represent a relatively homogeneous population, as they mostly originate from the 2018 season in which the Yamagata strain was circulating. For influenza A patients, we did not distinguish between seasonal (H3N2) and pandemic influenza (H1N1pd2009), which could potentially affect the clinical course. As we did not collect data on influenza vaccination status, so breakthrough infections could not be discriminated. The SARS-CoV-2 infections described here are caused by the original strain when no vaccinations were yet available.
In the present study, RSV, influenza A and B, and SARS -CoV-2 all caused severe respiratory infection requiring hospitalization. Especially in older patients > 65 years, symptoms of respiratory infections are rather weak and only 60 - 70% present any symptoms at all, such as cough and sore throat [12], while fever is not a useful diagnostic criterion in this group of patients, as shown by our group and others [20], [21], [22]. Neither in clinical symptoms nor in laboratory parameters can adult RSV patients be distinguished from patients with other respiratory viral infections [23,24]. However, in the latter study the neutrophil-to-lymphocyte ratio was at least one parameter to distinguish SARS-CoV-2 from RSV infections. While levels of LDH cannot help to discriminate RSV from influenza, LDH levels were found to be especially high in patients with SARS-CoV-2 and these may be related to increased tissue damage by SARS-CoV-2, which is supported by previous findings.
Among our hospitalized RSV patients, more than 85% also had a suggested risk factor for severe respiratory infections, which is significantly higher than in elderly out-patients with mild RSV courses [25]. Especially COPD and renal disease are associated with RSV infections in our hospitalized cohort, and an association between chronic lung disease and severe RSV infection has been suggested previously [26], [27], [28] - but not for SARS-CoV-2 patients [29,30]. The association of RSV with renal disease is not that clear. While we were able to demonstrate a significantly higher proportion of kidney disease in RSV compared to influenza [13], other studies only found a trend [27] or no association [31].
In our current study, RSV, Influenza A/B as well as SARS-CoV-2 patients have mean ages >70 years, but the average age is highest in the RSV group. This finding is common [22,23,31] whereas an opposite trend can be observed for influenza, which is in line with a population-based study from US [26]. One explanation for this observation could be the vaccination recommendations for the elderly and high-risk patients as well as the vaccination coverage rate: In a German surveillance study [32], the influenza vaccination rate among >80-year-olds was as high as >50% - well above the average of 38.8%. Since vaccination is known to have a protective effect on morbidity and mortality [33], [34], [35], this may have led to a selection bias towards younger patients at lower risk, which had a mitigating effect on the clinical observations in the influenza patients in our study. The increased age of RSV patients may per se also contribute to the worse outcomes in this patient group.
Our data show a significantly longer stay in hospital for patients with RSV infections (12.6 days) compared to an infection with influenza A (10.9) and B (8.7), only topped by those with SARs-CoV-2 (17.9). While, reported lengths of stay vary widely between studies and health systems [4,36], a recent Swedish study also found that the length of stay was the longest for SARS-CoV-2 patients followed by RSV patients [37].
Based on our data, the clinical course of the respiratory disease is worst in SARS-CoV-2 patients, followed by RSV infections and influenza. The risk for RSV patients to be admitted to the ICU was 13%, which is also comparable to other studies with 15% and 17% [4,38] and significantly increased compared to patients with influenza A / influenza B. In our study, patients with RSV infection also required mechanical ventilation more frequently than those with Influenza A/B, but significantly less frequently than patients with SARS-CoV-2. The relatively high overall rate of ventilation may be due to advanced age in our cohort and thus, these rates are well comparable to patients <70 years [25], but higher than in younger patients with rates of 3–4% [39,40].
In our study we cannot say whether patients died from the viral infection or with the viral infection. In the above-mentioned Swedish study, the 30days-mortality was highest for SARS-CoV-2 (13%) compared to RSV (7%) and influenza (5%). Although we have only recorded hospital mortality, there are parallels to our data here; however, in other studies the mortalities of RSV and influenza was comparable [41] or in some also higher for RSV [19].
The strengths of the present study are the relatively broad database, the inclusion of patients based on virus PCR and the coverage of 4 consecutive years with comparative study tools. A disadvantage of the study is the monocentric and retrospective design. Although the diagnosis was made by PCR, the indication for testing was left to the treating physician; as already described, some of the patients did not show typical symptoms, so that a certain test bias is to be expected here. Finally, data comparison with infections by the original SARS-CoV-2 strain is in the meantime more historical in nature. However, such data can also contribute to our knowledge about the extraordinary nature of such pandemic events when they coincide with seasonal viral infections.
In conclusion, our data puts RSV in the spotlight as a leading source of severe respiratory disease in the elderly. As vaccinations are available now not only against influenza but also SARS-CoV-2, it can be expected that its role and impact on this population will diminish while RSV will not lose any of its potential to cause severe and even fatal infections in this age group. In contrast, even more severe RSV waves are likely after lifting infection control measures related to the COVID-19 pandemic. Thus, a better awareness of the impact of RSV infections on elderly high-risk patients is required.
Funding
Funding was received by a grant from Janssen Cilag, Germany.
Declaration of Competing Interest
None
References
- 1.Shi T., McAllister D.A., O'Brien K.L., Simoes E.A.F., Madhi S.A., Gessner B.D., Polack F.P., Balsells E., Acacio S., Aguayo C., Alassani I., Ali A., Antonio M., Awasthi S., Awori J.O., Azziz-Baumgartner E., Baggett H.C., Baillie V.L., Balmaseda A., Barahona A., Basnet S., Bassat Q., Basualdo W., Bigogo G., Bont L., Breiman R.F., Brooks W.A., Broor S., Bruce N., Bruden D., Buchy P., Campbell S., Carosone-Link P., Chadha M., Chipeta J., Chou M., Clara W., Cohen C., de Cuellar E., Dang D.A., Dash-Yandag B., Deloria-Knoll M., Dherani M., Eap T., Ebruke B.E., Echavarria M., de Freitas Lázaro Emediato C.C., Fasce R.A., Feikin D.R., Feng L., Gentile A., Gordon A., Goswami D., Goyet S., Groome M., Halasa N., Hirve S., Homaira N., Howie S., Jara J., Jroundi I., Kartasasmita C.B., Khuri-Bulos N., Kotloff K.L., Krishnan A., Libster R., Lopez O., Lucero M., Lucion F., Lupisan S., Marcone D., McCracken J.P., Mejia M., Moisi J.C., Montgomery J.M., Moore D.P., Moraleda C., Moyes J., Munywoki P., Mutyara K., Nicol M.P., Nokes D.J., Nymadawa P., da Costa Oliveira M.T., Oshitani H., Pandey N., Paranhos-Baccalà G., Phillips L.N., Picot V.S., Rahman M., Rakoto-Andrianarivelo M., Rasmussen Z.A., Rath B.A., Robinson A., Romero C., Russomando G., Salimi V., Sawatwong P., Scheltema N., Schweiger B., Scott J.A.G., Seidenberg P., Shen K., Singleton R., Sotomayor V., Strand T.A., Sutanto A., Sylla M., Tapia M.D., Thamthitiwat T., Thomas E.D., Tokarz R., Turner C., Venter M., Waicharoen S., Wang J., Watthanaworawit W., Yoshida L.M., Yu H., Zar H., Campbell H., Nair H. RSV global epidemiology network. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017 Sep 2;390(10098):946–958. doi: 10.1016/S0140-6736(17)30938-8. Epub 2017 Jul 7. PMID: 28689664; PMCID: PMC5592248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen-Van-Tam J.S., O'Leary M., Martin E.T., Heijnen E., Callendret B., Fleischhackl R., Comeaux C., Tran T.M.P., Weber K. Burden of respiratory syncytial virus infection in older and high-risk adults: a systematic review and meta-analysis of the evidence from developed countries. Eur. Respir. Rev. 2022 Nov 15;31(166) doi: 10.1183/16000617.0105-2022. PMID: 36384703; PMCID: PMC9724807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jansen A.G., Sanders E.A., Hoes A.W., van Loo A.M., Hak E. Influenza- and respiratory syncytial virus-associated mortality and hospitalisations. Eur. Respir. J. 2007 Dec;30(6):1158–1166. doi: 10.1183/09031936.00034407. Epub 2007 Aug 22. Erratum in: Eur Respir J. 2008 Mar;31(3):691. PMID: 17715167. [DOI] [PubMed] [Google Scholar]
- 4.Falsey A.R., Hennessey P.A., Formica M.A., Cox C., Walsh E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005 Apr 28;352(17):1749–1759. doi: 10.1056/NEJMoa043951. PMID: 15858184. [DOI] [PubMed] [Google Scholar]
- 5.Fowlkes A., Giorgi A., Erdman D., Temte J., Goodin K., Di Lonardo S., Sun Y., Martin K., Feist M., Linz R., Boulton R., Bancroft E., McHugh L., Lojo J., Filbert K., Finelli L. Viruses associated with acute respiratory infections and influenza-like illness among outpatients from the Influenza Incidence Surveillance Project, 2010-2011. J. Infect. Dis. 2014 Jun 1;209(11):1715–1725. doi: 10.1093/infdis/jit806. Epub 2013 Dec 12. PMID: 24338352; PMCID: PMC5749912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talbot H.K., Falsey A.R. The diagnosis of viral respiratory disease in older adults. Clin. Infect. Dis. 2010 Mar 1;50(5):747–751. doi: 10.1086/650486. PMID: 20121411; PMCID: PMC2826599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai W., Dürrwald R., Biere B., Schweiger B., Haas W., Wolff T., Buda S., Reiche J. Determination of respiratory syncytial virus epidemic seasons by using 95% confidence interval of positivity rates, 2011-2021, Germany. Influenza Other Respir. Viruses. 2022 Sep;16(5):854–857. doi: 10.1111/irv.12996. Epub 2022 Apr 29. PMID: 35485999; PMCID: PMC9343324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stamm P., Sagoschen I., Weise K., Plachter B., Münzel T., Gori T., Vosseler M. Influenza and RSV incidence during COVID-19 pandemic-an observational study from in-hospital point-of-care testing. Med. Microbiol. Immunol. 2021 Dec;210(5–6):277–282. doi: 10.1007/s00430-021-00720-7. Epub 2021 Oct 4. PMID: 34604931; PMCID: PMC8487758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terliesner N., Unterwalder N., Edelmann A., Corman V., Knaust A., Rosenfeld L., Gratopp A., Ringe H., Martin L., von Bernuth H., Mall M.A., Kallinich T. Viral infections in hospitalized children in Germany during the COVID-19 pandemic: association with non-pharmaceutical interventions. Front. Pediatr. 2022 Aug 11;10 doi: 10.3389/fped.2022.935483. PMID: 36034546; PMCID: PMC9403271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.M. Grobben, H.G. Juncker, K. van der Straten, A.H.A. Lavell, M. Schinkel, D.T.P. Buis, M.F. Wilbrink, K. Tejjani, M.A.F. Claireaux, A. Aartse, C. de Groot, D. Pajkrt, M.K. Bomers, J.J. Sikkens, M.J. van Gils, J.B. van Goudoever, B.J. van Keulen. Decreased passive immunity to respiratory viruses through human milk during the COVID-19 pandemic. microbiol spectr. 2022 Aug 31;10(4):e0040522. doi: 10.1128/spectrum.00405-22. Epub 2022 Jun 28. PMID: 35762813; PMCID: PMC9431045. [DOI] [PMC free article] [PubMed]
- 11.Ackerson B., Tseng H.F., Sy L.S., Solano Z., Slezak J., Luo Y., Fischetti C.A., Shinde V. Severe morbidity and mortality associated with respiratory syncytial virus versus influenza infection in hospitalized older adults. Clin. Infect. Dis. 2019 Jul 2;69(2):197–203. doi: 10.1093/cid/ciy991. PMID: 30452608; PMCID: PMC6603263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Influenza (Teil 1): Erkrankungen durch saisonale Influenzaviren https://www.rki.de/DE/Content/Infekt/EpidBull/Merkblaetter/Ratgeber_Influenza_saisonal.html (15.6.2022).
- 13.Ambrosch A., Klinger A., Luber D., Arp C., Lepiorz M., Schroll S., Klawonn F. Symptomatologie und klinischer Verlauf bei hospitalisierten Erwachsenen mit Virusinfektionen durch Influenza A und Respiratory Syncytial-Virus (RSV) [Clinical Characteristics and Course of Infections by Influenza A- and Respiratory Syncytial Virus (RSV) in Hospitalized Adults] Dtsch Med Wochenschr. 2018 May;143(9):e68–e75. doi: 10.1055/s-0044-102004. GermanEpub 2018 Mar 6. PMID: 29510433. [DOI] [PubMed] [Google Scholar]
- 14.Ambrosch A., Rockmann F., Klawonn F., Lampl B. Effect of a strict hygiene bundle for the prevention of nosocomial transmission of SARS-CoV-2 in the hospital: a practical approach from the field. J. Infect. Public Health. 2020 Dec;13(12):1862–1867. doi: 10.1016/j.jiph.2020.10.005. Epub 2020 Oct 20. PMID: 33144023; PMCID: PMC7574781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robert Koch Institut: COVID-19-dashboard https://experience.arcgis.com/experience/478220a4c454480e823b17327b2bf1d4 (10.08.2022).
- 16.Malosh R.E., Martin E.T., Callear A.P., Petrie J.G., Lauring A.S., Lamerato L., Fry A.M., Ferdinands J., Flannery B., Monto A.S. Respiratory syncytial virus hospitalization in middle-aged and older adults. J. Clin. Virol. 2017 Nov;96:37–43. doi: 10.1016/j.jcv.2017.09.001. Epub 2017 Sep 7. PMID: 28942341; PMCID: PMC5889293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruyndonckx R., Coenen S., Butler C., Verheij T., Little P., Hens N., Beutels P., Ieven M., Goossens H. GRACE project group. Respiratory syncytial virus and influenza virus infection in adult primary care patients: association of age with prevalence, diagnostic features and illness course. Int. J. Infect. Dis. 2020 Jun;95:384–390. doi: 10.1016/j.ijid.2020.04.020. Epub 2020 Apr 19. PMID: 32320810; PMCID: PMC7167228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A.R. Falsey, E.E. Walsh, S. House, Y. Vandenijck, X. Ren, S. Keim, D. Kang, P. Peeters, J. Witek, G. Ispas. Risk factors and medical resource utilization of respiratory syncytial virus, human metapneumovirus, and influenza-related hospitalizations in adults-A global study during the 2017-2019 epidemic seasons (Hospitalized acute respiratory tract infection [HARTI] study). Open Forum Infect Dis. 2021 Oct 5;8(11):ofab491. doi: 10.1093/ofid/ofab491. PMID: 35559130; PMCID: PMC9088513. [DOI] [PMC free article] [PubMed]
- 19.Milucky J., Pondo T., Gregory C.J., Iuliano D., Chaves S.S., McCracken J., Mansour A., Zhang Y., Aleem M.A., Wolff B., Whitaker B., Whistler T., Onyango C., Lopez M.R., Liu N., Rahman M.Z., Shang N., Winchell J., Chittaganpitch M., Fields B., Maldonado H., Xie Z., Lindstrom S., Sturm-Ramirez K., Montgomery J., Wu K.H., Van Beneden; C.A. Adult TAC Working Group. The epidemiology and estimated etiology of pathogens detected from the upper respiratory tract of adults with severe acute respiratory infections in multiple countries, 2014-2015. PLoS ONE. 2020 Oct 19;15(10) doi: 10.1371/journal.pone.0240309. PMID: 33075098; PMCID: PMC7571682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michiels B., Thomas I., Van Royen P., Coenen S. Clinical prediction rules combining signs, symptoms and epidemiological context to distinguish influenza from influenza-like illnesses in primary care: a cross sectional study. BMC Fam. Pract. 2011 Feb 9;12:4. doi: 10.1186/1471-2296-12-4. PMID: 21306610; PMCID: PMC3045895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrat F., Tachet A., Rouzioux C., Housset B., Valleron A.J. Evaluation of clinical case definitions of influenza: detailed investigation of patients during the 1995-1996 epidemic in France. Clin. Infect. Dis. 1999 Feb;28(2):283–290. doi: 10.1086/515117. PMID: 10064245.H. [DOI] [PubMed] [Google Scholar]
- 22.Ambrosch A., Rockmann F. Effect of two-step hygiene management on the prevention of nosocomial influenza in a season with high influenza activity. J. Hosp. Infect. 2016 Oct;94(2):143–149. doi: 10.1016/j.jhin.2016.07.006. Epub 2016 Jul 18. PMID: 27515458. [DOI] [PubMed] [Google Scholar]
- 23.Ben Shimol A., Dahan S., Alon N., Soffer S., Hod K., Brosh-Nissimov T., Shoenfeld Y., Dagan A. Can laboratory evaluation differentiate between coronavirus disease-2019, influenza, and respiratory syncytial virus infections? A retrospective cohort study. Croat. Med. J. 2021 Dec 31;62(6):623–629. doi: 10.3325/cmj.2021.62.623. PMID: 34981695; PMCID: PMC8771226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.L. Prozan, E. Shusterman, J. Ablin, A. Mitelpunkt, A. Weiss-Meilik, A. Adler, G. Choshen, O. Kehat. Prognostic value of neutrophil-to-lymphocyte ratio in COVID-19 compared with Influenza and respiratory syncytial virus infection. Sci. Rep. 2021 Nov 2;11(1):21519. doi: 10.1038/s41598-021-00927-x. PMID: 34728719; PMCID: PMC8563769. [DOI] [PMC free article] [PubMed]
- 25.Korsten K., Adriaenssens N., Coenen S., Butler C., Ravanfar B., Rutter H., Allen J., Falsey A.R., Pirçon J.Y., Gruselle O., Pavot V., Vernhes C., Balla-JhagjhoorsingS S., Öner D., Ispas G., Aerssens J., Shinde V., Verheij T., Bont L., Wildenbeest J. RESCEU investigators. Burden of respiratory syncytial virus infection in community-dwelling older adults in Europe (RESCEU): an international prospective cohort study. Eur. Respir. J. 2021 Apr 1;57(4) doi: 10.1183/13993003.02688-2020. PMID: 33060153. [DOI] [PubMed] [Google Scholar]
- 26.G. Matias, R. Taylor, F. Haguinet, C. Schuck-Paim, R. Lustig, V. Shinde. Estimates of hospitalization attributable to influenza and RSV in the US during 1997-2009, by age and risk status. BMC Public Health. 2017 Mar 21;17(1):271. doi: 10.1186/s12889-017-4177-z. PMID: 28320361; PMCID: PMC5359836. [DOI] [PMC free article] [PubMed]
- 27.P. Loubet, N. Lenzi, M. Valette, V. Foulongne, A. Krivine, N. Houhou, G. Lagathu, S. Rogez, S. Alain, X. Duval, F. Galtier, D. Postil, P. Tattevin, P. Vanhems, F. Carrat, B. Lina, O. Launay; FLUVAC Study Group. Clinical characteristics and outcome of respiratory syncytial virus infection among adults hospitalized with influenza-like illness in France. Clin. Microbiol. Infect. 2017 Apr;23(4):253–259. doi: 10.1016/j.cmi.2016.11.014. Epub 2016 Nov 27. PMID: 27903461; PMCID: PMC7128342. [DOI] [PMC free article] [PubMed]
- 28.E.E. Walsh, D.R. Peterson, A.R. Falsey. Risk factors for severe respiratory syncytial virus infection in elderly persons. J. Infect. Dis. 2004 Jan 15;189(2):233–8. doi: 10.1086/380907. Epub 2004 Jan 9. PMID: 14722887. [DOI] [PubMed]
- 29.Piroth L., Cottenet J., Mariet A.S., Bonniaud P., Blot M., Tubert-Bitter P., Quantin C. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir. Med. 2021 Mar;9(3):251–259. doi: 10.1016/S2213-2600(20)30527-0. Epub 2020 Dec 17PMID: 33341155; PMCID: PMC7832247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O'Donnell L., Chernyak Y., Tobin K.A., Cerfolio R.J., Francois F., Horwitz L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020 May 22;369:m1966. doi: 10.1136/bmj.m1966. PMID: 32444366; PMCID: PMC7243801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chuaychoo B., Rattanasaengloet K., Banlengchit R., Horthongkham N., Athipanyasilp N., Totanarungroj K., Muangman N. Characteristics, complications, and mortality of respiratory syncytial virus compared with influenza infections in hospitalized adult patients in Thailand. Int. J. Infect. Dis. 2021 Sep;110:237–246. doi: 10.1016/j.ijid.2021.07.045. Epub 2021 Jul 22. PMID: 34303842. [DOI] [PubMed] [Google Scholar]
- 32.Vaccination rates among adults in Germany - updates from the KV vaccination surveillance and the online survey of hospital personnel. Epidemiol. Bullet. 2020 https://edoc.rki.de/bitstream/handle/176904/7485/EB-47-Impfquoten%20bei%20Erwachsenen%20in%20Deutschland.pdf?sequence=1&isAllowed=y 47/ [Google Scholar]
- 33.Bao W., Li Y., Wang T., Li X., He J., Wang Y., Wen F., Chen J. Effects of influenza vaccination on clinical outcomes of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ageing Res. Rev. 2021 Jul;68 doi: 10.1016/j.arr.2021.101337. Epub 2021 Apr 1. PMID: 33813014. [DOI] [PubMed] [Google Scholar]
- 34.Taniguchi K., Ikeda S., Hagiwara Y., Tsuzuki D., Klai M., Sakai Y., Crawford B., Nealon J. Epidemiology and burden of illness of seasonal influenza among the elderly in Japan: a systematic literature review and vaccine effectiveness meta-analysis. Influenza Other Respir. Viruses. 2021 Mar;15(2):293–314. doi: 10.1111/irv.12814. Epub 2020 Sep 30PMID: 32997395; PMCID: PMC7902263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yedlapati S.H., Khan S.U., Talluri S., Lone A.N., Khan M.Z., Khan M.S., Navar A.M., Gulati M., Johnson H., Baum S., Michos E.D. Effects of influenza vaccine on mortality and cardiovascular outcomes in patients with cardiovascular disease: a systematic review and meta-analysis. J. Am. Heart Assoc. 2021 Mar 16;10(6) doi: 10.1161/JAHA.120.019636. Epub 2021 Mar 13PMID: 33719496; PMCID: PMC8174205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colosia A.D., Yang J., Hillson E., Mauskopf J., Copley-Merriman C., Shinde V., Stoddard J. The epidemiology of medically attended respiratory syncytial virus in older adults in the United States: a systematic review. PLoS ONE. 2017 Aug 10;12(8) doi: 10.1371/journal.pone.0182321. PMID: 28797053; PMCID: PMC5552193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hedberg P., Karlsson Valik J., van der Werff S., Tanushi H., Requena Mendez A., Granath F., Bell M., Mårtensson J., Dyrdak R., Hertting O., Färnert A., Ternhag A., Naucler P. Clinical phenotypes and outcomes of SARS-CoV-2, influenza, RSV and seven other respiratory viruses: a retrospective study using complete hospital data. Thorax. 2022 Feb;77(2):154–163. doi: 10.1136/thoraxjnl-2021-216949. Epub 2021 Jul 5PMID: 34226206; PMCID: PMC8260304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.K. Widmer, M.R. Griffin, Y. Zhu, J.V. Williams, H.K. Talbot. Respiratory syncytial virus- and human metapneumovirus-associated emergency department and hospital burden in adults. Influenza Other Respir. Viruses. 2014 May;8(3):347–52. doi: 10.1111/irv.12234. Epub 2014 Feb 7. PMID: 24512531; PMCID: PMC3984605. [DOI] [PMC free article] [PubMed]
- 39.Widmer K., Zhu Y., Williams J.V., Griffin M.R., Edwards K.M., Talbot H.K. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J. Infect. Dis. 2012 Jul 1;206(1):56–62. doi: 10.1093/infdis/jis309. Epub 2012 Apr 23PMID: 22529314; PMCID: PMC3415933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker E., Ison M.G. Respiratory viral infections among hospitalized adults: experience of a single tertiary healthcare hospital. Influenza Other Respir. Viruses. 2014 May;8(3):282–292. doi: 10.1111/irv.12237. Epub 2014 Feb 3PMID: 24490751; PMCID: PMC4181476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sieling W.D., Goldman C.R., Oberhardt M., Phillips M., Finelli L., Saiman L. Comparative incidence and burden of respiratory viruses associated with hospitalization in adults in New York City. Influenza Other Respir. Viruses. 2021 Sep;15(5):670–677. doi: 10.1111/irv.12842. Epub 2021 Jan 26PMID: 33501772; PMCID: PMC8013984. [DOI] [PMC free article] [PubMed] [Google Scholar]