Abstract
Background
Hyperbaric oxygen therapy (HBOT) has been proposed to address COVID-19- associated respiratory failure. However, its biochemical effects are poorly known.
Method
50 patients with hypoxemic COVID-19 pneumonia were divided into C group (standard care) and H group (standard care plus HBOT). Blood was obtained at t = 0 and t = 5 days. Oxygen saturation (O2 Sat) was followed up. White blood cell (WC) count, lymphocytes (L) and platelets
(P) and serum analysis (glucose, urea, creatinine, sodium, potassium, ferritin, D dimer, LDH and CRP) were carried out. Plasma levels of sVCAM, sICAM, sPselectin, SAA and MPO, and of cytokines (IL-1β, IL-1RA, IL-6, TNFα, IFNα, IFNγ, IL-15, VEGF, MIP1α, IL-12p70, IL-2 and IP-10) were measured by multiplex assays. Angiotensin Converting Enzyme 2 (ACE-2) levels were determined by ELISA.
Results
The average basal O2 Sat was 85 ± 3%. The days needed to reach O2 Sat >90% were: H: 3 ± 1 and C: 5 ± 1 (P < 0,01). At term, H increased WC, L and P counts (all, H vs C: P < 0,01). Also, H diminished D dimer levels (H vs C, P < 0,001) and LDH concentration (H vs C, P < 0.01]. At term, H showed lower levels of sVCAM, sPselectin and SAA than C with respect to basal values (H vs C: ΔsVCAM: P < 0,01; ΔsPselectin: P < 0,05; ΔSAA: P < 0,01). Similarly, H showed diminished levels of TNFα (ΔTNFα: P < 0,05) and increased levels of IL-1RA and VEGF than C respect to basal values (H vs C: ΔIL-1RA and ΔVEGF: P < 0,05).
Conclusion
Patients underwent HBOT improved O2 Sat with lower levels of severity markers (WC and platelets count, D dimer, LDH, SAA). Moreover, HBOT reduced proinflammatory agents (sVCAM, sPselectin, TNFα) and increased anti-inflammatory and pro-angiogenic ones (IL-1RA and VEGF).
Keywords: COVID-19, Hyperbaric oxygen therapy (HBOT), Cell adhesion molecules, Cytokines, Hypoxemia
Abbreviations
- Angiotensin Converting Enzyme 2
(ACE-2)
- C- Reactive Protein
(CRP)
- Hyperbaric oxygen therapy
(HBOT)
- Hypoxia-inducible factor 1α
(HIF1α)
- Interferon
(IFN)
- Interferon gamma-induced protein 10
(IP-10)
- Interleukin
(IL)
- Lactate dehydrogenase
(LDH)
- Lymphocytes
(L)
- Macrophage inflammatory protein
(MIP-1α)
- Myeloperoxidase
(MPO)
- Oxygen saturation
(O2 Sat)
- Platelets
(P)
- Serum amyloid A
(SAA)
- Soluble Intercellular Adhesion Molecule 1
(sICAM-1)
- Soluble P selectin
(sPsel)
- Soluble Vascular Cell Adhesion Molecule 1
(sVCAM-1)
- Tumor Necrosis Factor alpha
(TNFα)
- Vascular Endothelial Growth Factor
(VEGF)
- White blood cell
(WC)
1. Introduction
COVID-19 is an acute respiratory infection caused by the SARS-CoV-2 which continues to be a pandemic worldwide [1,2]. Approximately, 15–20% of hospitalized patients present with hypoxemic respiratory failure and require oxygen supplementation. However, respiratory failure actually still represents the main cause of death [3,4].
Hyperbaric oxygen therapy (HBOT) has been proposed as an adjuvant treatment to address COVID-19-associated hypoxemia [5,6]. HBOT involves the patient's exposure to 100% of oxygen inside a chamber at a pressure higher than 1 atm absolute (ATA). This procedure increases the partial pressure of oxygen at the blood-alveolar interface, enhancing oxygen diffusion to circulation and tissues. In addition, hyper-oxygenated arterial blood may also exert anti-inflammatory effects, which has been partially explored until now [7].
HBOT is a FDA approved medical treatment indicated for replacing any form of oxygen deficiency such as arterial gas embolism, carbon monoxide poisoning, severe anemia, decompression sickness, and diabetic foot ulcer [8]. The only major contraindications to HBOT are untreated pneumothorax and respiratory failure requiring mechanical ventilation. HBOT at 1.45 ATA has shown to be safer than treatment at pressures mostly exceeding 2.0 ATA, preventing neurological or lung complications among patients with COVID-19 [9]. In addition, this type of equipment presents lower costs and risks of fire accidents, ease of operation, transportation and disinfection.
Nowadays, COVID-19 is not a currently accepted indication for HBOT. However, preliminary clinical evidence of HBOT treatment in hypoxemic COVID-19 patients demonstrated clinical improvement, e.g., reduce intensive care unit (ICU) admission and prevent transition to mechanical ventilation [6,10]. Moreover, recent studies from our group and others pointed out that HBOT could be a decisive treatment for improving outcomes in patients with COVID-19 pneumonia, especially at early stages, and it could also be beneficial during the intubation period [11,12]. However, the mechanisms underlying these effects are poorly known, which limits its use for the treatment of this medical condition.
The objective of this study was to investigate the clinical response to HBOT and to analyze changes in the circulating concentration of markers of inflammation and infection, angiogenesis related factors and cardiovascular risk markers in patients with hypoxemic COVID-19 pneumonia subjected to hyperbaric oxygenation in order to identify potential effects of HBOT on these processes.
2. Materials and methods
2.1. Study design and participants
Patients (n = 50) with hypoxemic COVID-19 pneumonia were recruited from Hospital D. F. Santojanni (Buenos Aires, Argentina) between May–August 2021 in a randomized controlled study comparing the control group receiving standard care (C group) versus standard care plus HBOT (H group) in equal proportion. None of the participants in this study had been previously vaccinated. Eligible patients were aged over 18 years with confirmed diagnosis of COVID-19 by PCR on nasal swab, suffered from pneumonia with oxygen dependence -defined as the need for continuous oxygen supply to maintain pulse oxygen saturation (O2 Sat) ≥90% or arterial gas with PO2 ≥60 mm Hg- and had no previous hospitalization within the last 6 months. Exclusion criteria were: 1- age less than 18 years, 2- patients refusing to sign the informed consent or persons unable to give consent, 3- pregnancy and lactation, 4- requirement of mechanical ventilation, 5- patients unable to maintain sitting position for more than 2 h, 5- patients with contraindications for HBOT: a-acute respiratory distress syndrome (ARDS), which was excluded by physical examination, lung imaging studies, and determination of arterial oxygen level; and b-severe chronic obstructive pulmonary disease, c-emphysema, d-air cysts or bullae, e−untreated pneumothorax. All patients had a baseline imaging study (computed axial tomography of the chest), performed on hospital admission. The protocol followed the principles of the World Medical Association Declaration of Helsinki. After its approval by the Hospital Ethics Committee, all participants signed their written informed consent. This study was carried out in compliance with Good Clinical Practice (GCP). Personal data were protected and encrypted.
All participants received standard treatment for COVID- 19: antibiotic therapy (ceftriaxone 2 g/day and azithromycin 500 mg/day for 7 days), dexamethasone 8 mg/day and paracetamol 1 g/6 h in case of high temperature. Oxygen was supplied with a reservoir mask. Patients were regularly monitored following guidelines from the National Ministry of Health of Argentina [13]. Patients assigned to the treatment group (H) additionally receive daily HBOT sessions of 90 min for 5 days. HBOT was carried out employing a one-person hyperbaric chamber (Revitalair®, Buenos Aires, Argentina) at 1.45 ATA with an inspired fraction close to 100% of oxygen. Between sessions, chambers were disinfected with quaternary ammonium salts. This protocol was based on previous clinical results with patients (n = 9 for each group, total: 18 patients) with COVID- 19 associated hypoxemia (Keller et al., personal communication 2020).
O2 Sat was assessed before and after each HBOT session by removing the oxygen mask and allowing patients to breathe room air for ≥5 min while monitoring O2 Sat with a pulse oximeter. In the control group, each morning O2 Sat was measured in a same way, allowing patients to breathe room air for ≥5 min without oxygen mask. A fractional inspired oxygen of 21% was used to define room air.
To assess the clinical evolution, the time in days necessary for normalization O2 Sat was individually recorded. Normalization of oxygen requirement (oxygen independence) was defined as ≥90% pulse oximetry value breathing ambient air. In addition, vital parameters (systolic and diastolic blood pressure, heart rate, respiratory rate, axillary temperature) and general laboratory (hematocrit, white blood cell and platelets count, blood glucose, urea, creatinine, ionogram, ferritin, D dimer, LDH, C-reactive protein) were monitored. Adverse events were actively examined, through constant questioning and evaluation of patients before, during and after the HBOT sessions, asking them for the presence of ear pain, sensation of auditory obstruction, changes in hearing, changes in respiratory pattern, and any other subjective symptoms reported by the patient.
2.2. Blood analysis
Venous blood samples were collected at baseline (t = 0) and at the end of the study (t = 5 days). When plasma was needed, blood was collected in Vacutainer tubes containing EDTA (Becton, Dickinson, USA). Samples were immediately centrifuged for 10min at 1200 ×g and plasmas were stored at −70 °C until Multiplex and ACE-2 analysis. The rest of the laboratory determinations were performed immediately after obtaining the sample. Samples were processed by independent operators without knowledge of their origin.
Hematological determinations (Hematocrit, white blood cell (WC), lymphocytes (L) and platelets (P) count and serum analysis (glucose, urea, creatinine, sodium, potassium, ferritin, D dimer, lactate dehydrogenase (LDH) and C-Reactive Protein (CRP) were measured using an automatic biochemistry analyzer (Hemocytometry and Flow Cytochemistry Advia 2120 Analyzer). Biochemistry determination were measured using the following methodology: Glucose (UV Enzymatic), Urea (Kinetic), Creatinine (Kinetic/Colorimetric - Jaffe), Ionogram (Ion Selective Electrode), CRP (Immunoturbidimetry) and Ferritin (Immunoturbidimetry).
Plasma levels of Soluble Vascular Cell Adhesion Molecule 1 (sVCAM-1), Soluble Intercellular Adhesion Molecule 1 (sICAM-1), Soluble P selectin (sPsel), Serum Amyloid A (SAA) and Myeloperoxidase (MPO), and of a panel including interleukins (IL): IL-1β, IL-1RA, IL-6, IL-15, IL-12p70 and IL-2; interferons (IFN): IFNα and IFNγ; Tumor Necrosis Factor alpha (TNFα), Vascular Endothelial Growth Factor (VEGF), and the chemokines Macrophage inflammatory protein (MIP-1α), also known as CCL3, and Interferon gamma-induced protein 10 (IP-10), also named CXCL10, were measured by multiplex magnetic bead assays (Merck Millipore) employing a Magpix™ equipment (Merck-Millipore). The panels used were respectively the Human Cardiovascular Disese (CVD) Magnetic Bead Panel 2 (HCVD2MAG-67K) and the Human Cytokine/Chemokine/Growth Factor Panel A (HCYTA-60K), both from Merck Millipore, MO, USA. Each assay was performed according to the manufacturers’ instructions. Standard curves and samples were tested in duplicate. Standards were plotted and concentrations were determined using xPONENT software version 4.2. Plasma concentrations of CVD markers were expressed as ng/ml and those of Cytokine/Chemokine/Growth Factors, as pg/ml.
Angiotensin Converting Enzyme 2 (ACE-2) levels were determined employing a comercial sandwich ELISA (DY933: Human ACE-2 DuoSet ELISA, R&D Systems, USA). Standards and samples were loaded in duplicate. Data processing was performed using GraphPad Prism version 6.00 for Windows (GraphPad Software, San Diego, CA, USA). The standard curve between 0,3 and 20 ng/ml was generated using a four parameter logistic curve-fit. The best fit line was determined by regression analysis. Plasma ACE-2 levels were determined by interpolation. Results were expressed as ng/ml.
2.3. Safety controls
All patients were followed up for 28 days after their inclusion in the study. The length of hospitalization, the presence of complications, oxygen requirement, need for new hospitalizations, and mortality were evaluated.
2.4. Statistical analysis
Data are showed as arithmetic mean ± standard deviation (SD) and range (minimum-maximum). Sample comparisons between patients underwent HBOT (H) or control group (C) respect to their respective baseline measures, were performed using paired Student's t-test. Additionally, the mean relative variation respect to each baseline measure for treated patients (ΔH) or control ones (ΔC)) were calculated and comparisons between ΔH and ΔC were performed using unpaired Student's t-test. All analyses were performed using GraphPad Prism 6.0 software package (San Diego, CA, USA) for Windows. A P value < 0.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics of participants
The average age of the participants was 56 years. Demographic and clinical characteristics are shown in Supplementary Table 1. Supplementary Table 2 shows baseline laboratory outcomes. In addition, no comorbidities (respiratory, cardiovascular, neurological, renal, metabolic, etc.) that marked a difference between the groups were detected. No noteworthy ethnic group differences were detected (all corresponded to ad-mixed white population characteristic of Argentina). No prior baseline drug use was detected in the included patients (considering antihypertensives, antidiabetics, sedatives, hypnotics, and antibiotics). Two patients in each group reported occasional consumption of acid secretion inhibitors (omeprazole). All patients corresponded to a score (WHO ordinal scale) 4, compatible with the presence of hospitalization criteria and oxygen supplementation requirement but without criteria for ventilatory assistance (invasive or non-invasive). As shown, the comparison between the groups did not show significant differences in terms of clinical status, general compromise, laboratory, age, comorbidities or history.
3.2. Effects of HBOT on oxygen saturation
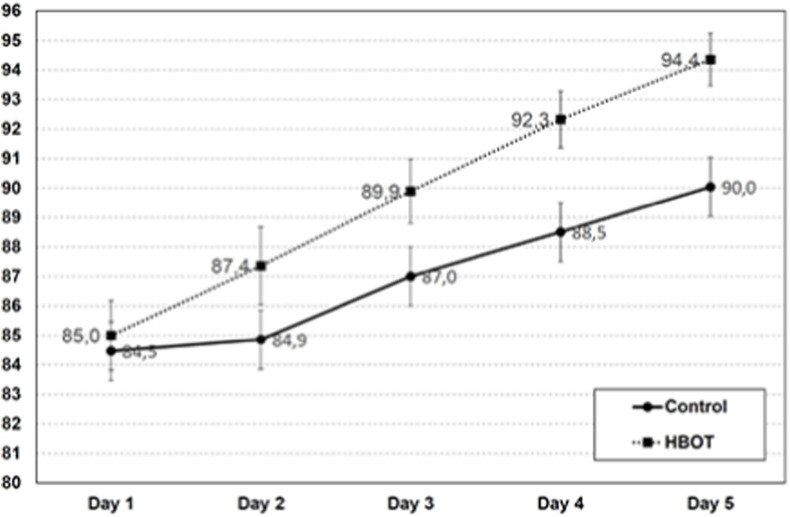
As shown in Supplementary Table 1, the average basal O2 Sat was 85 ± 3% for the whole group (H: 85 ± 3%, C: 84 ± 4), with no significant difference between both groups (P = 0.57). Supplementary Table 3 shows the average O2 Sat recording at pre and post HBOT sessions for the HBOT group (H) and at equivalent times for the control group (C) during 5 days. Except for the O2 Sat measured before the first HBOT session (Pre 1), the rest of the readings of O2 Sat exhibited statistically significant differences between H and C groups. Fig. 1 shows the faster increase in O2 Sat in group H compared to C. The ascending slope for O2 Sat in the HBOT group was significantly higher than in the control group: H = 2,4 ± 0,7 (1,1–3,7), C = 1,5 ± 0,7 (0,7 - 3,0) %/day, (H vs C, P < 0.0001). The days needed to reach O2 Sat >90% were: H: 3 ± 1 and C:5 ± 1 (H vs C, P < 0,01). These results agreed with preliminary data of the group (Keller et al., personal communication 2020).
Fig. 1.
Effect of HBOT on oxygen saturation
Graphs show the daily oxygen saturation (SatO2) breathing ambient air (FiO2 = 0.21%) pre HBOT session (H) or at equivalent time for the control group (C). Group H shows higher 02 Sat than group C since the second day of HBOT treatment (statistical differences are shown in Table 3). The days needed to reach 02 Sat >90% were: H: 3 + 1 and C: 5 ± 1.
3.3. Laboratory and clinical outcomes after HBOT treatment
Table 1 shows the values of laboratory outcomes at the end of the study for the two study groups. Patients underwent HOBT showed increased white cells count, lymphocytes and platelets count (for all, H vs C, P < 0,01). Also, HBOT group diminished urea levels (H = 35 ± 9, C = 40 ± 7, H vs CP < 0.05), D dimer levels (H = 233 ± 81, C = 402 ± 66 ng/ml; P < 0,00001) and LDH concentration (H = 237 ± 92, C: 329 ± 44 UI/L, H vs C P < 0,0001]. No significant differences were detected between groups in hematocrit, blood glucose, creatinine, sodium, potassium, ferritin and CRP levels. Even though adverse events were actively sought, none were reported.
Table 1.
Laboratory outcomes at the end of the study.
| Control | HBOT | P | |
|---|---|---|---|
| HT (%) | 36 ± 5 (26–45) | 37 ± 3 (32–40) | 0,47 |
| WC (/mm3) | 4314 ± 2178 (1610–9840) | 5981 ± 2461 (2710–10020) | <0,01 |
| Lymph. (/mm3) | 1212 ± 290 (900–1720) | 1444 ± 519 (800–2400) | <0,01 |
| Plat(/mm3) | 236143 ± 75630 (124000–369000) | 291786 ± 85361 (193000–436000) | <0,01 |
| BG (mg/dl) | 101 ± 46 (72–230) | 98 ± 10 (84–114) | 0,77 |
| Urea(mg/dl) | 40 ± 7 (22–50) | 35 ± 9 (21–48) | 0,03 |
| Creatin. (mg/dl) | 0,88 ± 0,18 (0,58 - 1,20) | 0,96 ± 0,24 (0,53 - 1,40) | 0,18 |
| Sodium (mEq/L) | 137 ± 5 (128–144) | 136 ± 3 (133–147) | 0,70 |
| Potassium (mEq/L) | 3,97 ± 0,25 (3,70 - 4,40) | 3,81 ± 0,33 (3,40 - 4,30) | 0,06 |
| Ferritin (mg/ml) | 586 ± 107 (357–684) | 540 ± 113 (336–672) | 0,12 |
| LDH (UI/L) | 329 ± 44 (209–381) | 237 ± 92 (129–389) | <0,0001 |
| D dimer (ng/mL) | 402 ± 66 (220–492) | 233 ± 81 (141–390) | <0.00001 |
| CPR(mg/L) | 38,5 ± 18,7 (12,3–62,2) | 37,2 ± 14,1 (13,7–58,5) | 0,77 |
Abbreviations: Creatin. (Creatinine), HT (Hematocrit), WC (White cell), Lymph (Lymphocytes), Plat (Platelets), BG (Blood glucose), LDH (lactate dehydrogenase), CRP (C-reactive protein). Statistical differences between groups (P < 0.05) are highlighted in the right column.
3.4. Plasma levels of cardiovascular disease risk markers
Plasma levels of soluble forms of endothelial adhesion molecules such as sVCAM, sICAM, sPselectin and inflammation related proteins, as SAA and MPO, have been largely associated with a higher risk of cardiovascular disease (CVD). In particular, severe cases of COVID-19 have been mostly associated with endothelial hyperactivation and dysfunction. For this reason, we evaluated the influence of HBOT on circulating levels of CVD markers in patients with COVID-pneumonia. Concentration data in absolute values are shown in Table 2 and variations respect each baseline are displayed in Fig. 2 .
Table 2.
Plasma levels of Cardiovascular Disese Risk Markers.
| Conc. (ng/ml) | BC | C | BH | H | P |
|---|---|---|---|---|---|
| sVCAM | 901.0 ± 166.2 (701.1–123.0) | 937.5 ± 167.3 (732.1–1216.0) | 960.2 ± 135.7 (718.9–1140.0) | 877.2 ± 248.7 (525.3–1238.0) | 0.0216 (#) |
| sPselectin | 138.0 ± 25.8 (103.8–195.7) | 143.8 ± 26.2 (101.9–181.7) | 142.7 ± 23.7 (101.9–176.4) | 136.5 ± 35.7 (76.5–192.0) | Ns |
| sICAM | 177.8 ± 51.30 (116.2–259.8) | 195.5 ± 59.46 (118.8–332,5) | 192.1 ± 55.21 (122.2–272.1) | 203.8 ± 54.24 (96.0–265.9) | Ns |
| SAA | 16816 ± 3598 (11345–26353) | 19974 ± 4336 (12043–28487) | 17618 ± 3926 (11575–24874) | 15487 ± 5362 (8281–23329) | 0.0357 (#) |
| 0.0097 (##) | |||||
| MPO | 57.41 ± 8.54 (42.10–79.60) | 59.49 ± 5.88 (50.60–71.10) | 58.60 ± 6.16 (44.20–65.80) | 60.44 ± 11.41 (37.80–81.00) | Ns |
Data are described as: mean ± SD (minimum-maximum). Abbreviations: BC: baseline control group, C: control group at t = 5 days, BH: baseline HBOT group, H: HBOT group at t = 5 days (n = 22–25 per group). P values for each analyte are listed and derive from paired t tests (H vs BH:#; C vs BC: ##). Significant differences are highlighted. Rest comparisons are ns.
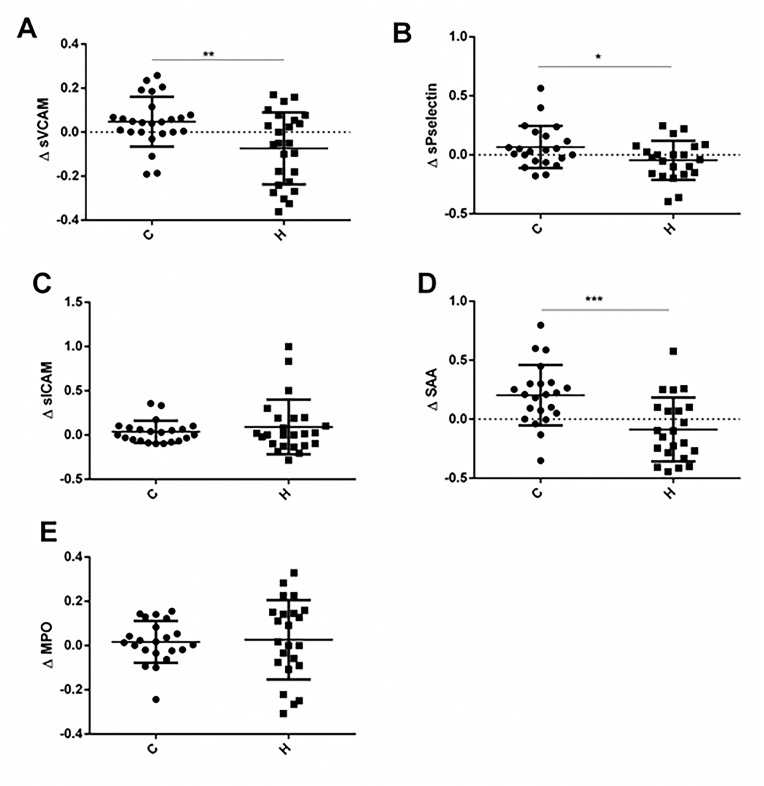
Fig. 2.
Variations in plasma concentration of Cardiovascular Disease Markers
A: Soluble Vascular Cell Molecule (sVCAM), B: Soluble P selectin (sPselectin), C. Intercellular Adhesion Molecule-1 (sICAM-1), D: Acute Phase Amyloid A (SAA) and E: Myeloperoxidase (MPO). Graphs show the mean variations of each plasma concentration at the end of the study relative to the respective baseline measure for treated patients (AH) or control ones (AC)). Significant differences between AH and AC are shown (AH vs AC, P < 0.05 and P < 0.01).
The most remarkable effect of HBOT was on SAA levels. Patients underwent HBOT diminished circulating SAA levels in comparison to baseline (BH: 17618 ± 3926 ng/ml, H: 15487 ± 5362 ng/ml; H vs BH: P = 0.035). Interestingly, patients with standard treatment only, increased them (BC: 16816 ± 3598 ng/ml, C: 19974 ± 4336 ng/ml; C vs BC: P = 0.0097). To analyze the magnitude of the effect, we compared the means of the individual relative variation of SAA levels respect to baselines, for treated patients (ΔH) and control ones (ΔC), Fig. 2A. At the end of the study, treated patients showed a mean concentration of SAA 29,1% lower than the control group (ΔH vs ΔC, P = 0.0007).
Moreover, patients that received HBOT (H) diminished plasma levels of sVCAM in comparison to baseline (BH) [BH = 960.2 ± 135.7; H = 877.2 ± 248.7, H vs BH: P = 0.021]. Patients from the control group at the end of the study (C) did not modify sVCAM levels respect to baseline (BC). Comparing the mean relative variation for each group, the H group showed a mean concentration of sVCAM 15% lower than the control group (ΔH vs ΔC, P = 0.0038), Fig. 2B.
With respect to sPselectin levels, differences between both groups was noticed after comparing the variation index: the H group showed 11.3% lower values than C (ΔH vs ΔC: P = 0.0353), Fig. 2C. HBOT affected neither sICAM nor MPO levels.
3.5. Plasma levels of cytokine/Chemokine/growth factor
Table 3 shows the mean plasma concentrations of the 12 cytokines, chemokines and growth factors.
Table 3.
Plasma levels of cytokines, chemokines and growth factors.
| Conc. (pg/ml) | BC | C | BH | H | P |
|---|---|---|---|---|---|
| IL-1β | 9.440 ± 7.50 (0.282–27.61) | 9.519 ± 6.12 (0.530–20.50) | 8.489 ± 5.660 (0.288–16.47) | 9.649 ± 7.97 (0.280–27.56) | Ns |
| IL-1RA | 5.608 ± 2.725 (2.04–11.30) | 5.762 ± 2.884 (2.000–11.94) | 5.380 ± 2.504 (2.16–9.85) | 5.565 ± 3.31 (1.000–11.93) | Ns |
| IL-6 | 0.899 ± 0.6469 (0.162–3.079) | 0.870 ± 0.5573 (0.077–2.512) | 1.102 ± 0.716 (0.200–3.169) | 0.918 ± 0.483 (0.200–1.751) | Ns |
| TNFα | 16.31 ± 4.55 (10.09–25.63) | 17.35 ± 4.14 (11.13–27.18) | 17.61 ± 5.98 (8.40–32.95) | 17.11 ± 6.95 (9.60–35.67) | Ns |
| IL-12p70 | 3.33 ± 1.53 (1.00–6.10) | 3.60 ± 1.36 (1.30–6.50) | 3.19 ± 1.08 (1–305.00) | 3.77 ± 1.30 (1.80–5.50) | Ns |
| MIP1α | 20.71 ± 10.30 (2.70–38.00) | 21.62 ± 9.92 (7.30–39.20) | 20.52 ± 8.368 (4.50–36.90) | 23.13 ± 10.38 (4.50–34.40) | Ns |
| IP10 | 290.20 ± 65.49 (156.7–377.7) | 305.50 ± 45.15 (160.0–371.1) | 284.50 ± 70.99 (130.3–376.0) | 298.30 ± 58.23 (190.00–391.30) | Ns |
| IFNα | 98.86 ± 34.22 (29.60–153.5) | 100.7 ± 36.43 (36.45–184.5) | 108.7 ± 37.57 (41.35–184.5) | 99.09 ± 35.96 (46.42–158.9) | Ns |
| IFNγ | 0.956 ± 0.720 (0.30–2.20) | 1.375 ± 1.125 (0.30–3.30) | 0.956 ± 0.713 (0.10–2.40) | 1.153 ± 0.786 (0.10–2.20) | 0.0535 (##) |
| IL-15 | 6.681 ± 2.21 (2.50–9.90) | 8.194 ± 3.26 (4.50–14.70) | 7.55 ± 2.80 (4.00–15.40) | 7.47 ± 2.40 (3-60-10.70) | 0.034 (##) |
| IL-2 | 0.520 ± 0.408 (0.05–1.43) | 0.490 ± 0.333 (0.04–1.35) | 0.442 ± 0.345 (0.10–1.50) | 0.581 ± 0.442 (0.04–1.60) | Ns |
| VEGF | 8.143 ± 4.53 (2.600–17.150) | 8.895 ± 6.012 (2.000–26.110) | 7.910 ± 2.965 (2.600–13.520) | 11.96 ± 8.275 (2.313–34.580) | Ns |
Data are described as: mean ± SD (minimum-maximum). Abbreviations: BC: baseline control group, C: control group at t = 5 days, BH: baseline HBOT group, H: HBOT group at t = 5 days (n = 20–24 per group). P values for each analyte are listed and derive from paired t tests (H vs BH: #; C vs BC: ##). Significant differences are highlighted. Rest of comparisons are ns (# and ##).
Even though HBOT did not show to modify IL-1β levels in COVID-pneumonia patients, this treatment was effective in regulating the pro-inflammatory effects of IL-1 (both α and β isoforms) by promoting the increase of circulating IL-1RA, its natural receptor antagonist. Comparing the mean relative variations, the H group showed a mean concentration of IL-1RA 10.9% higher than the control group (ΔH vs ΔC, P = 0,0467), Fig. 3 A. In line, treated patients also showed a mean plasma concentration of TNFα 14.6% lower than the control group (ΔH vs ΔC, P = 0,0239), Fig. 3B. HBOT did not induce modifications in the mean plasma concentration of the other analyzed pro-inflammatory cytokines (IL-6 and IL-12p70) and chemokines (MIP1α andIP10). On the other hand, the C group did not experiment variations in the levels of these proinflammatory cytokines and chemokines respect to the respective baselines.
Fig. 3.
Variations in plasma levels of IL-1RA, TNFα and VEGF
A: Interleukin 1 receptor antagonist (IL-1RA), B: Tumor Necrosis Factor alpha (TNFα), C. Vascular Endothelial Growth Factor (VEGF). Graphs show the mean variations of each plasma concentration at the end of the study relative to the respective baseline measure for treated patients (AH) or control ones (AC)). Significant differences between AH and AC are shown (AH vs AC, P < 0.05).
During the study, the concentration of IFNα was unaffected, either in treated patients (H) or control (C) group. Furthermore, HBOT did not modify plasma levels of IFNγ and IL-15. Interestingly, the control group showed a significant increase in IL-15 levels (BC: 6.681 ± 2.21, C: 8.194 ± 3.26; BC vs C: P = 0.034). IL-15 is implicated in NK cell development, cytotoxic activity and IFNγ production. Of note, the control group also showed a tendency to higher levels of IFNγ (BC: 0.956 ± 0.720, C: 1.375 ± 1.125; BC vs C: P = 0.053) which would agree with the effect on IL-15. Considering that patients from the control group, did not recover clinical and laboratory outcomes after 5 days of hospitalization, the present results may mirror an enhanced anti-viral innate response which could not be necessary in the H group. IL-2 is crucial during the LT expansion phase. During the period of the study, IL-2 levels remained constant in both groups.
Finally, the influence of HBOT on VEGF circulating levels was analyzed. Interestingly, our results showed that the H group presented values 48.9% higher than C (ΔH vs ΔC: P = 0,0366), Fig. 2C.
3.6. Plasma levels of Angiotensin Converting Enzyme 2 (ACE-2)
ACE-2 is a membrane bound enzyme ubiquitously expressed that catalyzes the cleavage of the pro-inflammatory angiotensin II (AngII) into angiotensin 1-7, regulating the renin-angiotensin- aldosterone system (RAS), reviewed by Santos et al. [14]. On the other hand, ACE-2 constitutes the SARS-CoV-2 receptor, and thus, facilitates cell infection. Considering its capacity of sequestering SARS-CoV-2 away from mACE2, low soluble ACE2 (sACE-2) levels might predict SARS-CoV-2 infection severity. Additionally, sACE2 levels can cleave circulating AngII and, as a consequence, downregulates its pro-inflammatory actions [15,16].
In the present study, we investigated whether HBOT affect plasma ACE-2 levels. The obtained results showed very low levels of ACE-2 at baseline (BH and BC) and after HBOT (H group) or in the control group (C group), without significant differences among the groups (Supplementary Table 4). These results supported other reports which described that, during the onset of infection, ACE-2 would suffer a degradation as a result of its interaction with the virus [17]. Moreover, the distribution of the ACE2 levels vary across a full spectrum of values in unexposed healthy people, suggesting that the low levels of ACE-2 in infected patients would not just be a by-product of the infection [16].
3.7. Safety controls
The mean length of hospitalization for the HBOT group (H) was 4 ± 1 days (4–5) and for the control group (C) was 6 ± 1 (5–7); H vs C: P = 0.05782. Therefore, in our working conditions, no statistical differences in days of hospitalization were detected between both groups. No patient was admitted to the ICU during hospitalization. Moreover, no intercurrences or complications (including need for intubation and mechanical ventilation) were detected in the patients analyzed. There were no fatal cases. Follow-up up to 28 days after inclusion in the study did not show cases of readmission and/or complications, so differences between the groups cannot be analyzed in this regard.
4. Discussion
One of the main limitations of HBOT in the clinical practice is the low number of studies analyzing biochemical markers that allow gaining more insight into its mechanisms of action. The COVID-19 pandemic created the necessity of optimizing times of respiratory improvement in hospitalized patients, avoiding heath complications and facilitating new hospital admissions. In this context, some clinical trials in the world have analyzed the benefits of the use of HBOT in hypoxemia secondary to SARS-Cov2 infection. In general, they reported improvements in clinical factors and indexes such as arterial blood gas analysis, liver function tests, complete blood count and computed tomography of lungs [6,18]. In our country, coauthors of the present work, participated in a multicenter, open-label randomized controlled trial that supported the safety and efficacy of HBOT in the treatment of COVID- 19 and severe hypoxemia [12].
The present study confirmed previous clinical reports since patients diagnosed with hypoxemia associated to COVID-19, who received HBOT in addition to standard normobaric oxygen supplementation during 5 days, required significantly less time than the control group to normalize arterial oxygen saturation, with no significant adverse effects.
Unlike the usual immune response against viral infections, SARS-CoV2 induces a decrease in the lymphocyte count. In addition, the virus has been shown to affect thrombocytopoiesis; therefore, COVID-19 often causes a decreased number of platelets [19]. Coronavirus infection can also lead to severe thrombotic events. Thus, increased serum concentration of D-dimer, a product generated during fibrinogen into fibrin conversion, constitutes another severe risk factor [20]. Infected patients usually also showed alterations in some liver enzymes [21]. In addition to the improvement in oxygen saturation levels, this study reported that patients underwent HBOT showed significant increases in lymphocyte and platelet counts, diminishing levels of D dimer and LDH concentration. This data suggest that HBOT would also contribute to the recovery of the viral process itself.
It was recently proposed that SAA could constitute an early and prognostic marker in COVID. Patients with severe forms of COVID-19 and associated mortality, showed significant higher plasma SAA concentrations in comparison to patients with mild disease or controls. These data suggested that the acute increase in SAA concentrations in patients with COVID-19 not only reflects an acute-phase response to infection, but may also be associated with the development of disease complications such as cytokine storm, multi-organ failure, and mortality. On the other hand, patients at the recovery phase, showed a faster rate of decline of plasma SAA levels than those of CRP [22,23]. In agreement with the literature, the present study reported not only that patients treated with HBOT showed significant lower levels of plasma SAA compared to the untreated control group, but also that patients that did not receive this treatment, increased SAA levels. These data support our previous observations suggesting a direct effect of HBOT on the recovery from the viral infection. In line, it has been proposed that hyperoxygenation of arterial blood together with plasma-dissolved oxygen during HBOT may have a direct virucidal impact on COVID-19 mediated by an increased production of oxygen free radicals and antiviral peptides, such as defensins and cathelicidins, in response to Hypoxia-inducible factor 1α (HIF1α) upregulation [24].
COVID-19 is a systemic disease with severe implications on the vascular and coagulation system. For this reason, soluble forms of adhesion molecules have been proposed as predictive markers of severity [25,26]. VCAM-1 is normally absent on resting endothelial cells but could be significantly induced following TNF-α and IL-1 stimulation and, in this way, it plays a key role in leukocyte infiltration. VCAM-1 has been also implicated in the activation of endothelial cell NADPH dual oxidase 2 (NOX2), which leads to the formation of intercellular gaps, suggesting its role in thrombosis by exposing tissue factors. Various works reported increased levels of circulating sVCAM-1 in early stages of SARS-CoV2 infection [27,28].
In COVID-19, several reports showed an increased expression of P-selectin on the platelet surface which promotes the inflammatory hypercoagulable endotheliopathy [29,30]. sP-sel is recognized as an in vivo biomarker of platelet activation and endothelial inflammation. In COVID-19, plasma levels of sP-sel have been associated with disease severity, reviewed by Agrati et al. [31]. Moreover, Campello et al. [32] reported higher levels of P-selectin + extracellular vesicles (EVs) in the plasma of hospitalized COVID- 19 patients compared to healthy controls, even 30 days after discharge from hospital.
The results of our study showed that patients that received HBOT diminished both sVCAM-1 and sPsel levels in comparison to the control group. These results demonstrated that HBOT induced after five sessions a lower level of endothelial activation in COVID-19 patients. Of note, we also detected lower mean plasma concentration of TNFα and maintenance of IL-1 levels in agreement with the majority of clinical studies, reviewed by De Wolde et al. [33]. This anti-inflammatory effect of HBOT is likely mediated by the inhibition of NF-kB, a transcription factor for pro-inflammatory genes. Moreover, in this study we also detected an increase mean concentration of circulating IL-1RA in treated patients with respect to controls. This result suggests that HBOT would exert, in addition, a direct anti-inflammatory action.
Nowadays, HBOT is a widely accepted treatment for increasing wound healing. The main mechanism of action proposed to explain its clinical benefits would be the induction of VEGF expression [34,35]. VEGF synthesis is initiated in response to a hypoxic environment, which induces migration and proliferation of endothelial cells and formation of new blood vessels. To explain the mechanisms by which HBOT leads to an increase in VEGF, it was postulated that the return to normoxia after a mild hyperoxia stimulus is sensed as a hypoxic trigger, process known as “Normobaric Oxygen Paradox”, which in turn induces HIF-1α activation and then VEGF synthesis [36].
In our study, we detected that patients underwent HBOT increased the levels of VEGF in plasma respect to baseline, as was reported by others [33] and this effect was not seen in the control group. It has been reported that VEGF enhances eNOS activity via arginine uptake in endothelial cells [37,38]. In consequence, in the context of SARS-Cov2 infection, it is likely that the increase of VEGF in response to HBOT, modulates endothelial function through nitric oxide (NO) synthesis. NO gives powerful anti-inflammatory and anti-oxidant effects under normal physiological conditions. It contributes to vessel homeostasis by inhibiting vascular smooth muscle contraction, reduces the release of inflammatory mediators, down-regulates platelet aggregation as well as leukocyte adhesion to the endothelium by reducing the expression of cell adhesion molecules and their ligands. NO also mediates at least some of the pro-angiogenic effects of VEGF [39]. We did not measure the levels of NO in the current study. However, recent results from our group demonstrated that HBOT increased NO circulating levels in HBOT treated rats (manuscript in preparation).
5. Limitations of the study
This study has two main limitations. The first one is the small number of participants. The sample size was calculated considering a reduction of at least 20% in the time required to reach arterial saturation of 90%, an alpha error of 5% and a power analysis of 90%. In this way, the necessary number of individuals was 42. We recruited n = 50, considering possible patient losses. However, since we had no prior data on outcomes linked to the analyzed biomarkers, further trials with larger number of patients may be necessary to verify these results.
On the other hand, even though the present work used hyperbaric oxygenation at lower pressures in comparison to other works that have demonstrated safety even with longer treatments [[40], [41], [42]], the present study describes a short treatment and 28 days of follow-up, which constitutes a possible limitation to the detection of toxicities that occur at remote times. This possibility cannot be completely ruled out.
6. Conclusions
The present work showed that patients with hypoxemic COVID-19 pneumonia that received HBOT, improved O2 Sat and presented lower levels of severity markers of the disease (increase of white cells and platelets count, diminished levels of D dimer, LDH and SAA). Moreover, this work demonstrated that HBOT reduced circulating levels of proinflammatory agents (sVCAM, sPselectin, TNFα) and increased anti-inflammatory and pro-angiogenic ones (IL-1RA and VEGF). These results indicate a clear beneficial effect of HBOT on acute COVID-19 treatment and could explain in part the recent reported benefits of HBOT for improving long COVID-related fatigue, pain and neurocognitive processes [43]. In sum, this study gives a deeper information to explain the mechanism of action of HBOT in humans, which will be useful for other clinical applications of this therapy.
Funding
This work was supported by Universidad de Buenos Aires, Argentina (Strategic Development Project.PDE 31_2021) with the contribution of the requesting company Oxavita SRL, manufacturer of HBOT chambers. Funding sources were not involved in the design of the study, collection, analysis, interpretation of data, writing and publication of results.
Author contributions
GK: Conducting and supervision of clinical research (presentation to Ethical Committee, responsible of informed consents, clinical follow up, HBOT management), acquiring and analysis of clinical data, interpretation and curation of clinical data; revision of manuscript, preparation of figures and tables. IC: Participation in clinical research: patients follow up and HBOT management. JC: Participation in analytical laboratory studies; acquiring and analysis of laboratory data. GDG: Conceptualization of the study; revision of manuscript. SM: Conceptualization and supervision of the study; funding acquisition; conducting laboratory determinations; acquiring and analysis of laboratory data; interpretation and curation of laboratory data; writing the manuscript; preparation of figures and tables. All authors reviewed the paper and approved the submitted version.
Declaration of competing interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmed.2023.107155.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Hu A., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krishnan A., Hamilton J.P., Alqahtani S.A., Woreta T.A. A narrative review of coronavirus disease 2019 (COVID-19): clinical, epidemiological characteristics, and systemic manifestations. Intern. Emerg. Med. 2021;16:815–830. doi: 10.1007/s11739-020-02616-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehraeen E., Karimi A., Barzegary A., Vahedi F., Afsahi A.M., Dadras O., Moradmand- Badie B., Seyed Alinaghi S.A., Jahanfar S. Predictors of mortality in patients with COVID- 19-a systematic review. Eur. J. Integr. Med. 2020;40 doi: 10.1016/j.eujim.2020.101226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie J., Covassin N., Fan Z., Singh P., Gao W., Li G., Kara T., Somers V.K. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin. Proc. 2020;95:1138–1147. doi: 10.1016/j.mayocp.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Hawa A.A.A., Charipova K., Bekeny J.C., Johnson-Arbor K.K. The evolving use of hyperbaric oxygen therapy during the COVID-19 pandemic. J. Wound Care. 2021;30:S8–S11. doi: 10.12968/jowc.2021.30.Sup2.S8. Sup.2. [DOI] [PubMed] [Google Scholar]
- 6.Oliaei S., Seyed Alinaghi S., Mehrtak M., Karimi A., Noori T., Mirzapour P., Shojaei A., MohsseniPour M., Mirghaderi S.P., Alilou S., Shobeiri P., Azadi Cheshmekabodi H., Mehraeen E., Dadras O. The effects of hyperbaric oxygen therapy (HBOT) on coronavirus disease-2019 (COVID-19): a systematic review. Eur. J. Med. Res. 2021;26:96–108. doi: 10.1186/s40001-021-00570-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ortega M.A., Fraile-Martinez O., García-Montero C., Callejón-Peláez E., Sáez M.A., Álvarez-Mon M.A., García-Honduvilla N., Monserrat J., Álvarez-Mon M., Bujan J., Canals M.L. A general overview on the hyperbaric oxygen therapy: applications, mechanisms and translational opportunities. Medicina (Kaunas, Lithuania) 2021;57:864–889. doi: 10.3390/medicina57090864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Undersea. Hyperbary Medicime Society . In: Hyperbaric Oxygen Therapy Indications. fourteenth ed. Moon R., editor. Besnet Publishing Company; North Palm Beach, FL, USA: 2019. [Google Scholar]
- 9.Gawdi R., Cooper J.S. StatPearls Publishing; 2022. Hyperbaric Contraindications, Treasure Island (FL) [PubMed] [Google Scholar]
- 10.Paganini M., Bosco G., Perozzo F.A.G., Kohlscheen E., Sonda R., Bassetto F., Garetto G., Camporesi E.M., Thom S.R. The role of hyperbaric oxygen treatment for COVID-19: a review. Adv. Exp. Med. Biol. 2021;1289:27–35. doi: 10.1007/5584_2020_568. [DOI] [PubMed] [Google Scholar]
- 11.Thibodeaux K., Speyrer M., Raza A., Yaakov R., Serena T.E. Hyperbaric oxygentherapy in preventing mechanical ventilation in COVID-19 patients: a retrospective case series. J. Wound Care. 2020;29:S4–S8. doi: 10.12968/jowc.2020.29.Sup5a.S4. [DOI] [PubMed] [Google Scholar]
- 12.Cannellotto M., Duarte M., Keller G., Larrea R., Cunto E., Chediack V., Mansur M., Brito D.M., García E., Di Salvo H.F., Verdini E., Domínguez C., Jorda-Vargas L., Roberti J., Di Girolamo G., Estrada E. Hyperbaric oxygen as an adjuvant treatment for patients withCOVID-19 severe hypoxaemia: a randomised controlled trial. Emerg. Med. J. 2022;39:88–93. doi: 10.1136/emermed-2021-211253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Ministry of Health of Argentina . 2020. Recomendaciones para el abordaje terapéutico de COVID- 19. Buenos Aires, Argentina. [Google Scholar]
- 14.Santos R.A.S., Oudit G.Y., Verano-Braga T., Canta G., Steckelings U.M., Bader M. The renin-angiotensin system: going beyond the classical paradigms. Am. J. Physiol. Heart Circ. Physiol. 2019;316:H958–H970. doi: 10.1152/ajpheart.00723.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maza M.D.C., Úbeda M., Delgado P., Horndler L., Llamas M.A., van Santen H.M., Alarcón B., Abia D., García-Bermejo L., Serrano-Villar S., Bastolla U., Fresnon M. ACE2 serum levels as predictor of infectability and outcome in COVID-19. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.836516. M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García-Ayllón M.S., Moreno-Pérez O., García-Arriaza J., Ramos-Rincón J.M., Cortés- Gómez M.A., Brinkmalm G., Andrés M., León-Ramírez J.M., Boix V., Gil J., Zetterberg H., Esteban M., Merino E., Sáez-Valero J. Plasma ACE2 species are differentially altered in COVID-19 patients. Faseb. J. 2021;35(8) doi: 10.1096/fj.202100051R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harch P.G. Hyperbaric oxygen treatment of novel coronavirus (COVID-19) respiratory failure. Med. Gas Res. 2020;10:61–62. doi: 10.4103/2045-9912.282177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu P., Zhou Q., Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann. Hematol. 2020;99:1205–1208. doi: 10.1007/s00277-020-04019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lippi G., Favaloro E.J. D-Dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb. Haemostasis. 2020;120:876–878. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kayaaslan A., Guner R. COVID-19 and the liver: a brief and core review. World J. Hepatol. 2021;13:2013–2023. doi: 10.4254/wjh.v13.i12.2013. R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pieri M., Ciotti M., Nuccetelli M., Perrone M.A., Caliò M.T., Lia M.S., Minieri M., Bernardini S. Serum Amyloid A Protein as a useful biomarker to predict COVID-19 patients severity and prognosis. Int. Immunopharm. 2021;95 doi: 10.1016/j.intimp.2021.107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L., Yang L.M., Pei S.F., Chong Y.Z., Guo Y., Gao X.L., Tang Q.Y., Li Y., Feng F.M., CRP S.A.A. LDH, and DD predict poor prognosis of coronavirus disease (COVID- 19): a meta-analysis from 7739 patients. Scand. J. Clin. Lab. Invest. 2021;81:679–686. doi: 10.1080/00365513.2021.2000635. [DOI] [PubMed] [Google Scholar]
- 24.Baugh M.A. HIV: reactive oxygen species, enveloped viruses and hyperbaric oxygen. Med. Hypotheses. 2000;55:232–238. doi: 10.1054/mehy.2000.1048. [DOI] [PubMed] [Google Scholar]
- 25.Tong M., Jiang M., Xia D., Xiong Y., Zheng Q., Chen F., Zou I., Xiao W., Zhu Y. Elevated expression of serum endothelial cell adhesion molecules in COVID-19 patients. J. Infect. Dis. 2020;222:894–898. doi: 10.1093/infdis/jiaa349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L., Huang M., Shen J., Wang Y., Wang R., Yuan C., Jiang L., Huang M. Serum levels of soluble platelet endothelial cell adhesion molecule 1 in COVID-19 patients are associated with disease severity. J. Infect. Dis. 2021;223:178–179. doi: 10.1093/infdis/jiaa642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bauer W., Ulke W.J., Galtung N., Strasser-Marsik L.C., Neuwinger N., Tauber R., Somasundaram R., Kappert K. Role of cell adhesion molecules for prognosis of disease development of patients with and without COVID-19 in the emergency department. J. Infect. Dis. 2021;223:1497–1499. doi: 10.1093/infdis/jiab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao S., Luo N., Liu J., Zha H., Ai Y., Luo J., Shi S., Wu K. Elevated serum levels of progranulin and soluble vascular cell adhesion molecule-1 in patients with COVID-19. J. Inflamm. Res. 2021;14:4785–4794. doi: 10.2147/JIR.S330356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canzano P., Brambilla M., Porro B., Cosentino N., Tortorici E., Vicini S., Poggio P., Cascella A., Pengo M., Veglia F., Fiorelli S., Bonomi A., Cavalca V., Trabattoni D., Andreini D., Omodeo Salè E., Parati G., Tremoli E., Camera M. Platelet and endothelial activation as potential mechanisms behind the thrombotic complications of COVID-19 patients. JACC Basic Transl Sci. 2021;6:202–218. doi: 10.1016/j.jacbts.2020.12.009. M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrett T.J., Cornwell M., Myndzar K., Rolling C.C., Xia Y., Drenkova K., Biebuyck A., Fields A.T., Tawil M., Luttrell-Williams F., Yuriditsky E., Smith G., Cotzia P., Neal M.D., Kornblith L.Z., Pittaluga S., Rapkiewicz A.V., Burgess H.M., Mohr I., Stapleford K.A., Voora D., Ruggles K., Hochman J., Berger J.S. Platelets amplify endotheliopathy in COVID-19. Sci. Adv. 2021;7:eabh2434. doi: 10.1126/sciadv.abh2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agrati C., Sacchi A., Tartaglia E., Vergori A., Gagliardini R., Scarabello A., Bibas M. The role of P-selectin in COVID-19 coagulopathy: an updated review. Int. J. Mol. Sci. 2021;22:7942–7954. doi: 10.3390/ijms22157942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campello E., Radu C.M., Simion C., Spiezia L., Bulato C., Gavasso S., Tormene D., Perin N., Turatti G., Simioni P. Longitudinal trend of plasma concentrations of extracellular vesicles in patients hospitalized for COVID-19. Front. Cell Dev. Biol. 2022;9 doi: 10.3389/fcell.2021.770463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Wolde S.D., Hulskes R.H., Weenink R.P., Hollmann M.W., Van Hulst R.A. The effects of hyperbaric oxygenation on oxidative stress, inflammation and angiogenesis. Biomolecules. 2021;11:1210–1257. doi: 10.3390/biom11081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sureda A., Batle J.M., Martorell M., Capó X., Tejada S., Tur J.A., Pons A. Antioxidant response of chronic wounds to hyperbaric oxygen therapy. PLoS One. 2016;11 doi: 10.1371/journal.pone.0163371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang X., Liang P., Jiang B., Zhang P., Yu W., Duan M., Guo L., Cui X., Huang M., Huang X. Hyperbaric oxygen potentiates diabetic wound healing by promoting fibroblast cell proliferation and endothelial cell angiogenesis. Life Sci. 2020;259 doi: 10.1016/j.lfs.2020.118246. [DOI] [PubMed] [Google Scholar]
- 36.Fratantonio D., Virgili F., Zucchi A., Lambrechts K., Latronico T., Lafère P., Germonpré P., Balestra C. Increasing oxygen partial pressures induce a distinct transcriptional response in human PBMC: a pilot study on the "normobaric oxygen paradox. Int. J. Mol. Sci. 2021;22:458–471. doi: 10.3390/ijms22010458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouloumié A., Schini-Kerth V.B., Busse R. Vascular endothelial growth factor up-regulates nitric oxide synthase expression in endothelial cells. Cardiovasc. Res. 1999;41:773–780. doi: 10.1016/s0008-6363(98)00228-4. [DOI] [PubMed] [Google Scholar]
- 38.Shashar M., Chernichovski T., Pasvolsky O., Levi S., Grupper A., Hershkovitz R., Weinstein T., Schwartz I.F. Vascular endothelial growth factor augments arginine transportand nitric oxide generation via a KDR receptor signaling pathway. Kidney Blood Press. Res. 2017;42:201–208. doi: 10.1159/000476016. [DOI] [PubMed] [Google Scholar]
- 39.Wallace J.L. Nitric oxide as a regulator of inflammatory processes. Mem. Inst. Oswaldo Cruz. 2005;100(Suppl 1):5–9. doi: 10.1590/s0074-02762005000900002. [DOI] [PubMed] [Google Scholar]
- 40.Chen X., You J., Ma H., Zhou M., Huang C. Efficacy and of hyperbaric oxygen therapy for fibromyalgia: a systematic review and meta-analysis. BMJ Open. 2023;13 doi: 10.1136/bmjopen-2022-062322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korhonen K. Hyperbaric oxygen therapy in acute necrotizing infections with a special reference to the effects on tissue gas tensions. Ann. Chir. Gynaecol. Suppl. 2000;214:7–36. [PubMed] [Google Scholar]
- 42.Kjellberg A., Hassler A., Boström E., El Gharbi S., Al-Ezerjawi S., Kowalski J., Rodriguez-Wallberg K.A., Bruchfeld J., Ståhlberg M., Nygren-Bonnier M., Runold M., Lindholm P. Hyperbaric oxygen therapy for long COVID (HOT-LoCO), an interim safety report from a randomised controlled trial. BMC Infect. 2023;23:33–41. doi: 10.1186/s12879-023-08002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zilberman-Itskovich S., Catalogna M., Sasson F., Elman-Shina K., Hadanny A., Lang E., Finci S., Polak N., Fishlev G., Korin C., Shorer R., Parag Y., Sova M., Efrati S. Hyperbaric oxygen therapy improves neurocognitive functions and symptoms of post-COVID condition: randomized controlled trial. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-15565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.