Abstract
Background
The therapeutic drugs available for ulcerative colitis (UC) have recently increased. However, use of topical therapy for UC in Japan has not been determined. This study aimed to investigate real-world use of topical therapy for UC in Japan using a web-based survey designed for UC patients.
Methods
A web-based questionnaire on UC management was administered to 773 patients over a 2-day period in September 2019. The responses regarding topical therapy use were analyzed.
Results
Questionnaire responses were obtained from 323 UC patients. Of these, the mean disease duration was 12.2 years, and 220 patients (68.1%) had used topical therapy, of whom 68 (21.1%) were currently using this treatment. The frequency of using the prescribed topical therapy was appropriate in 36.8% of patients, only when needed in 38.6%, and rarely in 24.5%. Among all topical therapy users, 64.4% reported that budesonide foam was easy to use, which was significantly higher than the rates for mesalazine suppositories (43.6%), mesalazine enemas (12.9%), and glucocorticoid enemas (13.9%; P < 0.05). Regarding treatment effects, 68.9% of patients were satisfied with the budesonide foam, which was a significantly higher rate of satisfaction than those for mesalazine suppositories (44.6%), mesalazine enemas (30.2%), glucocorticoid enemas (36.1%), and glucocorticoid suppositories (41.9%; P < 0.05).
Conclusions
Although topical therapy use was common in this Japanese UC population, patient adherence was not very high. Of all the topical therapy types, budesonide foam, which has recently become available, was rated highly by these patients.
Keywords: web-based survey, ulcerative colitis, topical therapy
Introduction
Ulcerative colitis (UC) is a refractory disease of unknown cause.1 The quality of life of patients with UC is significantly affected by symptoms such as diarrhea, abdominal pain, fever, and bloody stools.2 Various therapeutic drugs have recently become available for refractory UC, but topical therapies, as well as systemic therapies, are most effective for UC with localized lesions. However, compared with systemic therapies, including oral drugs, topical therapies may be less accepted or not appropriately used by the patients.
Recently, budesonide foam was approved for use in Japan,3,4 whereas budesonide enemas and mesalazine foam have not yet been approved; budesonide foam is the only topical therapy consisting of budesonide and the only agent administered in the form of foam. The actual circumstances of topical therapy for UC are not well understood in Japan. Therefore, a web-based survey targeting UC patients was conducted.
MATERIALS AND METHODS
Survey Instruments
A total of 773 patients with UC who were registered with Cross Marketing Co., Ltd., (Tokyo, Japan) were requested by email to participate in this survey (Treatment status of Topical Therapy for UC patients in Japan [3T survey]), which was conducted via the internet on September 27–28, 2019. The questionnaire included questions regarding age, sex, height, body weight, duration of disease, area of residence, smoking status, lesion site, history of intestinal resection, occupation, therapeutic drugs used, medical institution visited for treatment, frequency of doctor visits, current level of satisfaction with treatment, severity of diarrhea/abdominal pain, stools with blood/mucus, past experience with topical therapy, method of topical therapy, and feasibility of using and satisfaction with each topical therapy (Table 1). The survey protocol was approved by the Ethics Review Board of Iwate Medical University. All patients agreed to participate in this survey.
Table 1.
Question Matters
| Age | □years |
| Sex | □Male□Female |
| Height | □cm |
| Weight | □kg |
| Duration of disease | □years |
| Smoking status | □Present □Past □Never |
| Extent of disease | □Rectal or Distal □Extensive □Unknown |
| History of intestinal resection | □Yes □Never |
| Occupation status | □Full-time □Part-time □Student □Housework □Other □Unemployed |
| Therapeutic drug | □Yes □No (about each drug) |
| Visiting hospital | □University hospital □General hospital □Clinic □Not attending |
| Visiting frequency | □Once every two weeks □Once every month □Once every 2 months □Once every 3 months □Less frequencies □Not attending anywhere |
| Current level of satisfaction with the treatment | □Very satisfied □Satisfied □Obscure □Unsatisfied □Very unsatisfied |
| Diarrhea/abdominal pain | □Very mild □Mild □Obscure □Severe □Very severe |
| Bloody stools/mucous stools | □None □Rarely □Sometimes □Frequently |
| Past experience of topical therapy | □Yes □Never (about each drug) |
| Frequency of use of topical therapy | □Used on regular basis □Used on demand □Rarely used |
| Reason of never using treatment | □Therapy was never prescribed by a physician □Never felt that treatment was needed □Hesitant to used anally administrate agents |
| Feasibility about each topical therapy | □Very difficult □Difficult □Obscure □Easy □Very easy |
| Satisfaction about efficacy with each topical therapy | □Very satisfied □Satisfied □Obscure □Unsatisfied □Very unsatisfied |
Statistical Analysis
Statistical analyses were performed between the groups using the chi-square test or Fisher’s exact test for categorical data. A difference with a P value of <0.05 was considered statistically significant.
RESULTS
Patient Background Characteristics
Questionnaire responses were obtained from 323 patients with UC, all of whom were eligible for and included in the analyses. The patients, comprising 71.2% males, had a mean age of 51.8 ± 12.3 years and a mean disease duration of 12.2 ± 9.3 years. The lesion site was the rectum and left colon in 54.8% of patients. Only 20 patients (6.2%) had received treatments such as biologics for refractory conditions, and 68 (21.1%) had received topical therapies (Table 2). Regarding the medical institution where they were treated, 4.6% of the patients visited a university hospital, 55.1% a general hospital, and 29.4% a local clinic, while 0.9% of the patients did not visit any institution. The frequency of medical visits was once every 2 weeks for 3.4% patients, once every month for 29.1%, once every 2 months for 36.2%, once every 3 months for 25.7%, and other for 5.6%.
Table 2.
Demographics of the Patients
| Number of patients | 323 |
| Sex (male) | 71.2% (230/323) |
| Age (mean ± SD) | 51.8 ± 12.3 years |
| Height (mean ± SD) | 166.2 ± 8.1 cm |
| Weight (mean ± SD) | 62.5 ± 12.5 kg |
| Disease duration (mean ± SD) | 12.2 ± 9.3 years |
| Smoking habit | |
| Current | 13.0% (42/323) |
| Past | 38.7% (125/323) |
| Never | 48.3% (156/323) |
| Extent of disease | |
| Rectal or distal | 54.8% (177/323) |
| Extensive | 35.3% (114/323) |
| Unknown | 9.9% (32/323) |
| History of resection | 6.8% (22/323) |
| Occupation status | |
| Full-time | 52.9% (171/323) |
| Part-time | 13.6% (44/323) |
| Student | 0% (0/323) |
| Housework | 2.2% (7/323) |
| Other | 4.0% (13/323) |
| Unemployed | 27.2% (88/323) |
| Current therapies | |
| Infliximab | 2.2% (7/323) |
| Infliximab BS | 0% (0/323) |
| Adalimumab | 2.2% (7/323) |
| Golimumab | 0.9% (3/323) |
| Tofacitinib | 0% (0/323) |
| Vedolizumab | 0.9% (3/323) |
| Tacrolimus | 0% (0/323) |
| Azathioprine | 10.8% (35/323) |
| Oral glucocorticoid | 6.5% (21/323) |
| Oral mesalazine | |
| Time-dependent type | 37.2% (120/323) |
| pH-dependent type | 28.8% (93/323) |
| MMX type | 15.8% (51/323) |
| Salazosulfapyridine | 7.1% (23/323) |
| Budesonide foam | 3.7% (12/323) |
| Mesalazine suppository | 10.5% (34/323) |
| Mesalazine enema | 4.3% (14/323) |
| Glucocorticoid enema | 1.2% (4/323) |
| Glucocorticoid suppository | 1.2% (4/323) |
BS, biosimilar, MMX, multimatrix.
Disease Status
According to the patients’ responses to the questionnaire, 14% reported symptoms including diarrhea and abdominal pain, and 8% were not satisfied with their current treatment. Disease symptoms were generally controlled in the majority of patients, and the level of satisfaction with therapy was high (Fig. 1). On the other hand, 24.8% and 33.1% of patients reported frequent blood and mucous, respectively, in their stools, suggesting that a residual intestinal lesion might exist in the rectum (Fig. 2). Supplementary Table 1 indicates the satisfaction and feasibility rates in each topical treatment groups. As shown in the table, the satisfaction and feasibility rates were not significantly different between symptomatic subjects and asymptomatic subjects.
Figure 1.
Presence of diarrhea and abdominal pain and level of satisfaction with treatment.
Figure 2.
Presence of blood/mucus in stools.
Frequency of Topical Therapy Use
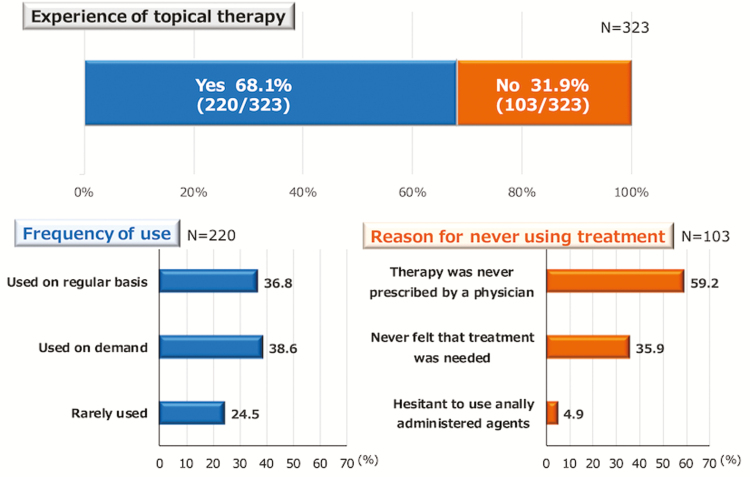
Of the total patients, 220 (68.1%) had experience with using topical therapy for UC. The topical therapy was administered on a regular basis in 36.8% of the patients, only when needed in 38.6%, and rarely in 24.5% (Fig. 3). Among the 103 patients who had never used a topical therapy for UC, the reasons for not using such treatment were as follows: the patient had never been prescribed topical therapy by a physician (59.2%), had never felt that such treatment was necessary (35.9%), and was hesitant to use an anally administered agent (4.9%). Among 220 patients with experiences of topical therapy, 60 patients (27.3%) and 80 patients (36.4%) had bloody stools and mucous stools, respectively, at the time of the survey. Of 103 patients without an experience of topical therapy, 20 (19.4%) had bloody stools and 27 (26.2%) had mucous stools. The frequencies of bloody stool and mucous stool were not different according to the experience of topical therapy.
Figure 3.
Frequency of topical therapy use. For each topical therapy, the administration method was assessed in the 220 patients who had any experience with topical therapy for UC. In the remaining 103 patients who had never used topical therapy, the reasons for not using such therapy are provided.
Feasibility of Using Topical Therapy
Regarding the feasibility of the five topical therapy products available in Japan, 64.4% of the patients responded that budesonide foam was easy to use, which was a significantly higher rate than those for mesalazine suppository (43.6%), mesalazine enema (12.9%), and glucocorticoid enema (13.9%) use (P < 0.05; Fig. 4). As for the form of administration, 64.4%, 44.8%, and 14.9% of the patients responded that foam (budesonide foam), suppositories (mesalazine and glucocorticoid suppositories), and enemas (mesalazine and glucocorticoid enemas) were easiest to use, respectively; significant differences were observed among these rates (foam vs. suppository: P = 0.0302; foam vs. enema: P < 0.0001; suppository vs. enema: P < 0.0001; Fig. 4).
Figure 4.
Feasibility of using the different topical therapies. For five topical therapy products, the feasibility of use was assessed among the patients who had experience with these products. The percentages of patients who responded that treatment was easy to use (very easy or easy) were compared among the different treatment types. Statistically significant differences were observed for budesonide foam versus mesalazine suppository, mesalazine enema, and glucocorticoid enema (P < 0.05).
Satisfaction With Topical Therapy
Regarding treatment satisfaction, 68.9% of the patients were satisfied with budesonide foam, which was a significantly higher rate than those for mesalazine suppositories (44.6%), mesalazine enemas (30.2%), glucocorticoid enemas (36.1%), and glucocorticoid suppositories (41.9%; P < 0.05; Fig. 5).
Figure 5.
Level of satisfaction with the different topical therapies. For five topical therapy products, satisfaction was assessed among the patients who had experience using these products. The percentages of patients who were satisfied with the treatment effects (very satisfied and satisfied) were compared among the different treatment types. Statistically significant differences were observed for budesonide foam versus mesalazine suppository, mesalazine enema, glucocorticoid enema, and glucocorticoid suppository (P < 0.05).
According to the generic product name, 68.9% were satisfied with budesonide (budesonide foam), 36.9% with mesalazine (mesalazine suppositories and enemas), and 38.8% with glucocorticoids (glucocorticoid suppositories and enemas); a significant difference in satisfaction was observed between budesonide and mesalazine (P = 0.0002) and between budesonide and glucocorticoids (P = 0.0009) but not between mesalazine and glucocorticoids (P = 0.8015; Fig. 6). According to the administration form, 68.9% of the patients were satisfied with using a foam (budesonide foam), 43.6% with suppositories (mesalazine and glucocorticoid suppositories), and 32.4% with enemas (mesalazine and glucocorticoid enemas); significant differences in satisfaction were observed among these forms (foam vs. suppository: P = 0.0045; foam vs. enema: P < 0.0001; suppository vs. enema: P = 0.0420; Fig. 7).
Discussion
We previously reported the actual circumstances of treatment for inflammatory bowel disease (IBD) and the correlations of treatment with quality of life and symptoms in IBD patients in Japan using a web-based questionnaire survey.2 There are not many reports available on the use of topical therapy for UC. Therefore, we decided to investigate the actual circumstances of the treatments, including topical therapies, for UC patients in Japan using a similar approach. For UC with localized lesions, both systemic and topical therapies are considered useful and are recommended by international, as well as Japanese, guidelines.5,6
In this survey, 68.1% of the patients had experience with topical therapy use, and 21.1% were currently using the therapy. According to a study by Seibold et al7 conducted in Switzerland, 25.6% of patients were currently using topical therapy for UC, which was similar to our rate. The patients who responded to our questionnaire reported being generally satisfied with their treatment, with good control of their diarrhea and abdominal pain. However, more than 20% of the patients still reported blood/mucus in their stools, suggesting that more patients should be using topical therapies. This recommendation is supported by our data that approximately 20% of patients without an experience of topical therapy complained of hematochezia.
Among the patients prescribed topical therapies, the frequency of treatment use was appropriate in 36.8%, only as needed in 38.6%, and rarely in 24.5%. According to a previous report,8 the level of adherence to IBD treatments was low and was associated with the rate of relapse. However, the adherence to topical therapies has not yet been evaluated in an actual clinical setting, and patient adherence might be particularly low for topical therapies.
Among the topical therapies for UC, mesalazine suppository was approved in Japan in 2013,9 followed by budesonide foam in 2017.3,4 Mesalazine foam10–12 and budesonide enemas13–15 have not yet been approved in Japan. Of all the UC topical therapy types available in Japan, budesonide foam is the only product consisting of budesonide and the only one available as a foam, and it was rated highly by the patients in terms of both feasible use and satisfaction. Richter reported that the mesalazine suppository was the most commonly used product for local treatment.16 However, this may change in Japan in the future. In a multi-institutional collaborative open-label randomized controlled trial, the efficacy of mesalazine enemas was significantly higher than that of budesonide enemas.17 However, only a limited amount of evidence is available from comparative studies on the various products used for topical therapy for UC, and no study has compared budesonide foam with the other topical products.18,19 Although the efficacy and safety of budesonide foam have recently been reported,20–25 large-scale and high-quality comparisons on these products are needed in future studies.
There were three limitations to this survey. First, as this survey obtained responses via the internet, there may have been selection bias among the included patients. Furthermore, the patients comprised more males than females. However, we consider that the selection bias was relatively low in this survey as the patient group was similar to UC populations in Japan and Asian studies26–29 in terms of sex ratio and age distribution, in general, and there was hardly any bias in the location of the responders. Second, there may be an issue with the accuracy of the responses considering that it was a web-based self-report survey. Regarding web-based surveys on IBD, Randell et al30 reported consistency among the evaluations made by the patients and physicians of CCFA Partners, and Kelstrup et al also reported consistency between patient evaluations and medical records in a web-based survey.31 Hence, the consistency of web-based surveys for IBD was considered to be relatively high, although consistency between the present survey results and medical records has not yet been verified. Third, the comparisons among the topical therapy products in this survey were subjective evaluations made by patients. Hence, rigorous and objective evaluations of disease activities were not performed, and the circumstances for use might have varied greatly for each product. This is a serious limitation. However, these data were obtained from an actual clinical setting and provide a comparison of the various available topical therapies for UC, an area in which very few studies have been conducted.
Despite these limitations, this web-based survey revealed a high frequency of topical therapy use among Japanese patients with UC, although the adherence level was not very high. Among the topical therapy products approved for UC in Japan, budesonide foam, which became available recently, was rated highest by this patient group in terms of satisfaction and feasible use.
Supplementary Material
Acknowledgments
This survey was supported by Cross Marketing Co., Ltd. (Tokyo, Japan).
Funding: None.
Disclosures: T.M.: Honoraria: Takeda Pharmaceutical, Abbvie, Kyorin Pharmaceutical Co., Sekisui Medical, Mochida Pharmaceutical, Tanaba-Mitsubishi Pharmaceutical, Jansen Pharmaceutical, Nippon Kayaku Co., and EA Pharma. Commercial research funding: Jansen Pharmaceutical, Takeda Pharmaceutical, Kissei Pharmaceutical.
Author Contribution: T.M. and S.Y. participated in designing the survey and drafting the manuscript. Y.T. participated in drafting the manuscript. S.N. participated in the data analysis and literature review. All authors read and approved the final manuscript.
References
- 1. Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. [DOI] [PubMed] [Google Scholar]
- 2. Matsumoto T, Yanai S, Toya Y, et al. . Internet-orientated assessment of QOL and actual treatment status in Japanese patients with inflammatory bowel disease: the 3I survey. J Crohns Colitis. 2015;9:477–482. [DOI] [PubMed] [Google Scholar]
- 3. Naganuma M, Aoyama N, Suzuki Y, et al. . Twice-daily budesonide 2-mg foam induces complete mucosal healing in patients with distal ulcerative colitis. J Crohns Colitis. 2016;10:828–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Naganuma M, Aoyama N, Tada T, et al. . Complete mucosal healing of distal lesions induced by twice-daily budesonide 2-mg foam promoted clinical remission of mild-to-moderate ulcerative colitis with distal active inflammation: double-blind, randomized study. J Gastroenterol. 2018;53:494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harbord M, Eliakim R, Bettenworth D, et al. . Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: Current management. J Crohns Colitis. 2017;11:769–784. [DOI] [PubMed] [Google Scholar]
- 6. Matsuoka K, Kobayashi T, Ueno F, et al. . Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018;53: 305–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seibold F, Fournier N, Beglinger C, et al. ; Swiss IBD Cohort Study Group . Topical therapy is underused in patients with ulcerative colitis. J Crohns Colitis. 2014;8:56–63. [DOI] [PubMed] [Google Scholar]
- 8. Kane SV, Cohen RD, Aikens JE, Hanauer SB. Prevalence of nonadherence with maintenance mesalamine in quiescent ulcerative colitis. Am J Gastroenterol. 2001;96:2929–2933. [DOI] [PubMed] [Google Scholar]
- 9. Watanabe M, Nishino H, Sameshima Y, et al. . Randomised clinical trial: evaluation of the efficacy of mesalazine (mesalamine) suppositories in patients with ulcerative colitis and active rectal inflammation—a placebo-controlled study. Aliment Pharmacol Ther. 2013;38:264–273. [DOI] [PubMed] [Google Scholar]
- 10. Pokrotnieks J, Marlicz K, Paradowski L, et al. . Efficacy and tolerability of mesalazine foam enema (Salofalk foam) for distal ulcerative colitis: a double-blind, randomized, placebo-controlled study. Aliment Pharmacol Ther. 2000;14:1191–1198. [DOI] [PubMed] [Google Scholar]
- 11. Cortot A, Maetz D, Degoutte E, et al. . Mesalamine foam enema versus mesalamine liquid enema in active left-sided ulcerative colitis. Am J Gastroenterol. 2008;103:3106–3114. [DOI] [PubMed] [Google Scholar]
- 12. Malchow H, Gertz B; CLAFOAM Study group . A new mesalazine foam enema (claversal foam) compared with a standard liquid enema in patients with active distal ulcerative colitis. Aliment Pharmacol Ther. 2002;16:415–423. [DOI] [PubMed] [Google Scholar]
- 13. Danielsson A, Hellers G, Lyrenäs E, et al. . A controlled randomized trial of budesonide versus prednisolone retention enemas in active distal ulcerative colitis. Scand J Gastroenterol. 1987;22:987–992. [DOI] [PubMed] [Google Scholar]
- 14. Löfberg R, Ostergaard Thomsen O, Langholz E, et al. . Budesonide versus prednisolone retention enemas in active distal ulcerative colitis. Aliment Pharmacol Ther. 1994;8:623–629. [DOI] [PubMed] [Google Scholar]
- 15. Hanauer SB, Robinson M, Pruitt R, et al. . Budesonide enema for the treatment of active, distal ulcerative colitis and proctitis: a dose-ranging study. U.S. Budesonide enema study group. Gastroenterology. 1998;115: 525–532. [DOI] [PubMed] [Google Scholar]
- 16. Richter JM, Kushkuley S, Barrett JA, Oster G. Treatment of new-onset ulcerative colitis and ulcerative proctitis: a retrospective study. Aliment Pharmacol Ther. 2012;36:248–256. [DOI] [PubMed] [Google Scholar]
- 17. Hartmann F, Stein J; BudMesa-Study Group . Clinical trial: controlled, open, randomized multicentre study comparing the effects of treatment on quality of life, safety and efficacy of budesonide or mesalazine enemas in active left-sided ulcerative colitis. Aliment Pharmacol Ther. 2010;32:368–376. [DOI] [PubMed] [Google Scholar]
- 18. Christophi GP, Rengarajan A, Ciorba MA. Rectal budesonide and mesalamine formulations in active ulcerative proctosigmoiditis: efficacy, tolerance, and treatment approach. Clin Exp Gastroenterol. 2016;9:125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cohen RD, Dalal SR. Systematic review: rectal therapies for the treatment of distal forms of ulcerative colitis. Inflamm Bowel Dis. 2015;21:1719–1736. [DOI] [PubMed] [Google Scholar]
- 20. Sandborn WJ, Bosworth B, Zakko S, et al. . Budesonide foam induces remission in patients with mild to moderate ulcerative proctitis and ulcerative proctosigmoiditis. Gastroenterology. 2015;148:740–750.e2. [DOI] [PubMed] [Google Scholar]
- 21. Zeng J, Lv L, Mei ZC. Budesonide foam for mild to moderate distal ulcerative colitis: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2017;32:558–566. [DOI] [PubMed] [Google Scholar]
- 22. Bosworth BP, Sandborn WJ, Rubin DT, Harper JR. Baseline oral 5-ASA use and efficacy and safety of budesonide foam in patients with ulcerative proctitis and ulcerative proctosigmoiditis: analysis of 2 phase 3 studies. Inflamm Bowel Dis. 2016;22:1881–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rubin DT, Sandborn WJ, Bosworth B, et al. . Budesonide foam has a favorable safety profile for inducing remission in mild-to-moderate ulcerative proctitis or proctosigmoiditis. Dig Dis Sci. 2015;60:3408–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gross V, Bar-Meir S, Lavy A, et al. ; International Budesonide Foam Study Group . Budesonide foam versus budesonide enema in active ulcerative proctitis and proctosigmoiditis. Aliment Pharmacol Ther. 2006;23: 303–312. [DOI] [PubMed] [Google Scholar]
- 25. Brunner M, Vogelsang H, Greinwald R, et al. . Colonic spread and serum pharmacokinetics of budesonide foam in patients with mildly to moderately active ulcerative colitis. Aliment Pharmacol Ther. 2005;22:463–470. [DOI] [PubMed] [Google Scholar]
- 26. Asakura K, Nishiwaki Y, Inoue N, et al. . Prevalence of ulcerative colitis and Crohn’s disease in Japan. J Gastroenterol. 2009;44:659–665. [DOI] [PubMed] [Google Scholar]
- 27. Ng SC, Tang W, Ching JY, et al. ; Asia–Pacific Crohn’s and Colitis Epidemiologic Study (ACCESS) Study Group . Incidence and phenotype of inflammatory bowel disease based on results from the Asia-Pacific Crohn’s and colitis epidemiology study. Gastroenterology. 2013;145:158–165.e2. [DOI] [PubMed] [Google Scholar]
- 28. Ng SC, Kaplan GG, Tang W, et al. . Population density and risk of inflammatory bowel disease: a prospective population-based study in 13 countries or regions in Asia-Pacific. Am J Gastroenterol. 2019;114:107–115. [DOI] [PubMed] [Google Scholar]
- 29. Kim HJ, Hann HJ, Hong SN, et al. . Incidence and natural course of inflammatory bowel disease in Korea, 2006-2012: a nationwide population-based study. Inflamm Bowel Dis. 2015;21:623–630. [DOI] [PubMed] [Google Scholar]
- 30. Randell RL, Long MD, Cook SF, et al. . Validation of an internet-based cohort of inflammatory bowel disease (CCFA partners). Inflamm Bowel Dis. 2014;20:541–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kelstrup AM, Juillerat P, Korzenik J. The accuracy of self-reported medical history: a preliminary analysis of the promise of internet-based research in inflammatory bowel diseases. J Crohns Colitis. 2014;8:349–356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.