Abstract
Management and treatment of pressure ulcers (PUs) are met with great difficulty due to various factors that cause vulnerability of the soft tissue such as location, limited mobility, increased friction and shearing forces, as well as other comorbidities that may delay or halt wound healing. The topical autologous blood clot therapy (TABCT) is a point‐of‐care treatment used as a blood clot to assist in recreating and repairing the extracellular matrix (ECM). The mechanism of action consists of reconstruction of the ECM by incorporating into the ulcer, providing protection from further external destruction, while assisting in advancement through the wound healing phases via interaction of necessary growth factors, mediators, and chemokines. This study aims to assess the efficacy of the TABCT in the treatment of PUs in comparison to standard of care (SOC) treatment. Twenty‐four patients, 18 years or older, with PUs ranging from stage 1 to 4, were included in this study. TABCT was created by using the patient's own peripheral blood in a point of care setting. Efficacy in percent area reduction (PAR) on weeks 4 and 12 with TABCT over SOC was assessed. Treatment using TABCT in PUs resulted in 77.9% of the patients achieving a 50% PAR on week 4. The mean PAR on week 12 was 96.23% with 45% of the wounds treated with TABCT achieving complete wound closure. TABCT exhibited efficacy in PAR of PUs. In addition, TABCT use prompted granulation tissue formation over vital structures, such as bone, which is often present in later stage PUs. The potential of bringing an affordable, cost‐effective, advanced biologic bedside treatment that is efficacious in resolution of these complex wounds has the potential to drastically reduce the burden of treatment on the health system.
Keywords: autologous, blood clot, chronic, pressure ulcers, wound care
Abbreviations
- ACDA
acid citrate dextrose adenine
- ECM
extracellular matrix
- IQR
interquartile range
- ITT
intent to treat
- NPWT
negative pressure wound therapy
- PAR
percent area reduction
- PP
per protocol
- PU
pressure ulcer
1. INTRODUCTION
Pressure ulcers (PUs) continue to be a huge burden on the health care system, with a substantial financial concern affecting mostly patients in intensive care units and long‐term care settings. 1 PUs are mostly seen in locations (such as the sacrum, ischial tuberosity, greater trochanter, lateral malleolus, and heel) in which prolonged pressure and shearing forces contribute to obstruction of local vascularity. This obstruction prohibits vascular perfusion and lymphatic drainage resulting in aggregation of metabolic waste, proteins, and enzymes in infected tissues, causing further injury. 2 Tissue injury and ischemia often begin in the deep layers of soft tissue and extend superficially. Other external forces, such as poor skin status, decreased mobility, autonomic dysreflexia, and poor nutrition further contribute to impaired cellular viability. 3 The PU becomes stalled in a dysfunctional inflammatory healing phase resulting in chronicity and increased severity of the soft tissue deficit. As the nature of the ulcer worsens, deterioration of the extracellular matrix (ECM) occurs, leading the wound environment to a state incapable of providing adequate healing properties. Continued degradation results in further soft tissue damage and exposure of vital structures, such as bone, tendon, and neurovascular bundles. 4 In some complex cases, the standard of care approach is insufficient resulting in a need for more advanced treatments to manage the PU.
Currently, there's an unmet need for an advanced treatment that assists in regenerating the ECM while providing an environment that would be capable of harbouring necessary elements for adequate wound healing. Use of a topical dressing that provides a moist wound bed, pressure redistribution, reduction in drainage, and supportive care may provide success in increasing healing potential. 5 Topical autologous blood clot therapy (TABCT) creates a temporary scaffold that resembles the ECM, reestablishing communication between cells and components necessary for healing in the wound environment. It also creates a protective barrier which maintains a moist wound environment, can assist with autolytic debridement, prevent further bacterial ingress, and cover exposed vital structures. 6 A TACBT point‐of‐care treatment for cutaneous wounds has been found to be safe and effective in treating chronic wounds of different etiologies in wound care patients. 7 This study aimed to evaluate the efficacy and superiority of TABCT in treating PUs in a real‐world point‐of‐care setting.
2. MATERIALS AND METHODS
This was a multicenter, observational study in which patient data were collected from a registry study database from August 1, 2021, through April 15, 2021 (NCT04699305). The inclusion criteria were patients ≥18 years of age with confirmed PUs located anywhere in the body and available baseline and 4‐week wound measurements. Patients or their legally authorized representative gave written informed consent prior to the patient's participation in the study. TABCT was created based on the manufacturer's instructions. 8 In brief, 18 mL of blood was withdrawn from the patient into Acid Citrate Dextrose Adenine (ACDA) vacuum tubes. The blood was gently mixed with calcium coagulant and kaolin in a coagulation mould to create a blood clot. The formation of the clot took approximately 8 min. The clot was applied to the wound and attached by steri‐strips to the clot‐embedded gauze, to prevent any movement of the clot. The clot was covered with a non‐adherent layer and a foam. Re‐application was performed weekly based on the investigator's decision.
2.1. Statistical analysis
Demographic and patient history data, where available, were described with the mean and standard deviation for normally distributed continuous data and the median and interquartile range (IQR) for non‐normally distributed continuous data. Categorical data was described using frequencies and graphs. Assumptions of normality were evaluated using boxplots, histograms, and a Shapiro‐Wilks normality test. The primary outcome “percent area reduction” (PAR) among PU patients was skewed, towards healing.
2.2. Effectiveness
Wound healing was operationalized as a 40% reduction in PAR by 4 weeks of treatment. Achievement of 40% PAR at 4 weeks is a strong predictor of 12‐week wound healing as identified by numerous clinicians. 9 Wounds that have not attained a 40% reduction by 4 weeks are not expected to heal by 12 weeks, establishing this early marker as a reliable endpoint for research purposes. To quantify the extent to which TABCT is associated with wound healing, a Wilcoxon signed‐rank test was conducted, and effect size (r) was calculated. Assumptions of independence and symmetric distribution of differences were confirmed. To establish the 95% confidence interval of TABCT's effect, an Agresti‐Coull confidence interval was constructed. 10
2.3. Superiority
To determine whether TABCT is superior to the existing standard of care, the success rates of TABCT were compared with known values of healing identified in epidemiological studies using a one‐sided binomial probability test. TABCT was compared with the success rates identified in two observational studies. 11 , 12 In an evaluation of 270 PUs with a mean size of 5.14 cm2, Palese et al 11 identified a complete healing rate of 56.7% at 10 weeks. Pressure ulcerations less than 3.1 cm2 were noted to require substantially less healing time, healing in 19.2 days on average. Conversely, an observational study of NHS patients found that only 50% of all PUs were healed within 1 year of presentation. 12 Ultimate healing rates ranged from 21% for stage 4 to 100% for stage 1 PUs. Time to heal varied from 1 to 10 months, depending on the stage of the ulcer. Success of TABCT therapy was defined as achieving a (PAR) of 40% or more at the 4‐week visit. To comprehensively evaluate the potential for TABCT to produce healing rates which exceed the standard of care methods, successful TABCT therapy was compared with both the 50% and 56.7% healing rates observed in the above‐mentioned studies. 11 , 12
2.4. Clinical significance ‐ measures of effect
To quantify the size of the effect of TABCT, an odds ratio was calculated using the definition of success identified above. This provides the odds of patients being treated with TABCT achieving the benchmark for healing identified for this study, as compared with those who do not receive TABCT treatment.
3. RESULTS
Review of the registry study database identified 24 patients, 22 of which had sufficient data for inclusion. This population was 62.5% male and 83.33% Caucasian (Table 1). The mean wound size at baseline was 20.7 ± 30.3 cm2 (range 0.2 to 129). The mean wound duration was 13.4 ± 17.1 months (range 1 to 60). Eleven (50%) of the PUs were stage 2–4 (Stage 2 = 3, Stage 3 = 4, and Stage 4 = 4). The mean PAR at 4‐weeks was 77.9% ± 24.8% (range 22% to 100%). Eighty six percent (86.3%) of the patients exceeded 40% PAR at week 4. Two patients had an increase in wound size after multiple applications at week 4. All 22 patients achieved a PAR greater than 40% by week 12. The mean 12‐week PAR for all 22 patients was 96.2% ± 9.2%. Complete wound closure was achieved by 8 (36.4%) patients by week 4 and 10 patients (45.5%) by week 12.
TABLE 1.
Baseline demographics
| Characteristic | n | % |
|---|---|---|
| Sex | ||
| Male | 15 | 62.5% |
| Female | 9 | 37.5% |
| Race | ||
| White | 20 | 83.2% |
| NonWhite | 4 | 16.8% |
3.1. Effectiveness
The mean 4‐week PAR of 77.9% for PUs in this study was a statistically significant increase to the surrogate marker of healing of 40% PAR at 4‐weeks (z = 3.76, P ≤ 0.001, r = 0.89) The Wilson confidence interval for this effect ranges from .6078 to .9416 or 60.78% to 94.16%, indicating that TABCT is substantially associated with complete wound healing.
3.2. Superiority
When compared with Palese's 11 findings of 56.7%, the (86.3%) of the patients exceeded 40% PAR at week 4 was found to be superior (P ≤ 0.001). This was also the case when compared with the observational study of NHS patients, 12 reflecting a 12‐month healing rate of 50% (P ≤ 0.001). In both comparisons, TABCT was identified as the statistically significant superior treatment, achieving a greater proportion of 40% PAR at 4 weeks than the current standard of care models identified in this study and complete wound closure of 45.5% of the wounds by week 12. In addition, patients evaluated in the observational studies experienced smaller wounds and wounds of a shorter duration. The mean wound size for patients in this registry was 20.66 cm2 at baseline, which far exceeds the benchmarks identified as less likely to heal in epidemiological studies. 11 , 12
3.3. Case studies
3.3.1. Case study #1
A 41‐year‐old female with a past medical history of paraplegia, multiple sclerosis, sarcopenia, anemia, metabolic encephalopathy, and vitamin D deficiency developed multiple stage 4 PUs on the sacrum and bilateral ischium. The wounds were initially treated with negative pressure wound therapy (NPWT), Dakin's moist to dry dressings, and 4 months of hyperbaric oxygen therapy. Despite these modalities, the ulcers remained open for 4 years. At the time of the first TABCT application, the ulcerations measured: sacrum = 42 cm2, left ischium = 6 cm2, and right ischium = 8.45 cm2. The ulcerations consisted of a fibrogranular wound bed with macerated wound edges and undermining noted. TABCT was applied six times over the course of 2 months. During that time the patient used a low air loss mattress and offloaded the wounds entirely. Following TABCT applications, tissue granulation was observed with a significant reduction in wound size. At the end of the 2‐month treatment period with TABCT, the sacral wound measured 3 cm2 (92.9% PAR), the left ischium wound measured 0.04 cm2 (99.3% PAR), and complete wound closure was achieved for the right ischial wound. (Figure 1).
FIGURE 1.
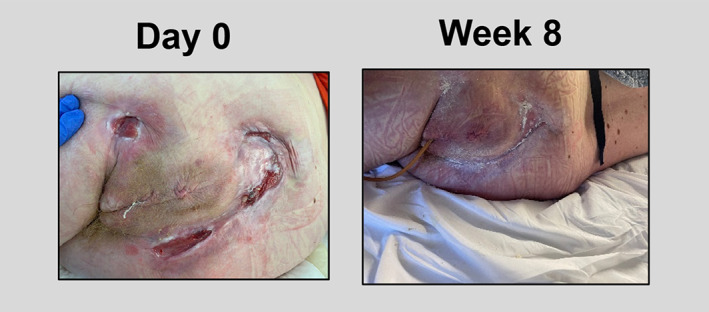
At day 0 ulcers consisted of a fibrogranular wound bed with macerated wound edges and undermining. Ulcers were measured as: sacrum = 42 cm2, left ischium = 6 cm2, and right ischium = 8.45 cm2. At the end of week 8 using TABCT, the wound bed exhibited an increase tissue granulation. The ulcers measured: sacrum = 3 cm2, left ischium = 0.04 cm2, and right ischium = complete wound closure.
3.3.2. Case study #2
A 64‐year‐old male with a past medical history of septic shock, two intraspinal abscesses, hypertension, hyperlipidemia, hypothyroidism, and iron and protein anemia deficiencies was treated for PUs on the ischium bilateral, which had been present for 1 month. The ulceration on the right appeared significantly worse than the left. The ulceration on the left consisted of a fibronecrotic wound base measuring approximately 23 cm2 that was partially healed, while the right ulceration had eschar encompassing the entire wound bed and measured approximately 25 cm2. Previous treatment of the wound was consistent with standard of care, which was unsuccessful in promoting healing. Following TABCT application, the left PU achieved complete wound closure in 1 week. Additional weekly applications over a course of 9 weeks were performed on the right side, resulting in wound closure. (Figure 2).
FIGURE 2.
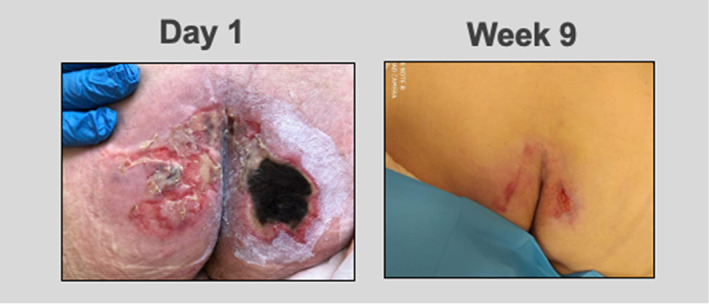
At day 1 two pressure ulcers noted to the ischium, right appeared worse than the left due to presence of an eschar encompassing the entire wound bed. The right measured 25 cm2 and the left 23 cm2. At the end of week 1 using TABCT, the left PU achieved complete wound closure. At the end of week 9 using TABCT, the right PU achieved complete wound closure.
3.3.3. Case study #3
A 36‐year‐old, paraplegic male developed a stage 3 pressure ulceration to the lumbosacral area. Offloading and NPWT use were unsuccessful. Initial wound measurements were 12.5 × 12.6 × 3.8 cm with exposed bone noted. The central aspect of the wound bed consisted of a fibronecrotic base with a fibrogranular periphery and mild undermining noted. TABCT was applied five times over the course of 9 weeks. With each application was a reduction of fibrotic and necrotic tissue, and an increase in granulation tissue was evident. After the fifth application, the area of ulceration measured approximately 7 × 6.5 × 1.1 cm (71.1% PAR) with an overall reduction in‐depth of 72%. Minimal fibrotic tissue was noted to the central most aspect of the wound bed consistent with a significant reduction in overall the fibronecrotic presence of the wound bed. (Figure 3).
FIGURE 3.
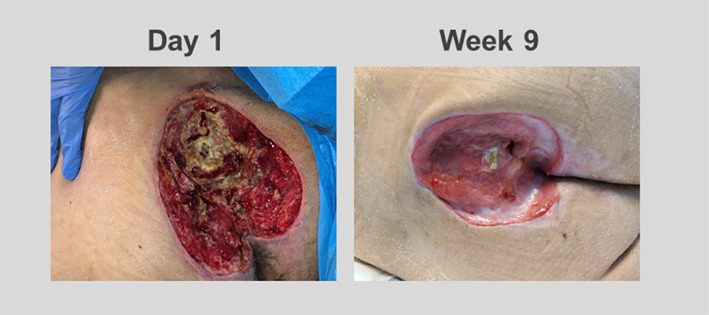
At day 1, an ulcer was noted to the lumbosacral area with a fibronecrotic wound bed and fibrogranular periphery measured 12.5 × 12.6 × 3.8 cm with exposed bone. After 5 applications of TABCT over the course of 9 weeks, the ulcer decreased in size to 7 × 6.5 × 1.1 cm. There was an increase in tissue granulation present at the wound bed.
4. DISCUSSION
TABCT was found to be effective and superior to standard of care in the treatment of PUs. A mean PAR of 77.9% and 96.2% on weeks 4 and 12, respectively, were achieved with TABCT use. In addition, a complete wound healing rate of 45.9% was achieved at 12‐weeks. The 4‐ and 12‐week mean PAR was statistically significantly increased compared with the healing rates reported in two prior epidemiological studies of PU management. 11 , 12 Although the 12‐week healing rate was smaller than that previously reported, the PUs included in this study were larger and of longer duration, wound characteristics known to be associated with delayed or less chance of complete healing. 11 , 12 , 13 , 14 Maintenance of a moist wound environment, removal of nonviable tissue, and protection from the outside environment and pathogens are key components of wound healing. 15 A functional ECM is also essential for dynamic reciprocity and wound resolution. 16 Use of TABCT provides all these essential aspects of wound healing at the point‐of‐care with minimal risk to the patient.
Point‐of‐care TABCT promotes wound closure by providing a temporary ECM scaffold, delivering and recruiting components necessary for healing to occur, assisting in autolytic debridement, protecting the wound from pathogen entry, and providing a moist wound environment. 17 TABCT has been proposed to contain and attract many growth factors, that is, platelet‐derived growth factor (PDGF), fibroblast growth factor (FGF), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), insulin‐like growth factor (IGF), and transforming growth factor (TGF), necessary for the progression of the wound towards healing. 6 The importance of those growth factors in wound healing has been extensively described. 18 , 19 Growth factors in the wound area act as mitogens, stimulating the proliferation of cells, 20 attract inflammatory cells and fibroblasts into the wound area, can stimulate angiogenesis, and showed to have a profound effect on the production and degradation of the ECM. 19 After an injury, their levels in the wound area is upregulated which suggests the need for high levels to promote the wound towards healing. 18
TABCT also provides a temporary ECM scaffold that functions as a biological delivery system, controlling the interaction and release of growth factors and cytokines within the wound overtime, helping to establish functional dynamic reciprocity within the wound. 6 , 16 This plays a major role in creation of a stronger and more permanent scaffold. In addition, TABCT treatment is suggested to mediate macrophage transition from the M1 inflammatory to the M2 anti‐inflammatory phenotype. 21 The ability of the wound to progress into the proliferative phase depends on this transition, which is dysfunctional in chronic wounds contributing to delayed healing. 6 These effects emphasise the major advantage of TABCT in progression of stagnant wounds from the inflammatory phase to the proliferative phase of wound healing. 17 TABCT also alters the secretion of extracellular proteases in the wound, preventing further distraction of the ECM. 22 , 23 Delivery of components within whole blood and the physical barrier provided by TABCT also assist in autolytic debridement, maintenance of a moist wound environment, and protection of the wound from pathogen entry and desiccation of exposed structures, further assisting in progression towards wound resolution. 17
TABCT has been shown to be safe and effective in the treatment of other complex wounds, including those with exposed bone and tendon, arterial compromise and exposed tendon and bone. 7 , 24 , 25 In an observational pilot study of 29 patients, 10% of which had PUs and almost half of which had wounds present for greater than 1‐year duration reported a 4‐week PAR of 65% and a 12‐week PAR of 94%. 19 A second pilot study of TABCT use in the treatment of complex wounds, including five Stage III PUs, reported complete wound healing in 78% wounds with an average of 4 (range: 1–7) TABCT applications. 7 Only two patients failed to heal. However, these patients achieved a 77% and 82% reduction in wound sizes, respectively. A case series of TABCT use in complex wounds with exposed bone and tendon in patients with various factors resulting in delayed healing, including end stage renal disease, peripheral vascular disease, rheumatoid arthritis, and osteomyelitis, reported results ranging from 80% reduction in wound size to complete resolution between 8 and 23 weeks with one to 11 applications of TABCT. 25 Similar healing results were observed in the current study, specific to the use of TABCT for treatment of PUs.
5. LIMITATIONS
Limitations of the current study is use of registry data with lack of a control or comparison group. However, a statistically significant difference was noted in the current study when compared with two prior epidemiological studies in wound types of the same aetiology that were not as severe. 11 , 12 Use of registry data also allows for inclusion of patients who often do not meet inclusion criteria in other clinical trials because of the presence of multiple comorbidities and wounds of larger size and greater duration. Thus, registry studies are more likely to produce estimates of effectiveness that are lower than those observed in controlled trials and more generalizable to patients with chronic wounds routinely encountered in clinical practice. The statistical significance of the results in this study, in patients with multiple comorbidities with large wounds of long duration, would likely have a larger effect in the efficacy of TABCT compared with standard of care in a controlled trial with typically cited inclusion and exclusion criteria.
6. CONCLUSION
TABCT, a point‐of‐care treatment, creates an environment autologously that enhances the healing of complex and difficult to heal PUs in an efficient and timely manner. With consistent application of the autologous blood clot to the injured area, reconstruction of the ECM occurs, aiding in organized recruitment of growth factors, mediators, and cytokines necessary to advance the wound out of its chronic nature and towards the state of healing and complete wound closure. TABCT superiority over a standard of care treatment positions it as a promising treatment in PU and complex cases.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Landau Z, Whitacre KL, Leewood C, Hawkins J, Wachuku CD. Utilization of a topical autologous blood clot for treatment of pressure ulcers. Int Wound J. 2023;20(3):806‐812. doi: 10.1111/iwj.13927
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Mervis JS, Phillips TJ. Pressure ulcers: pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. 2019;81(4):881‐890. [DOI] [PubMed] [Google Scholar]
- 2. Aburayan WS, Booq RY, BinSaleh NS, et al. The delivery of the novel drug ‘Halicin’ using electrospun fibers for the treatment of pressure ulcer against pathogenic bacteria. Pharmaceutics. 2020;12(12):1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Niemiec SM, Louiselle AE, Liechty KW, Zgheib C. Role of micrornas in pressure ulcer immune response, pathogenesis, and treatment. Int J Mol Sci. 2020;22(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. www.worldwidewounds.com/2005/august/Schultz/Extrace-Matric-Acute-Chronic-Wounds.html
- 5. Brown‐Etris M, Milne CT, Hodde JP. An extracellular matrix graft (oasis® wound matrix) for treating full‐thickness pressure ulcers: a randomized clinical trial. J Tissue Viability. 2019;28(1):21‐26. [DOI] [PubMed] [Google Scholar]
- 6. Snyder RJ, Schultz G, Wachuku C, Rashid AM, Ead JKK. Proposed mechanism of action of topically applied autologous blood clot tissue: a quintessential cellular and tissue based therapy. J Am Podiatr Med Assoc. 2020;13:20‐140. [DOI] [PubMed] [Google Scholar]
- 7. Kushnir I, Kushnir A, Serena TE, Garfinkel D. Efficacy and safety of a novel autologous wound matrix in the management of complicated, chronic wounds: a pilot study. Wounds. 2016;28(9):317‐327. [PubMed] [Google Scholar]
- 8. ActiGraft Instruction for Use LBL‐IFU‐0002 Rev.05.
- 9. Edsberg LE, Wyffels JT, Ha DS. Longitudinal study of stage III and stage IV pressure ulcer area and perimeter as healing parameters to predict wound closure. Ostomy Wound Manage. 2011;57(10):50‐62. [Google Scholar]
- 10. Agresti A, Coull BA. Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat. 1998;52(2):119‐126. [Google Scholar]
- 11. Palese A, Saiani L, Pota I, Laquintana D, Stinco G, Di Giulio P. What is the healing time of stage II pressure ulcers? Findings from a secondary analysis. Adv Skin Wound Care. 2015;28(2):69‐75. [DOI] [PubMed] [Google Scholar]
- 12. Guest JF, Fuller GW, Vowden P, Vowden KR. Cohort study evaluating pressure ulcer management in clinical practice in the UKfollowing initial presentation in the community: costs and outcomes. BMJ Open. 2018;8(7):e021769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bosanquet DC, Harding KG. Wound duration and healing rates: cause or effect? Wound Repair Regen. 2014;22(2):143‐150. [DOI] [PubMed] [Google Scholar]
- 14. Argolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers: predicting which ones will not heal. Am J Med. 2003;115:627‐631. [DOI] [PubMed] [Google Scholar]
- 15. Snyder RJ, Fife C, Moore Z. Components and quality measures of DIME (devitalized tissue, infection/inflammation, moisture balance, and edge preparation) in wound care. Adv Skin Wound Care. 2016;29(5):205‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011;19(2):134‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Snyder RJ, Driver V, Cole W, et al. Topical Autologous Blood Clot Therapy: an Introduction and Development of Consensus Panel to Guide Use in the Treatment of Complex Wound Types. WOUNDS. Accepted April 2022. [PubMed]
- 18. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835‐870. [DOI] [PubMed] [Google Scholar]
- 19. Greenhalgh DG. MD the role of growth factors in wound healing. J Trauma Injury Infect Crit Care. 1996;41(1):159‐167. [DOI] [PubMed] [Google Scholar]
- 20. Sporn MB, Roberts AB. Peptide growth factors and inflammation, tissue repair, and cancer. J Clin Invest. 1986;78(2):329‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Snyder RJ, Lantis J, Kirsner RS, Shah V, Molyneaux M, Carter MJ. Macrophages: a review of their role in wound healing and their therapeutic use. Wound Repair Regen. 2016;24(4):613‐629. [DOI] [PubMed] [Google Scholar]
- 22. Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17(2):153‐162. [DOI] [PubMed] [Google Scholar]
- 23. Maquart FX, Monboisse JC. Extracellular matrix and wound healing. Pathol Biol. 2014;62(2):91‐95. [DOI] [PubMed] [Google Scholar]
- 24. Naude L, Idensohn P, Bruwer F, et al. An observational pilot study to collect safety and efficacy data on wound care using whole blood clot technology on hard‐to‐heal wounds. Wound Int. 2021;12(2):42‐53. [Google Scholar]
- 25. Gurevich M, Wahab N, Wachuku C, Ead KJ, Snyder RJ. ActiGraft treatment in complex wounds with exposed structure – a case series. Ann Rev Res. 2021;7(1):555701. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


